Abstract
Background
In the progression of severe sepsis, sepsis-induced myocardial dysfunction (SIMD) contributes to severity of illness and ultimate mortality. Identification of SIMD causing depressed cardiac function during critical illness has implications for ongoing patient management. However, assessing pediatric cardiac function traditionally relies on echocardiographic qualitative assessment and measurement of left ventricular ejection fraction (EF) or fractional shortening (FS). These metrics are often insensitive for detecting early or regional myocardial dysfunction. Strain echocardiography (SE) is a contemporary echocardiographic modality that may be more sensitive to perturbations in cardiac function. This investigation hypothesizes that SE metrics correlate with severity of illness in pediatric sepsis despite normal FS.
Design
Single-center retrospective observational study.
Setting
Tertiary 36-bed medical/surgical pediatric intensive care unit.
Patients
Pediatric patients admitted with sepsis.
Interventions
None.
Measurements and Main Results
Twenty-three children with sepsis received an echocardiogram in the study period. Patients with sepsis demonstrated abnormal peak systolic longitudinal strain for age (mean = −0.13 ±0.07, p < 0.01) and low normal peak systolic circumferential strain (mean = −0.17± 0.14 P= 0.02) compared to internal controls as well as previously published normal values. Depressed strain was demonstrated in the septic patients despite having normal FS (mean = 0.41, 95% CI 0.38 – 0.43). On initial echocardiographic imaging, worsening peak systolic longitudinal strain was associated with increasing lactate (p= 0.04).
Conclusion
Pediatric patients with sepsis demonstrate evidence of depressed SE parameters not shown by FS that correlate with clinical indices of sepsis severity. Whether SE could eventually assist in grading pediatric sepsis severity and affect management is an area for potential future investigation.
Keywords: sepsis-induced myocardial dysfunction, shock, pediatric, ultrasound, cardiomyopathy
Introduction
Sepsis is a major cause of morbidity and mortality worldwide [1]. Over the past two decades, the incidence of sepsis in the United States has tripled, and is now the tenth leading cause of death in the general population [1] [2]. Similarly, the prevalence of pediatric sepsis has increased from 3.7% to 4.4% in the past decade with small improvements in outcome [3]. In the progression of severe sepsis and septic shock, sepsis induced myocardial dysfunction (SIMD) is being increasingly recognized [4] [5] with evidence suggesting an association between myocardial dysfunction in sepsis and mortality [6] [7]
Classically SIMD is described as a reduced left ventricular ejection fraction (LVEF) and acute dilation of the left ventricle [8] [12]. Recent studies have also demonstrated biventricular systolic and diastolic dysfunction in clinical models of sepsis [9] [10]. This corroborates with biomarkers of cardiac injury, as elevation in cardiac troponin I (cTnI) has been documented in children with meningococcal septicemia [11]. Although SIMD can potentially contribute to poor outcomes, aggressive and early treatment of children with septic shock can reverse left ventricular (LV) global dysfunction and myocardial wall motion abnormalities [8] [12].
Echocardiography is the most common clinical method of assessing myocardial function in sepsis with fractional shortening (FS) and ejection fraction (EF) being the most commonly used quantitative metrics of global systolic function. Two-dimensional strain echocardiography (SE) is a contemporary angle-independent method for evaluating cardiac function by tracking cardiac tissue deformation [13]. The utility of SE in measuring ventricular function has been demonstrated in different cardiovascular diseases such as ischemic-cardiomyopathy [14], chemotherapy-induced cardiotoxicity [15], hypertrophic cardiomyopathy and preeclampsia [16].
Although a previous study has demonstrated depressed echocardiographic strain in pediatric sepsis [17], the connection between cardiac strain mechanics, illness severity, and clinical outcomes remains unclear. Accordingly, this study hypothesizes that; 1) children and infants with sepsis will manifest reduced strain parameters reflecting depressed LV function, and 2) depressed strain parameters in children with sepsis will correlate with markers of illness severity (lactate) and indicators of therapy intensity ( IS/VIS).
Methods
Study Participants
This investigation was approved by the Johns Hopkins University Institutional Review Board. Children less than 19 years of age admitted to the pediatric intensive care unit between January 1, 2005 and July 10, 2014 were evaluated for study inclusion. Eligible patients were identified using ICD-9 codes from the Johns Hopkins billing database. Patients with diagnoses consistent with sepsis (sepsis, septic shock, bacteremia, septicemia, circulatory shock, viremia, fungemia, pneumonia and urosepsis) and who received an echocardiogram during that admission were considered for inclusion into the study. Patients with known cardiomyopathy, myocarditis, pericarditis, endocarditis, cardiothoracic surgery/trauma, Kawasaki’s disease, and congenital heart disease were excluded. In addition, patients with a history of oncologic diagnoses and exposure to cardiotoxic chemotherapy were also excluded. Following identification of study patients, chart review was performed to confirm that each individual in the cohort met the diagnostic criteria set in the Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 3rd Edition [18].
Clinical Data and Outcomes
Following determination of study population, demographic and clinical data including hemodynamics, and laboratory biomarkers were recorded. Pediatric Risk of Mortality-III (PRISM III) score was calculated at admission, while Multiple Organ Dysfunction Score (MODS) was calculated on the day of echocardiogram acquisition [19] [20]. Inotrope score (IS) and vasoactive-inotrope score (VIS) were also calculated at the time of echocardiogram acquisition [21] [22]. IS was assessed separate from VIS due to the differential effects of vasoactives and inotropes on cardiac contractility.
Outcome data including length of ICU stay and mortality were also collected from the patients in the cohort. Biomarkers of organ dysfunction including: lactate, coagulation studies, liver function tests, and creatinine were collected both at admission and within 24 hrs. of echocardiography. Lactate collected within 24 hrs. of echocardiography was used as a surrogate for illness severity. VIS and IS within 24 hrs. of echocardiography were used as surrogates of therapy intensity.
Echocardiography
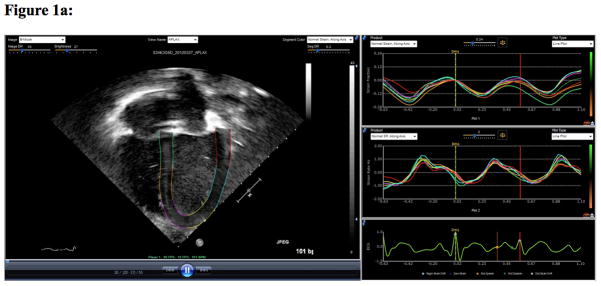
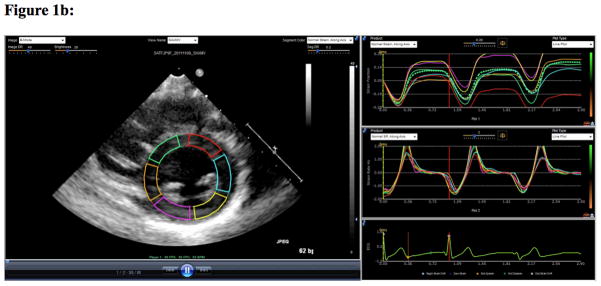
Images were obtained using IE 33, Sonos 5500 or 7500 ultrasound machines equipped with pediatric phased array transducers (Philips Medical Systems, Andover, MA). SE analysis was performed using echocardiography post-processing software (EchoInsight, Epsilon Imaging, Ann Arbor, MI). The apical four-chamber view was used to calculate the peak systolic longitudinal strain (LS) and peak systolic longitudinal strain rate (LSR) (Figure 1a). The parasternal short axis view was used to calculate the peak systolic circumferential strain (CS) and peak systolic circumferential strain rate (CSR), as well as the fractional shortening (FS) (Figure 1b). While LS and LSR reflect the function of longitudinally oriented sub-endocardial fibers [23], CS and CSR reflect function of circumferentially oriented sub-endocardial fibers [24]. Two physicians analyzed the echocardiographic studies (BH, IP). Physicians were blinded to outcomes data as well as biomarkers of illness severity at the time of strain analysis.
Figure 1.
Figure 1a. This figure demonstrates strain analysis using echocardiogram post-processing software (EchoInsight, Epsilon Imaging, Ann Arbor, MI). The apical four chamber view was used to calculate the global peak systolic longitudinal strain (LS-%) and the global peak systolic longitudinal strain rate (LSR−1/sec).
Figure 1b. This figure demonstrates strain analysis using echocardiogram post-processing software (EchoInsight, Epsilon Imaging, Ann Arbor, MI). The parasternal short axis view was used to calculate the peak systolic circumferential strain (CS - %) and peak systolic circumferential strain rate (CSR−1/sec)
Controls
Internal control data was derived from echocardiographic studies performed on healthy pediatric patients at The Johns Hopkins Children’s Center. Indications for these echocardiographic studies in the internal control group were; evaluation of a murmur, syncope and chest pain. Echocardiographic studies for the internal control group were noted to have normal function with no structural defect by official report. In addition to the internal normative controls, echocardiographic studies from septic children were compared to age matched normative controls published by Dr. Marcus and colleagues to confirm consistency of results [25]
Statistical Analysis
Descriptive statistics were performed on patient demographics (age, sex, height and weight), hemodynamics (HR, BP, and CVP), Laboratory biomarkers, vasopressor requirements, FS as well as SE parameters (LS, LSR, CS and CSR). Data normality was assessed using kernel density plots as well as Shapiro-Wilk tests. Categorical variables were analyzed using the chi-square test. Differences in means for normally distributed variables were compared using a non-paired t-test, while variables with a non-normal distribution were compared using the two-sample Wilcoxon rank-sum test. Collinearity of covariates was evaluated with variance inflation factors (VIF).
Missing data was handled by comparing SE parameters between patients who had missing data and those who had a complete dataset to limit bias. Once no difference was determined between these groups, patients with missing variables were excluded from the analysis. The association between SE parameters and illness severity was evaluated using univariate linear regression. Data was analyzed using STATA 13 (StataCorp LP, College Station TX) and a p-value less than 0.05 was considered statistically significant.
Results
One hundred and forty-six infants and children were admitted to the Johns Hopkins Hospital Pediatric Intensive Care Unit (PICU) from January 1, 2005, to July 10, 2014 with a diagnosis consistent with sepsis, severe sepsis or septic shock. This cohort was cross-referenced to an existing database of all echocardiographic studies done at The Johns Hopkins Hospital Children’s Center, identifying 77 patients that had an echocardiographic study performed during admission. Out of the 77 patients identified, 23 patients met inclusion criteria. Only the initial echocardiographic study obtained during the sepsis admission was utilized for the statistical analysis described below.
Demographics
Out of the 23 patients identified, 52.2% were male. Ages ranged from 1 week to 19 years with a mean age of 7.21 years (Table 1). The majority of the presenting symptoms included fever, respiratory distress and altered mental status. When compared to both control populations, patients with sepsis were noted to have significantly elevated heart rates and depressed blood pressure (Table 1).
Table 1. Patient morphometric and physiological data.
Patient height weight and hemodynamic data between the septic cohort and internal as well as historic controls. Significant difference are seen in heart rate and blood pressure between the septic cohort and controls
| Variables | (n) | Septic patients Mean (95% CI) | (n) | Internal Controls Mean (95% CI) | p | (n) | Age matched Historical controls Mean (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| Age | 23 | 7.21 (4.07 – 10.35) | 10 | 2.77 (1.02 – 4.52) | 0.12 | 23 | NA | NA |
| Height (cm) | 23 | 102.8 (64.7 – 140.87) | 10 | 87.3 (67.9 – 106.7) | 0.34 | 23 | 114 (93.51 – 135.01) | 0.56 |
| Weight (kg) | 23 | 24.42 (13.8 – 34.9) | 10 | 14.05 (8.3–19.78) | 0.14 | 23 | 29.3 (18.4 – 40.2) | 0.51 |
| Heart Rate at admission (bpm) | 23 | 163.2 (152–174) | 10 | 111 (78–143) | < 0.01** | 23 | 93.2 (83.6 – 102) | < 0.01 ** |
| Systolic BP at admission (mm/Hg) | 23 | 66.6 (59–74) | 10 | 96.6 (83.7 – 109.5) | < 0.01** | 23 | 94.43 (84–105) | < 0.01** |
| Diastolic BP at admission (mm/Hg) | 23 | 39 (33.6–45) | 10 | 57 (47–67) | 0.02* | 23 | 62 (55.3–68.9) | < 0.01** |
| CVP | 9 | 9.14 (1.57–15.67) | NA | NA | NA | NA | NA | NA |
Central venous pressure = CVP
In this study population, the median PRISM-III score was 15 (range, 2 – 17). Eleven patients (47%) required vasopressor or inotrope infusions for more than 12 h during admission. These patients had a median VIS score of 8 (range 2 – 42) and a median inotrope score (IS) of 8 (range 5 – 32). Median ICU length of stay was 16 days (range= 6 – 69) and one patient died during admission (Table 2). The average time from PICU admission and sepsis diagnosis to echocardiogram was 2.1 days with a range varying from 1 to 15 days. The average time from serum lactate measurement to echocardiogram was 9 hrs (range 0.5–36 hrs) (Table 2).
Table 2. Illness severity and outcomes in patients with sepsis.
displays illness severity scores, biomarkers of end organ perfusion as well as outcomes for the septic cohort. Lactate, VIS/IS score and MODS score were calculated on day of echocardiogram acquisition while PRISM III was calculated within 24 hrs of admission
| Illness severity | n | median (Q1– Q3) | number of patients with event | |
|---|---|---|---|---|
| Shock index | 23 | 2.73 (1.89 – 3.32) | NA | |
| IS | 23 | 8 (7.25 – 23.5) | 9/23 | |
| VIS | 23 | 8 (5 – 22) | 11/23 | |
| MODS score | 8 | 10 (7.5 – 11.5) | NA | |
| PRISM III score | 18 | 15 (10 – 16) | NA | |
|
| ||||
| Serum biomarkers | ||||
|
| ||||
| Lactate | 20 | 2.35 (1.25 – 3.05) | NA | |
| creatinine | 23 | 0.5 (0.3 – 0.7) | NA | |
| HCT | 23 | 28.9 (26.2 – 32.6) | NA | |
| WBC | 23 | 10.42 (6.3 – 19.14) | NA | |
| PLT | 23 | 233 (147 – 328) | NA | |
| PT | 22 | 13.5 (11.4 – 14.8) | NA | |
| PTT | 22 | 33.4 (27.6 – 39.4) | NA | |
| Fibrinogen | 22 | 426 (227 – 507) | NA | |
| Time from admission to echo (days) | 23 | 1 (0–2) | NA | |
| Time from lactate to echo (hrs) | 20 | 4 (2–12) | NA | |
|
| ||||
| Outcomes | ||||
|
| ||||
| Length of ICU stay (days) | 23 | 16 (12.5 – 37) | NA | |
| Mortality | 23 | NA | 1/23 | |
Vasoactive–Inotropic Score (VIS), Pediatric Risk of Mortality-III (PRISM), Multiple Organ Dysfunction Score (MODS), Hematocrit (HCT), Platelet count (PLT), Partial thromboplastin time (PTT), prothrombin time (PT)
Echocardiography parameters
All patients demonstrated normal LV dimensions and cardiac morphology. Compared to internal controls, patients with sepsis did not demonstrate a significant difference in conventional two-dimensional echocardiographic measures of cardiac function (FS = 41% vs 37.6%, p = 0.29) (Figure 2). However, LS was significantly worse in the septic patients when compared to internal controls, [mean of − 0.13 (95% CI = −0.11, −0.14) in the sepsis group vs. −0.19 (95% CI= −0.21, −0.17) in the control group (p < 0.01)]. CS was also significantly worse in the sepsis group when compared to control group [mean of −0.17 (95% CI = −0.13, −0.20) in sepsis group vs. −0.21 (95% CI= −0.23, −0.19) in the control group, (p = 0.03)] (Table 3). In the patients with sepsis, the LSR was −1.03 s−1 (SD= 0.34) while the CSR was −1.45 s−1 (SD =0.42) (Table 3).
Figure 2.
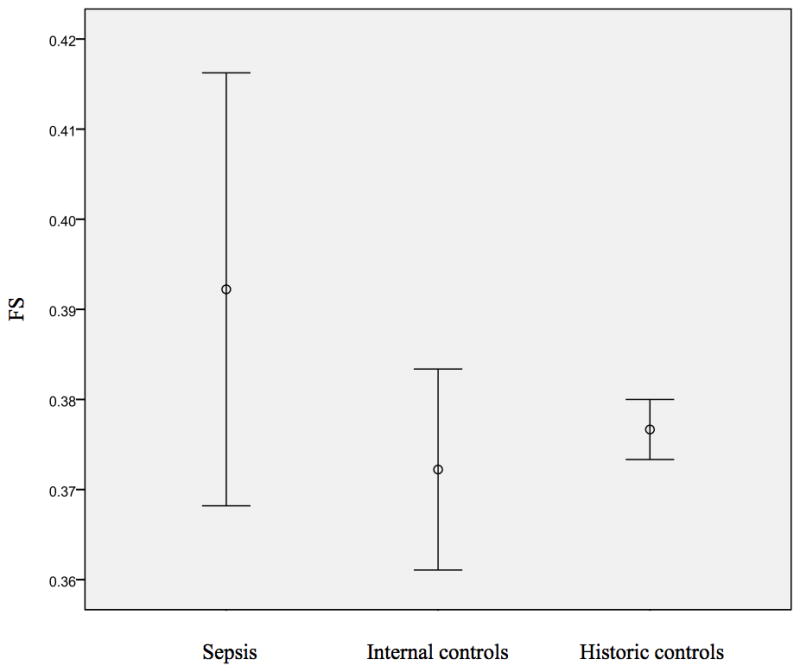
This figure demonstrates the average fractional shortening (FS) for the 23 patients with sepsis as compared to the internal and age matched historic controls [23].
Table 3. Echocardiographic indices of study patients.
compares fractional shortening (FS) as well as echocardiographic stain parameters between the septic cohort and internal as well as historic controls. Significant differences are seen in peak systolic longitudinal and circumferential strain between the septic cohort and controls while no differences are seen in FS between septic and control group.
| Variables | (n) | Septic patients mean (95% CI) | (n) | Internal Controls mean (95% CI) | p | (n) | Age matched Historical controls mean (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| FS | 23 | 0.41 (0.38 – 0.43) | 10 | 0.38 (0.36 – 0.40) | 0.29 | 23 | 0.38 (0.37 – 0.38) | .08 |
| LS | 23 | −0.13 (−0.14 – −0.11) | 10 | − 0.19 (−0.21 – −0.17) | < 0.01** | 23 | − 0.20 (−0.21 – −0.19) | < 0.01** |
| CS | 22 | −0.17 (−0.20 – −0.14) | 10 | −0.21 (−0.23 – −0.19) | 0.03* | 22 | −0.21 (−0.22 – −0.20) | 0.02* |
| CSR | 23 | −1.45 (−1.66 – −1.24) | 10 | −1.35 (−1.47 – −1.21) | 0.4 | 23 | NA | NA |
| LSR | 22 | −1.03 (−1.19 – −0.86) | 10 | −1.14 (−1.30 – −0.99) | 0.27 | 22 | NA | NA |
Central venous pressure (CVP), left ventricular end-diastolic dimension (LVEDD), fractional shortening (FS), strain echocardiography (SE), peak systolic longitudinal strain (LS), peak systolic circumferential strain (CS), peak systolic longitudinal strain rate (LSR), peak systolic circumferential strain rate (CSR)
When SE from the sepsis cohort was compared to age matched historical controls described by Dr. Marcus and colleagues [25] (Table 3), Similar results were demonstrated showing significantly depressed LS (−0.13 %, p < 0.01) as well as CS (−0.17 %, p = 0.02) in patients with sepsis (Figure 3).
Figure 3.
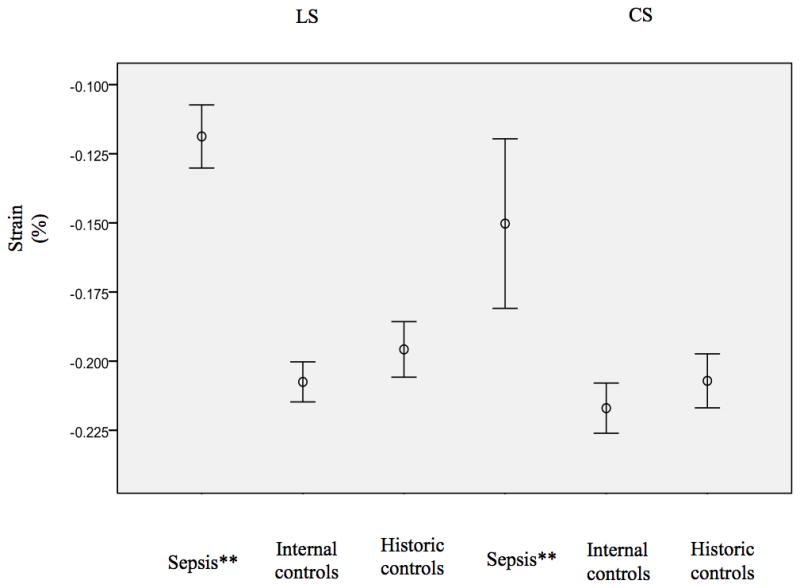
This figure demonstrates the peak systolic longitudinal (left) circumferential (right) strain for the 23 patients with sepsis as compared to the internal and age matched historic controls (average and standard error of the mean). Significant depression seen in the septic group (**)
Markers of sepsis severity
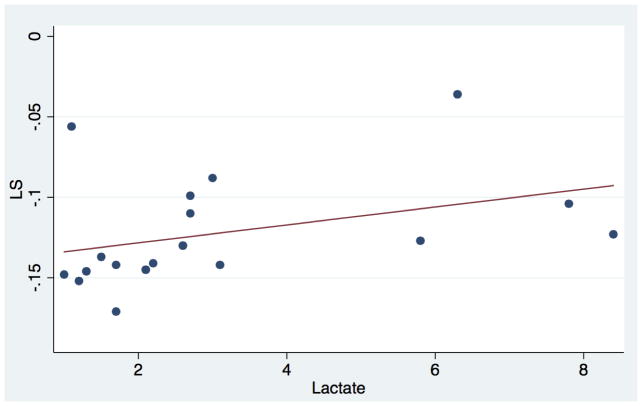
Direction and shape of the association between strain mechanics and surrogates of sepsis severity were evaluated using scatter plots with Lowess smoothing (Figure 4). In a univariate analysis, increased serum lactate was associated with worse LS (r = 0 .49, p = 0.04) as well as LSR (r= 0.5, p=0.03) (Table 4). When comparing SE parameters to markers of therapy intensity, there was no significant association between SE metrics and VIS or IS (Table 4)
Figure 4.
This figure demonstrates the association between lactate and LS. As seen in the scatter plot there is a trend between increasing lactate and worsening LS % in the septic cohort.
Table 4. Associations between SE and markers of illness severity / therapy intensity.
This table represents univariate the associations between SE parameters and metrics of illness severity as well as therapy intensity. As seen above there is a significant association between lactate and SE (LS &LSR).
| Variables | Lactate | VIS | IS | |||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| LS % | 0.48* | 0.04* | −0.06 | 0.87 | 0.43 | 0.25 |
| LSR −1/sec | 0.50* | 0.03* | 0.01 | 0.9 | −0.08 | 0.84 |
| CS % | −0.02 | 0.92 | −0.22 | 0.53 | −0.15 | 0.72 |
| CSR −1/sec | 0.12 | 0.66 | −0.39 | 0.21 | −0.63 | 0.091 |
Longitudinal strain (LS), longitudinal strain rate (LSR), circumferential strain (CS), circumferential strain rate (CSR), Vasoactive–Inotropic Score (VIS), Inotropic score (IS)
Patient Outcomes
The indirect association between length of stay and SE parameters were evaluated with uni-variate linear regression. Univariate analysis demonstrated no significant association between SE parameters and ICU length of stay. Mortality was not assessed due to the limited number of patients who died (n=1).
Inter-rater reliability
The inter-observer agreement between investigators interpreting studies was appropriate for both LS (r = 0.89, p < 0.01, Bland-Altman limits of agreement −0.03 to 0.03) and CS (r = 0.9, p < 0.001, Bland-Altman limits of agreement −0.07 to 0.03). Increased heterogeneity was seen in the inter-rater agreement of LSR (r = 0.72, p=0.03, Bland-Altman limits of agreement −0.34 to 0.33) and CSR (r = 0.6, p=0.07, Bland-Altman limits of agreement −0.87 to 0.21).
Discussion
This study demonstrates that pediatric patients with sepsis manifest significantly reduced LS and CS despite normal FS. Moreover, this is the first study to illustrate the association between strain mechanics and clinical indices of sepsis severity such as serum lactate levels in a pediatric cohort. These findings are consistent with available literature describing the improved sensitivity of SE for subtle cardiac dysfunction in sepsis compared to conventional cardiac function metrics [26–28].
The majority of contemporary literature validating SE has been described in adult ischemic cardiomyopathy. Previous studies have shown associations between SE and infarct size and ejection fraction [29, 30]. Additionally SE has been used to predict LV remodeling, clinical events [31] and response to reperfusion strategies [32, 33].
Currently, there are a limited number of studies evaluating the utility of SE in septic shock. A recent study by Dr. Orde and colleagues reported a higher incidence of RV and LV dysfunction demonstrated by SE metrics when compared with conventional echo metrics (EF/FS) in a cohort of adults with sepsis [34]. In addition, the same group demonstrated a significant association between depressed SE parameters and severity of illness (Sequential Organ Failure Assessment (SOFA) score as well as outcomes (3 and 6 month mortality) [34]. The specificity of SE in sepsis was also evaluated by Dr. Dalla and colleagues who demonstrated that changes in SE seen in sepsis are independent of increased adrenergic state seen with critical illness [35].
At this time, there is only a single pediatric study that demonstrates reduced strain and strain rate parameters in a cohort of 15 septic pediatric patients [17]. This current study further corroborates published pediatric data on SE metrics seen in sepsis, and is the first study to demonstrate correlation between abnormal SE parameters and indices of sepsis severity in the pediatric population.
Strain Echocardiography and Lactate
The relevance of serum lactate in sepsis as a biomarker of tissue hypoxia is well described in the medical literature [36]. Additionally improved lactate clearance in resuscitation has been associated with decreased mortality in sepsis patients [36, 37]. Consequently recommendations in the Surviving Sepsis Guidelines emphasize the importance of lactate clearance during the resuscitation of septic patients [18]. Associations between depressed SE parameters and lactate in this study may lend insights on the mechanical contribution of cardiac dysfunction to hyperlactatemia in the context of the current literature [34, 35, 42]. Therefore further investigation into the utility of SE as a noninvasive assessment tool for cardiac function in children with sepsis is potentially useful.
Strain and Therapy intensity
VIS and IS are metrics utilized for scoring the intensity of vasopressor and inotrope support in pediatric critical illness [21, 22]. IS was originally described in the postoperative congenital heart surgery population for quantifying inotropic support and is the sum of scalar-adjusted inotropic infusion doses at a given time point. VIS adds vasopressor infusions as well to the total assessed with IS [21, 22]. Previous small series have shown an association between worsening VIS score and higher mortality rates in pediatric sepsis cohorts.[40] In this study both VIS and IS were utilized to describe the total dosing of inotropes with and without vasopressors in pediatric sepsis. While no significant association was seen between IS and LS or CS, a trend towards significance was seen between IS and CSR (Table 4). Whether SE provides information on cardiac effects of therapy intensity could be further investigated.
Strain and outcomes
Currently two adult studies have shown a significant association between SE parameters and mortality in patients with sepsis [34, 39]. While Dr. Orde and colleagues demonstrated an increased odds of mortality (OR = 1.1, p = 0.02) in patients with sepsis with evidence of right ventricular dysfunction on SE [34], Dr. Chang and colleagues demonstrated that left ventricular LS ≥ −13 % had good performance in mortality prediction (ROC 0.79) in a cohort of 136 adults with sepsis [39]. In the pediatric cohort evaluated in this study, the influence of SIMD on mortality was not assessed due to limited number of events (mortality rate < 5%). Evaluating a larger population powered to assess morbidity and mortality is necessary since SIMD may potentially have an association with sepsis mortality [6] [7].
Limitations
Limitations of this study include risk of information bias based on the retrospective nature of the study design as well as the selection criteria utilized. Dr. Weiss and colleagues have described this issue previously in their work noting a significant difference in illness severity scores (p<0.01) depending on inclusion criteria [41]. To minimize this risk, the cohort was screened using multiple selection criteria including the 2012 Surviving Sepsis diagnostic guidelines [18]. Another potential limitation in this study is the utilization of control data from children who had echocardiographic imaging performed for a suspected pathology, despite being read as normal. However a comparison of the control data derived from patients seen at this institution did not differ from published SE data on otherwise healthy control patients[25].
Finally, the limited data on the indication for echocardiogram in this cohort increases the risk of bias by indication since study patients may have had cardiovascular symptomatology prompting imaging. This is an unavoidable effect of the retrospective nature of this study that utilizes a convenience sample of echocardiograms obtained as a part of routine clinical care. Although this risk is limited by use of restriction in the study design to include only patients with structurally normal hearts prior to their sepsis admission, it is clear that further prospective investigation utilizing probability sampling is necessary for a more complete SE-based survey of LV dysfunction among septic patients.
Conclusion
Pediatric patients with sepsis demonstrate evidence of depressed SE parameters not shown by conventional assessment of cardiac function (FS). Furthermore, SE metrics seem to correlate with clinical indices of sepsis severity. Whether SE has the potential to grade pediatric sepsis severity and augment management is an area for potential future investigation.
Acknowledgments
Support: T32 5T32GM075774-09
References
- 1.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Balamuth F, Weiss SL, Neuman MI, Scott H, Brady PW, Paul R, Alpern ER. Pediatric severe sepsis in US children’s hospitals. Pediatr Crit Care Med. 2014;15(9):798. doi: 10.1097/PCC.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care Med. 2002;6:500–508. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma AC. Sepsis-induced myocardial dysfunction. Shock. 2007;28(3):265–269. doi: 10.1097/01.shk.0000235090.30550.fb. [DOI] [PubMed] [Google Scholar]
- 6.Prabhu MM, Yalakala SK, Shetty R, Thakkar A, Sitapara T. Prognosis of Left Ventricular Systolic Dysfunction in Septic Shock Patients. JCDR. 2015;9(3):OC05. doi: 10.7860/JCDR/2015/10812.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weng L, Liu YT, Du B, Zhou JF, Guo X, Peng JM, Zhu WL. The prognostic value of left ventricular systolic function measured by tissue Doppler imaging in septic shock. Crit Care Med. 2012;16(3):R71. doi: 10.1186/cc11328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Fredrerick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 9.Munt B, Jue J, Gin K, Fenwick J, Tweeddale M. Diastolic filling in human severe sepsis: an echocardiographic study. Crit Care Med. 1998;26(11):1829–1833. doi: 10.1097/00003246-199811000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Poelaert J, Declerck C, Vogelaers D, Colardyn F, Visser CA. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 1997;23(5):553–560. doi: 10.1007/s001340050372. [DOI] [PubMed] [Google Scholar]
- 11.Landesberg G, Jaffe AS, Gilon D, Levin PD, Goodman S, Abu-Baih A, Beeri R, Weissman C, Sprung C, Landesberg A. Troponin Elevation in Severe Sepsis and Septic Shock: The Role of Left Ventricular Diastolic Dysfunction and Right Ventricular Dilatation. Crit Care Med. 2014;42(4):790–800. doi: 10.1097/CCM.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 12.Feltes TF, Pignatelli R, Kleinert S, Mariscalco MM. Quantitated left ventricular systolic mechanics in children with septic shock utilizing noninvasive wall-stress analysis. Crit Care Med. 1994;22(10):1647–1658. [PubMed] [Google Scholar]
- 13.Langeland S, Wouters P, Claus P, Leather H, Bijnens B, Sutherland G, Rademakers F, D’hooge J. Experimental assessment of a new research tool for the estimation of two-dimensional myocardial strain. Ultrasound Med Biol. 2006;32:1509–1513. doi: 10.1016/j.ultrasmedbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Gjesdal O, Hopp E, Vartdal T, Lunde K, Helle-Valle T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Global longitudinal strain measured by two-dimensional speckle tracking echo-cardiography is closely related to myocardial infarct size in chronic ischemic heart disease. Clin Sci (Lond) 2007;113:287-Valle. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- 15.Migrino RQ, Aggarwal D, Konorev E, Brahmbhatt T, Bright M, Kalyanaraman B. Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol. 2008;34:208–214. doi: 10.1016/j.ultrasmedbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahul S, Rhee J, Hacker MR, Gulati G, Mitchell JD, Hess P, Mahmood F, Arany Z, Rana S, Talmor D. Subclinical left ventricular dysfunction in preeclamptic women with preserved left ventricular ejection fraction: a 2D speckle-tracking imaging study. Circ Cardiovasc Imaging. 2012;5:734–739. doi: 10.1161/CIRCIMAGING.112.973818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu S, Frank LH, Fenton KE, Sable CA, Levy RJ, Berger JT. Two-dimensional speckle tracking imaging detects impaired myocardial performance in children with septic shock, not recognized by conventional echocardiography. Pediatr Crit Care Med. 2012;13(3):259–264. doi: 10.1097/PCC.0b013e3182288445. [DOI] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JC, Cook DJ, Christou NV. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Hirsch JC. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 22.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Hanley FL, Hickey PR, Wessel DL. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92(8):2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 23.Buckberg G, Hoffman JIE, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–2587. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 24.Burns AT, La Gerche A, D’hooge J, MacIsaac AI, Prior DL. Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur Heart J Cardiovasc Imaging. 2010;11(3):283–9. doi: 10.1093/ejechocard/jep214. [DOI] [PubMed] [Google Scholar]
- 25.Marcus KA, Mavinkurve-Groothuis AM, Barends M, van Dijk A, Feuth T, de Korte C, Kapusta L. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J Am Soc Echocardiogr. 2011;24(6):625–636. doi: 10.1016/j.echo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Abraham TP, Dimaano VL, Liang HY. Role of tissue Doppler and strain echocardiography in current clinical practice. Circulation. 2007;116(22):2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 27.Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging. 2009;25(Supp-1):9–22. doi: 10.1007/s10554-008-9414-1. [DOI] [PubMed] [Google Scholar]
- 28.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 29.Gjesdal O, Hopp E, Vartdal T, Lunde K, Helle-Valle T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Global longitudinal strain measured by two-dimensional speckle tracking echo-cardiography is closely related to myocardial infarct size in chronic ischemic heart disease. Clin Sci (Lond) 2007;113:287-Valle. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- 30.Delgado V, Mollema SA, Ypenburg C, Tops LF, van der Wall EE, Schalij MJ, Bax JJ. Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. Am Soc Echocardiogr. 2008;21:1244–21:1. doi: 10.1016/j.echo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Park YH, Kang SJ, Song JK, Lee EY, Song JM, Kang DH, Kim YH, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Prognostic value of longitudinal strain after primary reperfusion therapy in patients with anterior-wall acute myocardial infarction. Am Soc Echocardiogr. 2008;21:262–267. doi: 10.1016/j.echo.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Korosoglou G, Haars A, Humpert PM, Hardt S, Bekeredjian R, Giannitsis E, Kuecherer H, Katus HA. Evaluation of myocardial perfusion and deformation in patients with acute myocardial infarction treated with primary angioplasty and stent placement. Coron Artery Dis. 2008;19:497–506. doi: 10.1097/MCA.0b013e328310904e. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka H, Kawai H, Tatsumi K, Kataoka T, Onishi T, Nose T, Mizoguchi T, Yokoyama M. Improved regional myocardial diastolic function assessed by strain rate imaging in patients with coronary artery disease undergoing percutaneous coronary intervention. J Am Soc Echocardiogr. 2006;19:756–762. doi: 10.1016/j.echo.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Orde SR, Pulido JN, Masaki M, Gillespie S, Spoon JN, Kane GC, Oh JK. Outcome prediction in sepsis: speckle tracking echocardiography based assessment of myocardial function. Crit Care. 2014;18:R149. doi: 10.1186/cc13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalla K, Hallman C, Bech-Hanssen O, Haney M, Ricksten SE. Strain echocardiography identifies impaired longitudinal systolic function in patients with septic shock and preserved ejection fraction. Cardiovasc Ultrasound. 13(1):30. doi: 10.1186/s12947-015-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(8):1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 37.Arnold RC, Shapiro NI, Jones AE, Schorr C, Pope J, Casner E Emergency Medicine Shock Research Network (EMShockNet) Investigators. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. 2009;32(1):35–39. doi: 10.1097/shk.0b013e3181971d47. [DOI] [PubMed] [Google Scholar]
- 38.Silva PS, Fonseca MC, Iglesias SB, Carvalho WB, Bussolan RM, Freitas IW. Comparison of two different severity scores (Paediatric Risk of Mortality [PRISM] and the Glasgow Meningococcal Sepsis Prognostic Score [GMSPS]) in meningococcal disease: preliminary analysis. Ann Trop Paediatr. 2001;21(2):135–140. [PubMed] [Google Scholar]
- 39.Chang WT, Lee WH, Lee WT, Chen PS, Su YR, Liu PY, Tsai WC. Left ventricular global longitudinal strain is independently associated with mortality in septic shock patients. Intensive Care Med. 2015;41(10):1791–1799. doi: 10.1007/s00134-015-3970-3. [DOI] [PubMed] [Google Scholar]
- 40.Haque A, Siddiqui NR, Munir O, Saleem S, Mian A. Association between vasoactive-inotropic score and mortality in pediatric septic shock. Ind pediatrics. 2015;52(4):311–313. doi: 10.1007/s13312-015-0630-1. [DOI] [PubMed] [Google Scholar]
- 41.Weiss SL, Parker B, Bullock ME, Swartz S, Price C, Wainwright MS, Goodman DM. Defining pediatric sepsis by different criteria: Discrepancies in populations and implications for clinical practice. Pediatr Crit Care Med. 2012;13(4):e219–e226. doi: 10.1097/PCC.0b013e31823c98da. [DOI] [PubMed] [Google Scholar]
- 42.Lanspa MJ, Pittman JE, Hirshberg EL, Wilson EL, Olsen T, Brown SM, Grissom CK. Association of left ventricular longitudinal strain with central venous oxygen saturation and serum lactate in patients with early severe sepsis and septic shock. Crit Care. 2015;19(1):1–9. doi: 10.1186/s13054-015-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]