Abstract
The resurgence of interest in anhedonia within major depression has been fuelled by clinical trials demonstrating its utility in predicting antidepressant response as well as recent conceptualizations focused on the role and manifestation of anhedonia in depression. Historically, anhedonia has been conceptualized as a “loss of pleasure”, yet neuropsychological and neurobiological studies reveal a multifaceted reconceptualization that emphasizes different facets of hedonic function, including desire, effort/motivation, anticipation and consummatory pleasure. To ensure generalizability across studies, evaluation of the available subjective and objective methods to assess anhedonia is necessary. The majority of research regarding anhedonia and its neurobiological underpinnings comes from preclinical research, which uses primary reward (e.g. food) to probe hedonic responding. In contrast, behavioural studies in humans primarily use secondary reward (e.g. money) to measure many aspects of reward responding, including delay discounting, response bias, prediction error, probabilistic reversal learning, effort, anticipation and consummatory pleasure. The development of subjective scales to measure anhedonia has also increased in the last decade. This review will assess the current methodology to measure anhedonia, with a focus on scales and behavioural tasks in humans. Limitations of current work and recommendations for future studies are discussed.
Keywords: anhedonia, reward, major depression, effort, anticipation, scale, delay discounting, prediction error, monetary incentive delay, gambling, response bias
Introduction
Treatments for Major Depressive Disorder (MDD) are hampered by relatively low rates of remission with various antidepressants and adjunctive treatments (Warden et al, 2007), prompting a need to define other avenues of therapy. Towards this aim, there is a need to understand the various neurobiological substrates for depressive symptoms in order to detect predictive biomarkers for treatment selection. Identifying the symptoms that actually predict treatment failure is also a logical springboard from which to elucidate neurobiology-symptom links.
Two large trials, the Genome Based Therapeutic Drugs for Depression (GENDEP) and the Sequenced Treatment Alternatives to Relieve Depression (STAR*D), have provided an opportunity to explore factors related to antidepressant outcomes. In the GENDEP trial, Uher and colleagues (2008) identified 6 symptom dimensions that were needed to describe the structure of depressive symptoms: mood, anxiety, sleep, appetite, pessimism and interest-activity. In particular, low interest-activity, reflecting reduced enjoyment in addition to interest and activity, strongly predicted poor antidepressant outcome (Uher et al, 2011). Generalizability of this finding was enhanced with replication in the STAR*D data (Uher et al, 2012). Furthermore in the Treatment of Resistant Depression in Adolescents (TORDIA) trial, anhedonia (as measured by the anhedonia dimension on the Child Depression Rating Scale-Revised) was the only unique negative predictor of time to remission with a selective serotonin reuptake inhibitor (SSRI) and the number of depression-free days (McMakin et al, 2012).
Though anhedonia is a core feature of a Major Depressive Episode according to DSM-5 and is a key diagnostic criterion for the depressive subtype melancholia (APA, 2013), the assessment of this construct has received disproportionately less attention in the context of MDD. This may be partly due to the inconsistent conceptualization of anhedonia that has resulted in a paucity of adequate measures and tasks that tap into the different facets of a “pleasure response”. Despite increasing research in this domain, several key limitations remain in anhedonia research. The goal of this review is to evaluate the benefits and disadvantages of preclinical and clinical methods to measure anhedonia with a focus on human studies, and advance future directions for anhedonia research.
Conceptualizing anhedonia
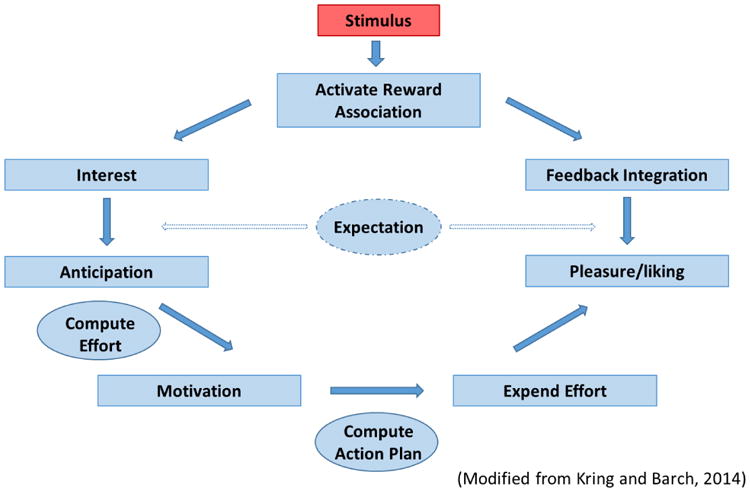
Traditionally, anhedonia has been defined as a “loss of pleasure” (Ribot, 1956), a definition that emphasizes the consummatory/enjoyment aspect of reward function. However, the assessment of anhedonia according to the Diagnostic and Statistical Manual of Axis I Disorders-5th edition (APA, 2013) reflects a broader conceptualization that includes interest as well as consummatory pleasure. Even within the Hamilton Depression Rating Scale, one of the most widely used depression scales, the single anhedonia item is measured as a dimensional construct representing desire, effort and consummatory pleasure: “loss of interest in activities”, “decrease in actual time spent on activities”, “experiencing pleasure” (Hamilton, 1960). This equivocal conceptualization makes anhedonia measurement imprecise. As Treadway & Zald (2011) assert “heterogeneity at the level of symptom definition is at least as problematic as…issues of comorbidity…” (p.3), and refining the construct is imperative if we hope to understand the neurobiological underpinnings of anhedonia. Consequently, it may be more helpful to address more precise facets of hedonic function (Der-Avakian and Markou, TINS). Based on a modified version of the reward process described by Kring and Barch (2014), we describe the process as initially building an stimulu-reward association, which then leads to interest/desire (wanting a reward), anticipation (state of readiness for a reward), motivation (initial energy expenditure to attain a reward), effort (sustained energy expenditure to attain reward), hedonic response (e.g. enjoyment of reward), and feedback integration (updating reward presence and values) (Figure 1). In this model, expectation of a reward is necessary for anticipation and important for feedback integration following reward outcome. Conceptually, it is helpful to define a linear reward process but it is important to emphasize that, behaviourally, these facets of reward can occur in parallel (i.e., one can feel interested and anticipating, and effort to attain reward can be in itself pleasurable).
Figure 1. Model of reward processing.
Growing neuroscientific evidence also supports unique aspects of reward processing that can give rise to loss of pleasure, or may even act independently of pleasure. This was evaluated in a reward task whereby MDD patients viewed cartoons for which they provided “liking” scores (and thus measures of consummatory pleasure). In order to view the liked cartoons once again, subjects were required to expend a specified level effort (number of clicks on a moving square). While MDD patients experienced similar levels of consummatory pleasure as healthy controls, lower levels of reward anticipation were associated with reduced motivation for effort expenditure in the MDD group; conversely, liking predicted motivation in the healthy control group (Sherdell et al, 2012). This supports distinct processes in MDD, whereby anticipation may be a limiting factor in the experience of reward in depression. An alternative explanation is that the ability to translate the prospect of reward into motivation is impaired. This fits evidence that MDD patients display a reduced ability to detect reward and incorporate experience of reward into reward-learning associations (reviewed in Pizzagalli, 2014). Consistent with this work are data showing reduced prediction error in depression, in response to a reward, suggesting patients are not characterized by the same level of neural feedback from rewards as healthy controls (Gradin et al, 2011; Kumar et al 2008). Taken together, it is clear that available tasks and tools need to reflect a refinement in anhedonia conceptualization in order to yield an adequate assessment of this core symptom of depression. Critically, there is mounting evidence that different facets of reward processing such as motivation, reward learning, effort-based reward-related decision making as well as anticipation and consummatory pleasure map onto partially dissociable neural pathways and signaling (Der-Avakian and Markou, 2012). In this vein, a more multi-faceted and precise definition of anhedonic behavior promises to improve our understanding of the pathophysiology of this cardinal feature of MDD, and identify sub-groups of patients that might preferentially benefit from particular treatment strategies.
Neurobiology of anhedonia
An in-depth discussion of the neurobiology of anhedonia is beyond the scope of this review, but has been well summarized in other articles (see Treadway & Zald, 2011; Der-Avakian & Markou, 2012 for review). Briefly, we assume that anhedonia can arise from impairments in various facets of reward processing, including desire for reward, anticipation of reward, effort to attain reward, consummatory pleasure, as well as cognitive aspects of learning stimulus-reward associations. Evidence suggests that there are specific neuroanatomical areas that underlie various facets of reward processing, including the prefrontal cortex (orbitofrontal cortex, ventromedial prefrontal cortex and anterior cingulate cortex), dorsal striatum (caudate and putamen), nucleus accumbens and amygdala. While dopamine has been the main neurotransmitter investigated in relation to reward, there is also mounting evidence that systems other than dopamine are critically involved in the reward process. Specifically, opioids, glutamate, gamma-aminobutyric acid (GABA) and serotonin also play a significant role. Opioids and GABA, for example, may be particularly important in the experience of consummatory pleasure (Barbano & Cador, 2007; van Zessen et al, 2012), while serotonin may be more associated with increased impulsivity and preference for immediate reward (Schweighofer et al, 2008).
Primary vs. secondary reward
The experience of pleasure can pertain to many stimuli that are instinctual (e.g. food, sex) versus non-instinctual (e.g. photography, reading). It could be argued that food and sex represent “primary” rewards (inherent rewards), whereas photography or money are secondary rewards (no inherent reward in itself and for which reward value must be learned). This distinction of “reward type” may complicate anhedonia research in two ways. First, neurobiological response to a primary reward may overlap with secondary reward, but may also have independent actions (Beck et al, 2010; Sescousse et al, 2013). In a meta-analysis of 87 studies to determine the overlapping and distinct brain areas activated in response to monetary, erotic and food rewards, there were some differences in activation within a common network, which included the ventromedial prefrontal cortex, ventral striatum, amygdala, anterior insula and mediodorsal thalamus (Sescousse et al, 2013). Notably, a study evaluating fluid and monetary incentives demonstrated that fluids were not as susceptible to contextual framing as secondary reward, and were more related to the experience of satiety or temperature (McClure et al, 2007), which also may affect distinctions in neural activation. In particular, the primary rewards (food and erotic) resulted in activation of the anterior insula and amygdala (in the case of erotic reward), while the monetary secondary reward activated the orbitofrontal cortex. Furthermore, the authors suggest that even within the neocortex there may be a difference in how primary and secondary rewards are encoded. Accordingly, the more phylogenetically recent anterior portion of the OFC was more likely to respond to monetary reward. While the same group previously demonstrated that the posterior OFC is more activated in response to primary stimuli (e.g. erotic) (Sescousse et al, 2010), this requires replication due to some inconsistencies in findings (Sescousse et al, 2013). In contrast to the view of distinct activity with reward type, in a meta-analysis of 206 studies, Bartra and colleagues (2013) reported that both primary and secondary rewards activated the same brain regions including the striatum and ventromedial prefrontal cortex. Based on these findings, the authors suggest that assertions of domain-based differences in functional activity should be interpreted with caution.
Second, the distinction of incentive type limits the translation from preclinical to clinical studies, considering most preclinical work uses primary reward as incentive while most human studies employ secondary rewards. Importantly, investigation of primary reward provides a more direct comparison with animal studies. The following sections will expand on this idea through a review of the preclinical and clinical methods that have been developed to measure anhedonia and will also evaluate their generalizability and ability to measure the different facets of anhedonia. Indeed, one of the ultimate difficulties in measuring anhedonia lies in devising measures that tap into the objective or subjective nature of this construct.
Preclinical methods
Animal models for depression that induce anhedonia include chronic unpredictable mild stress (CMS), social defeat, and early-life stress (reviewed in Duman, 2010). All evoke depression-like behaviour that occurs following ongoing stressors, thereby providing greater face validity based on the chronic nature of depression than models such as learned helplessness (Willner, 1984; Nestler & Hyman, 2010). This is supported by findings that chronic but not acute antidepressant administration relieves anhedonia in these models (Tsankova et al, 2006; Papp et al, 1996; Levantopoulos et al, 2009). Only tasks that have been employed to measure reward in animal depression models will be discussed herein, including the sucrose preference test, place preference test, and intracranial self-stimulation (ICSS). These tasks assess interest in reward and levels of consummatory pleasure cannot be ascertained.
Sucrose preference
The anhedonia-inducing CMS model was initially validated using the sucrose preference task. In this task, rats are given the option of seeking out a sucrose solution or plain water. Since sucrose is more palatable, choosing to drink plain water instead of the sucrose water after a period of food deprivation is considered to reflect levels of anhedonia (Willner et al, 2005). After CMS, sucrose preference decreases compared to a baseline (no-stress) condition. Antidepressant effects have frequently been measured using this model to demonstrate reversal of anhedonia through reinstatement of sucrose preference (Willner et al, 1987; Papp et al, 2003; Hamani et al, 2012).
Ultimately, the sucrose preference test is a proxy of an animal's interest in a pleasurable stimulus, from which “liking” can only be inferred. Considering anhedonia encompasses a subjective quality that cannot be gleaned from rodents (e.g., desire, motivation, liking), a primary concern for using sucrose as a reward is whether not preferring sucrose water actually reflects anhedonia. This test is considered a proxy of liking because prior studies demonstrate that a decrease in sucrose consumption within the CMS model: (1) is not related to calorie content, and (2) does not reflect a global decrease in consummatory behavior (Willner et al, 1992). Furthermore, CMS-exposed rodents treated with an antidepressant exhibit place preference (described below) for sucrose, suggesting reinstatement of motivation to attain a rewarding stimulus (Willner et al, 1992).
Place preference
The objective of this task is to evaluate an animal's preference between environments where a stimulus was or was not previously presented (Carr & White, 1983; Bevins & Besheer, 2005). Conditioned place preference is widely used to assess the rewarding properties of a drug in the context of addiction research (Shinohara et al, 2014; Collier et al, 2013; Aguilar et al, 2009). In depression models, animals are freely exposed to a rewarding stimulus (e.g. food) only on one side of a chamber for several days and then assessed for side preference in the absence of the reward. Control animals show a strong preference for the area where a reward was received, while animals with depression-like phenotypes do not – an effect that can be reversed by antidepressants (Muscat et al, 1992). It has been suggested this task probes the incentive-motivational aspect of acquiring a reward, while other researchers have suggested that performance on a place preference task may merely reflect reinforcement learning of a reward (Huston et al, 2013).
Intracranial self-stimulation
In ICSS trials, animals learn to press a lever in order to receive electrical stimulation to a specific brain area along the brain reward pathway (e.g., posterior lateral hypothalamus, medial forebrain bundle). In depression models, the stimulation threshold for bar pressing increases compared to control animals, suggesting a decrease in the rewarding properties of stimulation. However, treatment with antidepressants can reverse this effect in CMS rats (Moreau et al, 1992). Performance is considered to be a metric of motivation; since this task can be trained on different schedules (e.g., fixed interval, progressive ratio), the degree of effort a stressed vs. control animal will put forth to receive reward can be evaluated. It is also a measure of the rewarding properties of a stimulus, which is why it continues to be a gold standard method to assess a drug's abuse potential with strong predictive validity (Horton et al, 2013.).
In all of the above tasks, only primary reward incentive is used to assess levels of anhedonia. Furthermore, facets of anhedonia, such as interest and liking, cannot be determined using animal models. These constructs can only be inferred by the animals' response to a reward. Finally, the reproducibility of the sucrose and ICSS tests (as indicators of the effects of stress on anhedonia) following CMS has been questioned (Harris et al, 1997; Nielsen et al, 2000; Hatcher et al, 1997). For example, in a study to evaluate the hedonic properties of sucrose preference and ICSS, Nielsen and colleagues (2000) used the CMS model of depression in different strains of rats. They found sucrose preference was demonstrated with certain rat strains and only a subgroup of rats exhibited attenuated ICSS behaviour.
In summary, animal models have utility in measuring observable aspects of reward processing, including motivation and effort. Indeed, preclinical models have been integral to elucidating the neurobiology of reward function (see Schultz, 2013 for review). The extent to which data from animal models can be generalized to human populations remains unclear. For example, in animal models of depression, SSRIs have demonstrated positive effects on sucrose consumption (Wilner, 1997), whereas SSRI non-response was predicted by anhedonia in human studies (Uher et al, 2011; McMakin et al, 2012). Highlighting convergence with human studies, recent work suggests the phenotype of anhedonia in animal models may actually map onto treatment resistance (Christensen et al, 2011). Further complicating the picture, translational studies using the sucrose test in humans, for example, have largely been negative (Berlin et al, 1998; Dichter et al, 2010; McCabe et al, 2009) (see “Behavioural Tasks” below).
Reward tasks not tested in animal models of depression
Tasks measuring prediction error, reward bias, learning and effort have been used preclinically, although not in animal models of depression (Shultz et al, 1998; Der-Avakian et al, 2013; Ineichen et al, 2012; Bari et al, 2010; Salamone et al, 1994). However, they have been utilized in human MDD studies. Consequently, their use in animal MDD models could provide more informative translational data. Description of preclinical tasks not employed in depression research is beyond the scope of this article; therefore, details regarding prediction error, signal detection, probabilistic reward learning, and reward effort tasks as they pertain to human studies are presented in the clinical section below (see “Task-based methods”).
Clinical methods
Anhedonia measurement in depressed humans utilizes direct symptom scales or behavioral tasks. While scale methods provide direct evidence of patients' experience, combining behavioral methods with neuroimaging offers an opportunity to disentangle different components of reward processing and identify neurobiological underpinnings associated with such abnormalities.
“First generation” questionnaire-based methods
The four main validated self-report measures of anhedonia used in clinical research are the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al, 1995), the Fawcett-Clark Pleasure Capacity Scale (FCPS) (Fawcett et al, 1983), the Revised Chapman Physical Anhedonia Scale (CPAS) and the Chapman Social Anhedonia Scale (CSAS) (Chapman et al, 1976) (Table 1). Ideally, a scale quantifying anhedonia in the context of MDD should be able to measure different aspects of anhedonia, detect state versus trait differences, and take into account varying cultural beliefs and preferences (generalizability). Although all of these scales have been validated in clinical populations, they differ in their ability to meet these criteria.
Table 1. First generation scales to measure anhedonia.
| Scale | Authors | Description | Reliability (Cronbach-α) | Populations Tested |
|---|---|---|---|---|
| Fawcett-Clark Pleasure Capacity Scale (FCPCS) | Fawcett, Clark, Sheftner Arch Gen Psy 1983; 40:79-84 | 36-item, 9-point scale (extreme and lasting displeasure to extreme and lasting pleasure) | .85 | Major Depressive Disorder |
| SHAPS | Snaith, Hamilton, Morley, Humayan, Hargreaves and Trigwell Br J Psy 1995; 167:99 | 14-item scale (strongly disagree to strongly agree)
|
.74 MDD | Healthy controls Major Depressive Disorder |
| Revised Social Anhedonia Scale | Eckbald et al, 1982 (Original in: Chapman, Chapman & Raulin. J Abn Psy 1976) |
61 Item true-false scale
|
.79 male and female | Healthy controls, schizophrenia, personality disorder, major depressive disorder, alcohol abuse |
| Revised Physical Anhedonia Scale | (Original in: Chapman, Chapman & Raulin. J Abn Psy 1976) | 40-Item true-false scale
|
Male: .82 Female: .78 |
Healthy controls, schizophrenia, major depressive disorder, alcohol abuse |
The aspects of anhedonia measured differ among the four scales. Whereas the CPAS and CSAS measure various aspects of anhedonia (motivation, effort, and consummatory pleasure) in addition to personality traits, both the FCPS and SHAPS focus exclusively on consummatory pleasure. Factor analysis of the FCPS and SHAPS revealed a unitary structure that primarily loaded onto hedonic capacity (Nakonezny et al, 2010; Leventhal et al, 2006), whereas the CPAS did not significantly relate to hedonic capacity (Leventhal et al, 2006). In this aspect, the CPAS and CSAS may be more likely to detect individual differences where hedonic capacity is not expected to significantly correlate with a task outcome. For example, in one study, high scores on the CPAS and CSAS were correlated with low willingness to expend effort for a reward task, whereas the SHAPS did not (Treadway et al, 2009).
It is also still unclear whether anhedonia is a stable construct over time in depressed patients (trait) or a symptom that fluctuates depending on severity or even antidepressant mechanism (state). Therefore, a scale that is able to assess responses “right now” versus “over time” or “in general” would be ideal for measurement in MDD. The CPAS and CSAS measure anhedonia “in general” and the items reflect anhedonia as a personality trait instead of specific aspects of hedonic function (e.g. “when I move to a new city, I feel a strong need to make new friends” on the CSAS). In contrast, both the FCPS and SHAPS measure state anhedonia (FCPS right now, SHAPS “last two days”), which may be more beneficial in capturing information in the context of a depressive episode. For example, in a sample of inpatients anhedonia scores based on the FCPS were stable over 7 months despite recovery in two thirds of patients (Clark et al, 1984). In another naturalistic study in chronic MDD patients, anhedonia (based on the FCPS) did not change over a 1-year follow-up despite reductions in depressive symptoms (Schrader, 1997). It is unclear whether the same effect would be observed in other samples and to what extent anhedonia is related to failure of antidepressants to target this symptom. Importantly, a trait measure will not be as sensitive to the acute and perhaps early changes that can occur with antidepressant treatment. Consistent with this notion, both the SHAPS and FCPS have demonstrated ability to measure acute changes in anhedonia (Martinotti et al, 2012; Willner et al, 2005).
In order for a scale to effectively evaluate a construct, it needs to avoid unnecessary measurement of other overlapping but different constructs (e.g., mood and anxiety in the context of MDD; divergent validity), while retaining the ability to demonstrate a correlation with similar constructs (convergent validity). Both the SHAPS and FCPS exhibit good convergent and discriminant validity: they moderately correlate with depression severity as would be expected, but do not correlate with measures of anxiety (Leventhal et al, 2006; Nakonezny et al, 2010). The SHAPS is also positively correlated with quality of life and functioning (Nakonezny et al 2010). Contrary to this, the CPAS has a weak correlation with depression severity, while both the CPAS and CSAS have strong associations with non-affective aspects of personality and psychotic disorders (Leventhal et al, 2006), likely due to the development of these scales for use in schizophrenia. In addition, the Chapman scales include items that are not clearly related to anhedonia (CPAS: “I have often felt uncomfortable when my friends touch me; CSAS: “My emotional responses seem very different from those of other people”).
All of these scales incorporate questions relating to both primary and secondary reward. The SHAPS, in particular, devised items based on the domains of pastimes, social interaction, food/drink, achievement and sensory experience. This contrasts to the CPAS and CSAS, which separate physical and social anhedonia. While they may be separate facets of anhedonia, it would be more feasible for clinical use if a questionnaire incorporated different domains of anhedonia in a single scale. However, as discussed, the SHAPS encompasses a unitary construct of consummatory anhedonia and does not separate based on domain.
The CPAS, CSAS as well as the FCPS lack generalizability due to the high degree of cultural bias demonstrated from questions such as “I always find organ music dull and unexciting” (CPAS) and “you are skiing down a mountain very fast while still in good control of yourself” (FCPS). In contrast, the SHAPS was developed to avoid cultural bias and so contains items with wider applicability (“I would be able to enjoy my favourite meal”). As a result, one could argue that it does not capture the events or activities that are likely to elicit strong hedonic responses. The ability to accurately tap into the subjective nature of what individuals find pleasurable or interesting is, perhaps, the greatest challenge in measuring anhedonia.
“Second Generation” Anhedonia Scales
In the last 8 years there has been a resurgence in refining anhedonia scales with at least 4 new published self-report questionnaires (Table 2), which attempt to take into account different facets of reward function instead of only consummatory pleasure. All of these scales take approximately 5-10 minutes to complete.
Table 2. Second generation scales to measure anhedonia.
| Scale | Authors | Description | Reliability (Cronbach-α) | Populations Tested |
|---|---|---|---|---|
| Temporal Experience of Pleasure Scale | Gard, Gard, Kring & John, 2006 | 18-item, 6 point scale (very false for me to very true for me
|
Total scale: .78 TEPS-ANT: .72 TEPS-CON: .64 |
Healthy students |
| Motivation and Pleasure Scale | Llerena et al, 2013 | 15-item, 5 point scale
|
.90 | Schizophrenia |
| Specific Loss of Interest Scale | Winer et al, 2014 | 23-item, 4 point scale
|
.94 | Healthy controls |
| Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) | Gooding and Pflum, 2014 | 17-item, 6 point scale
|
.86 | Healthy controls |
| Dimensional Anhedonia Rating Scale | Rizvi et al, 2015 | 17-item, 5 point scale
|
0.92-0.96 | Community sample Healthy controls MDD |
The Temporal Experience of Pleasure Scale (TEPS) has 18 items and two subscales designed to distinguish between anticipatory and consummatory pleasure (Gard et al, 2006). Items only reflect physical anhedonia as the authors believed this would result in more homogenous and interpretable results. The scale was validated using a student population, and extended, to the best of our knowledge, only to schizophrenia and bipolar disorder psychiatric populations (Gard et al, 2007; Tso et al, 2014). Although the authors report that items were developed to avoid cultural bias, certain questions could still be seen as culture specific (“When I'm on my way to an amusement park, I can hardly wait to ride the roller coasters”). Furthermore, the scale also includes questions that may be too vague to elicit a strong hedonic response (“Looking forward to a pleasurable experience is in itself pleasurable”). Finally, during the validation trial, the anticipation scale had an internal consistency reliability of 0.64, which is below the recommended 0.70. Additional validation trials in psychiatric groups should be completed to confirm the psychometric and factor analytic properties. A significant advantage of this scale is that its two-factor structure that separates anticipatory and consummatory aspects of reward.
More recently, Llerena and colleagues (2013) developed the Motivation and Pleasure Scale-Self Report (MAP-SR) to measure negative symptoms in schizophrenia (LLerena et al, 2013). The MAP-SR contains 15 questions across four domains: social pleasure, recreational or work pleasure, feelings and motivations about close, caring relationships, motivation and effort to engage in activities. The questions are answered on a 4-point Likert scale, where a high score is indicative of low anhedonia. Notably, within each domain, both intensity and frequency are gauged (“…. what is the most pleasure you have experienced this week…”, “…how often have you experienced pleasure from being with other people”). A unique feature of the MAP-SR is that it contains items querying prediction of future reward (“in the next few weeks, how much pleasure do you expect you will experience…”). Scale items avoid issues around generalizability by presenting broader questions (“What is the most pleasure you experienced from your hobbies…”), but therefore do not have specificity that may produce a strong reward response. Furthermore the motivation and effort to engage in activities is solely centred around those at work or school. The scale demonstrates high internal consistency reliability (0.90), as well as appropriate convergent and divergent validity. Thus far, normative data do not exist and the scale has only been validated in schizophrenia.
The Specific Loss of Interest Scale (SLIPS) was primarily designed to measure levels of interest over time (2 week period) (Winer et al, 2014). Each of the 23-items has a 3-point scale, with higher scores denoting increasing levels of anhedonia. The SLIPS demonstrated high reliability (0.94) as well as convergent validity with the SHAPS, moderate correlation with the TEPS anticipatory scale and small correlation with the TEPS consummatory scale. A 1-factor solution mostly encompassing social anhedonia items was reported. This is consistent with the disparate correlations observed with the TEPS, which measures physical anhedonia. Justification for the two week time point is not clear, especially considering the stability of anhedonia as a construct over time has not been established.
The Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) (Gooding et al, 2014) is a 17-item self-report scale that focuses on measuring anhedonia related to social interactions. The focus on social anhedonia is derived from research reporting high levels of social anhedonia as being a risk factor for the development of schizophrenia-spectrum disorders. Although the revised CSAS measures the same construct as the ACIPS, it does not distinguish between anticipation and consummatory pleasure, and it is unclear whether the CSAS would be useful in youth or adolescent groups due to the age bias of the items. The ACIPS was specifically designed to address these limitations as well as achieve a shorter scale, which would be more useful in clinical settings. While the ACIPS theoretically has an anticipatory and consummatory component, this was not reflected in the 3-factor structure identified in the preliminary validation trial, which mapped onto “intimate social interactions”, “social interactions within a group context”, and “pleasure derived from social bonding and making connections with others” (Gooding et al, 2014). The internal consistency was high for the overall scale (0.86), and the total scores were significantly correlated with both the anticipatory and consummatory subscales of the TEPS, more so than with the revised CSAS. Test-retest reliability of the ACIPS was good in a healthy population (r=0.78). Although the authors devised scale items in order to avoid age, gender, and cultural biases, these factors were not evaluated in the validation trial.
Finally, the Dimensional Anhedonia Rating Scale (DARS) is a 17-item self-report questionnaire that was designed to assess anhedonia in MDD, and particularly to increase scale generalizability while maintaining specificity (Rizvi et al, 2015). Towards this aim, respondents provide their own examples of rewarding experiences across the domains of hobbies, social activities, food/drink, and sensory experience (increase in item specificity). They subsequently answer a set of standardized questions with the timeframe of “right now” evaluating interest, motivation, effort, and enjoyment of reward within each domain. The reliability and validity of the DARS was evaluated in three studies, two in community samples, and the third in an MDD and healthy control sample (Rizvi et al, 2015). Principal components analysis demonstrated a four component solution that mapped onto reward domain. Internal consistency reliability of the total DARS score and subscales was high across studies (0.92-0.96 and 0.75-0.92, respectively). The DARS also demonstrated good convergent and divergent validity with the SHAPS and depression scores, respectively. In MDD patients, the DARS also showed utility over the SHAPS in predicting treatment resistant status. The timeframe of the DARS enables repeat testing that can aid in assessing the stability of anhedonia over time. However, further research is required to confirm the component structure and test-retest reliability, which is currently being evaluated in the Canadian Biomarker Integration Network for Depression research program (Kennedy et al, 2012).
Additional scales to measure aspects of anhedonia
BIS/BAS
The Behavioral Inhibition System and the Behavioral Activation System (BIS/BAS) was developed to measure personality traits of behavioral approach and inhibition based on Gray's theory of behaviour, the concept being that there are two distinct neurological systems for aversive motivation and appetitive motivation (Carver & White, 1994). Accordingly, the BIS system is activated in response to non-rewarding, novel stimuli that can lead to negative outcomes and inhibits behavior towards these stimuli. The BAS system, in contrast, is activated by rewarding stimuli. The BIS/BAS is a 24-item self-report scale with 4 factors: one for the BIS and 3 subscales for the BAS (drive, reward response and fun seeking). Psychometric properties of the BIS/BAS reflect convergent validity with depression and anxiety, as well as other personality measures (e.g., positive correlation between BIS and neuroticism) (Campbell et al, 2004). In the context of mood disorders, high BIS sensitivity and low BAS sensitivity have been associated with depression (Hundt et al, 2007; Kasch et al, 2002). Interestingly, low BAS sensitivity at baseline is predictive of poor depression outcomes in MDD (McFarland et al, 2006); however, in bipolar disorder high BAS sensitivity is predictive of time to manic episodes, while the BIS is predictive of depressive episodes (Alloy et al, 2008).
Motivation Scales
Considering motivation is a key component of reward function, it follows that adequate measurement of this construct is necessary to obtain a full assessment of anhedonia. There is a general paucity of motivation scales available, with most assessment of motivation extracted from quality of life and health surveys.
Apathy Evaluation Scale
In the psychological context, apathy is conceptualized as an overall lack of motivation based on decreased goal-directed behavior or thoughts, as well as affective flattening or indifference (Marin et al, 1991). Indeed, a lack of desire to pursue a reward or pleasure from a reward is common to both apathy and anhedonia, while emotional blunting/indifference is specific to apathy. An anhedonic individual may still view social relationships as being important despite a lack of drive to pursue them while an apathetic individual no longer finds social relationships to be a valuable endeavor. The Apathy Evaluation Scale (AES; also known as Marin Apathy Scale) (Marin et al, 1991), is a 14-item scale that can be administered as a self-report, observer-rated (e.g., caregiver, spouse, family member), or clinician-rated scale. In the validation trial involving Alzheimer's, depression and stroke patients, all three versions of the AES demonstrated high internal consistency reliability (α=0.86-0.94) and adequate test-retest reliability (r=0.76-0.94). However, the informant version was not able to distinguish between apathy and depression, while the self-report and clinician rated version did. This could be due to several factors, including the level of variability in responses as well as differences among how the groups understood the questions. Furthermore, specifically in depressed subjects, high apathy was correlated with functional impairment (Rothschild et al, 2014).
The Motivation and Energy Inventory (MEI)
To our knowledge, this is the only published motivation scale to be developed specifically for MDD. The self-report inventory is designed to measure changes in lassitude and energy with antidepressant treatment. The original scale includes 27-items with 3 subscales measuring social motivation, physical energy and mental energy with adequate internal reliability across the subscales (Fehnel et al, 2004). Poor motivation was weakly correlated with higher depression severity and moderately correlated with decreased quality of life and functioning. Subsequently, the scale has been shortened to an 18-item version that has been used in clinical trials to evaluate efficacy of bupropion XR (Hewett et al, 2009).
Clinical Scale Summary
The SHAPS remains the gold standard for measuring anhedonia in depression. It is recommended that any scale should be validated in the population of interest prior to use. While the CPAS and CSAS have been used in to assess anhedonia in studies of depression, only the SHAPS, FCPS and DARS have undergone empirical scale validation procedures in MDD samples. Although the SHAPS only measures consummatory pleasure, it does so across several domains and with limited cultural biases. Considering consummatory reward is not consistently impaired in depression, the SHAPS may be useful to identify subgroups within MDD or test specific effects of treatment, but may not have the sensitivity to be utilized as a predictor of treatment response. Further study is needed to determine whether deficits in reward responding are dependent on reward type (e.g. social, food, physical), for which the DARS may be able to tease apart any potential differences given its component structure is based on reward type. Most importantly, in light of emerging evidence indicating that different facets of reward processing are subserved by partially dissociable neurobiological systems (Der-Avakian & Markou, 2012), it will be imperative for future scale development to evaluate desire, motivation, effort, and consummatory pleasure across different domains of anhedonia, as well as measuring the stability of the construct.
Behavioural Tasks
Various tasks have been developed to objectively assess facets of reward processing in MDD. These include paradigms probing anticipation and consumption of positive stimuli, delay discounting, reward response bias, prediction error, reversal learning, and effort to acquire rewards (Table 3). For the purposes of this review, we will discuss how tasks can be used to evaluate the following aspects of reward function: association/valuation, expectation, anticipation, effort, outcome, and feedback integration (see Figure 1). Interest will not be discussed in this section as at present, levels of interest are probed using questionnaire based methods as opposed to behavioural tasks.
Table 3. Behavioural tasks to measure facets of anhedonia in depression.
| Task | Anhedonia process measured | Reward | Studies in MDD |
|---|---|---|---|
| Delay Discounting |
|
Money |
Pulcu et al, 2013 Takahashi et al, 2008 Dombrovski et al, 2012 Lempert & Pizzagalli, 2010 |
| Monetary Incentive Delay |
|
Money |
Knutson et al, 2008 Dichter et al, 2012 Ossewaarde et al, 2011 Pizzagalli et al, 2009 Stoy et al, 2012 |
| Gambling Paradigms |
|
Money |
Capuron et al, 2012 Must et al, 2013 |
| Reward Response Bias |
|
Money |
Henriques & Davidson, 2000 Pizzagalli et al, 2005 Pizzagalli et al, 2008 Vrieze et al, 2013 Pechtel et al, 2013 Liu et al, 2011 |
| Prediction Error |
|
Money, water |
Kumar et al, 2008 Forbes et al, 2009 Cohen et al, 2009 Gradin et al, 2011 Chase et al, 2013 |
| Probabilistic Reversal Learning |
|
Money |
Murphy et al, 2003 Taylor Tavares et al, 2008 Hasler et al, 2009 |
| Effort |
|
Money, humour |
Treadway et al, 2012 Sherdell et al, 2012 |
Association and Valuation
At the outset of the reward process, association of a stimulus with a reward needs to be established in addition to a value judgment on the rewarding properties of a stimulus. The ability to make reward associations is best tested using reward response bias tasks, while valuation of future reward can be tested using delayed discounting.
Reward Response Bias
Tasks based on signal detection theory measure a participant's ability to accurately discriminate between target and nontarget stimuli (“discriminability” or “sensitivity”), as well as the tendency to define an ambiguous stimulus as the target (response bias) (Henriques & Davidson, 2000). When the reward associated with correctly identifying a stimulus is manipulated, there is a change in response bias towards the stimulus associated with the larger (or more frequent) reward. Response bias is a key component of signal detection theory and allows investigators to assess disorder-related sensitivity to reward or punishment. Findings of a lack of response bias towards reward in depression are interpreted as reflecting impairment in an approach-related positive affect system (low reward engagement) (Pizzagalli et al, 2008).
The stimuli and reward contingencies employed in signal detection tasks can vary. For example, Henriques & Davidson (2000) used word recognition as the discriminating stimulus. Individuals with MDD and healthy controls were given three blocks of trials (neutral, reward and punishment) and viewed a series of target words, followed by a color distraction task, and then discrimination trials. Participants were presented with the reward contingencies at the beginning of each block (no reward in neutral block, $0.10/correct word identification in reward block, deduction of $0.10/failure to identify target word in punishment block), so they did not have to learn reward contingencies. Relative to healthy controls, MDD participants showed a significantly lower response bias during the reward condition, and therefore, failed to maximize their earnings. Highlighting the specificity of these findings, there was no group effect observed for the punishment condition (Henriques & Davidson, 2000).
Subsequently, Pizzagalli and colleagues (2005, 2008) extended this research using a different task to evaluate response bias as a function of reward, depression and anhedonia (see also Tripp and Alsop, 1999). During each trial a mouth-less cartoon face is presented, followed by a brief presentation (100 ms) of a long or short mouth. The goal is to identify whether the mouth presented was long or short. Unbeknownst to the participants, correct identifications for one stimulus (e.g., the long mouth) are rewarded with monetary rewards three times more frequently than correct identification of the other stimulus. Among healthy controls, such differential reinforcement schedules elicit a reliable response bias (i.e., a preference for the stimulus that has been paired with more frequent reward). Individuals with elevated depressive symptoms were characterized by blunted response bias (Pizzagalli et al., 2005). While this effect was correlated with levels of anhedonia based on the melancholic subscores of the Beck Depression Inventory (BDI; Beck et al, 1961), they did not correlate with CPAS or CSAS scores. Test-retest reliability of this task was satisfactory. In subsequent studies a similar pattern of results emerged whereby MDD patients demonstrated reduced reward learning associated with high levels of anhedonia (Pizzagalli et al., 2008; Vrieze et al, 2013). Reduced reward learning also predicted poor antidepressant response after 8 weeks (Vrieze et al, 2013), and was observed in fully remitted MDD patients compared to healthy controls (Pechtel et al, 2013), suggesting that blunted reward learning might be a trait-like characteristic of depression. Of note, Pizzagalli and coworkers recently developed a rodent version of this task, which is conceptually analogous to that used in humans, opening new avenues for cross-species translational research (Der-Avakian et al., 2013; Pergadia et al., 2014).
The main differences between the Henriques & Davidson (2000) and Pizzagalli et al (2005) tasks pertain to the stimuli used as well as the reward contingencies. The first task described used word recognition as the targets, while the second task used discrimination of a non-verbal stimulus. Consequently, the Henriques & Davidson task is more susceptible to confounding due to the verbal working memory impairment observed in depression (Walsh et al, 2007); non-affective visual discrimination impairment is not consistently reported in MDD (Feinberg et al, 1986). Furthermore, in the word recognition task, the reward contingency for a correct response was known for all trials, while in the cartoon face task, participants had to learn which response was associated with greater reward. Therefore, the second task incorporates probabilistic reward learning. Moreover, whether participants know about probabilistic contingencies may not impact the lack of response bias to reward in MDD (Liu et al, 2011). Importantly, for signal detection tasks the reward is always fixed (unlike for delay discounting and other probabilistic reward tasks), limiting the ability to assess the effect of depression on subjective evaluation of different reward magnitudes.
Delay discounting
Decision making concerning future reward is another aspect of reward function that can be measured in depression. Delay discounting is a phenomenon where the value of a future reward decreases as the time to acquiring it increases (Green et al, 1997). It is typically measured using a monetary choice task of immediate versus delayed reward (“would you prefer $2 now or $10 in 30 days”) (Kirby et al, 1999; Richards et al, 1999). Monetary values and time delays vary across studies; in some studies a range of reward magnitudes is used (Pulcu et al, 2013), while in others a fixed reward value is used (Lempert & Pizzagalli, 2010); however, the immediate reward is always smaller than the delayed reward.
Indeed, the extent to which individuals value future reward varies considerably and can be acutely affected by different factors. The main issue regarding these tasks is that high impulsivity and low working memory capacity have been noted to result in a bias towards immediate reward, thereby confounding the results (Crean et al, 2000; Li et al, 2013; Hinson et al, 2003). However, impulsivity as assessed on established personality scales in healthy controls did not affect delayed discounting (McLeish & Oxoby, 2007), and a preclinical study where impulse control in rats was decreased by inactivation of the ventral medial prefrontal cortex did not affect delayed discounting (Feja & Koch, 2014). This suggests impulsivity, in particular, may only relate to discounting rates in subsamples of individuals. Still other studies have shown that the uncertainty of the future and psychosocial stress can both result in increased delayed discounting (Kimura et al, 2013; Lempert & Pizzagalli, 2010).
In depression, there have been few studies of delayed discounting, although they consistently show an increased discounting rate in MDD compared to healthy controls (Pulcu et al, 2013; Dombrovski et al, 2012; Takahashi et al, 2008), demonstrating a preference for immediate rewards. This effect was sustained between acutely depressed patients compared to remitted MDD patients and healthy controls, whereby depressed patients demonstrated increased discounting of large future rewards (Pulcu et al, 2013). This suggests that preference for immediate smaller rewards may be a state phenomenon in depression for at least a subset of patients. Interestingly, higher levels of anhedonia (rated by the SHAPS) among a student sample were associated with reduced discounting of large future reward even after controlling for working memory capacity and impulsiveness (Lempert & Pizzagalli, 2010). The authors propose this may be due to a decreased responsiveness to immediate reward in anhedonic individuals.
Anticipation
Many tasks can be modified to evaluate anticipation of reward. Typically this is measured using brain imaging during the delay just before a reward is received. The monetary incentive delay (MID) task and gambling paradigms are frequently used for this purpose.
Monetary Incentive Delay
Developed by Knutson and colleagues (2000), the monetary incentive delay (MID) task was designed to disentangle anticipatory vs. consummatory phases of reward processing, and has been extensively used across clinical and non-clinical populations in conjunction with functional neuroimaging. It involves three trial types where participants gain (reward trial), lose (punishment trial), or no win/lose (no-incentive trial). Each trial requires participants to quickly respond to a target preceded by an incentive cue and followed by feedback. For example, participants receive a reward in a reward trial or avoid losing in a punishment trial if they respond to the target within an individually titrated response window. In healthy individuals, increased striatal activity during reward anticipation has been reported (Knutson et al, 2000; Kumar et al, 2014; Dillon et al, 2008), as well as increased activity in the anterior cingulate (Dillon et al, 2008). Similar to healthy controls, depressed patients demonstrate increased activation in the anterior cingulate gyrus during anticipation (Knutson et al, 2008), an effect that persists in remission (Dichter et al, 2012). However, striatal activity is not consistently observed (Knutson et al, 2008; Dichter et al, 2012; Stoy et al, 2012). In two reports, decreased ventral striatal activity was normalized with an SSRI or SNRI (Ossewaarde et al, 2011; Stoy et al, 2012). With respect to consummatory reward outcome, in healthy controls increased activation in the orbitofrontal cortex and medial prefrontal cortex is observed (Knutson et al 2001; Dillon et al, 2008), while in depressed patients in a current episode outcomes are associated with decreased nucleus accumbens and caudate activity (Pizzagalli et al, 2009); among individuals with remitted depression, activation in response to reward outcome is blunted in the orbitofrontal cortex, insula and thalamus (Dichter et al, 2012). Limitations of task-based neuroimaging using the monetary incentive delay task are the assumption that anticipation and consummatory pleasure are temporally independent and that the task elicits these reward processes in all participants, which can lead to inconsistencies in findings.
Gambling Paradigms
These tasks can be used to evaluate several aspects of reward function including risk-taking, anticipation of reward, hedonic response to reward and punishment, prediction error, and feedback integration (Steele et al, 2007; Capuron et al, 2012; Adida et al, 2011). One of the most common gambling tasks is the Iowa Gambling Task (IGT) (Bechara et al, 1994), which involves choosing a card from 4 decks of cards. Two decks are associated with high gains but unpredictable high losses, while the other two decks are associated with smaller reward and lower losses over time (advantageous deck). A variant of this task also includes the reverse, where two decks are associated with high loss but periodic and unpredictable high reward, while the other two are associated with smaller loss (Bechara et al, 2000). Findings using the IGT in depression show that MDD patients are less likely to choose from the advantageous deck and fail to adjust their choices based on feedback, preferring immediate high short-term reward (Must et al, 2013), consistent with the delay discounting literature described above. Furthermore, in gambling tasks designed to measure hedonic response to gains, decreased activation in the ventral striatum to reward was correlated with greater anhedonia levels (Capuron et al, 2012). As with delay discounting limitations, it is difficult to dissociate risky behaviour/impulsivity from a desire for immediate reward.
Expectation
Expectation of reward can be based on available probability information or on previous stimulus-reward associations. The latter is the most frequently assessed in terms of prediction error - the brain signal that results from unexpected reward outcomes.
Prediction Error
Prediction error has been studied with respect to dopamine neuronal functioning in corticolimbic and nigrostriatal circuits (reviewed in Schultz, 2013). Research in this area has demonstrated that dopamine activity in response to reward acts as a metric to evaluate whether a stimulus is a reward (in the orbitofrontal cortex), as well as whether the expected reward was received (in the ventral striatum). Prediction error occurs when the reward magnitude is not accurately predicted: specifically, a phasic burst of dopamine is observed when the outcome is better than expected, whereas a dopamine “dip” is seen when the outcome is worse than expected. There is no response when a reward is fully predicted. Given growing evidence to support dopaminergic deficits in MDD (Dunlop & Nemeroff, 2007; Pizzagalli, 2014), the adaptation of this task in human models provides an opportunity to more fully understand deficits in reward learning among MDD patients. Several paradigms have been used to test prediction error in depression (Kumar et al, 2008; Cohen et al, 2009; Gradin et al, 2011; Steele et al, 2007; Chase et al, 2013;). The common thread among the tasks is to have a learned reward contingency change where the received reward is greater or less than expected.
Initial studies used a primary reward by depriving participants from water the night before and delivering drops of water when they correctly identified which of two pictures predicted receiving water. Participants were told the picture predicting outcomes might change (Kumar et al, 2008; Gradin et al, 2011). Water was delivered based on a probability contingency whereby one picture was associated with high reward probability (60-90%), and the other was associated with low probability (0-20%). Importantly, these associations changed over 100 trials to elicit a prediction error signal. In both studies prediction error signal in MDD patients was blunted compared to healthy controls (Kumar et al, 2008; Gradin et al, 2011). In addition, there were differences between MDD and schizophrenia patients, whereby MDD patients demonstrated reduced activation in striatal and midbrain regions that correlated with anhedonia severity, while schizophrenia patients demonstrated decreases in the caudate, thalamus, insula, amygdala and hippocampus that correlated with psychotic symptoms (Gradin et al, 2011).
A card guessing game task has also been employed to evaluate prediction error in depression (Chase et al, 2013; Forbes et al, 2009), which was adapted from Delgado and colleagues (2000) to probe striatal response to feedback. Participants can earn $1, lose $0.50 or win nothing: there are win trials (expectation of a win, followed by win), disappointment trials (expectation of win followed by no reward), loss trials (expectation of loss, followed by loss), or relief trials (expectation of loss followed by no loss). This task does not involve a learning component, so is more suited to assess feedback integration than prediction error which is dependent on developing a cue/reward or cue/punishment association. This may be why there were no prediction-related differences among MDD, bipolar and healthy controls, despite demonstrating differences across groups during reward-expectancy and anticipation (Chase et al, 2013).
A further modification to this task involves separating acquisition of reward contingencies and already learned associations (Cohen et al, 2009). Based on preclinical studies (Morris et al, 2006), this task features two phases: learning and choosing. In the learning phase, participants click a left or right button in response to a left or right visual cue, respectively, after which they were given feedback about winning either $.06 (safe trial with 100% contingency) or winning or losing $0.12 (risky trial with 75% chance of reward and 25% chance of loss). Once this phase is complete, the choosing phase begins with participants freely selecting one of the two cues presented to them from the first phase, which sometimes results in winning money and sometimes not. The reward contingencies are the same, although participants are not informed about this. MDD patients demonstrated greater nucleus accumbens activity to risky compared to safe cues, consistent with prediction error findings (Schultz et al, 1998). However, nucleus accumbens activity during the choose phase only occurred during feedback presentation, which the authors suggest reflects that the participants have already learned the task and so the nucleus accumbens is not needed (Cohen et al, 2009).
Effort
Effort-Expenditure for Rewards (EEfRT)
The EEfRT task (Treadway et al., 2009) was developed based on two streams of research: (1) Depression is not consistently associated with deficits in consummatory pleasure, but may be more related to anticipation or reward cost/benefit decision making, and (2) animal research has demonstrated neurobiological differences in the components of reward (e.g. anticipation, consummatory reward), whereby low dopamine levels appear to bias animals towards low rewards requiring low effort, and high levels of dopamine bias animals towards high rewards requiring high efforts (Salamone et al, 2007). There is also greater dopamine release in the nucleus accumbens when high cost/high rewards cannot be accurately predicted, but this effect is not observed for consummatory pleasure. Therefore, the dopaminergic dysfunction observed in depression (Dunlop & Nemeroff, 2007), would not be detected by the majority of reward tasks that emphasize consummatory reward response.
Based on the animal task developed by Salamone and colleagues (2007), the EEfRT task (Treadway et al, 2009) allows analysis of the predictive value of reward probability and magnitude on effort-based decision making. This computerized task lasts 20 minutes and has multiple trials, in which participants have 5 seconds to choose whether they want to perform an “easy” or “hard” task for monetary reward. The easy trials last a total of 15 seconds, require 30 button presses in 7 seconds (using a dominant finger) and are always associated with winning the same amount ($1.00) (low cost/low reward). The hard trials are twice as long, require 100 button presses in 21 seconds (using a non-dominant finger) and are associated with winning varying amounts of monetary reward ($1.24-$4.30) (high cost/low or high reward). Since participants are not guaranteed to win all trials, one of three probabilities of winning the trial (12%, 50%, and 88%) along with the monetary value associated with the easy vs. hard task is presented prior to choosing. Participants receive “win/no win” feedback once the trial is completed. The number of variables in the task and the speed with which participants have to choose limit the ability to develop a strategy for optimal performance, which helps to ensure that the task is measuring willingness to expend effort for a reward value.
In the initial study of this task in healthy volunteers, those with higher levels of anhedonia (based on the CPAS, melancholy subscale of the BDI and negative affect on the Positive and Negative Affect Schedule) were less willing to expend effort for rewards, an effect that was particularly pronounced when the uncertainty of obtaining the reward was highest (Treadway et al, 2009). The SHAPS, which as described above measures consummatory pleasure, did not correlate with task performance, further suggesting a distinction between reward-related effort and hedonic capacity. In a subsequent study in MDD vs. healthy controls, MDD participants were less likely to choose the high cost/high reward tasks and both probability and magnitude of reward were more predictive of choosing hard tasks among healthy controls (Treadway et al, 2012).
Importantly, a gender effect was noted in both studies, where men were more likely to choose high cost/high reward options. It is unclear whether this effect is related to aspects of the task or sex differences in effort-based decision making. As a component of anhedonia, effort to acquire reward may be correlated with general levels of energy. Consequently, it follows that in the EEfRT task performance was associated with physical anhedonia.
Other effort-based tasks
Sherdell and colleagues (2012) employed an effort-based task in MDD patients modified from Waugh and Gotlib (2008). Instead of using money as a reward, they used cartoons. The authors contend that humour does not have an inherent anticipatory effect as money nor does it have the satiety effects of juice, and therefore allows for a more precise evaluation of consummatory pleasure that can be measured over many trials.
Participants viewed two series of cartoons and rated their level of enjoyment. Subsequently, they had to choose to see a cartoon from one of the series again. The enjoyed cartoon was associated with greater expenditure of effort based on mouse clicks. Overall, there were no differences in in consummatory pleasure between MDD participants and healthy controls. However, among healthy controls, levels of cartoon liking predicted motivation to expend effort, while in MDD liking and motivation were dissociated (Sherdell et al, 2012). Furthermore, among MDD participants, levels of anticipatory pleasure predicted likelihood to expend effort for cartoons. However, it should be noted that the method to evaluate anticipation using a question from the Hamilton Rating Scale for Depression, is not solely a representation of anticipation.
Finally, Clery-Melin and colleagues (2011) developed an incentive force task to evaluate the effects of monetary incentive and emotional arousal on grip force in MDD patients and healthy controls. Participants viewed an emotional picture (positive, neutral, negative) and subsequently had to squeeze a grip for money (0.01, 0.1 and 1€). The authors reported a lack of money value on grip force in patients, while emotionally arousing pictures (irrespective of valence) did predict force production. These findings highlight an impairment in incentivized motivation in MDD. It also supports a distinction among stimulus types in generating effort to obtain a reward.
Outcome
The pleasurable endpoint of reward (consummatory pleasure) can be evaluated with most paradigms. Gambling and MID tasks, as described above are useful for assessing outcome with reward. Response to positive stimuli and the sweet taste test have also been used to evaluate reward outcome.
Evidence suggests that there is an overall affect dysregulation in depression that impairs response to both positive and negative stimuli. Some studies show a lack of recognition of happy faces and reduced activation to positive pictures, while others support an attentional bias to negative stimuli (Harmer et al, 2009; Leppanen, 2006; Bourke et al, 2010). Most commonly, the negative and positive stimuli are emotional faces (happy, angry, fearful), or emotional pictures with varying levels of salience (happy, neutral, sad) (reviewed in Pizzagalli, 2014). These tasks may reflect attentional biases or general emotional dysregulation rather than reward response.
The sweet taste test (STT) represents a direct human translational task to the sucrose task in animals. It involves having participants taste different concentrations of a sucrose solution and then rate them based on intensity and pleasantness (Kampov-Polevoy et al, 2006). Contrary to the traditional conceptualization of anhedonia as a “loss of pleasure,” the few studies that have been conducted using the SST or a variant of this task mostly show no difference in hedonic response to sucrose in MDD participants compared to healthy controls (Berlin et al, 1998; Dichter et al, 2010; McCabe et al, 2009). However, while ratings may not have been different, McCabe and colleagues (2009) reported decreased activation in the ventral striatum in response to tasting chocolate in depressed patients compared to healthy controls, indicating impairment in reward circuitry may exist without self-reported reduced liking.
An important distinction with respect to consummatory pleasure is related to “relative anhedonia”. This concept reflects the likely phenomenon that the liking of a stimulus is dependent on the reward value of a stimulus experienced before it. For example, liking of a neutral stimulus might be rated higher if it was preceded by an unpleasant stimulus versus a pleasurable one. Because depression is often characterized by a prolonged low-level of reward in daily life, absolute consummatory pleasure might be blunted in MDD. Consequently, patients may report higher “liking” of stimuli in reference to their low reward experience in a depressed state, as opposed to their higher reward experience when not depressed. This could result in inflated liking scores in depressed individuals that are not representative of the overall reduction in consummatory pleasure. [We thank an anonymous reviewer for raising this point].
Feedback Integration
The ability to integrate information regarding a rewarding stimulus is important in order to maintain updated and accurate associations and expectations of reward. Prediction error tasks and gambling paradigms can both be used to evaluate feedback integration, however, probabilistic reversal learning is a simple interpretable task to evaluate this construct.
Probabilistic reversal learning
There is substantial evidence of general cognitive impairment in MDD (Gualtieri et al, 2006; McClintock et al, 2010; Porter et al, 2003), although these deficits are not specific to depression. However, there are data to suggest that individuals with MDD are impaired in integrating negative feedback in order to optimize their performance on a task, an effect that may be depression specific (Elliott et al, 1997). Probabilistic reversal learning tasks were developed to evaluate the effects of feedback on reward response (Budhani & Blair, 2005; Swainson et al, 2000). Again tasks can vary, but the common elements are that participants are presented with a choice of two stimuli (e.g., pictures, two types of colored lines). Based on their first choice, the selected stimulus will be rewarded with a high probability, while the other will be rewarded with a low probability. Participants are asked to choose the one that most frequently is associated with reward and to continue choosing that stimulus despite any potential loss trials they may incur. They are also told that this rule may reverse, and thus the other stimulus might become more frequently rewarded, which should prompt them to select such stimulus. MDD patients demonstrate hypersensitivity to negative feedback and are more likely than healthy controls as well as depressed bipolar patients to switch to the incorrect stimulus following negative reinforcement (Murphy et al, 2003; Taylor Tavares et al, 2008). This effect was not observed in remitted MDD patients who underwent catecholamine depletion (Hasler et al, 2009), indicating that the effects of negative feedback is a state phenomenon of MDD.
Behavioural Tasks Summary
Tasks to measure aspects of anhedonia primarily tap into reward learning as opposed to the experience of desire, anticipation, motivation, effort and pleasure. The monetary incentive delay task, as well as the gambling task can be utilized to parse anticipation and consummatory pleasure, while the EffRT task maps onto the experience of effort. In all three of these tasks, MDD is characterized by impaired performance, which is exacerbated by the presence of anhedonia as measured by various symptom scales. In some studies, however, the SHAPS did not correlate with task performance, which is not surprising given the focus of this scale on consummatory pleasure. Critically, the “type” of anhedonia measured symptomatically may be relevant to the task at hand. For example, reduced effort in MDD was associated with physical anhedonia (Treadway et al, 2012). Neuroimaging data suggest that there is a lack of striatal activity during anticipation in MDD (Knutson et al, 2008; Dichter et al, 2012), although activity in this region is triggered during consummatory pleasure. Interestingly, anhedonia is associated with decreased striatal volume in MDD patients (Pizzagalli et al, 2009). In contrast, healthy controls recruit striatal activation during anticipation (Kumar et al, 2014; Dillon et al, 2008), and cortical regions during consummatory pleasure (Dillon et al, 2008).
A few key considerations with the tasks described should be highlighted. Firstly, they have not all been adequately validated. As a scale must undergo the rigors of validation prior to use, so should behavioural tasks in order to ensure the appropriate constructs are being evaluated and to ascertain the stability of repeated testing. Secondly, they mostly use secondary reward (money) as an incentive. This limits translation to and from animal models. In addition, tasks used in humans and animals are considerably different, which also hinders translation (for exceptions, see Der-Avakian et al., 2013 and Treadway et al., 2009 for two tasks that probe reward learning and effort-based decision making, respectively, in ways that are conceptually identical across species). Lastly, while there are many aspects of anhedonia and reward learning that can be tested, it will be difficult to have a task that is able to measure all components of reward effectively, both theoretically and logistically. Tasks that are overly complex are likely to be hindered by participants' learning effects or technical ability considering the vast majority of tasks are computerized. However, it is essential to systematically evaluate the different components of anhedonia in order to determine what features are episode- (state) versus disorder- (trait) specific. For example, response bias to reward appears to persist in remitted patients (Pechtel et al, 2013), while delayed discounting and the effects of negative feedback do not (Hasler et al, 2009; Pulcu et al, 2013).
Conclusion
While anhedonia has been evaluated in preclinical models for decades, behavioural measurement in humans is relatively nascent. However, anhedonia mechanisms may be a promising area for biomarker research in MDD, since they map onto specific and partially dissociable neurocircuitry and signaling pathways (Der-Avakian and Markou, 2012; Pizzagalli, 2014). It is important to emphasize that an ideal biomarker will be clearly linked to a clinical phenotype, which is why the development of scales and tasks in concert would be a useful endeavor. Currently used anhedonia questionnaires do not always correlate with reward task performance, which follows considering some scales only measure consummatory pleasure, or domains of anhedonia (e.g., physical vs. social), whereas tasks evaluate specific aspects of reward function (e.g. prediction error, anticipation). Additionally scales, albeit valuable, do not allow the disentanglement of different cognitive processes despite a similar outcome, and require introspection. For example, imagining versus remembering a pleasurable experience could have the same subjective hedonic effect, although via different mechanisms and activation of neural pathways. Therefore in order to discern the neurobiological correlates of anhedonia, combining behavioural tasks with neuroimaging is particularly relevant. To this end, task components need to be clearly distinguished in order to interpret functional imaging data. Furthermore, task development must demonstrate utility in depression as well as test-retest reliability.
As a final note, individuals with MDD who are anhedonic appear to respond differently than healthy controls to reward tasks, a finding that needs to be further evaluated in both human and animal studies. Development of preclinical models of depression that distinguish anhedonic animals from non-anhedonic animals would also provide more face validity when testing drug compounds, such as SSRIs, for which anhedonia is a predictor of non-response in human studies (Uher et al, 2011; McMakin et al, 2012). Similarly in human tasks, enriching study samples with anhedonic versus non-anhedonic participants from the outset may provide a clearer picture of the behavioural and neurocircuitry phenotype of MDD with anhedonia. For example, overall participants with MDD tend to prefer immediate smaller reward (ref.), while individuals with anhedonia do not (Lempert & Pizzagalli, 2010).
Measurement of anhedonia in depression is a complex process as it encompasses aspects of personality, learning and biases. Future research should focus on and prioritize direct translational models of anhedonia in depression, more refined scale and task development, as well as evaluation of potential differences between primary and secondary rewards. For increased generalizability of findings, it will be important to devise consistent methodology across studies.
Acknowledgments
The authors would like to thank Anna Cyriac for research support, as well as Dr. Lena Quilty and Dr. Jonathan Downar for advice and discussion related to the present work.
Footnotes
Conflict of Interest: SJR has no conflicts to report. Over the past three years, DAP has received consulting fees from Otsuka Pharmaceutical and Pfizer for projects unrelated to the present review. BAS has no conflicts to report. SHK has received grant/research support from: Bristol Myers Squibb, Canadian Institutes of Health Research, Clera Inc., Eli Lilly, GlaxoSmithKline, Janssen Ortho, Lundbeck, Ontario Brain Institute, Servier and St. Jude Medical. He is a consultant to AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Lundbeck, Pfizer, Servier, and St. Jude Medical.
References
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–65. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Aguilar MA, Rodríguez-Arias M, Miñarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–77. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME. Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–22. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacol. 2010;35:1290–301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck SM, Locke HS, Savine AC, Jimura K, Braver TS. Primary and secondary rewards differentially modulate neural activity dynamics during working memory. PLoS One. 2010;5:e9251. doi: 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Novelty reward as a measure of anhedonia. Neurosci Biobehav Rev. 2005;29:707–14. doi: 10.1016/j.neubiorev.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry. 2010;44:681–96. doi: 10.3109/00048674.2010.496359. [DOI] [PubMed] [Google Scholar]
- Budhani S, Blair RJ. Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J Child Psychol Psychiatry. 2005;46:972–81. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychol Assess. 2004;16:244–54. doi: 10.1037/1040-3590.16.3.244. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–53. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GD, White NM. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sciences. 1983;33:2551–2557. doi: 10.1016/0024-3205(83)90165-0. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. J Personality Soc Psychol. 1994;67:319–333. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–82. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15:839–54. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T, Bisgaard CF, Wiborg O. Biomarkers of anhedonic-like behavior, antidepressant drug refraction, and stress resilience in a rat model of depression. Neuroscience. 2011;196:66–79. doi: 10.1016/j.neuroscience.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Clark DC, Fawcett J, Salazar-Grueso E, Fawcett E. Seven-month clinical outcome of anhedonic and normally hedonic depressed inpatients. Am J Psychiatry. 1984;141:1216–20. doi: 10.1176/ajp.141.10.1216. [DOI] [PubMed] [Google Scholar]
- Cléry-Melin ML, Schmidt L, Lafargue G, Baup N, Fossati P, Pessiglione M. Why don't you try harder? An investigation of effort production in major depression. PLoS One. 2011;6:e23178. doi: 10.1371/journal.pone.0023178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Neuroelectric signatures of reward learning and decision-making in the human nucleus accumbens. Neuropsychopharmacol. 2009;34:1649–58. doi: 10.1038/npp.2008.222. [DOI] [PubMed] [Google Scholar]
- Collier AD, Echevarria DJ. The utility of the zebrafish model in conditioned place preference to assess the rewarding effects of drugs. Behav Pharmacol. 2013;24:375–83. doi: 10.1097/FBP.0b013e328363d14a. [DOI] [PubMed] [Google Scholar]
- Crean JP, de Wit H, Richards JB. Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Exp Clin Psychopharmacol. 2000;8:155–62. doi: 10.1037//1064-1297.8.2.155. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, D'Souza MS, Pizzagalli DA, Markou A. Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry. 2013;3:e297. doi: 10.1038/tp.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27:859–63. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiol. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Med. 2012;42:1203–15. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH. Models of depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Herrod JJ, Robbins TW, Paykel ES. Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J Neurol Neurosurg Psychiatry. 1997;63:74–82. doi: 10.1136/jnnp.63.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry. 1983;40:79–84. doi: 10.1001/archpsyc.1983.01790010081010. [DOI] [PubMed] [Google Scholar]
- Fehnel SE, Bann CM, Hogue SL, Kwong WJ, Mahajan SS. The development and psychometric evaluation of the Motivation and Energy Inventory (MEI) Qual Life Res. 2004;13:1321–36. doi: 10.1023/B:QURE.0000037502.64077.4d. [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Rifkin A, Schaffer C, Walker E. Facial discrimination and recognition in in schizophrenia and affective disorders. Arch Gen Psychiatry. 1986;43:276–9. doi: 10.1001/archpsyc.1986.01800030094010. [DOI] [PubMed] [Google Scholar]
- Feja M, Koch M. Ventral medial prefrontal cortex inactivation impairs impulse control but does not affect delay-discounting in rats. Behav Brain Res. 2014;264:230–9. doi: 10.1016/j.bbr.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–60. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Germans Gard M, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40:1086–1102. [Google Scholar]
- Gooding DC, Pflum MJ. Further validation of the ACIPS as a measure of social hedonic response. Psychiatry Res. 2014;215:771–7. doi: 10.1016/j.psychres.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ. The assessment of interpersonal pleasure: Introduction of the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) and preliminary findings. Psychiatry Res. 2014;215:237–43. doi: 10.1016/j.psychres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Reid I, Hall J, Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–64. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Mem Cognit. 1997;25:715–23. doi: 10.3758/bf03211314. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG, Benedict KB. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. J Neuropsychiatry Clin Neurosci. 2006;18:217–25. doi: 10.1176/jnp.2006.18.2.217. [DOI] [PubMed] [Google Scholar]
- Hamani C, Machado DC, Hipólide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–5. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–84. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav. 1997;63:91–100. doi: 10.1016/s0031-9384(97)00425-3. [DOI] [PubMed] [Google Scholar]
- Hasler G, Mondillo K, Drevets WC, Blair JR. Impairments of probabilistic response reversal and passive avoidance following catecholamine depletion. Neuropsychopharmacology. 2009;34:2691–8. doi: 10.1038/npp.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JP, Bell DJ, Reed TJ, Hagan JJ. Chronic mild stress-induced reductions in saccharin intake depend upon feeding status. J Psychopharmacol. 1997;11:331–8. doi: 10.1177/026988119701100408. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cog Emotion. 2000;14:711–724. [Google Scholar]
- Hewett K, Chrzanowski W, Schmitz M, Savela A, Milanova V, Gee M, Krishen A, Millen L, Leary MO, Modell J. Eight-week, placebo-controlled, double-blind comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. J Psychopharmacol. 2009;23:531–8. doi: 10.1177/0269881108089602. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:410–36. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Hundt NE, Nelson-Gray RO, Kimbrel NA, Mitchell JT, Kwapil TR. The interaction of reinforcement sensitivity and life events in the prediction of anhedonic depression and mixed anxiety-depression symptoms. Personality and Individual Differences. 2007;43:1001–1012. [Google Scholar]
- Huston JP, Silva MA, Topic B, Müller CP. What's conditioned in conditioned place preference? Trends Pharmacol Sci. 2013;34:162–6. doi: 10.1016/j.tips.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Ineichen C, Sigrist H, Spinelli S, Lesch KP, Sautter E, Seifritz E, Pryce CR. Establishing a probabilistic reversal learning test in mice: evidence for the processes mediating reward-stay and punishment-shift behaviour and for their modulation by serotonin. Neuropharmacology. 2012;63:1012–21. doi: 10.1016/j.neuropharm.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Alterman A, Khalitov E, Garbutt JC. Sweet preference predicts mood altering effect of and impaired control over eating sweet foods. Eat Behav. 2006;7:181–187. doi: 10.1016/j.eatbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kasch Karen L, Rottenberg Jonathan, Arnow Bruce A, Gotlib Ian H. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Downar J, Evans KR, Feilotter H, Lam RW, MacQueen GM, Milev R, Parikh SV, Rotzinger S, Soares C. The Canadian Biomarker Integration Network in Depression (CAN-BIND): advances in response prediction. Curr Pharm Des. 2012;18:5976–89. doi: 10.2174/138161212803523635. [DOI] [PubMed] [Google Scholar]
- Kimura K, Izawa S, Sugaya N, Ogawa N, Yamada KC, Shirotsuki K, Mikami I, Hirata K, Nagano Y, Hasegawa T. The biological effects of acute psychosocial stress on delay discounting. Psychoneuroendocrinology. 2013;38:2300–8. doi: 10.1016/j.psyneuen.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biol Psychiatry. 2008;63:686–92. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 2014;24:725–36. doi: 10.1016/j.euroneuro.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Berghorst LH, Nickerson LD, Dutra SJ, Goer FK, Greve DN, Pizzagalli DA. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neurosci. 2014;266C:1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–93. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–9. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Lempert KM, Pizzagalli DA. Delay discounting and future-directed thinking in anhedonic individuals. J Behav Ther Exp Psychiatry. 2010;41:258–64. doi: 10.1016/j.jbtep.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62:1545–58. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology. 2009;56:692–701. doi: 10.1016/j.neuropharm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Li N, Ma N, Liu Y, He XS, Sun DL, Fu XM, Zhang X, Han S, Zhang DR. Resting-state functional connectivity predicts impulsivity in economic decision-making. J Neurosci. 2013;33:4886–95. doi: 10.1523/JNEUROSCI.1342-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WH, Chan RC, Wang LZ, Huang J, Cheung EF, Gong QY, Gollan JK. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1045–52. doi: 10.1016/j.pnpbp.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Llerena K, Park SG, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ. The Motivation and Pleasure Scale-Self-Report (MAP-SR): reliability and validity of a self-report measure of negative symptoms. Compr Psychiatry. 2013;54:568–74. doi: 10.1016/j.comppsych.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–62. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Szczepanik J, Nugent A, Barhaghi K, Rallis D, Herscovitch P, Carson RE, Drevets WC. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33:1706–15. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Sepede G, Gambi F, Di Iorio G, De Berardis D, Di Nicola M, Onofrj M, Janiri L, Di Giannantonio M. Agomelatine versus venlafaxine XR in the treatment of anhedonia in major depressive disorder: a pilot study. J Clin Psychopharmacol. 2012;32:487–91. doi: 10.1097/JCP.0b013e31825d6c25. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–77. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN. Behavioral activation system deficits predict the six-month course of depression. J Affect Disord. 2006;91:229–34. doi: 10.1016/j.jad.2006.01.012. [DOI] [PubMed] [Google Scholar]
- McLeish KN, Oxoby RJ. Measuring impatience: Elicited discount rates and the Barratt Impulsiveness Scale. Pers Individ Dif. 2007;43:553–565. [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51:404–11. 3. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur Neuropsychopharmacol. 1992;2:43–9. doi: 10.1016/0924-977x(92)90035-7. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: the effects of feedback on task performance. Psychol Med. 2003;33:455–67. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- Muscat R, Papp M, Willner P. Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology (Berl) 1992;109:433–8. doi: 10.1007/BF02247719. [DOI] [PubMed] [Google Scholar]
- Must A, Horvath S, Nemeth VL, Janka Z. The Iowa Gambling Task in depression - what have we learned about sub-optimal decision-making strategies? Front Psychol. 2013;4:732. doi: 10.3389/fpsyg.2013.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakonezny PA, Carmody TJ, Morris DW, Kurian BT, Trivedi MH. Psychometric evaluation of the Snaith-Hamilton pleasure scale in adult outpatients with major depressive disorder. Int Clin Psychopharmacol. 2010;25:328–33. doi: 10.1097/YIC.0b013e32833eb5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Arnt J, Sánchez C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: interstrain and interindividual differences. Behav Brain Res. 2000;107:21–33. doi: 10.1016/s0166-4328(99)00110-2. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, Verkes RJ, Hermans EJ, Kooijman SC, Urner M, Tendolkar I, van Wingen GA, Fernández G. Two-week administration of the combined serotonin-noradrenaline reuptake inhibitor duloxetine augments functioning of mesolimbic incentive processing circuits. Biol Psychiatry. 2011;70:568–74. doi: 10.1016/j.biopsych.2011.03.041. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Boyer PA, Mocaër E. Effect of agomelatine in the chronic mild stress model of depression in the rat. Neuropsychopharmacology. 2003 Apr;28(4):694–703. doi: 10.1038/sj.npp.1300091. [DOI] [PubMed] [Google Scholar]
- Papp M, Moryl E, Willner P. Pharmacological validation of the chronic mild stress model of depression. Eur J Pharmacol. 1996;296:129–36. doi: 10.1016/0014-2999(95)00697-4. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47:1864–9. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergadia ML, Der-Avakian A, D'Souza MS, Madden PAF, Heath AC, Shiffman S, Markou A, Pizzagalli DA. Withdrawal of nicotine blunts reward responsiveness in humans and rats. JAMA Psychiatry. 2014;71:1238–45. doi: 10.1001/jamapsychiatry.2014.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. 2009;166:702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress and anhedonia: Toward a synthesis and integrated model. Ann Rev Clin Psychol. 2014 doi: 10.1146/annurev-clinpsy-050212-185606. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RJ, Gallagher P, Thompson JM, Young AH. Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry. 2003;182:214–20. doi: 10.1192/bjp.182.3.214. [DOI] [PubMed] [Google Scholar]
- Pulcu E, Trotter PD, Thomas EJ, McFarquhar M, Juhasz G, Sakian BJ, Deakin JF, Zahn R, Anderson IM, Elliott R. Psychol Med. 2013. Temporal discounting in major depressive disorder; pp. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot T. La psychologie des sentiment. Paris: Felix Alcan; 1896. [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SJ, Quilty LC, Sproule BA, Cyriac A, Michael Bagby R, Kennedy SH. Development and validation of the Dimensional Anhedonia Rating Scale (DARS) in a community sample and individuals with major depression. Psychiatry Res. 2015;229:109–19. doi: 10.1016/j.psychres.2015.07.062. [DOI] [PubMed] [Google Scholar]
- Rothschild AJ, Raskin J, Wang CN, Marangell LB, Fava M. The relationship between change in apathy and changes in cognition and functional outcomes in currently non-depressed SSRI-treated patients with major depressive disorder. Compr Psychiatry. 2014;55:1–10. doi: 10.1016/j.comppsych.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol Biochem Behav. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Schrader GD. Does anhedonia correlate with depression severity in chronic depression? Compr Psychiatry. 1997;38:260–3. doi: 10.1016/s0010-440x(97)90057-2. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–38. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, Doya K. Low-serotonin levels increase delayed reward discounting in humans. J Neurosci. 2008;28:4528–32. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Redouté J, Dreher JC. The architecture of reward value coding in the human orbitofrontal cortex. J Neurosci. 2010;30:13095–104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–96. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J Abnorm Psychol. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara F, Kihara Y, Ide S, Minami M, Kaneda K. Critical role of cholinergic transmission from the laterodorsal tegmental nucleus to the ventral tegmental area in cocaine-induced place preference. Neuropharmacology. 2014;79:573–9. doi: 10.1016/j.neuropharm.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–74. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, Adli M, Bauer M, Heinz A, Ströhle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677–88. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Oono H, Inoue T, Boku S, Kako Y, Kitaichi Y, Kusumi I, Masui T, Nakagawa S, Suzuki K, Tanaka T, Koyama T, Radford MH. Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects--an analysis based on Tsallis' statistics. Neuro Endocrinol Lett. 2008;29:351–8. [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Furey ML, Williams GB, Sahakian BJ, Drevets WC. Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage. 2008;42:1118–26. doi: 10.1016/j.neuroimage.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–8. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28:366–75. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tso IF, Grove TB, Taylor SF. Differential hedonic experience and behavioral activation in schizophrenia and bipolar disorder. Psychiatry Res. 2014;219:470–6. doi: 10.1016/j.psychres.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Farmer A, Maier W, Rietschel M, Hauser J, Marusic A, Mors O, Elkin A, Williamson RJ, Schmael C, Henigsberg N, Perez J, Mendlewicz J, Janzing JG, Zobel A, Skibinska M, Kozel D, Stamp AS, Bajs M, Placentino A, Barreto M, McGuffin P, Aitchison KJ. Measuring depression: comparison and integration of three scales in the GENDEP study. Psychol Med. 2008;38:289–300. doi: 10.1017/S0033291707001730. [DOI] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, Hauser J, Dernovsek MZ, Souery D, Bajs M, Maier W, Aitchison KJ, Farmer A, McGuffin P. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42:967–80. doi: 10.1017/S0033291711001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–94. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Ceccarini J, Pizzagalli DA, Bormans G, Vandenbulcke M, Demyttenaere K, Van Laere K, Claes S. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2013;34:575–86. doi: 10.1002/hbm.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–9. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh ND, Williams SC, Brammer MJ, Bullmore ET, Kim J, Suckling J, Mitterschiffthaler MT, Cleare AJ, Pich EM, Mehta MA, Fu CH. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry. 2007;62:1236–43. doi: 10.1016/j.biopsych.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The STAR*D Project results: a comprehensive review of findings. Curr Psychiatry Rep. 2007 Dec;9(6):449–59. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Gotlib IH. Motivation for reward as a function of required effort: Dissociating the ‘liking’ from the ‘wanting’ system in humans. Motiv Emot. 2008;32:323–330. [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology (Berl) 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willner P, Hale AS, Argyropoulos S. Dopaminergic mechanism of antidepressant action in depressed patients. J Affect Disord. 2005;86:37–45. doi: 10.1016/j.jad.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Winer ES, Veilleux JC, Ginger EJ. Development and validation of the Specific Loss of Interest and Pleasure Scale (SLIPS) J Affect Disord. 2014:152–154. 193–201. doi: 10.1016/j.jad.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A. Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci. 2004;24:4105–12. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


