Abstract
Objectives
Tendinopathy is a common, costly condition affecting both sporting and sedentary populations. Research into tendinopathy frequently involves the evaluation of tendinosis, a pathology characterized by a lack of inflammatory cells, collagen disruption, neovascularisation, altered cell numbers and morphology and increased glycosaminoglycans. Evaluation of these characteristics can be undertaken using the Bonar histopathology score, but the characteristics are heterogeneous throughout tendon specimens with no standardized method of determining the area to be evaluated. The objective of this study was to assess whether the Bonar score varies depending on the criteria used to define the area of evaluation.
Design
Case series.
Methods
Two independent assessors, with a third to resolve disputes, evaluated 103 areas from 35 tendon specimens using the Bonar score. Specimens were scored once each in the area of worst collagen disruption, degree of vascularization, and cell morphological changes. The inter-tester reliability of the updated Bonar scale was good (r2 = 0.71)
Results
The Bonar score was highest in the areas of worst cell morphological (CM) changes, followed by collagen disruption (CD) and lowest for the area of most extensive vascular proliferation (VS) (regression: CD vs. CM, p = 0.008, CM vs. VS, p < 0.001, CD vs. VS, p = 0.013). Suggested modifications to the Bonar score include the addition of a cellularity domain, specific definitions of hypo- and hypercellularity, and changes to the vascularity score to include pathological avascularity.
Conclusions
The updated Bonar score includes a standardized method of selecting the area of evaluation, which should provide increased reliability when assessing the extent of tendon degeneration.
1. Introduction
Tendinopathy with histopathological evidence of degeneration in the absence of inflammatory cells is referred to as tendinosis. Tendinopathy is common in sporting and sedentary populations,1 and is associated with significant pain, reduction of productivity and quality of life, and costly medical interventions.2 Thus research that increases our understanding of this condition and that evaluates possible treatments is important. Research in this field continues to employ histopathological evaluation of tendon tissue specimens as a standard method.3
The histopathological changes associated with tendinosis include cellular changes, collagen disruption, vascular proliferation, and an increase in glycosaminoglycans.4 and5 Histopathological changes in tendon have been recognized as providing insights into both aetiology and response to treatments.6 For instance, normal tendon and osseotendinous junction display a low blood flow, evidenced by minimal colour Doppler activity,7 whereas tendinopathic tendon displays increased colour Doppler activity and up to three times the number of intratendinous microvessels.7 and 8 Histological changes have also been hypothesized to relate to the progression of the condition, with altered cell morphology hypothesized to be an early but potentially reversible histological feature, and with proliferation of blood vessels occurring later.9,10 and 11 Different patterns of degeneration have been reported between full or partial thickness tears of the rotator cuff,12 and varying levels of tendinosis have been noted in Achilles tears, tendinopathy and control tissue.13 and 14 A change in cell morphology and proliferation was found more commonly than collagen disruption in people with asymptomatic patellar tendinosis.9 These studies provide insights into possible driving factors for tendinopathy. Robust reproducible grading is important in diagnosis and to compare patient outcomes and possible treatments. However, the features of pathology described above are often heterogenous throughout the biopsy sample, with relatively normal areas closely adjacent to areas of advanced pathology.
To date there is no standard method of determining the area of the tendon biopsy to be evaluated. Authors have used a number of different assessment methods,15 and16 and have reported a wide range in levels of agreement, varying from (Kappa) 0.17 to 0.87.13 and14 We are not aware of any report detailing how authors have determined which area of tendon to assess, which may in part explain the variation in agreement.
The aim of this study was to assess whether the Bonar score varies depending on which definition of highest morphological change is used (degree of collagen disruption, level of cell morphological change, or, degree of vascularity). We also critically assessed the current criteria used in the Bonar score.
2. Methods
This study was approved by the local clinical research ethics board in Australia (ETH.9/07.865) and the Clinical Research Ethics Board at the University of British Columbia (approval for the histological analysis). The study group comprised two mutually exclusive groups – Australian patients undergoing either gluteal tendon reconstruction surgery or total hip arthroplasty. Participant and surgery details have been previously reported.17 Two researchers experienced in using the Bonar score reviewed each domain of the grading system. By consensus, the appropriate level of magnification used for each domain was determined. The need for polarization, and need for additional categorisation of pathological changes with regard to cellularity and vascularity were reviewed. Hyper and hypo cellularity were defined. To determine the area of tendon which represented the area of worst pathology, two researchers, with a third to resolve disputes evaluated 35 sections of tendon. A separate Bonar score was calculated for each of the following areas: the area of worst collagen disruption; the area scoring the highest vascularity; and lastly, the area scoring the highest cell morphological change. The review of the Bonar score and suggestions for changes to the scale were completed by consensus. To determine the area that represented the region with the most advanced pathological change, regression with post hoc pair-wise analysis was undertaken on the Bonar scores, using STATA 10.1 (StataCorp, College Station, TX). The same tendon sections were scored on separate days by two examiners using the original Bonar score (inter-tester reliability, r2 = 0.32), and again using the revised Bonar score (inter-tester reliability, r2 = 0.71).
3. Results
The Bonar score is designed to assess, by light microscopy, tendon morphology on longitudinal sections – not cross sections or those cut at an oblique angle. In order to make the score consistent with the previously published hierarchy of tendon morphology,18 and19 “fiber” was replaced with “fibre bundle.” Secondly, we added a section on cellularity, with appropriate definitions for each grade. Hypercellularity was defined as >30 nuclei per field of view and hypo cellularity was defined as <20 nuclei per field of view. We found that the vascularity score required a classification to allow for areas of pathological avascularity. Previously these areas would have been scored as zero or normal when clearly they are not. We added two dichotomous evaluations – the presence or absence across the entire section of calcification and adipocytes among the fibre bundles. Finally, recommended levels of magnification for each feature were determined (Table 1).
Table 1.
Bonar Score: updated and with clarification of terms and grades.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
|
Cell morphology Four fields of view, 200× |
Inconspicuous elongated spindle shaped nuclei with no obvious cytoplasm at light microscopy | Increased roundness: nucleus becomes more ovoid to round in shape without conspicuous cytoplasm | Increased roundness and size; the nucleus is round, slightly enlarged and a small amount of cytoplasm is visible | Nucleus is round, large with abundant cytoplasm and lacuna formation (chondroid change) |
|
Collagen arrangement Polarized One field of view, 100× |
Collagen arranged in tightly cohesive well demarcated bundles with a smooth dense bright homogenous polarization pattern with normal crimping | Diminished fibre polarization; separation of individual fibre bundles but with maintenance of overall bundle architecture Non homogeneous polarization | Bundle changes: separation and loss of demarcation of fibre bundles, giving rise to expansion of the tissue overall and clear loss of normal polarization pattern | Marked separation of fibre bundles with complete loss of architecture |
|
Cellularity One field of view, 100× |
Mainly discrete cells | Hyper cellulara, in runs and/or increased cell numbers | Areas of hypoa as well as hypera cellularity | Area of assessment is mostly a-cellular |
|
Vascularity ≤ 10 fields of view, 400× |
Inconspicuous blood vessels coursing between bundles | Occasional cluster of vessel, <2 per 10 high power fields | 2–3 clusters of capillaries power 10 high power field | Areas with greater than 3 clusters per 10 high power fields, and/or areas of pathological a-vascularity |
|
Ground substance One field of view, 100× |
Not stainable ground substance | Stainable mucin between bundles but bundles still discrete | Stainable mucin within bundles with loss of clear demarcation of bundles | Abundant mucin throughout the section with inconspicuous collagen staining |
Hypocellular: < 20 nuclei per field; hypercellular: >30 nuclei per field. Plus 2.5 points for each of: calcification; adipocytes (intratendinous). Total score: a tendon with the most pathology will score 20. A tendon with no observable pathology will score 0.
A total of 103 areas of tendon were evaluated. Marked differences were seen in the Bonar score both within individual tendon sections (Fig. 1), and between the different areas. The total Bonar score was highest in the areas of worst cell morphological (CM) changes (mean (SD) = 14.4 (1.50)), followed by collagen disruption (CD:13.0 (2.56)), and was lowest for the area of greatest vascular proliferation (VS: 11.6 (1.68)) (regression: CD vs. CM, p = 0.008, CM vs. VS, p < 0.001, CD vs. VS, p = 0.013).
Figure 1.
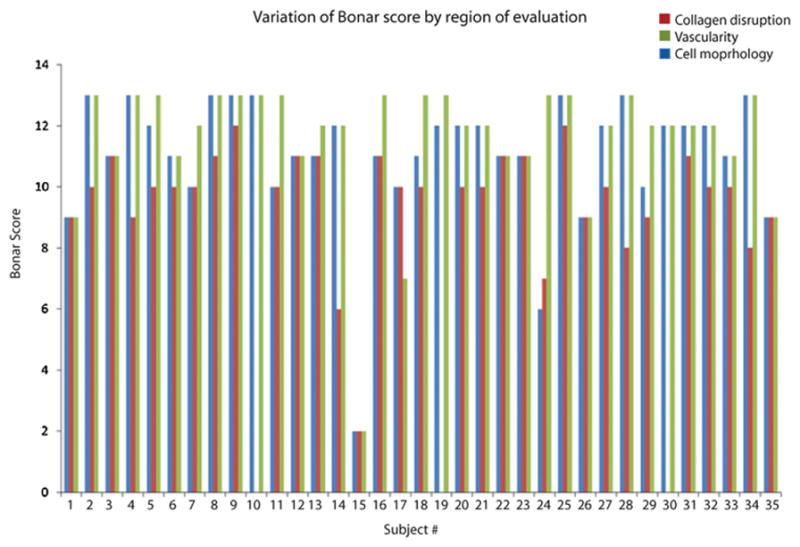
Individual participant Bonar scores, by category. Evaluating the area of worst cell morphological change resulted in the highest overall Bonar score. The area of collagen disruption also resulted in high scores. The area of highest vascularity resulted in the lowest scores. In some participants the score varied markedly depending on which area was assessed, e.g. participants #12 and #13 compared to #24, #29, and #30 (which had no areas of visible vessels).
4. Discussion
This study demonstrated that the extent of tendon degeneration as assessed by the Bonar score varies depending on the area of the biopsy which is graded. Because tendon functions primarily as a load-bearing structure requiring continuous force transmission throughout the length of the tendon, we suggest that a system which captures the area of most advanced degenerative change – in this study, identified as the regions showing the worst cell morphology – is appropriate. Given that cell morphological change also appears to be one of the earliest features of tendinosis, this system may have a greater possibility of capturing the early signs of tendon degeneration. In addition, defining a priori the region to be evaluated has the potential to improve the reliability of this standardized assessment. This point has previously been raised by Fasanella et al. in their discussion of the reliability of assessing human breast tissue histopathology.20
The Bonar score was originally designed for the assessment of patellar tendinopathy. However, the same scale has been used by other groups to assess pathology in other tendons with distinct biomechanical functions, e.g. the supraspinatus tendon.15 Although the normal mechanical environment and anatomy of different tendons is variable, the features of injury which are observed at various sites appear to be consistent as noted by Khan et al.5 We previously examined gluteal tendon pathology and found that the pathology conforms to descriptions of tendinosis at other major anatomical sites.17
By consensus, we determined and applied the following guidelines: the entire section should initially be viewed at 100× total magnification, in order to identify the area to evaluate based on the cell morphology domain. Collagen disruption should be assessed with polarization, one field of view at 100×. Cell morphology should be further evaluated over four fields of view, at 200× total magnification. Cellularity should be evaluated over one field of view, at 100× total magnification. Vascularity should be evaluated over ≤10 fields of view, at 400× total magnification. Individual vessels that course through the tendon section should only be counted once. Ground substance should be assessed over one field of view, 100× total magnification.
Using these magnifications and fields of view, the area (in mm2) under examination is the same for each domain (refer to Appendix A for calculations). In all cases, a higher magnification may be used to clarify the score, e.g. individual areas of cell morphology may be viewed at 400× to confirm the extent of cell morphological change. Likewise, to distinguish between longitudinally coursing intratendinous vessels vs longitudinal arrays of fibroblastic cell nuclei, a higher magnification may be necessary (Table 1).
To assist with determining the level of morphological change, when evaluating a field of view we suggest that a rule of 20% should be applied. That is if 20% of the tissue can be scored at the highest level, this score is applied to the entire field of view. When assessing cell morphology, if 20% of the field of view demonstrates grade 3 morphology, then the score for that tendon section is grade 3. To assist researchers we have made a table with examples of each classification within the Bonar score (Fig. 2), and the key features distinguishing the different grades are detailed in Appendix B (supplementary materials).
Figure 2.
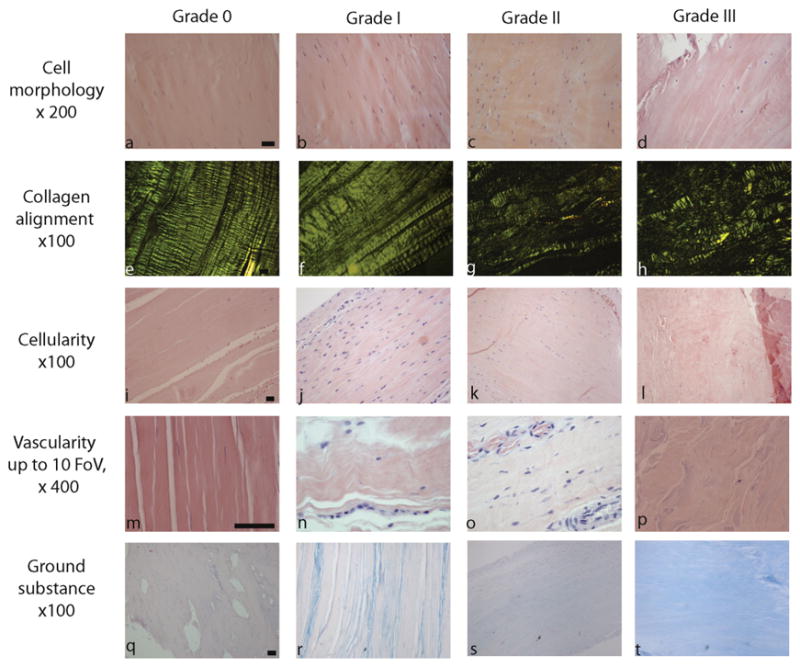
Pictorial representation of the Bonar score. Example micrographs of each grade are shown. The slides in the first four rows are stained with haematoxylin and eosin. The slides in the ground substance row are stained with Alcian blue. Magnification is reported for each row. Scale bars = 50 μm. Key differences between the grades are further described in Appendix B
The highest Bonar score was found in the area of highest cell morphology change. These data suggest that using the area of maximum cell change provides a score that is representative of the worst degree of tendinosis within the area of the specimen being evaluated. To our knowledge this has not previously been described. Some previous studies have noted that evaluation has been undertaken in the area of “…most advanced pathological changes…” however this area was not overtly defined.13 and 21 One earlier cross-sectional study of asymptomatic patellar tendon from active athletes noted that changes in cellular morphology were more extensive than changes in collagen alignment.9 Recent studies have focused on local cellular function in relation to understanding tendon pathology, including the local production and activation of matrix degrading enzymes such as the MMP and ADAMTS family.22 Until such time as a histopathological finding has been correlated with function or patient status, we suggest that cell morphological change be used to identify the area of evaluation.
Our review of the Bonar score makes a number of recommendations covering magnification levels, field of view and area to assess, definitions of hypo and hypercellularity, and clarification of how to rate areas that are acellular, avascular, calcified, or containing adipocytes. By specifying the level of magnification and number of fields of view, each domain within the Bonar score will be evaluated over the same area. In addition to providing greater confidence among users of the score, the results of our review have the potential to allow better comparison between studies and provide clinicians with a structure for reporting their results.
We suggest that any assessment that requires a degree of qualitative evaluation should be undertaken twice in a blinded manner. Ideally this should be done by two assessors, with a third assessor to resolve discrepancies23 in order to reduce the chance of bias.24 A further tool that is helpful is to create reference slides. These can be reviewed regularly throughout the assessment, providing a reference for calibration. For this purpose we have provided a sample pictorial Bonar score (Fig. 2).
In the current paper, we found that the characteristics of tendon pathology were heterogenous throughout the tendon specimens. When assessing the extent of tendon degenerative change, evaluating the area of maximal cell morphological change provides a Bonar score that is representative of the worst degree of tendinosis within the area of the specimen being evaluated. We suggest that when evaluating tendon degeneration, cell morphology should be assessed first, followed by assessment of the other domains (collagen alignment, vascularity, degree of cellularity) assessed in the same area.
It should also be acknowledged that the Bonar score assigns a pathological score to features that, in some situations, may actually represent normal adaptations which could also be heterogeneously represented throughout the specimen. For example, insertional regions and regions of tendon exposed to compression or shear commonly exhibit a more chondrocytic phenotype, which would be interpreted under the Bonar score as “cell morphological change”. Insertional areas are also typically less vascular, whereas it is normal for the blood supply to be enriched at particular points (e.g. the anterior surface of the Achilles tendon), and these regions can have an appearance of “hypervascularity” when assessed under the microscope, even in normal tendon. Finally, many healthy tendons contain tertiary bundles separated by regions of loose connective tissue which could be misinterpreted as collagen disorganization. The fact that the scale may inadvertently assign a pathological score to features that are normal needs to be kept in mind when conducting the Bonar assessment. It would therefore be prudent for biopsies to be taken consistently from the same region of tendon, and avoiding areas of loose connective tissue or insertional regions. Despite the limitations noted above, the Bonar score does consistently assign a higher grade to injured, as opposed to uninjured, tendon, even in tendons such as the supraspinatus which contain regions of fibrocartilage as well as more vascular, loose connective tissue. In one study, the Bonar score of cadaveric rotator cuff samples with no known shoulder pathology ranged from 0 to 4 (mean 1.9), whereas samples from patients with rotator cuff injuries ranged from 6 to 12 (mean 9.5).15
Hypervascularity in association with tendon overuse injury has been referred to as “angiofibroblastic tendinosis”25 – this finding is typically interpreted as evidence of repair activity within the tendon. Conversely, hypovascularity has been interpreted as more typical of a more advanced state of tissue pathology, in which repair activity is less apparent.26 Despite the potential that hyper- and hypo-vascularity have different implications for the reparative capacity of the tissue, for ease of use of the scale we elected to assess vascularity in one category. On a practical level, it is not possible for the selected region of interest to demonstrate both hyper-and hypo-vascularity, therefore the inclusion of both hyper- and hypovascularity on the same scale does not cause any problems when scoring tendons.
This study has several limitations. The results of this study are limited to human degenerative tissue changes. They are yet to be validated for acute tendinopathy, or for tendon pathology observed in animals. However, these results provide a basis on which further evaluation may be undertaken. A second limitation is that the Bonar score changes are based on the consensus of two researchers experienced in assessing tendon tissue, and with discussion with the remaining authors. Thus, others may not agree, however we consider this a step towards higher quality assessment of tendon tissue.
5. Conclusion
The updated Bonar score includes a standardized method of selecting the area of evaluation.
5.1. Practical implications
The modified Bonar score provides improved clarity and reproducibility when assessing the extent of tendon degenerative change.
Histopathology may be used to assess the response of degenerated tendon to treatments.
Supplementary Material
Acknowledgments
This study was funded by grants from the Canadian Institutes of Health Research and from WorksafeBC. Alex Scott was funded by a Clinical Scholar award from the Michael Smith Foundation for Health Research. The authors are grateful to Fiona Bonar for helpful advice in the evaluation of tendon degeneration.
References
- 1.Maffulli N, Longo UG. Conservative management for tendinopathy: is there enough scientific evidence? Rheumatology. 2008;47(4):390–391. doi: 10.1093/rheumatology/ken011. [DOI] [PubMed] [Google Scholar]
- 2.Virta L, Joranger P, Brox JI, et al. Costs of shoulder pain and resource use in primary health care: a cost-of-illness study in Sweden. BMC Musculoskelet Disord. 2012;13 doi: 10.1186/1471-2474-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43(2):131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 4.Nirschl RP. Rotator cuff tendinitis: basic concepts of pathoetiology. Instr Course Lect. 1989;38:439–445. [PubMed] [Google Scholar]
- 5.Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27(6):393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Cook J, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. [DOI] [PubMed] [Google Scholar]
- 7.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Br J Sports Med. 2008;43:409–416. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]; Knee Surg Sports Traumatol Arthrosc. 2001;9(4):233–238. doi: 10.1007/s001670000189. [DOI] [PubMed] [Google Scholar]
- 8.Scott A, Lian O, Roberts CR, et al. Increased versican content is associated with tendinosis pathology in the patellar tendon of athletes with jumper’s knee. Scand J Med Sci Sports. 2008;18(4):427–435. doi: 10.1111/j.1600-0838.2007.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook JL, Feller JA, Bonar SF, et al. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22(2):334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Scott A, Cook JL, Hart DA, et al. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56(3):871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 11.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;(415):111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 13.Maffulli N, Barrass V, Ewen SWB. Light microscopic histology of achilles tendon ruptures: a comparison with unruptured tendons. Am J Sports Med. 2000;28(6):857–863. doi: 10.1177/03635465000280061401. [DOI] [PubMed] [Google Scholar]
- 14.Tallon C, Maffulli N, Ewen SWB. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001;33(12):1983–1990. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Maffulli N, Longo UG, Franceschi F, et al. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008;466(7):1605–1611. doi: 10.1007/s11999-008-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millar NL, Wei AQ, Molloy TJ, et al. Heat shock protein and apoptosis in supraspinatus tendinopathy. Clin Orthop Relat Res. 2008;466(7):1569–1576. doi: 10.1007/s11999-008-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fearon AM, Scarvell JM, Cook JL, et al. Does ultrasound correlate with surgical or histologic findings in greater trochanteric pain syndrome? A pilot study. Clin Orthop Relat Res. 2010;468(7):1838–1844. doi: 10.1007/s11999-009-1174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Screen H, Bader D, Lee D, et al. Local strain measurement within tendon. Strain. 2004;40(4):157–163. [Google Scholar]
- 19.Kastelic J, Galeski A, Baer E. Multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 20.Fasanella S, Leonardi E, Cantaloni C, et al. Proliferative activity in human breast cancer: Ki-67 automated evaluation and the influence of different Ki-67 equivalent antibodies. Diagn Pathol. 2011;6(Suppl 1):S7. doi: 10.1186/1746-1596-6-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maffulli N, Testa V, Capasso G, et al. Similar histopathological picture in males with Achilles and patellar tendinopathy. Med Sci Sports Exerc. 2004;36(9):1470–1475. doi: 10.1249/01.mss.0000139895.94846.8d. [DOI] [PubMed] [Google Scholar]
- 22.Corps AN, Robinson AHN, Harrall RL, et al. Changes in matrix protein biochemistry and the expression of mRNA encoding matrix proteins and metalloproteinases in posterior tibialis tendinopathy. Ann Rheum Dis. 2012;71:746–752. doi: 10.1136/annrheumdis-2011-200391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards P, Clarke M, DiGuiseppi C, et al. Identification of randomized controlled trials in systematic reviews: accuracy and reliability of screening records. Stat Med. 2002;21(11):1635–1640. doi: 10.1002/sim.1190. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S, editors. Cochrane Handbook of Systematic Reviews of Interventions. Wiley-Blackwell; Chichester, West Sussex, England: 2012. [Google Scholar]
- 25.Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81(2):259–278. [PubMed] [Google Scholar]
- 26.Matthews TJ, Hand GC, Rees JL, et al. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88(4):489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


