Abstract
Background
Representation by age ensures appropriate translation of clinical trial results to practice, but historically, older patients were underrepresented in clinical trial populations. As the general population has aged, it is unknown whether clinical trial enrollment has changed in parallel.
Methods and Results
We studied time trends in enrollment, clinical characteristics, treatment, and outcomes by age among 76,141 NSTE ACS patients enrolled in 11 phase III clinical trials over 17 years (1994–2010). Overall, 19.7% of patients were ≥75 years; this proportion increased from 16% during 1994–1997 to 21% during 1998–2001 and 23.2% during 2002–2005, but declined to 20.2% in 2006–2010. The number of comorbidities increased with successive time periods irrespective of age. There were substantial increases in use of evidence-based medication in-hospital and at discharge regardless of age. While predicted 6-month mortality increased slightly over time, observed 6-month mortality declined significantly in all age strata (1994–1997 vs. 2006–2010: <65 years: 3.0% vs. 1.9%; 65–74 years: 7.5% vs. 3.4%; 75–79 years: 13.0% vs. 6.5%; 80–84 years: 17.6% vs. 8.2%; and ≥85 years: 24.8% vs. 12.6%).
Conclusions
The distribution of enrollment by age in phase III NSTE ACS trials was unchanged over time. Irrespective of age, post-myocardial infarction mortality decreased significantly over time, concurrent with increased evidence-based care and despite increasing comorbidities.
Clinical Trial Registration Information
ClinicalTrials.gov. Identifier: NCT00089895.
Keywords: coronary disease, myocardial infarction
Introduction
In current practice, individuals ≥75 years old account for more than one-third of non-ST-segment elevation acute coronary syndrome (NSTE ACS) episodes and the majority of overall mortality due to NSTE ACS, but represent <10% of NSTE ACS clinical trial populations.1,2 Substantial differences between trial and community patient characteristics exist and efforts are needed to increase enrollment of older patients in NSTE ACS trials to increase certainty about treatment effects across all age groups.3
We previously showed that along with significant increases in discharge evidence-based medications and use of invasive strategies, there was a significant decline in 6-month mortality despite enrollment of increasingly higher-risk patients over time.4 We explored whether these trends applied to patients of all ages by examining age-related temporal trends in enrollment, clinical characteristics, use of evidence-based hospital and discharge therapies, and clinical outcomes in NSTE ACS clinical trials using the databases of 11 phase III randomized clinical trials that enrolled patients from 1994 to 2010.
Methods
Study population
All phase III clinical trials of antithrombotic therapy in NSTE ACS in which the Duke Clinical Research Institute (DCRI) had a coordinating-center role, plus 3 trials conducted elsewhere, from which we had access to patient-level data, were included (N=11 trials).5–15 These trials are described in Table 1.
Table 1. Summary of clinical trials.
| Clinical Trials | Enrollment period | Enrollment criteria | Treatment studied | <65 years | 65–74 years | 75–79 years | 80–84 years | ≥85 years |
|---|---|---|---|---|---|---|---|---|
| GUSTO IIb* | 1994–1997 | Chest discomfort <12 h, ECG changes | Heparin, hirudin | 3850 (48.1%) | 2598 (32.4%) | 868 (10.8%) | 504 (6.3%) | 191 (2.4%) |
| PRISM | 1994–1997 | Chest pain <24 h, ECG changes, CK>2× ULN or CK-MB>ULN | Tirofiban, heparin | 1780 (55.2%) | 1002 (31.1%) | 271 (8.4%) | 134 (4.2%) | 38 (1.2%) |
| PRISM-PLUS | 1994–1997 | Chest pain <12 h, ECG changes, CK>ULN or CK-MB>ULN | Tirofiban, heparin, tirofiban plus heparin | 980 (51.2%) | 597 (31.2%) | 214 (11.2%) | 92 (4.8%) | 32 (1.7%) |
| PARAGON-A | 1994–1997 | Chest pain <12 h, ECG changes | Low-dose lamifiban with and without heparin, high-dose lamifiban with and without heparin | 1074 (47.1%) | 770 (33.7%) | 252 (11.0%) | 136 (6.0%) | 47 (2.1%) |
| PURSUIT | 1994–1997 | Chest pain <24 h, ECG changes, CK-MB>ULN | Placebo, low-dose eptifibatide, high-dose eptifibatide | 5717 (52.2%) | 3743 (34.2%) | 938 (8.6%) | 411 (3.8%) | 139 (1.3%) |
| PARAGON-B | 1998–2001 | Chest pain <12 h, ECG changes, CK-MB or troponin I or T>ULN | Lamifiban, heparin | 2709 (51.8%) | 1586 (30.4%) | 568 (10.9%) | 263 (5.0%) | 99 (1.9%) |
| GUSTO IV-ACS† | 1998–2001 | Chest pain <24 h, ECG changes, troponin I or T>ULN | Heparin, 24-h abciximab, 48-h abciximab | 3453 (44.3%) | 2577 (33.0%) | 1112 (14.3%) | 452 (5.8%) | 206 (2.6%) |
| SYNERGY‡ | 1998–2001, 2002–2005 | Chest pain <24 h, ECG changes, CK-MB or troponin I or T>ULN | Enoxaparin, unfractionated heparin | 3836 (38.4%) | 3601 (36.1%) | 1451 (14.5%) | 798 (8.0%) | 291 (2.9%) |
| EARLY ACS‡ | 2002–2005, 2006–2010 | Chest pain <24 h, ECG changes, CK-MB or troponin I or T>ULN | Early, routine administration of eptifibatide, delayed, provisional administration | 3907 (41.5%) | 3119 (33.2%) | 1331 (14.2%) | 763 (8.1%) | 283 (3.0%) |
| TRACER§ | 2006–2010 | Chest pain <24 h, ECG changes, CK-MB or troponin I or T>ULN | Placebo, voraxapar | 6759 (52.2%) | 3979 (30.7%) | 1329 (10.3%) | 685 (5.3%) | 192 (1.5%) |
| APPRAISE-2* | 2006–2010 | ACS (MI, with or without STE or UA) within 7 days, symptoms>10 min at rest, ECG changes or elevated biomarkers | Placebo, apixaban | 1807 (40.9%) | 1675 (37.9%) | 500 (11.3%) | 308 (7.0%) | 124 (2.8%) |
| 35872 (47.1%) | 25247 (33.2%) | 8834 (11.6%) | 4546 (6.0%) | 1642 (2.1%) |
ACS: acute coronary syndrome, CK: creatine kinase, ECG: electrocardiogram, MI: myocardial infarction, NSTE: non-ST-segment elevation, UA: unstable angina, ULN: upper limit of normal.
Patients with STE ACS not included.
NSTEMI/UA not undergoing planned early revascularization.
NSTEMI, unstable angina undergoing early invasive management.
Patients with transient STE ECG changes (<30 min) enrolled.
Study design
Baseline characteristics; in-hospital and discharge pharmacotherapy; coronary angiography and revascularization; and in-hospital, 30-day, 6-month, and 1-year outcomes were trended across 17 years and stratified by age. Four prespecified time periods (1994–1997; 1998–2001; 2002–2005; 2006–2010) within 5 age groups (<65 years, 65–74 years, 75–79 years, 80–84 years, and ≥85 years) were designated for display purposes. We excluded glycoprotein IIb/IIIa inhibitors and heparins from our analysis as these medications were part of protocol-driven randomized treatment in most of the trials we examined. The Duke University Medical Center Institutional Review Board approved the current study with a waiver of written informed consent and HIPAA authorization.
Endpoints
Study endpoints included in-hospital, 30-day, 6-month, and 1-year mortality; a composite of 30-day death or myocardial infarction (MI); in-hospital GUSTO bleeding (mild, moderate, and severe); and transfusion during index hospitalization. Observed 6-month mortality was compared with GRACE score-predicted mortality. MI was used as classified by the adjudication protocol of each trial.
Statistical methods
We summarized categorical variables using percentages and frequencies, and continuous variables using medians and first and third quartiles. GRACE risk scores were calculated for 6-month mortality, using previously described methods.16,17 Complete data for GRACE score determinations were available from GUSTO IIb, PARAGON-A and -B, PURSUIT, SYNERGY, EARLY ACS, and TRACER. Observed versus predicted mortality analyses were limited to these trials.
Regression models assessed trends over time according to age. Actual month since the beginning of 1994 and age as a continuous variable, as well as an age × time interaction term, were included in the models. For short-term (in-hospital and 30-day) binary outcomes (e.g., use of angiography or a medication, and mortality), we used hierarchical logistic regression, and for continuous outcomes (e.g., length of stay and GRACE score) we used linear mixed models. Length of stay was log-transformed for normality. To compare 6-month and 1-year mortality, we use Kaplan-Meier rates and Cox regression with a shared frailty for trial. These models assume and account for clustering of patient outcomes within trials. Statistical significance was set at P<0.05 (two-sided) without adjustments for multiple comparisons. Analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population
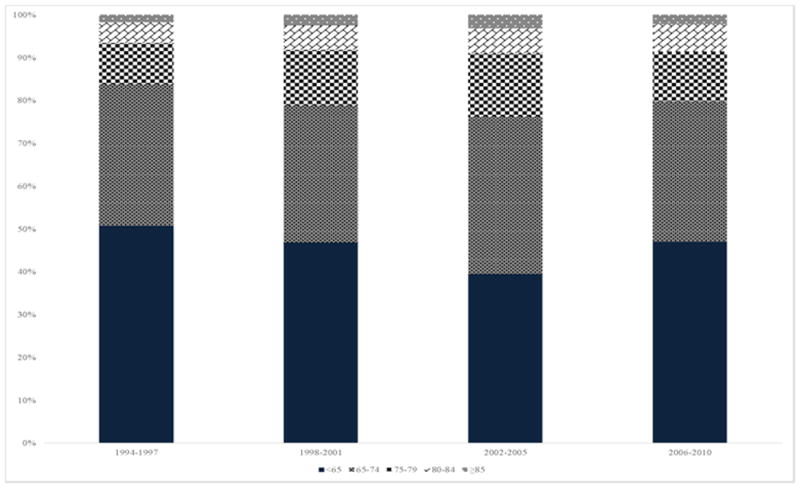
Of 76,141 patients, 29,697 (39%) presented with unstable angina and 46,196 (61%) with NSTE MI. Overall, 11.6% of patients were 75–79 years of age, 6.0% 80–84 years, and 2.1% ≥85 years. The distribution of enrollment by age according to time period is shown in Figure 1. Although the changes in distribution of enrollment by age over time were statistically significant in the overall population of >76,000 patients, the changes were qualitatively modest. Enrollment by region by time period is shown in Supplemental Figure 1, and the proportion of US enrollment by age by time is shown in Supplemental Table 1. The higher proportion of North American and US enrollment during the 2002–2005 time period reflects 1) the pattern of early enrollment in EARLY ACS during which US contribution predominated, and 2) enrollment in SYNERGY, in which US enrollment predominated.
Figure 1. Distribution of enrolled trial participants by age group over time.
Age-stratified temporal trends in baseline characteristics
Age-stratified temporal changes in baseline characteristics are presented in Table 2. Over time, there were clinically modest but statistically significant increases in the number of male (P<0.0001) and non-white participants (P<0.0001), and a marked increase in patients with diabetes (P<0.0001), hypertension (P<0.0001), and hypercholesterolemia (P<0.0001) in all age groups. In all age strata, the proportion of patients with prior percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) increased over time (both P<0.0001).
Table 2. Baseline characteristics stratified by age grouping and time period.
| Randomization Year | ||||
|---|---|---|---|---|
|
|
||||
| 1994–1997 | 1998–2001 | 2002–2005 | 2006–2010 | |
| Age <65 years | (N=13401) | (N=6377) | (N=4320) | (N=11774) |
|
| ||||
| Demographics | ||||
| Age, median (Q1-Q3) | 56 (49-60) | 55 (50-60) | 59 (52-62) | 58 (53-61) |
| Female sex | 27.1% (13401)* | 28.8% (6377) | 24.8% (4320) | 23.3% (11774) |
| White race | 87.3% (13236) | 91.5% (6376) | 83.4% (4226) | 84.0% (11378) |
| Past medical history | ||||
| Diabetes | 17.8% (13401) | 17.9% (6377) | 27.0% (4320) | 33.1% (11774) |
| Hypertension | 47.8% (13401) | 47.4% (6377) | 59.7% (4320) | 66.4% (11774) |
| Hypercholesterolemia | 46.5% (13401) | 43.0% (6377) | 59.3% (4320) | 60.1% (11774) |
| Chronic renal insufficiency | 0.5% (13401) | 0.7% (2709) | 5.5% (8566) | |
| Current smoking | 40.8% (13381) | 41.4% (6377) | 42.8% (4320) | 39.5% (11774) |
| Congestive heart failure | 6.0% (13401) | 5.4% (6377) | 6.3% (4320) | 11.2% (11774) |
| MI | 32.0% (13401) | 25.2% (6377) | 23.8% (4320) | 32.2% (11774) |
| PCI | 13.0% (13401) | 12.5% (6377) | 18.5% (4320) | 25.0% (11774) |
| CABG | 11.6% (13401) | 9.7% (6377) | 12.5% (4320) | 9.5% (11774) |
| PVD | 6.5% (13401) | 4.7% (2924) | 6.3% (4320) | 9.0% (11774) |
| Presenting clinical characteristics | ||||
| GRACE score risk for 6-month mortality, median (Q1-Q3)† | 85 (71-98) | 82 (67-95) | 96 (87-106) | 91 (81-101) |
| GRACE risk score for 6-month mortality ≥140† | 0.3% (10331) | 1.0% (2861) | 0.7% (4255) | 0.5% (9493) |
|
| ||||
| Age 65–74 years | (N=8710) | (N=4353) | (N=4014) | (N=8170) |
|
| ||||
| Demographics | ||||
| Age, median (Q1-Q3) | 70 (67-72) | 70 (67-72) | 70 (67-72) | 69 (67-72) |
| Female sex | 37.9% (8710) | 38.9% (4353) | 35.3% (4014) | 33.0% (8170) |
| White race | 92.9% (8649) | 94.9% (4352) | 88.8% (3965) | 87.7% (7946) |
| Past medical history | ||||
| Diabetes | 24.8% (8710) | 26.1% (4353) | 32.6% (4014) | 35.9% (8170) |
| Hypertension | 56.7% (8710) | 59.6% (4353) | 72.8% (4014) | 75.8% (8170) |
| Hypercholesterolemia | 42.4% (8710) | 41.2% (4353) | 62.2% (4014) | 61.6% (8170) |
| Chronic renal insufficiency | 1.2% (8710) | 2.2% (1586) | 11.4% (5654) | |
| Current smoking | 16.6% (8696) | 15.9% (4353) | 17.2% (4014) | 17.1% (8170) |
| Congestive heart failure | 11.1% (8710) | 9.9% (4353) | 10.2% (4014) | 14.5% (8170) |
| MI | 37.6% (8710) | 33.4% (4353) | 30.5% (4014) | 29.0% (8170) |
| PCI | 11.9% (8710) | 12.1% (4353) | 22.9% (4014) | 24.0% (8170) |
| CABG | 15.2% (8710) | 12.5% (4353) | 18.7% (4014) | 13.6% (8170) |
| PVD | 12.0% (8710) | 11.2% (1776) | 11.5% (4014) | 10.5% (8170) |
| Presenting clinical characteristics | ||||
| GRACE score risk for 6-month mortality, median (Q1-Q3)† | 114 (102-124) | 111 (99-124) | 116 (106-127) | 112 (103-123) |
| GRACE risk score for 6-month mortality ≥140† | 6.1% (6885) | 6.8% (1729) | 7.1% (3956) | 4.7% (6204) |
|
| ||||
| Age 75–79 years | (N=2543) | (N=1769) | (N=1611) | (N=2911) |
|
| ||||
| Demographics | ||||
| Age, median (Q1-Q3) | 77 (76-78) | 77 (76-78) | 77 (76-78) | 77 (76-78) |
| Female sex | 44.4% (2543) | 46.7% (1769) | 41.4% (1611) | 38.9% (2911) |
| White race | 94.2% (2528) | 97.2% (1768) | 90.8% (1594) | 90.0% (2833) |
| Past medical history | ||||
| Diabetes | 25.0% (2543) | 27.1% (1769) | 32.2% (1611) | 34.4% (2911) |
| Hypertension | 57.8% (2543) | 64.0% (1769) | 76.8% (1611) | 81.0% (2911) |
| Hypercholesterolemia | 35.4% (2543) | 35.8% (1769) | 59.0% (1611) | 59.4% (2911) |
| Chronic renal insufficiency | 2.2% (2543) | 2.6% (568) | 15.2% (1829) | |
| Current smoking | 9.0% (2542) | 6.8% (1769) | 9.6% (1611) | 8.5% (2911) |
| Congestive heart failure | 16.0% (2543) | 13.8% (1769) | 13.2% (1611) | 17.2% (2911) |
| MI | 37.4% (2543) | 36.2% (1769) | 30.4% (1611) | 32.9% (2911) |
| PCI | 9.4% (2543) | 12.2% (1769) | 22.7% (1611) | 24.3% (2911) |
| CABG | 13.4% (2543) | 13.1% (1769) | 20.7% (1611) | 15.4% (2911) |
| PVD | 13.5% (2543) | 13.7% (657) | 12.0% (1611) | 11.6% (2911) |
| Presenting clinical characteristics | ||||
| GRACE score risk for 6-month mortality, median (Q1-Q3)† | 130 (118-141) | 125 (115-141) | 130 (121-142) | 128 (118-138) |
| GRACE score ≥140† | 29.0% (1998) | 26.4% (640) | 30.6% (1584) | 21.4% (2313) |
|
| ||||
| Age 80–84 years | (N=1277) | (N=778) | (N=888) | (N=1603) |
|
| ||||
| Demographics | ||||
| Age, median (Q1-Q3) | 82 (81-83) | 82 (80-83) | 82 (80-83) | 82 (81-83) |
| Female sex | 50.0% (1277) | 50.6% (778) | 45.7% (888) | 47.1% (1603) |
| White race | 95.4% (1275) | 96.5% (778) | 90.6% (883) | 91.8% (1566) |
| Past medical history | ||||
| Diabetes | 21.5% (1277) | 24.8% (778) | 28.2% (888) | 32.5% (1603) |
| Hypertension | 57.0% (1277) | 57.1% (778) | 76.4% (888) | 82.5% (1603) |
| Hypercholesterolemia | 27.1% (1277) | 29.6% (778) | 53.3% (888) | 58.2% (1603) |
| Chronic renal insufficiency | 3.1% (1277) | 4.2% (263) | 21.3% (993) | |
| Current smoking | 6.3% (1275) | 4.8% (778) | 6.0% (888) | 5.9% (1603) |
| Congestive heart failure | 21.8% (1277) | 18.1% (778) | 17.5% (888) | 19.9% (1603) |
| MI | 41.6% (1277) | 42.5% (778) | 30.9% (888) | 32.8% (1603) |
| PCI | 6.6% (1277) | 11.2% (778) | 21.8% (888) | 23.6% (1603) |
| CABG | 9.0% (1277) | 10.7% (778) | 18.7% (888) | 16.2% (1603) |
| PVD | 14.8% (1277) | 12.3% (326) | 15.2% (888) | 11.2% (1603) |
| Presenting clinical characteristics | ||||
| GRACE score risk for 6-month mortality, median (Q1-Q3)† | 141 (130-153) | 138 (127-150) | 140 (129-151) | 137 (128-148) |
| GRACE score ≥140† | 52.7% (1017) | 44.1% (322) | 51.3% (875) | 43.4% (1237) |
|
| ||||
| Age ≥85 years | (N=447) | (N=326) | (N=341) | (N=528) |
|
| ||||
| Demographics | ||||
| Age, median (Q1-Q3) | 87 (86-88) | 86 (85-88) | 87 (86-89) | 86 (86-88) |
| Female sex | 57.3% (447) | 54.6% (326) | 47.2% (341) | 45.8% (528) |
| White race | 95.0% (443) | 97.2% (326) | 92.9% (338) | 93.0% (513) |
| Past medical history | ||||
| Diabetes | 16.8% (447) | 23.6% (326) | 23.2% (341) | 29.4% (528) |
| Hypertension | 55.0% (447) | 60.7% (326) | 75.1% (341) | 81.3% (528) |
| Hypercholesterolemia | 23.3% (447) | 21.8% (326) | 49.6% (341) | 55.3% (528) |
| Chronic renal insufficiency | 3.8% (447) | 5.1% (99) | 26.6% (316) | |
| Current smoking | 4.9% (446) | 2.8% (326) | 5.6% (341) | 3.2% (528) |
| Congestive heart failure | 21.5% (447) | 23.0% (326) | 19.1% (341) | 24.6% (528) |
| MI | 41.8% (447) | 40.8% (326) | 34.0% (341) | 35.8% (528) |
| PCI | 5.8% (447) | 8.0% (326) | 18.2% (341) | 21.0% (528) |
| CABG | 8.3% (447) | 8.3% (326) | 19.4% (341) | 17.0% (528) |
| PVD | 13.9% (447) | 14.2% (120) | 13.2% (341) | 12.5% (528) |
| Presenting clinical characteristics | ||||
| GRACE score risk for 6-month mortality, median (Q1-Q3)† | 151 (139-165) | 147 (134-159) | 151 (141-165) | 148 (138-157) |
| GRACE score ≥140† | 74.3% (366) | 68.9% (119) | 77.7% (336) | 70.0% (387) |
Percentage and denominator for each variable across time is reported.
GRACE score for 6-month death is not available for
GUSTO IV, PRISM, PRISM PLUS and APPRAISE-2. CABG: coronary artery bypass graft, MI: myocardial infarction, PCI: percutaneous coronary intervention, Q1-Q3: first and third quartiles.
Age-stratified temporal trends in pharmacological and invasive management
In-hospital use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEi/ARBs), beta-blockers, and lipid-lowering drugs increased in all age strata from 1994–2010 (all P<0.0001; Table 3). Similarly, there were significant increases over time in use of these medications at discharge (all P<0.0001). The trend over time × age interaction terms were statistically significant, indicating that changes in slopes between age groups differed over time, reflecting narrowing treatment gaps between age groups (Figure 2). Within all age groups, in-hospital aspirin use was high throughout the study period, while aspirin use at discharge increased between 1994–1997 and 1998–2001 and then remained high (Table 3, Figure 2).
Table 3. Concomitant medications and invasive treatment by age group and time period.
| Randomization Year | ||||
|---|---|---|---|---|
|
|
||||
| 1994–1997 | 1998–2001 | 2002–2005 | 2006–2010 | |
| Age <65 years | (N=13401) | (N=6377) | (N=4320) | (N=11774) |
|
| ||||
| In-hospital medications | ||||
| Aspirin | 96.7% (13278)* | 98.4% (6373) | 95.5% (4314) | 97.4% (11745) |
| ACE inhibitors/ARBs | 26.6% (13389) | 28.6% (6377) | 61.2% (4314) | 67.1% (4977) |
| Thienopyridines | 14.2% (9546) | 21.5% (6377) | 71.1% (4319) | 94.4% (11763) |
| Beta-blockers | 75.8% (13396) | 60.2% (6377) | 88.8% (4315) | 86.2% (11747) |
| GP IIb/IIIa inhibitors | 35.1% (9545) | 24.6% (6377) | 32.9% (4318) | 15.5% (11772) |
| Lipid-lowering drugs | 21.6% (13387) | 24.7% (2924) | 76.8% (4320) | 91.3% (11774) |
| Discharge medications | ||||
| Patients alive at discharge | 13230 | 6318 | 4278 | 11663 |
| Aspirin | 69.9% (13104) | 98.7% (2882) | 93.8% (4274) | 95.9% (11658) |
| ACE inhibitors/ARBs | 18.3% (13221) | 35.2% (2892) | 58.9% (4272) | 64.6% (4921) |
| Thienopyridines | 10.9% (9430) | 30.8% (2892) | 63.6% (4276) | 77.7% (11639) |
| Beta-blockers | 48.6% (13225) | 70.1% (2892) | 83.1% (4273) | 80.6% (11636) |
| Lipid-lowering drugs | 18.3% (13219) | 42.3% (2892) | 80.4% (4278) | 87.7% (11663) |
| Index hospital invasive treatments | ||||
| Catheterization | 62.2% (13400) | 54.3% (6375) | 95.0% (4320) | 77.9% (11772) |
| PCI | 23.5% (13400) | 23.0% (6375) | 53.2% (4320) | 51.2% (11774) |
| CABG | 11.3% (13396) | 8.6% (6375) | 18.5% (4317) | 8.8% (11772) |
| Length of stay, median (Q1-Q3) | 9 (6-13) | 8 (6-12) | 5 (4-9) | 5 (4-8) |
|
| ||||
| Age 65–74 years | (N=8710) | (N=4353) | (N=4014) | (N=8170) |
|
| ||||
| In-hospital medications | ||||
| Aspirin | 96.5% (8605) | 97.6% (4349) | 95.4% (4007) | 96.3% (8142) |
| ACE inhibitors/ARBs | 34.0% (8695) | 39.8% (4353) | 64.9% (4003) | 67.7% (4164) |
| Thienopyridines | 12.3% (6104) | 17.5% (4353) | 67.7% (4014) | 92.1% (8162) |
| Beta-blockers | 72.0% (8702) | 62.3% (4353) | 86.1% (4011) | 85.0% (8161) |
| GP IIb/IIIa inhibitors | 35.6% (6107) | 20.5% (4353) | 31.7% (4013) | 11.9% (8164) |
| Lipid-lowering drugs | 18.0% (8693) | 21.5% (1776) | 74.9% (4014) | 88.9% (8168) |
| Discharge medications | ||||
| Patients alive at discharge | 8382 | 4208 | 3917 | 8031 |
| Aspirin | 71.7% (8270) | 98.8% (1725) | 91.8% (3917) | 95.4% (8021) |
| ACE inhibitors/ARBs | 24.7% (8373) | 43.9% (1727) | 61.0% (3908) | 64.9% (4073) |
| Thienopyridines | 9.8% (5876) | 26.5% (1727) | 58.7% (3916) | 76.6% (8007) |
| Beta-blockers | 46.8% (8377) | 65.9% (1727) | 80.5% (3914) | 80.1% (8025) |
| Lipid-lowering drugs | 15.1% (8371) | 38.7% (1727) | 77.5% (3917) | 86.7% (8029) |
| Index hospital invasive treatments | ||||
| Catheterization | 56.0% (8702) | 47.3% (4352) | 93.9% (4014) | 73.6% (8168) |
| PCI | 18.3% (8708) | 17.2% (4353) | 46.5% (4014) | 45.2% (8170) |
| CABG | 15.2% (8705) | 11.3% (4353) | 19.5% (4013) | 10.0% (8168) |
| Length of stay, median (Q1-Q3) | 10 (7-16) | 10 (7-15) | 6 (4-10) | 6 (4-9) |
|
| ||||
| Age 75–79 years | (N=2543) | (N=1769) | (N=1611) | (N=2911) |
|
| ||||
| In-hospital medications | ||||
| Aspirin | 96.9% (2517) | 97.2% (1767) | 94.6% (1607) | 96.2% (2899) |
| ACE inhibitors/ARBs | 36.7% (2537) | 43.9% (1769) | 65.0% (1607) | 67.4% (1565) |
| Thienopyridines | 9.7% (1673) | 15.0% (1769) | 68.3% (1610) | 92.3% (2908) |
| Beta-blockers | 68.6% (2542) | 62.7% (1769) | 84.7% (1611) | 85.0% (2908) |
| GP IIb/IIIa inhibitors | 31.9% (1673) | 19.1% (1769) | 31.2% (1611) | 9.8% (2909) |
| Lipid-lowering drugs | 12.9% (2541) | 20.9% (657) | 73.4% (1611) | 87.6% (2910) |
| Discharge medications | ||||
| Patients alive at discharge | 2368 | 1672 | 1546 | 2822 |
| Aspirin | 70.3% (2340) | 99.0% (621) | 89.6% (1546) | 93.8% (2817) |
| ACE inhibitors/ARBS | 25.9% (2363) | 43.6% (624) | 61.8% (1542) | 63.7% (1512) |
| Thienopyridines | 6.4% (1554) | 26.8% (624) | 59.4% (1544) | 77.3% (2809) |
| Beta-blockers | 43.8% (2368) | 66.3% (624) | 76.9% (1546) | 78.9% (2820) |
| Lipid-lowering drugs | 10.7% (2367) | 36.5% (624) | 74.1% (1546) | 84.9% (2821) |
| Index hospital invasive treatments | ||||
| Catheterization | 47.0% (2539) | 44.5% (1768) | 92.1% (1611) | 75.3% (2910) |
| PCI | 13.0% (2543) | 16.0% (1767) | 44.6% (1611) | 47.0% (2911) |
| CABG | 13.0% (2539) | 10.3% (1768) | 17.9% (1611) | 9.3% (2910) |
| Length of stay, median (Q1-Q3) | 10 (7-16) | 10 (7-16) | 7 (4-10) | 6 (5-10) |
|
| ||||
| Age 80–84 years | (N=1277) | (N=778) | (N=888) | (N=1603) |
|
| ||||
| In-hospital medications | ||||
| Aspirin | 96.6% (1259) | 97.2% (778) | 94.6% (887) | 95.9% (1596) |
| ACE inhibitors/ARBs | 40.2% (1277) | 39.3% (778) | 64.4% (888) | 64.8% (907) |
| Thienopyridines | 9.4% (773) | 14.8% (778) | 69.3% (888) | 91.5% (1599) |
| Beta-blockers | 63.6% (1277) | 58.7% (778) | 86.0% (888) | 83.2% (1598) |
| GP IIb/IIIa inhibitors | 28.2% (772) | 21.0% (778) | 28.2% (888) | 8.6% (1602) |
| Lipid-lowering drugs | 6.8% (1277) | 19.3% (326) | 68.1% (888) | 85.8% (1603) |
| Discharge medications | ||||
| Patients alive at discharge | 1174 | 715 | 840 | 1532 |
| Aspirin | 70.6% (1155) | 98.3% (300) | 90.5% (840) | 94.5% (1529) |
| ACE inhibitors/ARBs | 27.9% (1174) | 44.0% (300) | 58.0% (840) | 62.5% (867) |
| Thienopyridines | 6.3% (712) | 25.0% (300) | 62.1% (840) | 77.5% (1527) |
| Beta-blockers | 44.2% (1174) | 63.3% (300) | 77.6% (840) | 79.5% (1526) |
| Lipid-lowering drugs | 5.7% (1174) | 32.0% (300) | 70.4% (840) | 84.3% (1532) |
| Index hospital invasive treatments | ||||
| Catheterization | 33.2% (1277) | 33.3% (778) | 85.2% (888) | 72.0% (1602) |
| PCI | 11.1% (1277) | 14.3% (778) | 43.9% (888) | 46.9% (1603) |
| CABG | 7.3% (1277) | 7.3% (778) | 15.0% (888) | 7.3% (1602) |
| Length of stay, median (Q1-Q3) | 10 (7-15) | 10 (7-15) | 7 (4-11) | 7 (5-10) |
|
| ||||
| Age ≥85 years | (N=447) | (N=326) | (N=341) | (N=528) |
|
| ||||
| In-hospital medications | ||||
| Aspirin | 95.4% (439) | 96.3% (326) | 96.5% (340) | 96.0% (527) |
| ACE inhibitors/ARBS | 35.3% (447) | 45.1% (326) | 65.2% (339) | 63.4% (333) |
| Thienopyridines | 6.6% (256) | 15.6% (326) | 69.5% (341) | 89.5% (526) |
| Beta-blockers | 66.0% (447) | 53.1% (326) | 83.0% (341) | 82.7% (526) |
| GP IIb/IIIa inhibitors | 32.0% (256) | 16.0% (326) | 23.8% (340) | 7.2% (528) |
| Lipid-lowering drugs | 5.6% (447) | 10.0% (120) | 63.3% (341) | 82.8% (528) |
| Discharge medications | ||||
| Patients alive at discharge | 397 | 297 | 313 | 494 |
| Aspirin | 74.9% (391) | 98.2% (110) | 91.3% (312) | 94.1% (493) |
| ACE inhibitors/ARBs | 26.2% (397) | 50.9% (110) | 57.6% (311) | 58.9% (314) |
| Thienopyridines | 5.7% (230) | 26.4% (110) | 60.9% (312) | 78.9% (492) |
| Beta-blockers | 46.1% (397) | 54.5% (110) | 75.7% (313) | 76.9% (493) |
| Lipid-lowering drugs | 4.8% (397) | 20.9% (110) | 66.1% (313) | 79.4% (494) |
| Index hospital invasive treatments | ||||
| Catheterization | 22.1% (447) | 27.3% (326) | 76.5% (340) | 64.8% (528) |
| PCI | 8.3% (447) | 10.7% (326) | 40.5% (341) | 39.8% (528) |
| CABG | 4.5% (447) | 4.3% (326) | 8.5% (340) | 2.7% (528) |
| Length of stay, median (Q1-Q3) | 9 (7-15) | 10 (7-15) | 6 (4-11) | 7 (5-10) |
Percentage and denominator for each variable across time is reported. ACE: angiotensin-converting enzyme; ARB: angiotensin II receptor blocker; CABG: coronary artery bypass graft; PCI: percutaneous coronary intervention; Q1-Q3: first and third quartiles.
Figure 2.
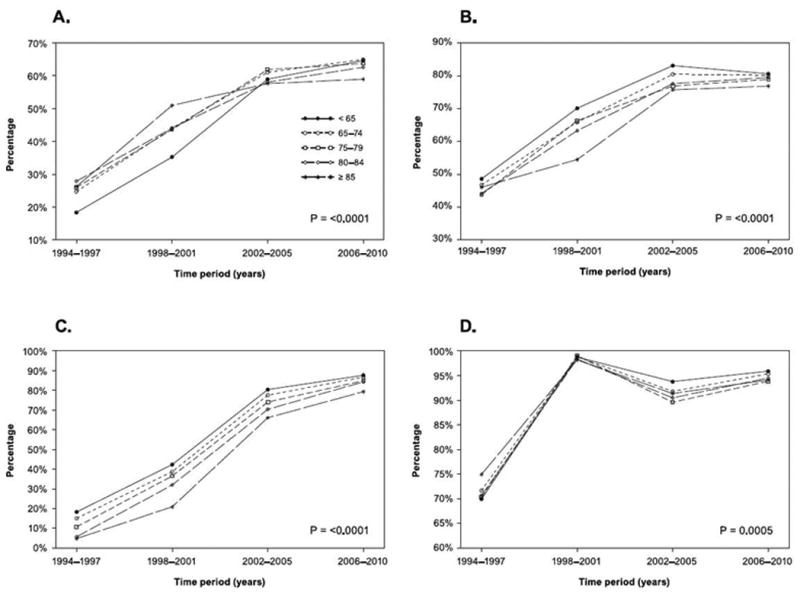
Selected discharge medication use by age group over time. A) Angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; B) Beta-blocker; C) Lipid-lowering drugs; D) Aspirin. Age × time interaction P-values are displayed. Significant P-values indicate that changes in use over time differed statistically according to age.
Coronary angiography and PCI use increased among patients in all age groupings over time (all P<0.0001). Regardless of time period, older patients less often received PCI than younger groups, but these differences narrowed over time (Table 3, Figure 3). Rates of CABG were generally stable over time, and were lowest among the two oldest age groups and highest among patients between 65–74 years. Median length of stay decreased by 3–4 days in all age groupings from 1994–1997 to 2006–2010 (P<0.0001; Table 3).
Figure 3.
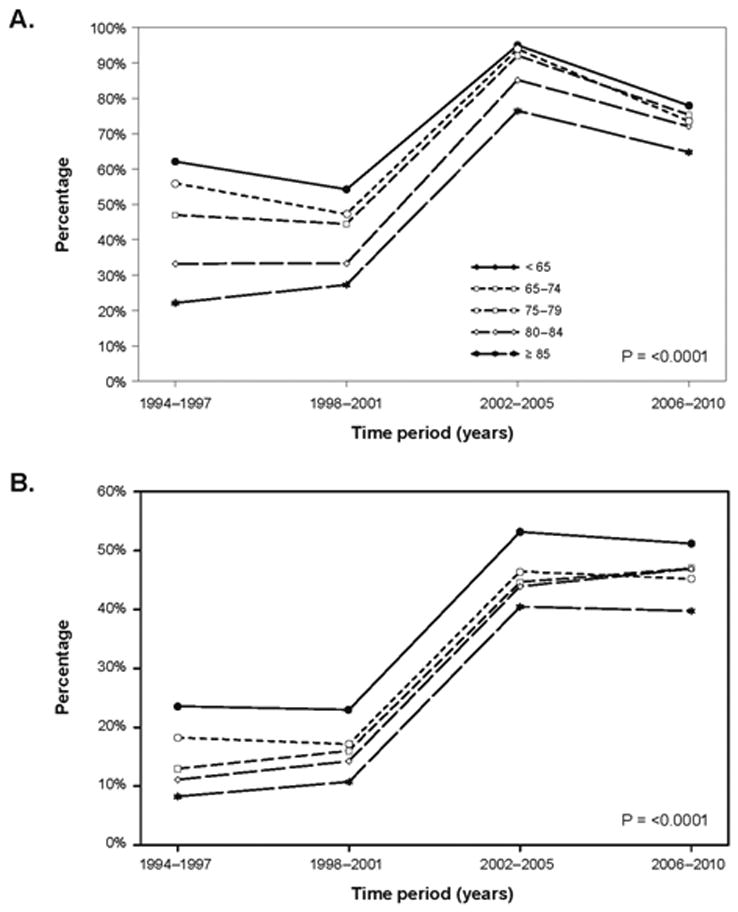
Use of an invasive strategy by age group over time. A) Angiography; B) Percutaneous coronary intervention. Age × time interaction P-values are displayed. Significant P-values indicate that changes in use over time differed statistically according to age.
Temporal trends in clinical outcomes by age
Rates and temporal patterns of observed in-hospital, 30-day, 6-month, and 1-year mortality over the 17-year period are shown in Table 4 and Supplemental Figure 2, respectively, and 30-day death or MI rates are displayed in Supplemental Table 2. Despite slightly increased predicted 6-month mortality within each age stratum over time, observed 6-month mortality fell in all age groups, and the changes in observed mortality rates over time did not vary significantly according to age (P for interaction=0.3345); Tables 2 and 4, Figure 4).
Table 4. Clinical outcomes by age according to randomization year.
| Randomization Year | ||||
|---|---|---|---|---|
|
|
||||
| 1994–1997 | 1998–2001 | 2002–2005 | 2006–2010 | |
| Age <65 years | (N=13401) | (N=6377) | (N=4320) | (N=11774) |
|
| ||||
| Death* | ||||
| In-hospital death | 1.3% (13401) | 0.9% (6377) | 0.9% (4318) | 0.8% (11758) |
| 30-day death | 1.6% (13401) | 1.4% (6377) | 1.2% (4320) | 0.9% (11774) |
| Kaplan-Meier rates† | ||||
| 6-month death | 331 (3.0%) | 82 (2.8%) | 108 (2.5%) | 212 (1.9%) |
| 6-month death among patients with GRACE score available‡ | 293 (2.9%) | 81 (2.9%) | 108 (2.5%) | 165 (1.8%) |
| 1-year death | 351 (3.8%) | 98 (3.6%) | 144 (3.4%) | 298 (2.7%) |
|
| ||||
| Age 65–74 years | (N=8710) | (N=4353) | (N=4014) | (N=8170) |
|
| ||||
| Death* | ||||
| In-hospital death | 3.8% (8709) | 3.3% (4353) | 2.4% (4012) | 1.5% (8157) |
| 30-day death | 4.3% (8710) | 3.9% (4353) | 2.7% (4014) | 1.7% (8170) |
| Kaplan-Meier rates† | ||||
| 6-month death | 557 (7.5%) | 105 (6.0%) | 214 (5.3%) | 269 (3.4%) |
| 6-month death among patients with GRACE score available‡ | 495 (7.2%) | 102 (5.9%) | 210 (5.3%) | 208 (3.4%) |
| 1-year death | 581 (9.2%) | 129 (7.8%) | 291 (7.3%) | 371 (5.0%) |
|
| ||||
| Age 75–79 years | (N=2543) | (N=1769) | (N=1611) | (N=2911) |
|
| ||||
| Death* | ||||
| In-hospital death | 6.8% (2542) | 5.5% (1769) | 4.0% (1611) | 2.8% (2903) |
| 30-day death | 7.7% (2542) | 6.3% (1769) | 5.0% (1611) | 3.2% (2911) |
| Kaplan-Meier rates† | ||||
| 6-month death | 284 (13.0%) | 62 (9.5%) | 138 (8.6%) | 180 (6.5%) |
| 6-month death among patients with GRACE score available‡ | 242 (12.2%) | 61 (9.6%) | 133 (8.4%) | 152 (6.7%) |
| 1-year death | 278 (14.6%) | 72 (11.6%) | 180 (11.2%) | 231 (8.6%) |
|
| ||||
| Age 80–84 years | (N=1277) | (N=778) | (N=888) | (N=1603) |
|
| ||||
| Death* | ||||
| In-hospital death | 8.1% (1277) | 8.1% (778) | 5.4% (888) | 3.8% (1593) |
| 30-day death | 8.9% (1277) | 9.5% (778) | 6.3% (888) | 3.4% (1603) |
| Kaplan-Meier rates† | ||||
| 6-month death | 194 (17.6%) | 42 (12.9%) | 91 (10.3%) | 125 (8.2%) |
| 6-month death among patients with GRACE score available‡ | 172 (17.0%) | 40 (12.5%) | 89 (10.2%) | 101 (8.3%) |
| 1-year death | 204 (21.1%) | 54 (18.2%) | 128 (14.5%) | 176 (12.1%) |
|
| ||||
| Age ≥85 years | (N=447) | (N=326) | (N=341) | (N=528) |
|
| ||||
| Death* | ||||
| In-hospital death | 11.2% (447) | 8.9% (326) | 8.2% (341) | 5.5% (523) |
| 30-day death | 13.0% (447) | 11.7% (326) | 11.1% (341) | 5.1% (528) |
| Kaplan-Meier rates† | ||||
| 6-month death | 98 (24.8%) | 21 (17.6%) | 60 (17.6%) | 63 (12.6%) |
| 6-month death among patients with GRACE score available‡ | 84 (23.1%) | 21 (17.7%) | 59 (17.6%) | 49 (13.1%) |
| 1-year death | 94 (26.5%) | 29 (26.6%) | 83 (24.4%) | 88 (18.8%) |
Percentage and denominator for in-hospital and 1-month mortality across time is reported.
Kaplan-Meier rates are reported for 6-month and 1-year mortality outcomes, as 6-month follow-up is not complete in GUSTO IV and PRISM and 1-year mortality is not complete in GUSTO IV, PRISM, and PRISM PLUS.
GRACE score was not available in GUSTO IV, PRISM, PRISM PLUS, and APPRAISE-2.
Figure 4.
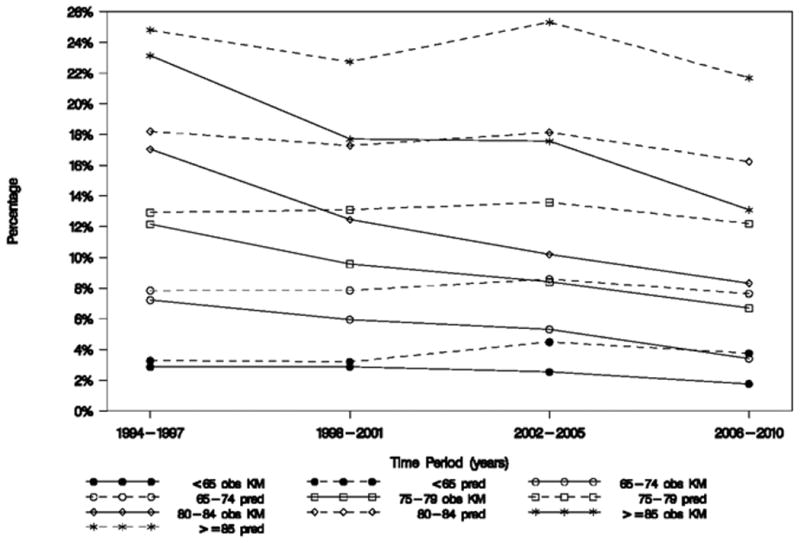
Predicted versus observed 6-month mortality. The age × time interaction P-value for observed mortality = 0.3345 and is from a model using trials that collected GRACE score data: GUSTO IIb, PARAGON-A and -B, PURSUIT, SYNERGY, EARLY ACS, and TRACER. An insignificant P-value indicates that changes in observed mortality rates over time did not vary significantly according to age. A P-value for GRACE model-predicted mortality was not calculated because age is a variable in the GRACE model.
Older groups had higher bleeding and transfusion rates than younger patients, but temporal patterns of GUSTO moderate (P for interaction=0.8860) and severe bleeding (P for interaction=0.4264) were similar by age group (Supplemental Table 3). Rates of blood transfusion were substantially higher than rates of severe bleeding during all time periods regardless of age, even after excluding CABG-treated patients (Supplemental Tables 3 and 4).
Discussion
This analysis of 76,141 patients revealed no overall change in the age distribution of enrollment in randomized clinical trials of NSTE ACS pharmacotherapies over the 17-year period studied, despite inclusion criteria in more recent trials that selected for older patients. Significant increases in use of evidence-based pharmacotherapies in-hospital and at discharge and in use of angiography and PCI and substantial decreases in length of stay occurred regardless of age. Observed mortality declined by approximately half in all age strata despite more comorbidities and a small increase in predicted 6-month mortality.
Age-stratified trends in enrollment and medical care
Despite efforts aimed at increasing enrollment of older patients in randomized clinical trials, particularly in the later trials in our series,14,15 enrollment by age did not change substantially over time. This may reflect physician bias in enrollment due to concern for bleeding, patient preference, or exclusion criteria related to renal function or other comorbidities that may disproportionately affect the elderly. Because safety and efficacy of pharmacotherapy can differ by patient age, underrepresentation of older patient groups could limit generalizability of clinical trial results to the aging population. To the extent that this creates concern among providers about extrapolating clinical trial results to treatment of older patients, under-treatment may result in both high ischemic risk and potentially high risk of adverse events in this group. To best generalize ACS clinical trials results to actual practice and particularly to ensure that treatment effects are known for the growing older segment of the population, every effort must be made to enroll eligible elderly patients and to avoid restrictive inclusion and exclusion criteria that limit their participation.
Age-stratified trends in treatment, mortality, and ischemic outcomes
Among those enrolled, use of evidence-based pharmacotherapies and angiography increased substantially within all age groupings over time. In general, gaps in care by age were narrowing over time, consistent with observations from registries,18,19 and mortality declines were more prominent among older groups. Concurrent with increase in use of evidence-based pharmacotherapy and catheterization and shorter lengths of stay, 6-month and 1-year mortality declined significantly, even though GRACE model-predicted mortality increased slightly over time.
Thirty-day death or MI fluctuated across time periods. This may reflect trial-to-trial variation in MI definition or changes in biomarkers used to define MI, but this is less likely given lower death or MI rates in more recent time periods in which troponins were more widely used in endpoint adjudication. The peak in death or MI rates during 2002–2005 may be due to greater use of an invasive strategy, resulting in more procedure-related MI in the trials enrolling during this time period. Because not all trials we examined distinguished between procedure-related and spontaneous MI, we could not directly assess this possibility. However, in previous work we reported a trend for increasing contribution of procedure-related infarction to total MI rates.4
Age-stratified trends in NSTE ACS bleeding outcomes
Bleeding and transfusion rates were higher among older age groups, but the temporal patterns of GUSTO moderate and severe bleeding and rates of transfusion were similar by age groupings. Bleeding and transfusion rates were lower in all age groupings and time periods after exclusion of CABG patients, but the disproportionately higher rates of transfusion relative to bleeding persisted in all age groups and point to a need for closer examination of transfusion practices.
Strengths and limitations
Strengths of this study include the large number of included patients, the 17-year time period evaluated, and the quality of information on treatments and outcomes. Despite the large number of patients, the analyses are based on a convenience sample of clinical trials conducted at the DCRI or by its colleagues, and for which we had access to patient-level data. However, trends over time, including enrollment by age, were comparable to other NSTE ACS trials conducted during the same period.20–25 Other recent large, multinational, phase III, NSTE ACS trials had comparable findings to the more recent trials in our series, although fewer patients aged ≥75 years were studied.26–29 Finally, overall rates of treatment and outcomes were comparable to those observed in practice registries.19,30,31
We acknowledge trial-to-trial heterogeneity, including in regional distribution of enrollment and endpoint definition (particularly MI) and reporting. We accounted for this by treating the trials as random effects in our mixed model analyses of time trends. Increases in use of some pharmacotherapies (e.g., statins and ACEi/ARBs) reflect their emergence as evidence-based treatments at later time points during our 17-year assessment. However, even for these agents, we observed trends for an increase in use that likely reflect the many efforts to improve use of guidelines-based therapy.
We used the GRACE 6-month mortality model in our predicted versus observed 6-month mortality comparisons. Other models, such as the Thrombolysis In Myocardial Infarction (TIMI) or Predicting Risk of Death in Cardiac Disease Tool (PREDICT) scores, could have been considered and have advantages and disadvantages in both research and clinical practice.32,33 The discriminative ability of the GRACE model and PREDICT score are superior to the model underpinning the TIMI risk score, likely due to a broader array of laboratory and clinical markers of risk.16,17,32–35 Conversely, the TIMI score is easily calculated at the bedside, whereas GRACE and PREDICT require programmed algorithms. Adding ejection fraction to PREDICT and TIMI models yielded incremental predictive value from these scores,32,34,35 but renders them less useful at presentation, although it may be helpful for reassessment in-hospital or at discharge. Because ejection fraction was not available consistently in our dataset, we could not use these modified scores.
Conclusions
Despite efforts aimed at more representative enrollment by age in randomized clinical trials, including elimination of upper age restrictions on enrollment, there was no substantial change in the distribution or enrollment by age in our 17-year series of trials. Use of evidence-based treatments increased in all age strata over the 17-year period, and perhaps due to better treatment, observed mortality fell by approximately half over this time despite slightly increasing predicted 6-month mortality rates.
Supplementary Material
Clinical Perspectives.
Representation by age is important to support translation of clinical trial results to treatment of older patients in practice, but historically, older patients have been underrepresented in clinical trial populations. Our examination of trends in enrollment of patients with non-ST-segment elevation acute coronary syndrome according to age in 11 phase III trials of antithrombotic therapy during 1994–2010 showed that despite relaxation of age criteria for inclusion in trials during more recent time periods, there were minimal changes in the age distribution of enrolled patients. The proportion of enrolled patients aged ≥75 years remained disproportionate to their representation in clinical practice. However, across all age groups, and despite increasing numbers of comorbidities, in parallel with significant increases in use of evidence-based pharmacotherapies in-hospital and an invasive treatment strategy, mortality fell by approximately half over the 17-year study period. The need to pool trial data to obtain an adequate sample size within the older patient groups to demonstrate these favorable trends highlights potential concerns about extrapolating the results from a single trial to the aging population in clinical practice—such concerns that may lead to underuse of guidelines-recommended care among older, inherently higher-risk patients who may derive greater ischemic benefit but also be at greater risk for adverse effects. To ensure that the clinical trial results that form the basis of evidence-based care adequately reflect the benefits and risks of treatment in the aging population, every effort must be made to ensure enrollment in clinical trials is representative by age.
Acknowledgments
We acknowledge statistical guidance from Karen S. Pieper.
Funding Sources: This work was supported by grant number T32GM086330 from the National Institute of General Medical Sciences and by the Merck/Schering-Plough Alliance, which were involved in study design or collection, management, analysis, and interpretation of the data. The following sponsors funded the individual trials: GUSTO IIb: Ciba-Geigy (Summit, NJ), Boehringer Mannheim (Indianapolis, IN) and Guidant (Redwood City, CA); PURSUIT: COR Therapeutics (San Francisco, CA) and Schering-Plough Research Institute (Kenilworth, NJ); PARAGON-A: F. Hoffmann-La Roche, Ltd. (Basel, Switzerland); PARAGON-B: F. Hoffmann-La Roche Ltd. (Basel, Switzerland); PRISM: Merck & Co, Inc. (Whitehouse Station, NJ); PRISM-PLUS: Merck & Co, Inc. (Whitehouse Station, NJ); GUSTO IV-ACS: Eli Lilly and Co., Inc (Indianapolis, IN); SYNERGY: Sanofi-aventis (Paris, France); EARLY ACS: Schering-Plough (Kenilworth, NJ); TRACER: Merck & Co, Inc. (Whitehouse Station, NJ); APPRAISE-2: Bristol-Myers Squibb (Princeton, NJ) and Pfizer (New York, NY).
R.D. Lopes reports research grants from Bristol Myers Squibb, Glaxo Smith Kline (GSK), Consultant/Advisory Board for Bayer Corporation US (Bayer AG/Bayer in Japan – subsidiaries), Boehringer Ingelheim, Bristol Myers Squibb, Glaxo Smith Kline (GSK), and Pfizer. H.D. White reports research grants from Sanofi-Aventis, Eli Lilly, The Medicines Company, NIH, Pfizer, Roche, Johnson & Johnson, Merck Sharpe & Dohme, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo Pharma Development, Bristol-Myers Squibb, Consultant/Advisory Board for Merck Sharpe & Dohme, Regado Biosciences. K.W. Mahaffey reports research grants from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Portola, Regado Biotechnologies, Sanofi, Schering-Plough (now Merck), The Medicines Company, Consultant/Advisory Board for Boehring Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, Ortho/McNeill, Pfizer, Polymedix, Sanofi, Bayer, Daiichi Sankyo, Johnson & Johnson. R.P. Giugliano reports research grants from Amgen, Daiichi Sankyo, Consultant/Advisory Board for Amgen, Daiichi Sankyo, Janssen, Lexicon, Merck, Sanofi-Aventis, Beckhman Coulter. P.W. Armstrong reports research grants from Merck & Co, Consultant/Advisory Board for Eli Lilly and AstraZeneca. R.A. Harrington reports research grants and Merck, AstraZeneca, BMS, Johnson & Johnson, Consultant/Advisory Board for CSL, BMS, Daiichi, MyoKardia, Gilead, Johnson & Johnson, and Merck. P. Tricoci and F. Van de Werf report research grants from Merck, Consultant/Advisory Board for Merck. J.H. Alexander reports research grants from Bristol Myers Squibb, Boehringer Ingelheim, CSL Behring, Oxygen Biotherapeutics, Perosphere, Regado Biosciences, Vivus Pharmaceuticals, Consultant/Advisory Board for Bristol Myers Squibb, Portola, and Regado Biosciences. K.P. Alexander reports research grants from Gilead Sciences, Inc., Sanofi-Aventis, Regeneron Pharmaceuticals, Consultant/Advisory Board for CytRx Corporation. L.K. Newby reports research grants from Amylin Inc., Bristol Myers Squibb, GlaxoSmithKline, Merck & Co., MURDOCK Study, Consultant/Advisory Board; Daiichi Sankyo, Genetech, Novartis, Roche Diagnostics, Janssen Pharmaceuticals, Navigant, and DSI-Lilly.
Footnotes
Disclosures: The remaining authors have no disclosures to report.
References
- 1.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286:708–13. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 2.Newby LK. Acute coronary syndromes in the elderly. J Cardiovasc Med (Hagerstown) 2011;12:220–2. doi: 10.2459/JCM.0b013e328343e9ce. [DOI] [PubMed] [Google Scholar]
- 3.Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–69. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 4.Chan MY, Sun JL, Newby LK, Lokhnygina Y, White HD, Moliterno DJ, Théroux P, Ohman EM, Simoons ML, Mahaffey KW, Pieper KS, Giugliano RP, Armstrong PW, Califf RM, Van de Werf F, Harrington RA. Trends in clinical trials of non-ST-segment elevation acute coronary syndromes over 15 years. Int J Cardiol. 2013;167:548–54. doi: 10.1016/j.ijcard.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 5.A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb investigators. N Engl J Med. 1996;335:775–82. doi: 10.1056/NEJM199609123351103. [DOI] [PubMed] [Google Scholar]
- 6.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998;339:436–43. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 7.International randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin or both in unstable angina. The PARAGON Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network. Circulation. 1998;97:2386–95. doi: 10.1161/01.cir.97.24.2386. [DOI] [PubMed] [Google Scholar]
- 8.Global Organization Network (PARAGON)-B Investigators. Randomized, placebo-controlled trial of titrated intravenous lamifiban for acute coronary syndromes. Circulation. 2002;105:316–21. doi: 10.1161/hc0302.102573. [DOI] [PubMed] [Google Scholar]
- 9.A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. N Engl J Med. 1998;338:1498–505. doi: 10.1056/NEJM199805213382103. [DOI] [PubMed] [Google Scholar]
- 10.Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) N Engl J Med. 1998;338:1488–97. doi: 10.1056/NEJM199805213382102. [DOI] [PubMed] [Google Scholar]
- 11.Simoons ML. Effect of glycoprotein IIb/IIIa receptor blocker abciximab on outcome in patients with acute coronary syndromes without early coronary revascularisation: the GUSTO IV-ACS randomised trial. Lancet. 2001;357:1915–24. doi: 10.1016/s0140-6736(00)05060-1. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson JJ, Califf RM, Antman EM, Cohen M, Grines CL, Goodman S, Kereiakes DJ, Langer A, Mahaffey KW, Nessel CC, Armstrong PW, Avezum A, Aylward P, Becker RC, Biasucci L, Borzak S, Col J, Frey MJ, Fry E, Gulba DC, Guneri S, Gurfinkel E, Harrington R, Hochman JS, Kleiman NS, Leon MB, Lopez-Sendon JL, Pepine CJ, Ruzyllo W, Steinhubl SR, Teirstein PS, Toro-Figueroa L, White H. Enoxaparin vs unfractionated heparin in high-risk patients with non-ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA. 2004;292:45–54. doi: 10.1001/jama.292.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, van 't Hof A, Berdan LG, Lee KL, Strony JT, Hildemann S, Veltri E, Van de Werf F, Braunwald E, Harrington RA, Califf RM, Newby LK. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009;360:2176–90. doi: 10.1056/NEJMoa0901316. [DOI] [PubMed] [Google Scholar]
- 14.Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- 15.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 16.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163:2345–53. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 17.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 18.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Eagle KA, Faxon DP, Fuster V, Gardner TJ, Gregoratos G, Russell RO, Smith SC. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina) Circulation. 2000;102:1193–209. doi: 10.1161/01.cir.102.10.1193. [DOI] [PubMed] [Google Scholar]
- 19.Peterson ED, Shah BR, Parsons L, Pollack CV, Jr, French WJ, Canto JG, Gibson CM, Rogers WJ. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1045–55. doi: 10.1016/j.ahj.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 21.Antman EM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, Salein D, Bayes De Luna A, Fox K, Lablanche JM, Radley D, Premmereur J, Braunwald E. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non-Q-wave myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) 11B trial. Circulation. 1999;100:1593–601. doi: 10.1161/01.cir.100.15.1593. [DOI] [PubMed] [Google Scholar]
- 22.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 23.Antman EM. Hirudin in acute myocardial infarction. Thrombolysis and Thrombin Inhibition in Myocardial Infarction (TIMI) 9B trial. Circulation. 1996;94:911–21. doi: 10.1161/01.cir.94.5.911. [DOI] [PubMed] [Google Scholar]
- 24.Cannon CP, McCabe CH, Wilcox RG, Langer A, Caspi A, Berink P, Lopez-Sendon J, Toman J, Charlesworth A, Anders RJ, Alexander JC, Skene A, Braunwald E. Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI 16) trial. Circulation. 2000;102:149–56. doi: 10.1161/01.cir.102.2.149. [DOI] [PubMed] [Google Scholar]
- 25.Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, Wolff AA, Skene A, McCabe CH, Braunwald E. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes: the MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–83. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- 26.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 27.Mehta SR, Tanguay JF, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, Faxon DP, Rupprecht HJ, Budaj A, Avezum A, Widimsky P, Steg PG, Bassand JP, Montalescot G, Macaya C, Di Pasquale G, Niemela K, Ajani AE, White HD, Chrolavicius S, Gao P, Fox KA, Yusuf S. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–43. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 28.Steg PG, Jolly SS, Mehta SR, Afzal R, Xavier D, Rupprecht HJ, López-Sendón JL, Budaj A, Diaz R, Avezum A, Widimsky P, Rao SV, Chrolavicius S, Meeks B, Joyner C, Pogue J, Yusuf S. Low-dose vs standard-dose unfractionated heparin for percutaneous coronary intervention in acute coronary syndromes treated with fondaparinux: the FUTURA/OASIS-8 randomized trial. JAMA. 2010;304:1339–49. doi: 10.1001/jama.2010.1320. [DOI] [PubMed] [Google Scholar]
- 29.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 30.Stone PH, Thompson B, Anderson HV, Kronenberg MW, Gibson RS, Rogers WJ, Diver DJ, Théroux P, Warnica JW, Nasmith JB, Kells C, Kleiman N, McCabe CH, Schactman M, Knatterud GL, Braunwald E. Influence of race, sex, and age on management of unstable angina and non-Q-wave myocardial infarction: The TIMI III registry. JAMA. 1996;275:1104–12. [PubMed] [Google Scholar]
- 31.Gale CP, Cattle BA, Woolston A, Baxter PD, West TH, Simms AD, Blaxill J, Greenwood DC, Fox KA, West RM. Resolving inequalities in care? Reduced mortality in the elderly after acute coronary syndromes. The Myocardial Ischaemia National Audit Project 2003-2010. Eur Heart J. 2012;33:630–9. doi: 10.1093/eurheartj/ehr381. [DOI] [PubMed] [Google Scholar]
- 32.Singh M, Reeder GS, Jacobsen SJ, Weston S, Killian J, Roger VL. Scores for post-myocardial infarction risk stratification in the community. Circulation. 2002;106:2309–14. doi: 10.1161/01.cir.0000036598.12888.de. [DOI] [PubMed] [Google Scholar]
- 33.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI Risk score for unstable angina/non–ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 34.Bawamia B, Mehran R, Qiu W, Kunadian V. Risk scores in acute coronary syndrome and percutaneous coronary intervention: a review. Am Heart J. 2013;165:441–50. doi: 10.1016/j.ahj.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Ohman EM, Granger CB, Harrington RA, Lee KL. Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA. 2000;284:876–8. doi: 10.1001/jama.284.7.876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


