Abstract
Objective
Acute kidney injury (AKI) is a severe complication of cardiac surgery associated with increased morbidity and mortality, yet AKI classification for neonates remains challenging. We characterized patterns of post-operative fluid overload (FO) as a surrogate marker for AKI and as a risk factor of poor post-operative outcomes in neonates undergoing cardiac surgery.
Design
Retrospective cohort study.
Setting
Single, congenital heart center destination program.
Patients
435 neonates undergoing cardiac surgery with cardiopulmonary bypass from January 2006 through December 2010.
Interventions
None
Measurements and Main Results
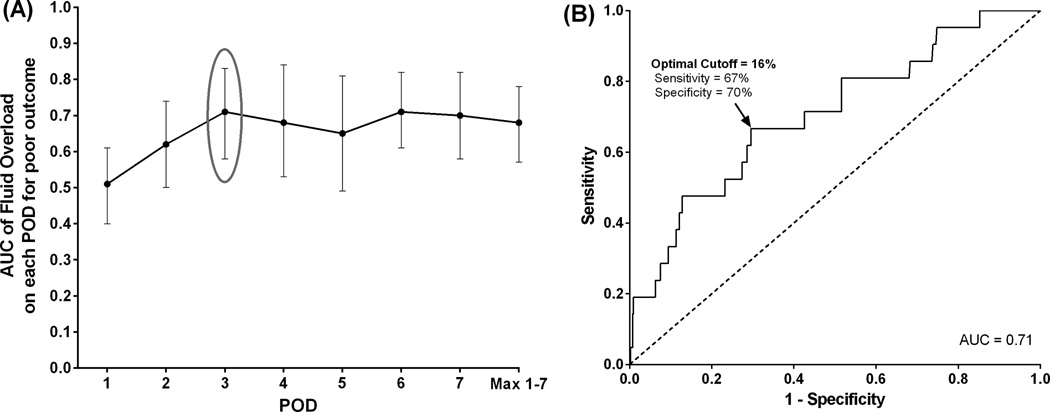
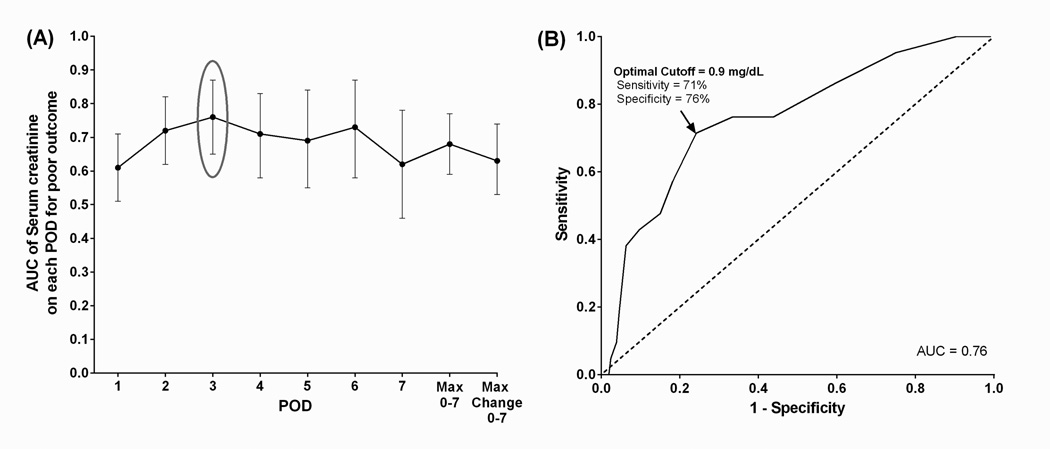
Demographics, diagnosis, and perioperative clinical variables were collected, including daily weights and serum creatinine (SCr) levels. A composite poor clinical outcome (death, need for renal replacement therapy (RRT), or extracorporeal life support (ECLS) within 30 post-operative days) was considered the primary outcome measure. Twenty-one neonates (5%) had a composite poor outcome with 7 (2%) requiring RRT, 8 (2%) requiring ECLS, and 14 (3%) dying between 3 and 30 days post-surgery. Neonates with a composite poor outcome had significantly higher maximum FO (>20%) and were slower to diurese. A receiver-operating characteristic curve determined that FO ≥ 16% and SCr ≥ 0.9 on post-operative day 3 were the optimal cutoffs for significant discrimination on the primary outcome (area under the curve = 0.71 and 0.76, respectively). In multivariable analysis, FO ≥ 16% (adjusted odds ratio [AOR] = 3.7) and SCr ≥ 0.9 (AOR = 6.6) on post-operative day 3 remained an independent risk factor for poor outcome. FO ≥ 16% was also significantly associated with cardiac arrest requiring cardiopulmonary resuscitation, prolonged intensive care unit stay, and chest re-exploration.
Conclusions
This study highlights the importance of monitoring fluid balance in the neonatal cardiac surgical population, and suggests that daily FO, a readily-available, non-invasive marker of renal function, may be a sensitive and specific predictor of adverse outcomes.
Keywords: Fluid Overload, Infant, Newborn, Creatinine, Cardiac Surgical Procedures, Acute Kidney Injury
INTRODUCTION
Acute kidney injury (AKI) is a well-recognized and potentially serious complication of pediatric cardiac surgery, affecting 10–45% of operative patients (1–6). Several published studies have identified risk factors for development of cardiac surgery-associated AKI (CS-AKI) including duration of cardiopulmonary bypass (CPB), age, Risk Adjusted Classification of Congenital Heart Surgery (RACHS-1) score, post-operative hypotension and vasopressor requirement (2, 4–6). Standardization of AKI definitions over the past 10 years with the introduction of Risk, Injury, Failure, Loss, and End-Stage Kidney Disease (RIFLE) (7), pediatric modified RIFLE (pRIFLE) (8) and Acute Kidney Injury Network (AKIN) (9) scoring has allowed for valuable comparisons among studies, contributing to our improved understanding of the epidemiology of AKI (10). The AKIN definition was employed in two recent studies of neonates undergoing biventricular cardiac surgery which found the incidence of CS-AKI to be 62%–64% (11, 12).
The use of serum creatinine (SCr) or urine output (UO) to establish AKI in neonates may be problematic for several reasons. First, it is widely recognized that a rise in SCr may lag by several days following the initial insult. Also, few neonates were included in the pediatric modified RIFLE study (8, 13). Neonates experience rapid changes in glomerular filtration rate (GFR) dependent on level of prematurity and post-natal age (14). In the first few days of life, SCr may be reflective of maternal renal function (15) and over half of documented neonatal AKI cases are non-oliguric (16).
There has been increasing recognition in other patient populations, particularly those requiring renal replacement therapy (RRT), that fluid overload (FO) is associated with morbidity and mortality (17–22). Most studies focus on the level of FO at the time of continuous RRT initiation; however two recent small pediatric studies demonstrated an association between early post-operative FO and worse outcomes following cardiac surgery (23, 24). Data from these studies has prompted clinician awareness of FO as a readily-available, non-invasive marker of renal function.
In this study, we sought to characterize the pattern of post-operative FO and evaluate the degree of FO, a surrogate marker for AKI, as a predictor of poor post-operative outcomes in a large cohort of neonates undergoing cardiac surgery with CPB.
MATERIALS AND METHODS
Study Population
A retrospective cohort study of all neonates (≤ 30 days old) who underwent cardiac surgery with CPB from January 2006 through December 2010 at the University of Michigan Congenital Heart Center was performed. Neonates with a history of previous sternotomy or extracorporeal life support (ECLS) had a higher likelihood of pre-operative renal injury and were thus excluded. This study was approved by the University of Michigan Health System Institutional Review Board.
Data Collection
Subjects were identified using an electronic perfusion database containing all patients undergoing CPB at this institution. Each medical record including demographic, clinical and laboratory data was reviewed. Demographic data included gestational age, age at time of surgery, gender, pre-operative weight, and planned surgical intervention. Prematurity was defined as gestational age less than 37 weeks. Surgical complexity was assigned according to the RACHS-1 consensus based scoring system (25). Additional clinical data included cardiac diagnosis, single-ventricle status, pre-operative SCr level, laboratory values, pre-operative inotropic support, pre-operative and post-operative mechanical ventilation, intra-operative data including duration of CPB, daily weights through post-operative day (POD) 7, need for RRT or ECLS. The most common indication for ECLS is failure to wean from CPB, followed by other indications such as combined respiratory/cardiac failure, low cardiac output syndrome, pulmonary hypertension, shunt occlusion, and respiratory failure.
All patients received routine standard of care in a dedicated cardiac surgery intensive care unit (ICU) during the study period which included the use of dextrose-containing crystalloid solutions (75–100 mL/kg/day) during the first 24–48 hours post-operatively, followed by the initiation of total parenteral nutrition. Bolus furosemide (1 mg/kg/dose q6h) was initiated within the first 24 hours post-operatively. Diruetic dosing was subsequently adjusted by the cardiac intensivist with the goal of achieving a daily net negative fluid balance by post-operative day 3. Indications for RRT included worsening fluid overload, azotemia, uncontrolled metabolic acidosis, and/or electrolyte abnormalities. The decision to initiation RRT was left to the consulting pediatric nephrologist in consultation with the patient’s primary cardiac surgeon and cardiac intensivist.
AKI Classification and FO Calculation
AKI classification was calculated based on the peak SCr any time within the first 72 post-operative hours using the proposed neonatal modification of the AKIN criteria (26). The baseline SCr level for each neonate was defined as the most recent SCr level measured prior to surgery. Weight based daily FO was defined as: [(Daily weight) – (Pre-operative weight)] / (Pre-operative weight) × 100 (27).
Patient Outcomes
Due to relatively low rates of RRT and death, a composite poor clinical outcome was used as primary outcome for analysis, including any of the following: death, need for RRT, or need for ECLS within 30 days of surgery. FO and SCr levels were determined daily for the first 7 PODs. Any patient who experienced a composite poor outcome within the first 48 post-operative hours was excluded from the risk factor analysis due to inability to calculate FO and document SCr level on POD 3. Secondary outcome measures including ICU length of stay (days), cardiac arrest requiring cardiopulmonary resuscitation (CPR), neurologic injury (stroke or seizure) and re-exploration of chest were also examined.
Statistical Methods
Data are presented as frequency (percentage) for categorical variables and median (interquartile range [IQR]) or mean ± standard deviation, as appropriate, for continuous variables. Demographics, clinical characteristics, and other clinical outcomes between neonates with and without a composite poor outcome were compared using Chi-square tests or Fisher’s exact tests for categorical variables, and t-test or Wilcoxon rank sum tests for continuous variables. Using receiver-operating characteristic (ROC) curves, the areas under the curves (AUCs) of each the first seven PODs were compared to determine the most predictive time point for poor outcome. The optimal cutoffs of FO and SCr on the selected POD for predicting poor outcome were then determined based on sensitivity and specificity from each ROC curve. To evaluate independent relations of FO and SCr (dichotomized by their optimal cutoffs) with poor outcome, a multivariable logistic regression was used, controlling for other variables significantly associated with a poor outcome in the univariate analysis (p < 0.05). Unadjusted odds ratios and adjusted odds ratio with their 95% confidence intervals were reported. All analyses were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC), with statistical significance set at p < 0.05 using a two-sided test.
RESULTS
Study Cohort
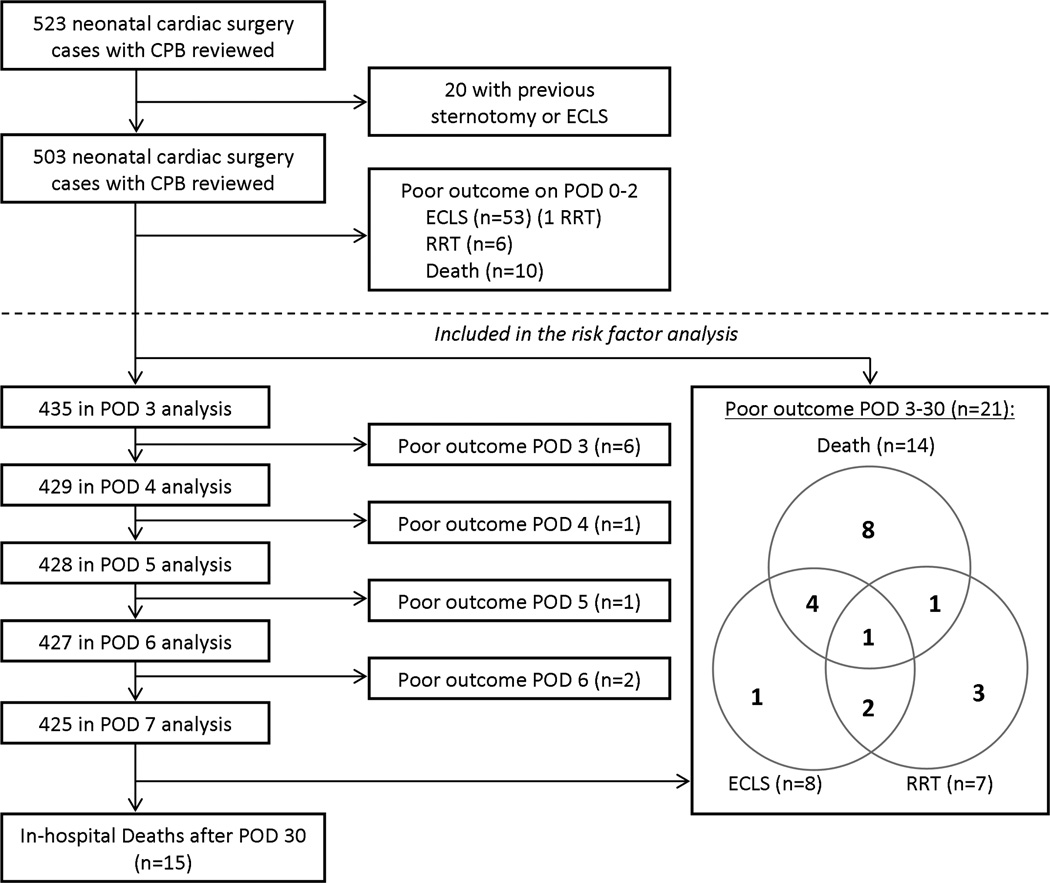
During the study period, 523 neonates underwent cardiac surgery with CPB, 20 of which were excluded due to a history of previous sternotomy or ECLS (Figure 1). Since FO and SCr on POD 3 were determined to be optimal for predicting poor outcome based on the AUCs from the ROC curves, an additional 68 neonates (13%) were excluded from analysis because they died (n=10), required RRT (n=6), or were placed on ECLS (n=53) within 48 post-operative hours. The remaining 435 neonates were included in the analysis.
Figure 1. Study cohort.
Of note, the majority of neonates were of term gestation with only 47 (11%) born prior to 37 weeks gestational age (Table 1). At the time of cardiac surgery, the neonates had a median age of 7 days and a mean weight of 3.2 kg. The median baseline SCr level for the entire cohort was 0.5 mg/dL. Pre-operative mechanical ventilation was required in 207 (48%) neonates and 132 (30%) required pre-operative inotropic support. One hundred, seventy-three neonates (40%) had functional single ventricle physiology, 124 (29%) were RACHS-1 category 5 or 6, and 107 (25%) had hypoplastic left heart syndrome (HLHS). Two hundred forty-one neonates (55%) underwent deep hypothermic circulatory arrest (DHCA) for aortic arch reconstruction. On POD 3, the median FO was 10% (IQR 3.3–18.4), median SCr level was 0.6 mg/dL (IQR 0.5–0.9), and the median peak SCr level during the first 72 post-operative hours was 0.8 mg/dL (IQR 0.7–1.0).
TABLE 1.
Demographic, Surgical, and Clinical Characteristics of All Neonates Following Cardiac Surgery according to Presence or Absence of Poor Outcome
| Poor outcomea | ||||
|---|---|---|---|---|
| Variables | All (n = 435) | Yes (n = 21) | No (n = 414) | p |
| Demographics | ||||
| Male sex | 262 (60.2) | 10 (47.6) | 252 (60.9) | 0.23 |
| Caucasian race | 296 (68.0) | 12 (57.1) | 284 (68.6) | 1.00 |
| Age at surgery (day) | 7 (5–10) | 6 (3–10) | 7 (5–10) | 0.05 |
| Age at surgery ≤ 3 days | 43 (9.9) | 8 (38.1) | 35 (8.5) | < 0.001 |
| Single ventricle | 173 (39.8) | 13 (61.9) | 160 (38.6) | 0.03 |
| RACHS-1 score: 5 or 6 | 124 (28.5) | 9 (42.9) | 115 (27.8) | 0.14b |
| Prematurity (gestational age < 37 week) | 47 (10.8) | 3 (14.3) | 44 (10.6) | 0.49 |
| Kidney abnormality | 32 (7.4) | 2 (9.5) | 30 (7.2) | 0.67 |
| Diagnosis: HLHS | 107 (24.6) | 9 (42.9) | 98 (23.7) | |
| d-TGA | 94 (21.6) | 2 (9.5) | 92 (22.2) | |
| Coarc with VSD | 71(16.3) | 2 (9.5) | 69 (16.7) | |
| TOF | 35 (8.0) | 1 (4.8) | 34 (8.2) | |
| Other | 128 (29.4) | 7 (33.3) | 121 (29.2) | |
| Pre-Operative | ||||
| Pre-operative weight, kg | 3.2 ± 0.6 | 3.1 ± 0.6 | 3.2 ± 0.6 | 0.28 |
| Pre-operative SCr, mg/dL | 0.5 (0.3–0.6) | 0.45 (0.4–0.6) | 0.5 (0.3–0.6) | 0.29 |
| Intubated | 207 (47.6) | 13 (61.9) | 194 (46.9) | 0.18 |
| Inotropic support | 132 (30.3) | 8 (38.1) | 124 (30.0) | 0.43 |
| Intra-Operative | ||||
| Cardiopulmonary bypass time (min) | 94 (69–129) | 115 (82–142) | 93.5 (69–128) | 0.17 |
| Aortic cross-clamp time (min) | 39 (28–58) | 38 (27–55) | 39 (28–58) | 0.92 |
| DHCA used | 241 (55.4) | 16 (76.2) | 225 (54.3) | 0.05 |
| Post-Operative | ||||
| FO on POD 3 (%) | 10.0 (3.3–18.4) | 18.6 (9.1–30.6) | 9.7 (3.0–17.6) | < 0.01 |
| SCr on POD 3 (mg/dl) | 0.6 (0.5–0.9) | 1.0 (0.8–1.3) | 0.6 (0.5–0.8) | < 0.0001 |
| FO ≥ 16 % on POD 3 | 136 (31.3) | 14 (66.7) | 122 (29.5) | < 0.001 |
| SCr ≥ 0.9 mg/dL on POD 3 | 115 (26.4) | 15 (71.4) | 100 (24.2) | < 0.0001 |
| ICU length of stay (day) | 7 (5–13) | 19 (8–28) | 7 (5–12) | < 0.001 |
| Hospital length of stay (day) | 17 (11–26) | 20 (11–37) | 16 (11–25) | 0.40 |
| Cardiac arrest requiring CPR | 34 (7.8) | 12 (57.1) | 22 (5.3) | < 0.0001 |
| Re-exploration of chest | 57 (13.1) | 10 (47.6) | 47 (11.4) | < 0.0001 |
Coarc = coarctation of the aorta, CPR = cardiopulmonary resuscitation, DHCA = deep hypothermic circulatory arrest, d-TGA = d-transposition of great arteries, FO = fluid overload, HLHS = hypoplastic left heart syndrome, ICU = intensive care unit, POD = post-operative day, RACHS-1 = risk adjustment for congenital heart surgery, SCr= serum creatinine, TOF = tetralogy of Fallot, VSD = ventricular septal defect.
For categorical variables, data are presented as N (% of outcome category)
Composite poor outcome includes renal replacement therapy, extracorporeal life support or death on POD 3–30.
Comparison was made as RACHS-1 category 1 to 4 vs. 5 or 6 and p-value was from Chi-square test.
Incidence of and Factors Associated with a Poor Outcome
Twenty-one neonates (5%) experienced a poor outcome during POD 3–30. Neonates with a poor outcome were more likely to be ≤ 3 days of age at surgery, have single ventricle physiology (62% vs. 39%; p = 0.03) and more frequently require DHCA (76% vs. 54%; p = 0.049) compared to those without a poor outcome. Percentage of FO measured on POD 3 was significantly higher in neonates who experienced a poor outcome (18.6% vs. 9.7%; p = 0.002). Likewise, neonates with a poor outcome had a higher SCr level on POD 3 (1.0 mg/dL vs. 0.6 mg/dL; p < 0.0001) and a higher peak SCr level during the first 72 post-operative hours (1.1 mg/dL vs. 0.8 mg/dL; p < 0.001). Gender, RACHS-1 score, prematurity, pre-operative weight, and pre-operative SCr level were not associated with having a poor outcome. While degree of FO peaked on POD 2 in both cohorts, neonates with a poor outcome reached a higher level of FO (median 23% vs. 17%, p = 0.05) and took significantly longer to achieve negative fluid balance (data not shown).
Predictive Ability of Fluid Overload and Serum Creatinine
ROC curves for each post-operative time point of FO and SCr measurement determined the optimal cut-off values of 16% for FO (AUC = 0.71) and 0.9 mg/dL for SCr (AUC = 0.76) on POD 3, each with a negative predictive value of 98% (Figures 2 and 3). Unadjusted OR of poor outcome using the optimal cut-off value FO ≥ 16% was 4.8 (95% CI, 1.9–12.9). Factors significantly differed between the neonates with and without a poor outcome from univariate analysis (p < 0.05) were included in the multivariable model: age at surgery ≤ 3 days, POD 3 FO ≥ 16%, SCr ≥ 0.9 mg/dL, single ventricle, and use of DHCA. Due to its strong correlation with single ventricle physiology (Chi-square statistic = 208, p < 0.0001), HLHS was not included in the multivariable analysis. Multivariable logistic regression demonstrates that age at surgery of ≤ 3 days, POD 3 FO ≥ 16% and SCr level ≥ 0.9 mg/dL still remained independently associated with having a poor outcome, controlling for single ventricle physiology and use of DHCA (Table 2).
Figure 2. Fluid overload and poor composite outcome by post-operative day.
(A) Area under the curve for each of the first seven PODs were compared to determine the most predictive time point for poor outcome. (B) The optimal cutoffs of FO on the selected POD for predicting poor outcome from (A) were then determined based on sensitivity and specificity from the ROC curve.
Figure 3. Serum creatinine and poor composite outcome by post-operative day.
(A) Area under the curve for each of the first seven PODs were compared to determine the most predictive time point for poor outcome. (B) The optimal cutoffs of SCr on the selected POD for predicting poor outcome from (A) were then determined based on sensitivity and specificity from the ROC curve.
TABLE 2.
Odds of Having a Poor Outcomea in Neonates Following Cardiac Surgery
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | pb | AOR | 95% CI | pc |
| Age at surgery ≤ 3 days | 6.61 | 2.45, 17.1 | <0.001 | 5.66 | 1.93, 16.6 | <0.01 |
| Single ventricle | 2.58 | 1.06, 6.65 | 0.03 | 2.74 | 0.96, 7.81 | 0.06 |
| DHCA used | 2.69 | 1.03, 8.34 | 0.05 | 2.67 | 0.85, 8.36 | 0.09 |
| FO on POD 3 ≥ 16% | 4.77 | 1.94, 12.9 | <0.001 | 3.74 | 1.33, 10.5 | 0.01 |
| SCr on POD 3 ≥ 0.9 mg/dL | 7.83 | 3.09, 22.4 | <0.0001 | 6.59 | 2.27, 19.1 | <0.001 |
AOR = adjusted odds ratio, CI = confidence interval, DHCA = deep hypothermic circulatory arrest, FO = fluid overload, OR = unadjusted odds ratio, POD = post-operative day, SCr = serum creatinine.
Composite poor outcome includes renal replacement therapy, extracorporeal life support or death on POD 3–30.
P-value from univariate logistic regression.
P-value from multivariable logistic regression.
Applying the neonatal modified AKIN criteria to our population, 308 (71%) developed CS-AKI, which was not associated with poor outcome (AUC = 0.58, Table 3).
TABLE 3.
Maximum AKIN Stage During First Three Post-operative Days
| Poor outcomea | ORb | 95% CI | p | |||
|---|---|---|---|---|---|---|
| Variables | All (n = 435) | Yes (n = 21) | No (n = 414) | |||
| No AKI | 127 (29.2) | 3 (14.3) | 124 (30.0) | |||
| Stage I | 116 (26.7) | 6 (28.6) | 110 (26.6) | 2.25 | 0.55,9.23 | 0.26 |
| Stage II | 114 (26.2) | 5 (23.8) | 109 (26.3) | 1.90 | 0.44,8.12 | 0.39 |
| Stage III | 78 (17.9) | 7 (33.3) | 71 (17.1) | 4.08 | 1.02,16.3 | 0.047 |
Abbreviations: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; CI, confidence ratio; OR, odds ratio.
Data are presented as N (% of outcome category)
Composite poor outcome includes renal replacement therapy, extracorporeal life support or death on POD 3–30.
Comparison was made as No AKI vs. AKI (Stage I, II, or III) and p-value was from Fisher’s exact test.
Secondary Clinical Outcomes in Neonates following Cardiac Surgery
Neonates with FO ≥ 16% had longer median length of stay both in the ICU (11 days vs. 7 days, p<.0001) and the hospital (20 days vs. 15 days, p<.0001). They were also more likely to experience a cardiac arrest requiring CPR (15% vs. 4%, p<.0001), develop thrombosis (10% vs. 4%, p=0.01), or require re-exploration of the chest (27% vs. 7%, p<.0001) (Table 4). For the patients who did not experience a composite poor outcome on POD 3–30, those with FO ≥ 16% had a higher proportion of death than those with FO < 16% (17% vs. 8%, p=0.01). Similarly, those with SCr ≥ 0.9 had a higher proportion of death than those with low SCr < 0.9 (15% vs. 10%, p=0.13).
TABLE 4.
Association of Fluid Overload and Serum Creatinine on POD 3 with other Clinical Outcomes in Neonates following Cardiac Surgery (n=435)
| Fluid Overload on POD 3 | Serum Creatinine on POD 3 | |||||
|---|---|---|---|---|---|---|
| Characteristics | ≥ 16% (n=136) |
< 16% (n=298) |
pa | ≥ 0.9 mg/dl (n=115) |
< 0.9 mg/dl (n=319) |
pa |
| ICU length of stay | 11 (7–18) | 7 (5–10) | <0.0001 | 10 (6–17) | 7 (5–11) | <0.0001 |
| Hospital length of stay | 20 (15–31) | 15 (10–24) | <0.0001 | 19 (12–36) | 16 (11–25) | 0.02 |
| Cardiac arrest requiring CPR | 21 (15.4) | 13 (4.4) | <0.0001 | 17 (14.8) | 17 (5.3) | 0.001 |
| Thrombosis | 14 (10.3) | 11 (3.7) | 0.01 | 8 (7.0) | 17 (5.3) | 0.52 |
| Re-exploration of the chest | 37 (27.2) | 20 (6.7) | <0.0001 | 19 (16.5) | 38 (11.9) | 0.21 |
Abbreviations: CPR, cardiopulmonary resuscitation; ICU, intensive care unit; POD = post-operative day.
Data are presented as N (%) for categorical variables and Median (25th percentile – 75th percentile) for continuous variables.
P-value from Chi-square test for categorical variables and Wilcoxon Rank Sum test for continuous variables.
DISCUSSION
This study examines the role of post-operative FO in a large cohort of patients undergoing neonatal cardiac surgery with CPB. Previous studies have demonstrated that FO > 10–20% is significantly associated with adverse outcomes in critically ill children (10, 27–28). In our study, all neonates subjected to CPB developed some degree of post-operative FO (median FO = 10%), and FO ≥ 16% was an independent risk factor for worse perioperative outcomes with a higher odds ratio than having a single ventricle or use of DHCA, (OR, 4.77 versus 2.58 or 2.69 respectively.) This parallels the Blinder study findings on the severity of AKI and odds ratio for mortality being higher than single ventricle (1). The degree of FO in our patients was much higher than reported in previous pediatric studies which included few neonatal patients. In a prospective observational study of 49 infants undergoing cardiac surgery, Hazel et al found that early post-operative FO was associated with poor outcomes in infants under 6 months of age, though FO was not a risk factor for poor outcome independent of illness severity based on RACHS-1 score or maximum vasoactive inotropic support (23). Hassinger and colleagues performed a secondary analysis on 98 pediatric cardiac surgery patients aged 2 weeks to 18 years, and found that early post-operative FO (> 5% up to midnight of POD 1) was independently associated with worse post-operative outcomes (24).
The search for a neonatal definition of AKI remains a challenge for the research community. Ricci and Ronco examined the SCr and UO components of the RIFLE and pRIFLE criteria noting that UO is one of the earliest clinical signs of acute renal injury and SCr alone may underestimate the severity of AKI due to its delayed increase in the setting of rapidly evolving AKI [29]. Morgan and colleagues examined risk factors for outcomes of CS-AKI in neonates undergoing biventricular cardiac surgery using a modified version of the AKIN SCr criteria (maximum SCr level measured at 3 time points – POD 1, POD 2–5, and POD 6+) (11). In their cohort of 264 neonates with a mean age of 17 days, CS-AKI occurred in 64% of the neonates, with 55% in AKIN stage 1, 20% in AKIN stage 2, and 25% in AKIN stage 3. Applying modified AKIN SCr criteria to our neonatal cohort resulted in a higher CS-AKI incidence of 71%, which is not surprising given the median age of our neonates was 7 days and 40% had single ventricle physiology. Although CS-AKI defined as AKIN stage 1 was not associated with poor outcomes in our study, SCr level ≥ 0.9 mg/dL was another independent risk factor for the poor outcome.
In this study, FO ≥ 16% was shown to be an independent risk factor of the composite poor outcome and is significantly associated with increased ICU length of stay, cardiac arrest requiring CPR, and re-exploration of the chest. Many factors play a role in post-cardiac surgery FO including hemodilution from CPB, fluid/ blood product administration, low oncotic pressure and capillary leak, low cardiac output, and/or impaired renal function. It is not currently clear whether preventing/treating FO would improve post-operative outcomes.
In the pediatric cardiac surgery population, peritoneal dialysis (PD) catheter implantation for abdominal decompression and dialysis access is sometimes employed. In pediatric patients on ECLS requiring continuous RRT at our institution, degree of fluid overload at RRT initiation was the most consistent predictor of survival suggesting that the prevention of significant FO is likely to be more effective at improving outcomes than attempting fluid removal once significant FO is established (30).
Management of volume status in neonates following cardiac surgery is complex and the role of diuretics is controversial. In a prospective trial, Ricci et al randomized infants with congenital heart disease to receive either furosemide or ethacrynic acid following elective cardiac surgery. They found that ethacrynic acid was slightly more effective at augmenting urine output without any significant differences in post-operative serum creatinine or incidence of AKI (based on pRIFLE) (31). In a study of 30 pediatric patients with congestive heart failure already receiving inotropic and diuretic therapy, administration of synthetic B-type natriuretic peptide resulted in improved diuresis without significant increase in SCr (32). A single-center randomized double-blind controlled trial of 80 infants undergoing biventricular cardiac surgery compared high-dose fenoldopam treatment with placebo during CPB and found decreased urinary levels of neutrophil gelatinase-associated lipocaline and cystatin C, reduced the use of diuretics and vasodilators during CPB, and a trend towards lower AKI incidence (50% vs. 72%, p = 0.08) (33). Michael and colleagues have suggested that aggressive use of diuretics and early initiation of RRT to prevent worsening of FO may improve survival of pediatric stem-cell transplant recipients (34). In a meta-analysis of 9 RCTs including 849 adult patients, furosemide was not associated with any significant clinical benefits in the prevention and treatment of acute renal failure (35). Using data from the Fluid and Catheter Treatment Trial, a multicenter, RCT evaluating a conservative versus liberal fluid-management strategy in 1,000 patients with acute lung injury, Grams et al found that post-AKI fluid balance was significantly associated with 60-day mortality and higher post-AKI furosemide doses had a protective effect on mortality but no significant effect after adjustment for post-AKI fluid balance (36).
Limitations
There are several limitations to the results of our study. This was a single-center study and a multicenter evaluation is needed to strengthen the evidence found here. Our study was limited by its retrospective cohort design with its inherent risk of confounding and bias. Since FO and SCr measurements on POD 3 were most predictive of poor outcome based on the ROC curves, we were forced to exclude neonates who experienced a composite poor outcome prior to POD 3. This exclusion of early poor outcomes reduced the power of our study. Although furosemide is routinely employed for the post-operative management of FO in our neonatal cardiac surgery population, the amount of diuretic administered was not incorporated into our analysis. Likewise, exposure to nephrotoxic agents was not examined and could have contributed to the development of AKI in a subset of our neonates. Degree of FO can be calculated by difference in daily weights or net fluid balance (subtracting total fluid out from total fluid in each day). We chose to employ the weight-based method which may be limited by the addition of surgical tubes and catheters during surgery, but incorporates insensible fluid losses and is easier to calculate at the bedside than the fluid balance method (23). Since FO is often the indication for initiation of RRT, the association of FO with a composite poor outcome which includes RRT may be somewhat confounded. UO data was not collected on all of the study subjects, thus AKI as calculated by the AKIN UO criteria could not be determined. Our neonates had a median age of 7 days, thus, it is likely that the pre-operative SCr levels were falsely elevated in some of the subjects due to the lingering influence of maternal creatinine. This could have led to an underestimation of the true incidence of AKI as calculated by the AKIN SCr criteria.
CONCLUSIONS
Post-operative FO is an independent risk factor for poor outcomes in neonates following cardiac surgery with CPB. Daily FO, a readily-available, non-invasive marker of renal function, may be a sensitive and specific predictor of adverse outcomes including need for ECLS, RRT, or death. Additional prospective study is warranted to determine if daily FO can be used to guide the perioperative management of this vulnerable patient population.
Acknowledgments
We thank Dr. David Selewski and Dr. Tim Cornell for providing a critical review of the manuscript.
This work was supported by funds from the Michigan Congenital Heart Outcomes Research and Discovery program and Department of Anesthesiology at the University of Michigan. NBB is supported by a career development award from the National Institutes of Health (K08 DK093785).
REFERENCES
- 1.Blinder JJ, Goldstein SL, Lee V-V, et al. Congenital heart surgery in infants: Effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143(2):368–374. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Chiravuri SD, Voepel-Lewis T, Devaney EJ, et al. The use of aprotinin in children undergoing operative repair of isolated atrial septal defects. Paediatr Anaesth. 2008;18(2):145–150. doi: 10.1111/j.1460-9592.2007.02361.x. [DOI] [PubMed] [Google Scholar]
- 3.Kist-van Holthe tot Echten JE, Goedvolk CA, Doornaar MB, et al. Acute renal insufficiency and renal replacement therapy after pediatric cardiopulmonary bypass surgery. Pediatr Cardiol. 2001;22(4):321–326. doi: 10.1007/s002460010238. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: A prospective multicenter study. Crit Care Med. 2011;39(6):1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picca S, Principato F, Mazzera E, et al. Risks of acute renal failure after cardiopulmonary bypass surgery in children: a retrospective 10-year case-control study. Nephrol Dial Transplant. 1995;10(5):630–636. [PubMed] [Google Scholar]
- 6.Sethi S, Goyal D, Yadav D, et al. Predictors of acute kidney injury post-cardiopulmonary bypass in children. Clin Exp Nephrol. 2011;15(4):529–534. doi: 10.1007/s10157-011-0440-2. [DOI] [PubMed] [Google Scholar]
- 7.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askenazi D. Evaluation and management of critically ill children with acute kidney injury. Curr Opin Pediatr. 2011;23(2):201–207. doi: 10.1097/MOP.0b013e328342ff37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan CJ, Zappitelli M, Robertson CM, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162(1):120–127. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 12.Alabbas A, Campbell A, Skippen P, et al. Epidemiology of cardiac surgery-associated acute kidney injury in neonates: a retrospective study. Pediatr Nephrol. 2013;28(7):1127–1134. doi: 10.1007/s00467-013-2454-3. [DOI] [PubMed] [Google Scholar]
- 13.Filler GM. The challenges of assessing acute kidney injury in infants. Kidney Int. 2011;80(6):567–568. doi: 10.1038/ki.2011.172. [DOI] [PubMed] [Google Scholar]
- 14.Miall LS, Henderson MJ, Turner AJ, et al. Plasma creatinine rises dramatically in the first 48 hours of life in preterm infants. Pediatrics. 1999 Dec;104(6):e76. doi: 10.1542/peds.104.6.e76. [DOI] [PubMed] [Google Scholar]
- 15.Brion LP, Fleischman AR, McCarton C, et al. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr. 1986;109(4):698–707. doi: 10.1016/s0022-3476(86)80245-1. [DOI] [PubMed] [Google Scholar]
- 16.Karlowicz MG, Adelman RD. Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol. 1995 Dec;9(6):718–722. doi: 10.1007/BF00868721. [DOI] [PubMed] [Google Scholar]
- 17.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 18.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004 Aug;32(8):1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie R, Seidel K, Symons J. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19(12):1394–1399. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005 Feb;67(2):653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayes LW, Oster RA, Tofil NM, et al. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009 Sep;24(3):394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Lane PH, Mauer SM, Blazar BR, et al. Outcome of dialysis for acute renal failure in pediatric bone marrow transplant patients. Bone Marrow Transplant. 1994 May;13(5):613–617. [PubMed] [Google Scholar]
- 23.Hazle MA, Gajarski RJ, Yu S, et al. Fluid Overload in Infants Following Congenital Heart Surgery. Pediatr Crit Care Med. 2013;14(1):44–49. doi: 10.1097/PCC.0b013e3182712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassinger AB, Wald EL, Goodman DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med. 2014;15(2):131–138. doi: 10.1097/PCC.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins KJ, Gauvreau K, Newburger JW, et al. Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg. 2002;123(1):110–118. doi: 10.1067/mtc.2002.119064. [DOI] [PubMed] [Google Scholar]
- 26.Koralkar R, Ambalavanan N, Levitan EB, et al. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011 Apr;69(4):354–358. doi: 10.1203/PDR.0b013e31820b95ca. [DOI] [PubMed] [Google Scholar]
- 27.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004 Aug;32(8):1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010 Feb;55(2):316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 29.Ricci Z, Ronco C. Neonatal RIFLE. Nephrol Dial Transplant. 2013 Sep;28:2211–2214. doi: 10.1093/ndt/gft074. [DOI] [PubMed] [Google Scholar]
- 30.Selewski DT, Cornell TT, Blatt NB, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40(9):2694–2699. doi: 10.1097/CCM.0b013e318258ff01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci Z, Haiberger R, Pezzella C, et al. Furosemide versus ethacrynic acid in pediatric patients undergoing cardiac surgery: a randomized controlled trial. Crit Care. 2015;19(2):1–9. doi: 10.1186/s13054-014-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahle WT, Cuadrado AR, Kirshbom PM, et al. Nesiritide in infants and children with congestive heart failure. Pediatr Crit Care Med. 2005;6(5):543–546. doi: 10.1097/01.pcc.0000164634.58297.9a. [DOI] [PubMed] [Google Scholar]
- 33.Ricci Z, Luciano R, Favia I, et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011 Jun;15(3):R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004 Jan;19(1):91–95. doi: 10.1007/s00467-003-1313-z. [DOI] [PubMed] [Google Scholar]
- 35.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006 Aug;333(7565):420–423. doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grams ME, Estrella MM, Coresh J, et al. Fluid balance, diuretic use, mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966–973. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]