Abstract
Background
Long-term survival (LTS) is uncommon for patients with pancreatic ductal adenocarcinoma (PDAC). We sought to identify factors that predict 10-year, LTS after resection of PDAC.
Methods
We identified all patients with PDAC that underwent resection at UCLA after 1990, and included all patients eligible for observed LTS (1/1/1990—12/31/2004). An independent pathologist reconfirmed the diagnosis of PDAC in patients with LTS. Logistic regression was used to predict LTS based on patient and tumor characteristics.
Results
Of 173 included patients, 53% were male, median age at diagnosis was 66, and median survival was 23 months. The rate of observed LTS was 12.1% (n = 21). Age, sex, number of lymph nodes evaluated, margin status, lymphovascular invasion, and adjuvant chemotherapy and radiation were not associated with LTS. The following were associated with LTS on bivariate analysis: low AJCC stage (Ia, Ib, IIa) (p = 0.034), negative lymph node status (p = 0.034), low grade (well-, moderately-differentiated) (p = .001), and absence of perineural invasion (p = 0.019). Only low grade (odds ratio 7.17, p = 0.012) and absent perineural invasion (odds ratio 3.28, p = 0.036) were independently associated with increased odds of LTS. Our multivariate model demonstrated good discriminatory power for LTS, as indicated by a c-statistic of 0.7856.
Conclusion
Absence of perineural invasion and low tumor grade were associated with greater likelihood of LTS. Understanding the tumor biology of LTS may provide critical insight into a disease that is typically marked by aggressive behavior and limited survival.
INTRODUCTION
The incidence of pancreatic cancer is rising, but the prognosis for the majority of patients remains strikingly poor. Overall 5-year survival for all patients with PDAC is approximately 7.1%.1, 2 By 2030, researchers project that pancreatic cancer will become the 2nd leading cause of cancer related death in the US after lung cancer, surpassing colorectal, breast, and prostate cancer.3 In 2015, it is estimated there will be 48,960 new cases and 40,560 deaths attributable to pancreatic cancer in the US—indicative of near universal lethality. Of these pancreatic cancers, approximately 96% will be pancreatic ductal adenocarcinoma (PDAC).2
Curative resection is attempted in the small subset of patients that present with localized disease; however, the overall 5-year survival rate for these patients remains no higher than 26%.2 Furthermore, unlike other solid malignancies for which 5-year survival rates are often equivocated with “cure” rates, patients that survive 5-years after PDAC resection continue to be at high risk for recurrence and disease-related mortality.4-6 Extended or long-term survival (LTS) after PDAC resection has been reported in a number of retrospective series, and has been defined variously as survival greater than 5 or 10 years depending on the study. However, in many of these series a histopathologic diagnosis of PDAC was not confirmed by a re-review of the pathology in a sizeable number of patients purported to achieve LTS. Many patients were instead found to harbor adenocarcinoma of various subtypes that are less aggressive than PDAC, and some were found not to harbor malignancy at all.7-9 This has led some experts to question whether LTS—let alone a cure—truly exists for patients with PDAC.10
The factors that impact prognosis in PDAC are well described. Small primary tumor size, negative surgical margins, absence of lymph node metastasis, a favorable lymph node ratio, and adjuvant chemotherapy and radiation are among the most notable.11-14 More recently, the marked impact of tumor grade on prognosis has also been recognized.11, 15 However, there is data to suggest that the group of patients that ultimately achieve LTS after PDAC resection do not reliably meet the above criteria for ideal prognosis; many may have advanced stage or other markers of poor prognosis.16, 17 It appears that our understanding of the tumor biology of patients with LTS after PDAC resection is therefore lacking. We undertook the present study for the following reasons: to provide an accurate, observed rate of LTS after resection of pathologically confirmed PDAC at our institution, and to perform a dedicated analysis to formally identify predictors of LTS.
METHODS
Patient Population and Definition of Long-term Survival
With approval from the UCLA institutional review board, we identified all patients that had surgical resection of PDAC at our institution after 1/1/1990. Patients diagnosed after 12/31/2004 were excluded such that all patients in the study population were eligible for the outcome of interest—10-year survival, or LTS—at the time of analysis. Patients that were found to have unresectable PDAC at the time of surgical exploration were excluded.
We considered a patient to have achieved LTS only if the following criteria were strictly met: confirmed overall survival for 10 years or more from the time of diagnosis, and a confirmed pathologic diagnosis of PDAC. We defined overall survival time as the length of time between the date of diagnosis and the date of death or last known contact. In order to ensure a conservative estimation of LTS, we presumed all patients lost to follow-up prior to achieving 10-year survival to be dead at the date of last known contact (n = 24). For all patients that appeared to achieve LTS, an independent pathologist (DD) re-reviewed the pathology specimen in order to confirm a true diagnosis of PDAC. We excluded cases in which there was a discrepancy between the original pathologic diagnosis and the subsequent re-review (n = 2). In one case, the specimen contained no residual tumor as a result of treatment effect, and was therefore not confirmed by re-review. This case was included, however, on the basis of two consecutive preoperative biopsies—including an open surgical biopsy—that concordantly demonstrated PDAC.
Covariates
We collected the following demographic and clinical variables: age, sex, type of operation, American Joint Committee on Cancer (AJCC) stage, and receipt of adjuvant chemotherapy and radiation. Pathologic data collected include, tumor size, margin status (negative = R0, positive = R1, R2), number of lymph nodes evaluated, tumor grade, and the presence or absence of both perineural and lymphovascular invasion. At our institution margin status is defined as positive only when tumor is definitively present at the surgical margin, not whenever tumor is within 1mm of the surgical margin. For analysis, we included the following as categorical variables: age (<50, 50-70, >70), AJCC stage (“low stage” = Ia, Ib, IIa; “high stage” = IIb, III, IV), number of lymph nodes evaluated (≤12, >12—according to international consensus),18 tumor grade (“low grade” = well-differentiated, moderately-differentiated; “high grade” = poorly-differentiated).
Statistics
We compared patients with and without LTS based on age, sex, AJCC stage, lymph node status, number of lymph nodes evaluated, tumor grade, margin status, perineural invasion, and lymphovascular invasion using chi-square tests. We tested the association between perineural invasion and lymph node status in a similar fashion. We then performed multivariate logistic regression to predict LTS controlling for these covariates. Lymph node status was not included in the regression due to collinearity with AJCC stage, of which it is an integral component. Adjuvant chemotherapy and radiation were also omitted from the multivariate regression, for the following reasons. Missing data regarding adjuvant therapy (primarily as a result of patients receiving adjuvant therapy at an outside institution) would have excluded a large number of patients from the regression. Furthermore, adjuvant chemotherapy with or without radiation has become standard practice at our institution and is almost uniformly recommended. Because most patients for whom information regarding adjuvant therapy was available did in fact receive adjuvant therapy, we believed its inclusion into the model might introduce a degree of selection bias.
We used the regression to calculated odds ratios and corresponding p-values. To test the model's discriminatory power, we plotted the receiver operating characteristic (ROC) curve. As a reference, we plotted this alongside the ROC curve for two additional logistic regression models predicting LTS—one controlling only for AJCC stage, the other controlling for AJCC stage and tumor grade. We report the c-statistic (area under the ROC curve) for these models.
RESULTS
Patient Demographics and Clinical Characteristics
173 patients met our inclusion criteria (Table 1). Overall, patients were mostly male (53%). The median age at diagnosis was 66. Pancreaticoduodenectomy was performed in 164 patients; the remainder had distal pancreatectomy. Stage IIb was the most common AJCC stage resected, representing 49.1% of patients. The overall median survival was 23 months and 5-year survival was 21.9%.
Table 1.
Patient Demographics and Tumor Characteristics
| Patient Characteristic | |
|---|---|
| Number of Patients, n | 173 |
| Sex, n (%) | |
| Male | 92 (53) |
| Female | 81 (47) |
| Median Age, years | 66 |
| Median Survival, months | 23 |
| 5-year survival, n (%) | 38 (22.0) |
| LTS, n (%) | 21 (12.1) |
| AJCC Stage, n (%) | |
| Ia | 24 (13.9) |
| Ib | 35 (20.2) |
| IIa | 27 (15.6) |
| IIb | 85 (49.1) |
| III | 0 (0) |
| IV | 2 (1.2) |
| Operation Performed, n (%) | |
| Pancreaticoduodenectomy | 164 (94.8) |
| Distal pancreatectomy | 9 (5.2) |
| Median tumor size, cm ± std. deviation | 2.5 ± 1.4 |
| Tumor Grade, n (%) | |
| well differentiated | 23 (13.3) |
| moderately differentiated | 76 (43.9) |
| poorly differentiated | 73 (42.2) |
| not reported | 1 (<1) |
| Lymph Node Status, n (%) | |
| Negative | 87 (50.3) |
| Positive | 86 (49.7) |
| Median Lymph Nodes Evaluated, n ± std. deviation | 10 ± 8.2 |
| Margin Status, n (%) | |
| Negative | 143 (82.7) |
| Positive | 30 (17.3) |
| Perineural Invasion, n (%) | |
| Absent | 66 (38.2) |
| Present | 105 (60.7) |
| not reported | 2 (1.1) |
| Lymphovascular Invasion, n (%) | |
| Absent | 113 (65.3) |
| Present | 57 (32.9) |
| not reported | 3 (1.7) |
The rate of observed LTS in our study was 12.1% (n = 21, Table 2). The most common AJCC stage of patients with LTS was Ib (33.3%). Median survival for patients with LTS was 138 months.
Table 2.
Clinical and Histopathologic Characteristics of Patients Achieving LTS
| Patient | Sex | Age | Operation | Survival (months) | AJCC Stage | Tumor Size | Grade | Margin Status | Perineural Invasion | Lymphovascular Invasion | # LN (+) | # LN Reviewed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 70 | PD | 221 | IIb | 2 | mod | - | - | - | 1 | 4 |
| 2 | M | 69 | PD | 174 | Ib | 3 | mod | - | - | - | 0 | 7 |
| 3 | M | 68 | PD | 174 | Ib | 2.5 | mod | - | - | - | 0 | 15 |
| 4 | M | 52 | PD | 173 | IIa | 0.6 | mod | - | - | - | 0 | 4 |
| 5 | F | 51 | PD | 161 | Ia | 1.5 | mod | - | - | - | 0 | 11 |
| 6 | M | 66 | PD | 150 | Ib | 5 | mod | - | - | - | 0 | 8 |
| 7 | F | 75 | PD | 149 | IIb | 3.2 | mod | + | + | + | 1 | 24 |
| 8 | M | 76 | PD | 142 | IIa | 4.5 | mod | - | - | - | 0 | 9 |
| 9 | F | 71 | PD | 142 | IIb | 1.2 | well | - | - | + | 1 | 20 |
| 10 | F | 79 | PD | 139 | Ib | 2.5 | mod | - | - | - | 0 | 1 |
| 11 | M | 63 | PD | 138 | IIb | 3.2 | mod | - | + | + | 9 | 16 |
| 12 | F | 59 | PD | 132 | IIb | 4 | mod | - | - | - | 1 | 22 |
| 13 | M | 56 | PD | 131 | IIa | 0.2 | mod | - | + | - | 0 | 18 |
| 14 | M | 76 | PD | 126 | Ia | 2 | mod | - | + | - | 0 | 7 |
| 15 | M | 56 | PD | 125 | IIb | 2.8 | poor | + | + | + | 8 | 47 |
| 16 | M | 36 | PD | 124 | IIa | 1.5 | mod | - | - | - | 0 | 7 |
| 17 | M | 68 | PD | 123 | Ia | 1 | well | - | - | - | 0 | 2 |
| 18 | F | 70 | PD | 122 | Ib | 3.6 | mod | - | + | + | 0 | 9 |
| 19 | F | 48 | PD | 122 | Ib | 4.5 | poor | - | + | - | 0 | 4 |
| 20 | F | 81 | PD | 122 | IIa | 5 | mod | + | - | - | 0 | 15 |
| 21 | M | 67 | PD | 120 | Ib | 2.7 | well | + | + | - | 0 | 8 |
PD = pancreaticoduodenectomy; mod = moderately differentiated; well = well differentiated; poor = poorly differentiated; LN = lymph nodes
Tumor Characteristics
The average tumor size for all patients was 2.5cm. The majority of patients had negative surgical margins (82.7%). Overall, 50.3% of patients had negative lymph nodes. The median number of lymph nodes evaluated was 10. 13.3% of patients had well-differentiated tumors, 43.9% had moderately-differentiated tumors, and 42.2% had poorly differentiated tumors. Perineural invasion was present in 60.7% of tumors, and lymphovascular invasion was present in 32.9% of tumors. By chi-square analysis, there was no correlation between perineural invasion and lymph node status (p = 0.640).
In the 21 patients that achieved LTS, 19 had tumors that were low grade, and 6 patients had positive lymph nodes. All patients with nodal involvement that achieved LTS had the extent of positive nodes limited to the pancreaticoduodenectomy specimen itself; 5/6 were limited to the peripancreatic fat, 1/6 had both peripancreatic and periduodenal nodal disease. One patient that achieved LTS had both positive lymph nodes and high tumor grade.
Bivariate Analysis
Age, sex, margin status, the number of lymph nodes evaluated, lymphovascular invasion, adjuvant chemotherapy, and adjuvant radiation were not associated with LTS (Table 3). However, low AJCC stage was significantly associated with LTS (p = 0.034); accordingly, negative lymph node status was also associated with LTS (p = 0.034). Low tumor grade was highly associated with LTS (p = 0.001), as was the absence of perineural invasion (p = 0.019).
Table 3.
Bivariate, Chi-Square Analysis to Identify Predictors of LTS
| Non-LTS n | LTS n | p value* | |
|---|---|---|---|
| Age | |||
| <50 | 14 | 2 | 0.969 |
| 50-70 | 84 | 11 | |
| > 70 | 54 | 8 | |
| Sex | |||
| Male | 80 | 12 | 0.698 |
| Female | 72 | 9 | |
| AJCC stage | |||
| Low (Ia, Ib, IIa) | 71 | 15 | 0.034 |
| High (IIb, III, IV) | 81 | 6 | |
| Lymph node status | |||
| Negative | 71 | 15 | 0.034 |
| Positive | 81 | 6 | |
| # Lymph nodes evaluated | |||
| ≤ 12 | 90 | 13 | 0.814 |
| > 12 | 62 | 8 | |
| Grade | |||
| Low (well-, moderately-differentiated) | 80 | 19 | 0.001 |
| High (poorly-differentiated) | 72 | 2 | |
| Margin status | |||
| Negative | 126 | 17 | 0.826 |
| Positive | 26 | 4 | |
| Perineural invasion | |||
| Absent | 53 | 13 | 0.019 |
| Present | 97 | 8 | |
| Lymphovascular invasion | |||
| Absent | 97 | 16 | 0.314 |
| Present | 52 | 5 | |
| Adjuvant chemotherapy | |||
| Yes | 71 | 15 | 0.330 |
| No | 13 | 1 | |
| Adjuvant radiation | |||
| Yes | 33 | 7 | 0.620 |
| No | 44 | 7 |
p value represents results of Pearson χ2 test, bolded values indicate p<0.05
Multivariate Analysis
The presence of low tumor grade was significantly and independently associated with increased odds of LTS (odds ratio (OR) 7.17, p = 0.012; Table 4). The absence of perineural invasion was also significantly and independently associated with increased odds of achieving LTS (OR 3.28, p = 0.036). However, after controlling for other variables in the model, low AJCC stage was no longer a statistically significant predictor of LTS (OR 2.9, p = 0.073). In an additional sensitivity analysis, inclusion of the year of operation into our multivariate model did not change our results, and the year of surgery was not associated with odds of LTS (p = 0.392).
Table 4.
Identification of Independent Predictors of LTS by Multivariate Logistic Regression
| Odds Ratio | p value* | |
|---|---|---|
| Age | ||
| 50-70 | 1.43 | 0.692 |
| > 70 | 1.43 | 0.704 |
| Sex (Male) | 0.93 | 0.896 |
| Low AJCC stage (Ia, Ib, IIa) | 2.9 | 0.073 |
| > 12 Lymph nodes reviewed | 1.21 | 0.416 |
| Low grade (well-, moderately-differentiated) | 7.17 | 0.012 |
| Margin status (negative) | 0.51 | 0.326 |
| Absent perineural invasion | 3.28 | 0.036 |
| Absent lymphovascular invasion | 0.83 | 0.344 |
p value represents results of multivariate logistic regression
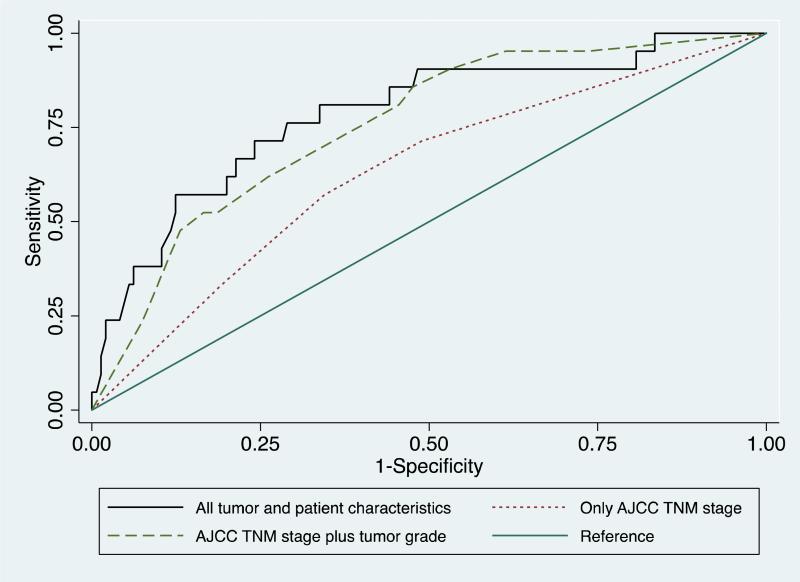
The multivariate model had good discriminatory power, as measured by the c-statistic (area under the ROC curve) of 0.7856 (Figure 1). Our model compared favorably with simpler models that controlled only for AJCC stage (c-statistic = 0.6314) and for AJCC stage plus tumor grade (c-statistic = 0.7548).
Figure 1. Receiver operating characteristic curves for logistic regression models predicting LTS following pancreatic resection for pancreatic adenocarcinoma.
Complete model, including perineural invasion, c-statistic = 0.7856. Model including AJCC stage plus tumor grade, c-statistic = 0.7548. Model including only AJCC stage, c-statistic 0.6314.
DISCUSSION
In this retrospective series of 173 patients, all patients were eligible to achieve an observed survival time of 10 years, and an independent pathologist confirmed the histopathologic diagnosis of PDAC in all of the patients that achieved LTS. We report an overall 12.1% observed rate of LTS after PDAC resection. The 5-year overall survival rate was 22%, indicating that nearly half of 5-year survivors did not survive to 10 years post-resection. Of the 21 patients that achieved LTS, 6 had advanced AJCC stage as defined by positive lymph nodes. We found that low AJCC stage, negative lymph node status, low tumor grade, and absent perineural invasion were associated with LTS. However, the results of our multivariate logistic regression indicate that only low tumor grade and absent perineural invasion are both independently and significantly associated with increased odds of LTS.
The literature regarding the actual rate of 10-year LTS is limited. The majority of studies that report on post-resection survival analyze only 5-year survivors, and when reported, LTS greater than 10-years is typically only described by Kaplan-Meier or actuarial estimation.9, 17, 19-21 The LTS rate by Kaplan-Meier estimation ranges from 9-13%.5, 22 An actual, observed rate of LTS was reported to as low as 4.1% in one series, but the accuracy of this rate may be questioned insofar as patients were included that were not eligible for 10-year observed survival.23 Another series observed a LTS rate of 5.1% after PDAC resection but with the following caveats: not all specimens were available for pathology re-review, and overall survival is likely worse as patients were enrolled in a randomized control trial studying adjuvant gemcitabine, which is now considered standard of care.10 Most recently, a large retrospective utilizing the National Cancer Database identified a 3.9% rate of LTS after PDAC resection. This rate is lower than that which we report, but represents a population that is heterogeneously treated.24 Thus the 12.1% rate of LTS after PDAC resection that we report may be considered a more accurate and valid estimate of LTS that is (a) based on observed survival, not estimated by Kaplan-Meier or actuarial methods, and (b) comprised only of confirmed diagnoses of PDAC after additional pathology review.
The positive impact of low tumor grade on overall PDAC prognosis—and conversely the marked negative impact of high tumor grade—is well established in the literature.10, 12, 17, 25-28 Data from the National Cancer Institute's (NCI) Surveillance, Epidemiology, and End Results (SEER) database has likewise demonstrated the prognostic importance of tumor grade.29 Using this data, a revision of the AJCC tumor node metastasis (TNM) staging system for PDAC was proposed to incorporate tumor grade (TNMG). The TNMG system improved survival discrimination between stages, and has been subsequently validated in a smaller cohort for our institution.15, 30 Tumor grade has also been independently associated with survival in a formal analysis of 5-year survivors, conducted in a manner similar to the current study.23 It is therefore consistent that we have found low tumor grade to have a highly significant and independent association with increased odds of LTS. Additionally, the addition of grade to the multivariate model including AJCC stage increased the c-statistic from 0.6314 to 0.7548.
Perineural invasion is widely described as an indicator of poor prognosis in PDAC, but recognition of its impact on survival is often overshadowed by the components of stage. We identified the absence of perineural invasion as independently associated with increased odds of LTS. A large series has similarly found the presence of perineural invasion to be associated with worse overall survival after controlling for lymph node status and grade.31 Perineural invasion may be particularly important as a marker for extended survival in patients with favorable disease. In a cohort of 40 patients with observed 5-year survival, perineural invasion was the only clinicopathologic factor found to influence additional survival.6 In one series, overall 5-year survival for patients with negative lymph nodes was markedly better in those with absent perineural invasion (75% versus 29%); in another, the greatest 5-year survival rates were only found in patients with absent perineural invasion, negative nodes, and no evidence of duodenal invasion.8, 32 Similarly, in our study, perineural invasion was an independent and significant predictor of LTS, and including this variable in our multivariate model improved the model's discriminatory power.
The major limitations of this study include its limited sample size, retrospective nature, and single institution experience. The observational nature of our data allows us to describe an association between low tumor grade and absent perineural and LTS, but does not establish causality. As a result of missing data, we are unable to fully assess the impact of adjuvant chemotherapy and adjuvant radiation on the odds of LTS. However, the survival benefit of adjuvant chemotherapy is well established, and it is standard practice at our institution to administer adjuvant chemotherapy. We expect that the vast majority of patients for whom data is missing did in fact receive adjuvant chemotherapy, which during the study period was predominantly a gemcitabine or fluorouracil based regiment. It is therefore unlikely that a statistically significant association between adjuvant chemotherapy and LTS would be detected in our patient population were all data available. Use of adjuvant radiation is less standard at our institution, and therefore we are unable to draw any conclusions regarding the association between adjuvant radiation and LTS in this study. Resection margin status has been implicated numerous times as a predictor of overall prognosis, but did not impact the odds of LTS in this series. The 17% rate of margin positivity (R1) we report is consistent with numerous other series that report a positive margin rate between 13-30%.5, 8, 9, 11, 17, 19, 23, 25, 28 It is lower, however, than a number of more recent studies reporting a 40-52% rate of R1 resection.12, 26, 33, 34 The low rate of margin positivity rate reported here is multi-factorial in origin, and in part a result of strict pre-operative patient selection; similarly, this may also explain in part the relatively low frequency of node positive disease in this cohort (49.7%). However, this discrepancy highlights a particular challenge regarding the definition of margin positivity in the PDAC literature. Our institution calls a margin positive only when tumor is definitively present at the margin, whereas many institutions have adopted a policy of calling a margin positive whenever tumor is present with 1mm of the inked edge. Relative to series using the latter definition, our study likely underreports the rate of margin positivity; this may in part explain why margin status was not associated with LTS in this cohort. Finally, we have defined LTS as overall survival; this fails to differentiate between patients that have been “cured” of disease from those alive with recurrence, and also fails to differentiate between those that succumb to PDAC from those that die of unrelated causes.
These limitations notwithstanding, our study is significant for the following reasons. We report an observed rate of LTS after PDAC that is methodologically conservative. To the best of our knowledge, this represents the first analysis dedicated solely to the identification of clinicopathologic features predictive of 10-year LTS after PDAC resection in patients whom the diagnosis is confirmed by a pathologic re-review. As opposed to the majority of previous studies that correlate prognostic factors with overall survival, this study identifies the specific attributes of the uncommon patients that achieve 10-year survival. From this perspective, tumor grade and perineural invasion are the only factors independently associated with LTS, neither of which are included in the traditional AJCC TNM staging system. However, as the majority of patients with PDAC manifest aggressive disease and limited survival, our focused analysis of patients with LTS is not an indictment of the current staging system, although our results do provide limited support to studies that call for the inclusion of tumor grade into the AJCC staging system. We also found there was no association between perineural invasion and lymph node status; although both features are well known markers of aggressive disease, they may reflect distinct aspects of tumor behavior. Indeed, 6 out of 21 patients that achieved LTS had positive nodes, indicating that positive lymph node status does not necessarily preclude LTS (particularly when limited to the peripancreatic fat).
Thus our results are in alignment with an emerging perspective that seeks to identify the unique aspects of tumor biology that may allow for rare patients with extended survival. Recent work has been done in an attempt to identify unique genetic and molecular characteristics that may account for LTS in patients with PDAC. Although it does not appear that differences in commonly mutated oncogenes can explain the tumor biology of patients with LTS, differences in the protein expression of tumors in patients with LTS have been identified.35, 36 Our results provide support for further inquiry into the mechanisms that may cause extended survival for patients with LTS. Additionally, our results underscore the need for further work to determine the difference between overall, disease-specific, and recurrence-free survival. As PDAC patients experience extended survival, teasing out the impact of causes of death other than cancer, will allow for more precise characterization of disease in this patient population. Further investigation is also necessary to determine whether major perioperative morbidity associated with pancreatectomy—such as pancreatic fistula or delayed gastric emptying—influences the survival of patients with tumor biology otherwise favorable to achieve LTS. Finally, there is burgeoning data to suggest the use of neoadjuvant therapy to downstage patients with borderline resectable PDAC;37 whether a uniform chemotherapy-first approach to all patients with PDAC (as opposed to the traditional surgery-first approach) will influence the rate of LTS after PDAC resection remains unknown.
In summary, we report a series of patients with resected PDAC in which the actual, observed rate of LTS is 12.1%. Our multivariate analysis identified low tumor grade and absent perineural invasion as the only factors significantly and independently associated with increased odds of LTS, neither of which are represented in the current staging system. Efforts to understand the unique characteristics and tumor biology of patients with LTS is an emerging avenue through which critical insight may be found to advance clinical approaches for the majority of patients with aggressive disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2012. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Annals of surgery. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Annals of surgery. 2008;247:456–62. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 6.Shimada K, Sakamoto Y, Nara S, Esaki M, Kosuge T, Hiraoka N. Analysis of 5-year survivors after a macroscopic curative pancreatectomy for invasive ductal adenocarcinoma. World journal of surgery. 2010;34:1908–15. doi: 10.1007/s00268-010-0570-9. [DOI] [PubMed] [Google Scholar]
- 7.Connolly MM, Dawson PJ, Michelassi F, Moossa AR, Lowenstein F. Survival in 1001 patients with carcinoma of the pancreas. Annals of surgery. 1987;206:366–73. doi: 10.1097/00000658-198709000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Annals of surgery. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han SS, Jang JY, Kim SW, Kim WH, Lee KU, Park YH. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas. 2006;32:271–5. doi: 10.1097/01.mpa.0000202953.87740.93. [DOI] [PubMed] [Google Scholar]
- 10.Sinn M, Striefler JK, Sinn BV, Sallmon D, Bischoff S, Stieler JM, et al. Does long-term survival in patients with pancreatic cancer really exist? Results from the CONKO-001 study. Journal of surgical oncology. 2013;108:398–402. doi: 10.1002/jso.23409. [DOI] [PubMed] [Google Scholar]
- 11.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 12.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–8. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2009;13:1337–44. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 14.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Annals of surgery. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rochefort MM, Ankeny JS, Kadera BE, Donald GW, Isacoff W, Wainberg ZA, et al. Impact of tumor grade on pancreatic cancer prognosis: validation of a novel TNMG staging system. Annals of surgical oncology. 2013;20:4322–9. doi: 10.1245/s10434-013-3159-3. [DOI] [PubMed] [Google Scholar]
- 16.Adham M, Jaeck D, Le Borgne J, Oussoultzouglou E, Chenard-Neu MP, Mosnier JF, et al. Long-term survival (5-20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: a series of 30 patients collected from 3 institutions. Pancreas. 2008;37:352–7. doi: 10.1097/MPA.0b013e31818166d2. [DOI] [PubMed] [Google Scholar]
- 17.Shin SH, Kim SC, Hong SM, Song KB, Lee JH, Park KM, et al. Can statistically determined prognostic factors predict the long-term survival of patients with pancreatic ductal adenocarcinoma following surgical resection?: Clinicopathological analysis of 82 long-term survivors. Pancreas. 2014;43:571–7. doi: 10.1097/MPA.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 18.Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad NA, Lewis JD, Ginsberg GG, Haller DG, Morris JB, Williams NN, et al. Long term survival after pancreatic resection for pancreatic adenocarcinoma. The American journal of gastroenterology. 2001;96:2609–15. doi: 10.1111/j.1572-0241.2001.04123.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. European journal of cancer. 2004;40:549–58. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Allen PJ. Pancreatic adenocarcinoma: putting a hump in survival. Journal of the American College of Surgeons. 2007;205:S76–80. doi: 10.1016/j.jamcollsurg.2007.06.331. [DOI] [PubMed] [Google Scholar]
- 22.Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764–72. doi: 10.1016/j.surg.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Cleary SP, Gryfe R, Guindi M, Greig P, Smith L, Mackenzie R, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. Journal of the American College of Surgeons. 2004;198:722–31. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Paniccia A, Hosokawa P, Henderson W, Schulick RD, Edil BH, McCarter MD, et al. Characteristics of 10-Year Survivors of Pancreatic Ductal Adenocarcinoma. JAMA surgery. 2015 doi: 10.1001/jamasurg.2015.0668. [DOI] [PubMed] [Google Scholar]
- 25.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Annals of surgery. 2004;240:293–8. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. discussion 210-1. [DOI] [PubMed] [Google Scholar]
- 27.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Annals of surgery. 2011;254:311–9. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 28.Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. Journal of surgical oncology. 2000;73:212–8. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. The American journal of pathology. 2002;160:1361–9. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasif N, Ko CY, Farrell J, Wainberg Z, Hines OJ, Reber H, et al. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Annals of surgical oncology. 2010;17:2312–20. doi: 10.1245/s10434-010-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin IH, Shama MA, Tanaka M, Abbruzzese JL, Curley SA, Hassan M, et al. Association of diabetes and perineural invasion in pancreatic cancer. Cancer Med. 2012;1:357–62. doi: 10.1002/cam4.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16–22. doi: 10.1007/BF02482964. [DOI] [PubMed] [Google Scholar]
- 33.Rau BM, Moritz K, Schuschan S, Alsfasser G, Prall F, Klar E. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery. 2012;152:S103–11. doi: 10.1016/j.surg.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Gebauer F, Tachezy M, Vashist YK, Marx AH, Yekebas E, Izbicki JR, et al. Resection margin clearance in pancreatic cancer after implementation of the Leeds Pathology Protocol (LEEPP): clinically relevant or just academic? World journal of surgery. 2015;39:493–9. doi: 10.1007/s00268-014-2808-4. [DOI] [PubMed] [Google Scholar]
- 35.Dal Molin M, Zhang M, de Wilde RF, Ottenhof NA, Rezaee N, Wolfgang CL, et al. Very Long-term Survival Following Resection for Pancreatic Cancer Is Not Explained by Commonly Mutated Genes: Results of Whole-Exome Sequencing Analysis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:1944–50. doi: 10.1158/1078-0432.CCR-14-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen R, Dawson DW, Pan S, Ottenhof NA, de Wilde RF, Wolfgang CL, et al. Proteins associated with pancreatic cancer survival in patients with resectable pancreatic ductal adenocarcinoma. Laboratory investigation; a journal of technical methods and pathology. 2015;95:43–55. doi: 10.1038/labinvest.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donahue TR, Isacoff WH, Hines OJ, Tomlinson JS, Farrell JJ, Bhat YM, et al. Downstaging chemotherapy and alteration in the classic computed tomography/magnetic resonance imaging signs of vascular involvement in patients with pancreaticobiliary malignant tumors: influence on patient selection for surgery. Archives of surgery (Chicago, Ill : 1960) 2011;146:836–43. doi: 10.1001/archsurg.2011.152. [DOI] [PubMed] [Google Scholar]