Abstract
Greater trochanteric pain syndrome (GTPS) is a pathology that can involve the trochanteric bursa or the tendons which attach to the greater trochanter. To clarify the potential importance of bursa versus tendon pathology and of substance P (SP) in contributing to pain in this condition tendon and bursa tissue biopsies were obtained from 34 patients with GTPS and 29 control subjects. Specimens were evaluated via light microscopy for histopathological and morphological differences, as well as using immunohistochemistry for macrophages (CD68), cells (CD45) and SP. Bursa [stroma score, mean (SD): 4.18 (1.65) vs. 2.53 (1.61), p = 0.051] and tendon [Bonar score, mean (SD): GTPS mean (SD) 12.65 (2.0), control (10.43 (4.84), p = 0.04] from subjects with GTPS demonstrated more extensive signs of pathology than specimens from control subjects. There was a significantly greater presence of SP in the bursa (frequency: 9/12 vs. 6/16, p = 0.047), but not in the tendon (8/12 vs. 8/15, p = 0.484) of subjects with GTPS compared to controls. An increased presence of SP in the trochanteric bursa may be related to the pain associated with GTPS.
Background
Greater trochanteric pain syndrome (GTPS) can cause debilitating pain on the lateral side of the hip, thigh and leg.[1] The incidence of primary GTPS is reported as up to 5.6 per 1000 patients per year. GTPS is present in approximately 20–35% of people with low back pain.[2, 3] High levels of disability have been reported in people with this condition [4, 5].
Published microscopic or molecular assessments of tendon and bursae from patients with GTPS are limited. Previous studies have been limited by the lack of a comparison group [6–9]. There remains ongoing speculation about the source of symptoms (tendon vs. bursa) in patients with GTPS [6–9]. Substance P (SP) has been implicated in the pain and pathology of tendinopathy and bursitis. Gotoh et al. [10] reported a correlation of pain levels with the presence of SP in the bursa of patients with rotator cuff tendinopathy. Schubert et al. [11] demonstrated an increased presence of SP-containing nerves in Achilles tendinosis pathology compared with controls (spontaneously ruptured Achilles without prior symptoms). The presence of SP within tendon is associated with failed healing and pain in a collagenase model of tendon injury [12].
In bursa, four types of synovium have been described: areolar, fibrous, adipose and vascular [13–16]. Subsynovial tissue and/or vascular synovium of bursae may contribute to nociception in a manner analogous to the synovial lining of the tibofemoral joint [17]; this nociceptive role could explain why bursectomy is sometimes helpful in refractory GTPS [18]. Furthermore, research examining the vascular and adipose subsynovial tissue may increase our understanding of the relationship between bursa and closely adjacent tendon pathology.
The aim of this study was to evaluate the pathology of the trochanteric bursae and gluteal tendons from patients with torn tendons associated with GTPS, in comparison with a control group.
Materials and methods
This study was conducted in two phases (retrospective, and prospective).
Participants
All participants were recruited through the Canberra Orthopaedic Group and the Canberra Hospital. Surgery was performed by three orthopaedic surgeons (PS, AB, DS) at Canberra Hospital or the Calvary John James Hospital.
Phase 1: Retrospective
Participants with refractory GTPS undergoing gluteal tendon reconstructive surgery and bursectomy were recruited between 2004 and 2007. Participants were eligible for gluteal tendon reconstruction if they had severe refractory lateral thigh pain that did not respond to conservative treatment and had evidence of gluteal tendon tears on imaging. Participants in the GTPS group had no clinical or radiological evidence of osteoarthritis of the hip. These participants had tissue collected at the time of surgery for standard pathological screening by a registered pathologist. Details regarding this group’s surgery and outcomes have been previously reported [7]. Fourteen control participants with a primary diagnosis of osteoarthritis of the hip undergoing a total hip arthroplasty were recruited. The control group underwent total hip arthroplasty for primary hip osteoarthritis between 2008 and 2009. Inclusion criteria for the control group were clinical and radiological evidence of hip osteoarthritis [19] and being scheduled for hip arthroplasty surgery. Exclusion criteria for both groups included systemic inflammatory disease (such as rheumatoid arthritis) or bone pathologies (such as Paget’s disease or metastatic disease). Patients undergoing combined hip arthroplasty and gluteal tendon reconstruction were excluded. GTPS and control participants were matched for sex (female) and age. Bursa and tendon tissue specimens were collected from both groups of participants.
Phase 2: Prospective
A second group of GTPS and control group participants was recruited between July 2007 and August 2009. Inclusion criteria were the same as previously, but with additional exclusion criteria for both groups: lumbar spine nerve root signs, or a history of surgery of the lumbar spine or the ipsilateral leg. In this phase, an additional exclusion criterion for controls was any lateral hip pain or tenderness to palpation of the greater trochanter. As in phase 1, GTPS and control participants were matched for sex (female) and age, and both bursa and tendon tissue specimens were collected from each participant. Approval for this study was provided by the ACT Health, the Australian National University and Calvary Health Care human research ethics committees (HREC). Following an explanation of the tissue collection procedure, and the risks and benefits of the research, the participants provided signed informed consent. As an exception to this procedure, in phase 1, the GTPS participants’ tissue had already been collected at the time of surgery and the HREC provided a waiver for the gaining of signed consent. Prior to analysis at the University of British Columbia, the secondary approval of the local clinical research ethics board was obtained.
Tissue sampling and processing
A direct lateral incision with longitudinal splitting of the fascia was used for the GTPS participants. The bursa was collected during the approach to the greater trochanter during gluteal tendon reconstruction and during the approach to the hip in the control groups’ surgery. The tendon specimen was collected prior to the tendon reconstruction in the GTPS groups and upon completion of the arthroplasty procedure in the control groups. All tendon specimens were approximately 0.5 cm × 1.5 cm × 0.5 cm and 0.2 cm × 0.5 cm × 0.2 cm in size, respectively. In all cases, a mid-tendon sample was used for the evaluation, avoiding the enthesis. Specimens were fixed in 10 % buffered formalin, processed and embedded into paraffin using routine laboratory methods. The 4- or 5-μm-thick sections were cut and mounted on slides.
Assessment of tissue sections
Bursa sections were stained with haematoxylin and eosin (H&E), Alcian blue and with inflammatory cell markers (phase 1: CD68+ macrophages; phase 2: CD45+ leucocytes). Tendon sections were stained with H&E and Alcian blue. All slides were coded to blind the assessors to the groups. Slides were examined independently by two assessors (AF, JT) using light microscopy. Scoring discrepancy was resolved by a third assessor (JD). A set proforma was used for the assessment of the bursa and tendon with reference slides available for calibration. For phase 2 of this study, immunohistochemistry for SP was conducted on all bursa and tendon tissue sections using rat spinal cord as positive control tissue (mouse monoclonal antibody, 70 min incubation, 1/5,000 dilution, Abcam). All slides were coded to blind the assessors to the groups. Slides were examined independently by two assessors (AF, AS) using light microscopy. In the case of a scoring discrepancy, the slide was reexamined and a consensus was reached. SP was assessed dichotomously (present or absent) in the bursa and tendon.
Scoring of bursa pathology
The bursa stroma was examined and evaluated using a semi-quantitative scoring system (Supplementary Table S1) and coded dichotomously for inflammatory cell aggregates (lymphocytes, plasma cells, histiocytes, neutrophils) [20], calcium deposits, chondroid metaplasia, mixed degeneration (fibrinoid, myxoid, and hyaline) [21], increased mucins [22], and inter-adipose septa. Using this scoring method, a stroma with no pathology or degenerative changes would score zero, while stroma with evidence of all of these characteristics would score six. Within the stroma, the widest inter-adipose septa within the specimen were identified by consensus between two of the researchers and a digital micrograph obtained. The width was measured using calibrated digital imaging software (Veritas, Arcturus). The presence or absence of synovium was recorded. Specimens with synovium were coded dichotomously for the presence or absence of synovial hyperplasia [20], vascular synovium [15, 16] and adipose synovium [23]. Using this scoring method, synovium with no pathology or degenerative changes would score zero, while those with evidence of pathology or degenerative changes would score three. The frequency of the four types of synovium (areolar, fibrous, adipose and vascular) was noted.
In the area of the synovium with the most macrophagelike synoviocytes, the CD68+ stained macrophage-like synoviocytes and fibroblast-like synoviocytes were counted and the ratio of one to the other calculated. Cell counts were performed by one researcher (AF) using Image J (http://rsbweb.nih.gov/ij/).
Representative samples from two different participants with adipose, areolar, fibrous and vascular synovium, and the inter-adipose septum were selected for illustrative purposes (Fig. 4). These were examined using second harmonic generation (SHG) microscopy (which highlights collagen-rich content components) and multi-photon emission fluorescence [24].
Fig. 4.
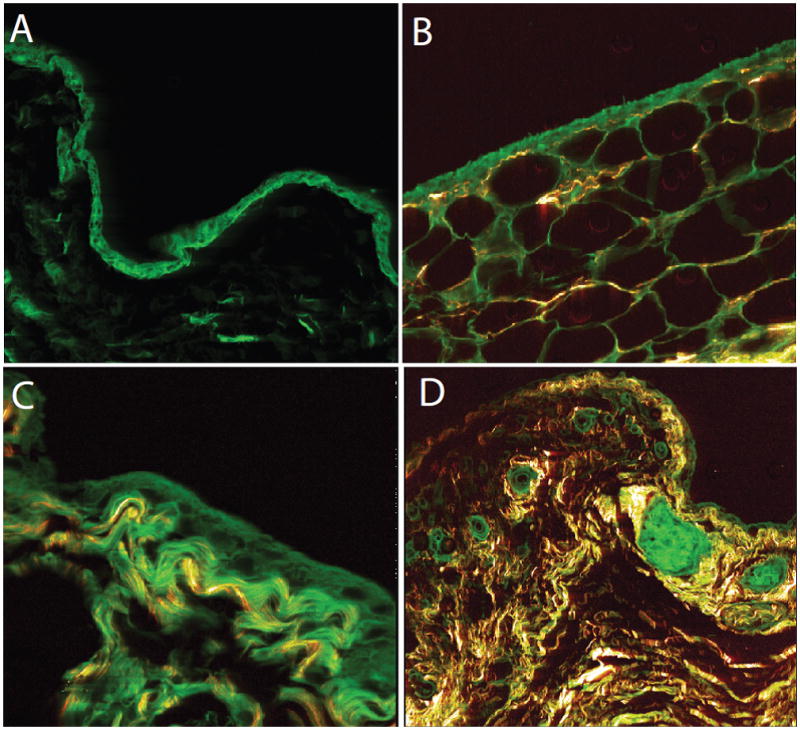
Synovial tissue in bursa: areolar (a), adipose (b), fibrous (c) and vascular (d) synovium. (a–c SHG x630, d SHG x200)
Assessment of tendon pathology
Tendon sections were assessed using a modified Bonar scale [25]. Each section was assessed in three areas: greatest collagen disruption, highest vascularity, and most chondroid changes. The median of these three scores was used. Additional points were allocated for calcification and for intra-tendinous adipocytes. Using this scoring method, a tendon with no pathology would score 0 and a tendon with maximum pathology would score 20.
Statistical methods
An intra-class correlation co-efficient (ICC) was calculated for intra-rater reliability for cell counts and measures of inter-adipose septum. Descriptive statistics (mean, SD and range) were used to summarise demographic data. Tendon and bursa scoring scales were assessed by group, using mean and standard errors. The Fisher exact test was used to determine differences for categorical data. For normally distributed numeric data, a student T test was used. For non-normal numeric data a two sample Wilcoxan rank sum was used.
Results
The final number of GTPS subjects for which tendon and bursa samples were available for analysis was 34. The number of control subjects was 29 (Fig. 1).
Fig. 1.
Enrolment of subjects in patient (GTPS) and control (OA) groups. GTPS greater trochanteric pain syndrome, OA osteoarthritis of the hip. Please see text for full description of inclusion and exclusion criteria
Bursa pathology
The total stroma score was higher in the GTPS group than in the control group, indicating the increased presence of bursa pathology with GTPS [T test, mean (SD) 4.18 (1.65) vs. 2.53 (1.61), p = 0.051]. Furthermore, a distinctive interadipose septa (Fig. 1) were more frequently seen in the bursa tissue from the GTPS group, 27/31, compared with controls, 15/27 (Fisher exact: p = 0.008).
Inter-adipose septum
Measurement of the thickness of the inter-adipose septum, via a laser capture microscope, showed no difference between the two test measurements, ICC (95 % CI) = 1.0 (1.00–1.00). Of the specimens that demonstrated inter-adipose septa, there was no group difference in the size of the inter-adipose septa.
The SHG imaging of the inter-adipose septa demonstrated that the inter-adipose septa are a well-defined structure, comprising primarily dense bundles of well-organised collagen (Fig. 2). The merged SHG and multiphoton emission fluorescence images illustrated that many of the collagenous and non-collagenous fibres within the septa course approximately parallel to each other.
Fig. 2.
Inter-adipose septum (IAS). a Multiphoton excitation fluorescences (MPEF) demonstrating highly organised, dense structure typical of IAS in the examined gluteal bursa tissue. b SHG signal captured from the same area as (a) demonstrates that the bulk of the IAS is composed of fibrillar collagen (red and yellow). c Merged MPEF/SHG image. Note the distinct presence of longitudinally oriented, non-collagenous fibrillar structures in the IAS. d The collagenrich fibrous band in the middle of the picture is the inter-adipose septum, and the latticework of collagen fibres can also be seen in and amongst the adipocytes, H&E staining, ×100 original magnification (a–c scale bar 25.4 μm. d scale bar 100 μm)
Inflammatory cell counts
The reliability of cell counting was tested using myofibroblasts and demonstrated excellent reliability, ICC (95 % CI) 0.910 (0.852–0.967).
No inflammatory cell aggregates or significant calcium deposits were seen in the bursa stroma in either population; the two groups had similar frequencies of mucins. There was no group difference for the ratio of CD68 positive (macrophage-like) to CD68 negative (fibrocyte-like) synoviocytes (Fig. 3a–d).
Fig. 3.
CD68 localisation. CD68 was prominent in the synovial layer of the gluteal bursa. a, b Some areas of synovium demonstrated little or no positive CD68 localisation, while other areas (c, d) showed intense immunoreaction (brown)
Presence and type of synovial lining
All four types of synovial lining were identified in the GTPS group (Fig. 4a–d); however, adipose synovium was absent in control bursa tissue. There was no difference in the frequency of areolar, fibrous or vascular synovium between groups. In the specimens with synovium, there was no group difference for the total synovial tissue change score (T test: p = 0.125).
Substance P
There was a significantly greater presence of SP in the bursa (9/12 vs. 6/16, Fisher’s exact test p = 0.047), but not in the tendon (8/11 vs. 9/16, p = 0.223) of subjects with GTPS compared with controls. Substance P was expressed in fibroblast-like cells embedded within the bursa stroma or within the tendon, as well as in close association with vessels both in bursa and in tendon.
Tendon pathology
Both groups displayed similar features of gluteus medius tendon pathology which were consistent with previous descriptions of tendinosis at other anatomical locations [18, 22]. These features included tenocyte rounding, areas of increased vascularity, collagen disruption and both hyperand hypo-cellularity (Fig. 5a–d). Degenerative tendon changes of a similar nature were observed in both GTPS and control specimens, although the GTPS group had a higher average Bonar score (GTPS mean (SD) 12.65 (2.0), control (10.43 (4.84): T test: p = 0.04). Synovial tissue attached to the tendon was noted in 16/27 of the GTPS group and 7/26 of the control group (Fisher exact: p = 0.017). In both groups, there was very little positive staining for inflammatory cells in the tendon or bursa (CD45).
Fig. 5.
Representative tendon from GTPS (a, b) and control (c, d) participants demonstrating the presence of tendinosis in both populations. a Hypercellularity, with grade 1–2 chondroid changes aligned along well-demarcated collagen bundles. b Hypocellularity, tenocytes with grade 2 and 3 chondroid changes. There is marked separation of collagen bundles, areas of complete loss of tendon architecture, and areas of altered extra cellular matrix (blue). c Tenocytes in runs, tenocytes with grade 0–1 chondroid changes aligned along well-demarcated collagen bundles. d Hypocellularity, tenocytes with grade 3 chondroid changes, poorly demarcated collagen bundles and areas of altered extra cellular matrix (blue). (H&E stain, ×200 original magnification)
Discussion
This paper reports that whereas both groups (GTPS and control) demonstrated tendon pathology, people with GTPS demonstrated higher levels of bursa pathology and an increased presence of SP in the bursa.
This study evaluated the histopathology of bursae and tendons in patients with GTPS who underwent gluteal tendon reconstruction, and control subjects who underwent a hip arthroplasty. We found an increased level of bursa pathology and an increased presence of SP in the bursa of patients with GTPS. This indicates that the bursa is a likely source of pain in GTPS and that treatment strategies directed at the bursa may be appropriate for this condition.
This study also reports the presence, and describes the morphology, of inter-adipose septum in the stroma of the trochanteric bursa. No previous study has been identified that describes and evaluates inter-adipose septa in human bursa stroma. Previous synovial tissue investigations have generally studied the fibro-fatty structure that supports the synovial lining, the bursa stroma; studies of subacromial bursae report increased biochemical substances in this tissue layer (cytokines and SP) [10, 21, 26] as well as increased neovascularisation [27], but more detailed studies on the bursa anatomy are lacking. One study in rats described inter-adipose septa in the fat pad of the retrocalcaneal bursa [28]. In our study, the inter-adipose septa within the bursa stroma were seen more frequently in the GTPS than the control groups, and had a tendency to be wider in the GTPS population of the first cohort. The SHG microscopy provides evidence that this structure is a wellorganised feature. Previous studies have not commented upon this well-defined feature of tissue organization. The inter-adipose septa within the trochanteric bursa stroma might act to protect the tendon from increased compressive or shear forces. This function could be similar to that of the septa in the heel pad, which assists in cushioning the heel [29, 30]. The highly organised nature of the inter-adipose septa suggests a possible adaptive response to a mechanical stimulus, rather than merely disorganised fibrous scarring.
This study found that people undergoing hip arthroplasty have pre-existing microscopic tendon and bursa pathology of a similar nature to those undergoing gluteal tendon reconstruction, although to a lesser degree. Hip arthroplasty surgery is an extremely effective operation for OA of the hip [31, 32]; however, GTPS following hip arthroplasty is not uncommon, leading to a less than optimal outcome. The preoperative incidence of GTPS in people undergoing hip arthroplasty has not, to our knowledge, been reported. In future, pre-operative screening for GTPS and/or imaging changes indicative of tendon or bursal pathology may assist in reducing the rate of postoperative GTPS. This could be achieved by screening for GTPS and performing a bursectomy and repairing tendons during hip arthroplasty if necessary.
The sample size was based on previous histology studies [22]. However, we acknowledge that this study may lack statistical power for some aspects of the histological analysis. The sample size for the SP immunohistochemistry, although sufficient to allow us to detect a statistically significant difference in the presence or absence of SP between the GTPS and control group, was relatively small, and it is thus not clear how representative this finding may be of the larger population of people with or without GTPS. A further limitation was that in phase 1, the control group was not explicitly screened for GTPS as this was not part of any standard clinical screening that we were aware of at the time. Our histological findings from this study provided some impetus for screening for GTPS in people undergoing hip arthroplasty; this was then carried out in phase 2, allowing the recruitment of a group of reference subjects who were known to be clinically free of GTPS.
Conclusions
These results confirm that in people with GTPS, the bursae demonstrated more extensive pathology and greater distribution of SP compared with controls and are thus of potential importance in the generation of pain.
Supplementary Material
Table 1.
Summary of Participant Details and Tissue Availability.
| GTPS1 n = 24 |
REF1 n = 14 |
T-Test p value |
GTPS2 n = 12 |
REF2 n = 20 |
T-test p value |
|
|---|---|---|---|---|---|---|
| Age | 57.0 (8.6) | 57.1 (13.8) | 0.978 | 54.5 (3.31) | 60.75 (3.16) | 0.208 |
| Number (%) of bursa specimens | 20/24 (83.3) | 14/14 (100) | 11/12 (91.7) | 16/20 (80.0) | ||
| Number (%) of tendon specimens | 18/24 (75) | 14/14 (100) | 11/12 (91.7) | 17/20 (85.0) |
GTPS: Greater trochanteric pain syndrome; REF; Reference group.
1 = First cohort, 2 = Second cohort.
Acknowledgments
This study was funded through the Australian National University as well as through a WorksafeBC Research at Work grant. AF was a recipient of an ANU PhD scholarship. AS is a recipient of a career investigator award from the Michael Smith Foundation for Health Research. JC was supported by the Australian Centre for Research into Sports Injury and its Prevention, which is one of the International Research Centres for Prevention of Injury and Protection of Athlete Health supported by the International Olympic Committee. We gratefully acknowledge the expert assistance of Dr T Abraham with second harmonic generation and multiphoton excitation fluorescence microscopy, Jennie Scarvell for assistance with supervising the study, and Al Burns and Damian Smith for tissue collection.
Footnotes
The authors declare no conflict of interest.
References
- 1.Williams BS, Cohen SP. Greater trochanteric pain syndrome: a review of anatomy, diagnosis and treatment. Anesth Analg. 2009;108:1662–1670. doi: 10.1213/ane.0b013e31819d6562. [DOI] [PubMed] [Google Scholar]
- 2.Rompe JD, Segal NA, Cacchio A, et al. Home training, local corticosteroid injection, or radial shock wave therapy for greater trochanter pain syndrome. Am J Sports Med. 2009;37:1981–1990. doi: 10.1177/0363546509334374. [DOI] [PubMed] [Google Scholar]
- 3.Swezey RL. Pseudo-radiculopathy in subacute trochanteric bursitis of the subgluteus maximus bursa. Arch Phys Med Rehabil. 1976;57:387–390. [PubMed] [Google Scholar]
- 4.Furia JP, Rompe JD, Maffulli N. Low-energy extracorporeal shock wave therapy as a treatment for greater trochanteric pain syndrome. Am J Sports Med. 2009;37:1806–1813. doi: 10.1177/0363546509333014. [DOI] [PubMed] [Google Scholar]
- 5.Fearon AM, Cook JL, Scarvell JM, et al. Greater trochanteric pain syndrome negatively affects work, physical activity and quality of life: a case control study. J Arthroplast. 2014;29(2):383–386. doi: 10.1016/j.arth.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Connell DA, Bass C, Sykes CA, et al. Sonographic evaluation of gluteus medius and minimus tendinopathy. Eur Radiol. 2003;13:1339–1347. doi: 10.1007/s00330-002-1740-4. [DOI] [PubMed] [Google Scholar]
- 7.Fearon AM, Scarvell JM, Cook JL, et al. Does ultrasound correlate with surgical or histologic findings in greater trochanteric pain syndrome? A pilot study. Clin Orthop Relat Res. 2010;468:1838–1844. doi: 10.1007/s11999-009-1174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva F, Adams T, Feinstein J, et al. Trochanteric bursitis: refuting the myth of inflammation. J Clin Rheumatol. 2008;14:82–86. doi: 10.1097/RHU.0b013e31816b4471. [DOI] [PubMed] [Google Scholar]
- 9.Walsh MJ, Walton JR, Walsh NA. Surgical repair of the gluteal tendons: a report of 72 cases. J Arthroplast. 2011;26:1514–1519. doi: 10.1016/j.arth.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Gotoh M, Hamada K, Yamakawa H, et al. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res. 1988;16:618–621. doi: 10.1002/jor.1100160515. [DOI] [PubMed] [Google Scholar]
- 11.Schubert TE, Weidler C, Lerch K, et al. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. doi: 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lui PP, Chan LS, Fu SC, et al. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med. 2010;38:757–764. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- 13.Key JA. The synovial membrane of joints and bursae. In: Cowdry EV, editor. Special cytology: the form and functions of the cell in health and disease. Paul B Hoeber; New York: 1928. pp. 737–756. [Google Scholar]
- 14.Woodley SJ, Mercer SR, Nicholson HD. Morphology of the bursae associated with the greater trochanter of the femur. J Bone Joint Surg Am. 2008;90:284–294. doi: 10.2106/JBJS.G.00257. [DOI] [PubMed] [Google Scholar]
- 15.Castor CW. The microscopic structure of normal human synovial tissue. Arthr Rheum. 1960;3:140–151. doi: 10.1002/art.1780030205. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohnsack M, Meier F, Walter GF, et al. Distribution of substance-P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: a neurohistological approach to anterior knee pain syndrome. Arch Orthop Trauma Surg. 2005;125:592–597. doi: 10.1007/s00402-005-0796-4. [DOI] [PubMed] [Google Scholar]
- 18.Baker CL, Jr, Massie RV, Hurt WG, et al. Arthroscopic bursectomy for recalcitrant trochanteric bursitis. Arthroscopy. 2007;23:827–832. doi: 10.1016/j.arthro.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthr Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 20.Krenn V, Morawietz L, Haupl T, et al. Grading of chronic synovitis—a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317–325. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 21.Blaine TA, Kim YS, Voloshin I, et al. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J Should Elb Surg. 2005;14:84S–89S. doi: 10.1016/j.jse.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Cook JL, Feller JA, Bonar SF, et al. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Firestein GS, Budd RC, Harris ED, et al. Kelley’s textbook of rheumatology. 8. Elsevier; Philadelphia: 2008. [Google Scholar]
- 24.Abraham T, Wadsworth S, Carthy JM, et al. Minimally invasive imaging method based on second harmonic generation and multiphoton excitation fluorescence in translational respiratory research. Respirology. 2011;16:22–33. doi: 10.1111/j.1440-1843.2010.01898.x. [DOI] [PubMed] [Google Scholar]
- 25.Fearon A, Dahlstrom JE, Twin J, et al. The Bonar score revisited: Region of evaluation significantly influences the standardized assessment of tendon degeneration. J Sci Med Sport. 2014;17:346–50. doi: 10.1016/j.jsams.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko JY, Wang FS, Huang HY, et al. Increased IL-1beta expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J Orthop Res. 2008;26:1090–1097. doi: 10.1002/jor.20631. [DOI] [PubMed] [Google Scholar]
- 27.Soifer TB, Levy HJ, Soifer FM, et al. Neurohistology of the subacromial space. Arthroscopy. 1996;12:182–186. doi: 10.1016/s0749-8063(96)90008-0. [DOI] [PubMed] [Google Scholar]
- 28.Shaw HM, Santer RM, Watson AH, et al. Adipose tissue at entheses: the innervation and cell composition of the retromalleolar fat pad associated with the rat Achilles tendon. J Anat. 2007;211:436–443. doi: 10.1111/j.1469-7580.2007.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahss MH, Michelson JD, Desai P, et al. Investigations into the fat pads of the sole of the foot: anatomy and histology. Foot Ankle. 1992;13:233–242. doi: 10.1177/107110079201300502. [DOI] [PubMed] [Google Scholar]
- 30.Cichowitz A, Pan WR, Ashton M. The heel: anatomy, blood supply, and the pathophysiology of pressure ulcers. Ann Plast Surg. 2009;62:423–429. doi: 10.1097/SAP.0b013e3181851b55. [DOI] [PubMed] [Google Scholar]
- 31.George LK, Ruiz D, Jr, Sloan FA. The effects of total hip arthroplasty on physical functioning in the older population. J Am Geriatr Soc. 2008;56:1057–1062. doi: 10.1111/j.1532-5415.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- 32.Bachmeier CJ, March LM, Cross MJ, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthr Cartil. 2001;9:137–146. doi: 10.1053/joca.2000.0369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






