Abstract
Introduction
Chronic rhinosinusitis (CRS) is likely a biologically heterogeneous disease process. Current guidelines propose subclassification using polyp status while others propose using mucosal eosinophilia. We hypothesized that appropriate CRS subclassification would increase homogeneity of baseline symptoms, and identify characteristic symptoms of each subtype.
Methods
57 CRS patients undergoing surgery prospectively completed a preoperative battery of 73 questions relating to symptoms including the SNOT-22 and PROMIS-29 general quality of life (QOL) measures. Eosinophilic cationic protein (ECP) levels were determined from ethmoid, uncinate, and polyp tissue homogenates using ELISA and normalized to total protein. Patients were classified as eosinophilic (eCRS) or non-eosinophilic (neCRS) using a 95-percentile threshold established from control tissue from 82 patients without CRS. Separate pairwise comparisons were performed on patient-reported symptoms using polyp and eosinophilic status.
Results
28 of 57 patients had CRS with polyps (CRSwNP). 27 of 57 had eCRS (n=21 CRSwNP, n=6 CRSsNP). CRSwNP patients had increased need to blow nose, frequency of nasal congestion, more severe difficulty breathing through nose, more severe nasal discharge, but less cough (p<0.05). eCRS had more bothersome loss of taste/smell, ear pain, sneezing, severe difficulty breathing through nose, and severe nasal congestion compared to neCRS (p<0.05).
Conclusion
Subclassifying CRS with symptoms alone is difficult with neither polyp status nor eosinophilia giving a distinctive clinical symptom profile. However, certain symptoms may help otolaryngologists identify CRS subtypes, which may help guide future treatments. Further validation and evaluation of prognosis following treatment is required to evaluate appropriate means of subclassifying CRS.
Keywords: Chronic rhinosinusitis, endoscopic sinus surgery, eosinophilic rhinitis and nasal polyposis, patient reported outcomes, quality of life
Introduction
The European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) defines chronic rhinosinusitis (CRS) as symptomatic inflammation of the nose and paranasal sinuses found on nasal endoscopy or computed tomography lasting longer than 12 weeks.1 Definitional symptoms include nasal congestion, facial pain/pressure, nasal drainage, and hyposmia.1 Despite this definition, CRS varies in its clinical presentation and response to treatment. This heterogeneity suggests that biological heterogeneity may underpin the clinically observed phenotypes.2–4 Current guidelines propose subclassification using polyp status,1. Further evidence with histologic evaluations of patients with CRS with nasal polyps (CRSwNP) have found a Type 2 inflammatory response with a predominance of eosinophils and interleukin 5 in roughly 80% of patients from Western countries compared to only 35–45% of Asian patients with CRSwNP.1,2,4–8 CRS without nasal polyps (CRSsNP) is more biologically heterogeneous with about 20% of patients demonstrating tissue eosinophilia.5,9
Despite these biological differences, the clinical diagnosis of both CRSwNP and CRSsNP is dependent on the same set of symptoms, and patient reported outcome measures are common to both clinical subtypes. Others have proposed using mucosal eosinophilia to subclassify CRS and found that increased eosinophilia is associated with increased inflammation, higher burden of disease, and a recalcitrant disease course.2,9–12 Unfortunately, mucosal eosinophilia is usually assessed on patients at the time of surgery. Theoretically, earlier identification of eosinophilic CRS (eCRS) may help predict which patients will respond to medical and surgical treatments, especially as there is a growing array of treatment options for Type 2 eosinophilic conditions, such as asthma and atopic dermatitis, in clinical trials.13–19
Considering the heterogeneity of CRS, our aim was to investigate baseline symptoms with both polyp- and eosinophilic-based classifications. We chose to quantify eosinophilic infiltration using eosinophilic cation protein (ECP). ECP is an eosinophilic-specific granular protein contained in circulating eosinophils and released in target tissues of inflammation.20 The function of ECP in CRS is unclear, but a relationship between ECP levels and tissue eosinophilia has been reported.21,22 Furthermore, eosinophil counts may not accurately quantitate degranulated eosinophils as has been suggested in conditions like asthma.23
Methods and Patients
Study population
Institutional Review Board approval was obtained from Northwestern University Feinberg School of Medicine. All patients (>18 years) with CRS undergoing surgery from October 2013 to January 2015 were invited to participate and informed consent was obtained. Patients were asked to complete a preoperative battery of 73 questions relating to clinical symptoms and general quality of life (QOL). Patients were also asked questions relating to medical history and demographics that included age, sex, ethnicity, smoking history, previous sinus surgery, asthma status, aspirin allergy, and aeroallergen sensitivity. Study data were collected and managed using REDCap electronic data capture tools hosted at Northwestern University.24 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.24 Exclusion criteria included cystic fibrosis, human immunodeficiency virus, congenital cilia dyskinesias, and systemic vasculitis or granulomatous processes. Control samples from patients without CRS who underwent endoscopic sinus surgery for indications such as sinonasal and skull base tumors, cerebrospinal fluid leaks and dacrocystorhinostomy were also accessioned from the tissue repository. None of the 82 control patients had asthma, 2 (2%) had atopy; 3 (4%) were using nasal steroids, and 3 (4%) used oral corticosteroids within 2 weeks of surgery. Symptoms were not assessed in the control patients due to the disparate reasons for which they received surgery.
Establishing ECP threshold for classifying eCRS
ECP levels were measured by enzyme-linked immunosorbent assay (ELISA) using the Mesacup ECP Test Kit (MBL; Woburn, MA) from uncinate and ethmoid tissue homogenates from an archived tissue bank of 82 control patients without CRS. The ECP concentration was measured as ng/mL and normalized to total protein concentration measured in each sample using the BCA assay (Thermo Scientific).25 Patients with CRS in our study cohort were then classified as eCRS or non-eosinophilic (neCRS) using a 95-percentile threshold of this normal range. In the same manner, mean ECP levels were calculated from uncinate, ethmoid, and/or polyp tissue obtained at surgery from our study cohort, and a patient’s eosinophilic status was based on the normative threshold.
QOL evaluation
Evaluation of CRS symptoms included the Sinonasal Outcomes Test (SNOT)-22 and additional questions that disambiguated compound symptoms (e.g nasal congestion/obstruction).26,27 We also separately asked how frequent (0, never; 1, rare; 2, sometimes; 3, usually; 4, always) and severe (0, not at all; 1, a little bit; 2, somewhat; 3, quite a bit; 4, very much) individual symptoms (facial pressure, facial pain, difficulty breathing through nose, nasal discharge, nasal congestion, post-nasal drip, smell loss, headaches, coughing, fatigue, nasal itching, sneezing, and eye itching) were in the past 14 days. Using a Visual Analog Scale (0–10cm), patients rated how troublesome their overall symptoms were at present and at one month prior to presentation.1 “0” is not troublesome and “10” is worst thinkable troublesome.1 To gauge how CRS had affected the patient’s overall QOL, the Patient Reported Outcomes Measurement Information System (PROMIS)-29 was used. The PROMIS-29 is an NIH-funded patient-reported outcome measure used to assess a patient’s overall QOL and has been used in other chronic diseases.28 Validated domains assessed by the PROMIS-29 are physical function, anxiety, depression, fatigue, sleep disturbance, satisfaction with social roles, pain interference, and pain intensity.
Statistical analysis
Data analysis was performed using IBM SPSS Statistics for Windows, v22.0 (IBM Corp., Armonk, NY) to investigate any differences in patient responses to the battery of 73 questions based on polyp-status and eosinophilic-status. Chi-square and Mann-Whitney U tests were respectively utilized to compare dichotomous and ordinal demographic and history items. Comparisons of patient responses to the general QOL and sinusitis specific QOL measures were also made using the Mann-Whitney U test. Some patients left certain items unanswered, so a fully conditional specification multiple imputation method was performed to replace missing data values from incomplete questionnaires. A p-value (p)<0.05 indicated statistical significance.
Results
Study population demographics
57 patients were included in our study (Table 1). There were 30 females (53%) and 27 males (47%) with a mean age of 46 years (range 23–74 years). In this cross-sectional study, there were no significant differences in age, race, sex, ethnicity, smoking history, asthma status, aspirin allergy, previous surgery, atopy by self-reported aeroallergen testing, and use of intranasal corticosteroids when subclassified by polyp or eosinophilic status. In the two weeks prior to surgery, use of oral corticosteroids was significantly higher in CRSwNP (25%) than CRSsNP (3%), and in eCRS (22%) compared to neCRS (3%).
Table 1.
The only significant difference (p<0.05) is that patients with CRSwNP compared to CRSsNP, and those with eCRS compared to neCRS, are more likely to have used oral corticosteroids two weeks before surgery.
| CRSsNP (n=29) n (%) |
CRSwNP (n=28) n (%) |
neCRS (n=30) n (%) |
eCRS (n=27) n (%) |
All Patients n (%) |
|
|---|---|---|---|---|---|
| Eosinophilic | 6 (20.7%) | 21 (75.0%) | 27 (100%) | 27 (47.4%) | |
| Nasal Polyps | 28 (100%) | 7 (23.3%) | 21 (77.8%) | 28 (49.1%) | |
| Female | 18 (62.1%) | 12 (42.9%) | 19 (63.3%) | 11 (40.7%) | 30 (52.6%) |
| Prior ESS | 4 (13.8%) | 8 (28.6%) | 4 (13.3%) | 8 (29.6%) | 12 (21.1%) |
| Atopy by skin/blood tests | 14 (48.3%) | 13 (46.4%) | 16 (53.3%) | 11 (40.7%) | 27 (47.4%) |
| Asthma | 9 (31.0%) | 10 (35.7%) | 9 (30.0%) | 10 (37.0%) | 19 (33.3%) |
| Aspirin Allergy | 2 (6.9%) | 4 (14.3%) | 1 (3.3%) | 5 (18.5%) | 6 (10.5%) |
| Smoker | 5 (17.2%) | 6 (21.4%) | 6 (20.0%) | 5 (18.5%) | 11 (19.3%) |
| Oral Steroid | 1 (3.4%) | 7 (25.0%) | 2 (6.7%) | 6 (22.2 %) | 8 (14.0%) |
| Nasal Steroid | 8 (27.6%) | 5 (17.9%) | 7 (23.3%) | 6 (22.2%) | 13 (22.8%) |
| Age (x̄, s2) | (46.7, 16.5) | (45.9, 11.5) | (48.8,14.9) | (43.2, 13.3) | (46.3, 14.3) |
Abbreviations: CRS with polyps (CRSwNP); CRS without polyps (CRSsNP); noneosinophilic CRS (neCRS); eosinophilic CRS (eCRS); endoscopic sinus surgery (ESS). Percentages are expressed as the percent of each column.
Establishing a threshold for eosinophilia
The range of ECP from tissue of 82 control patients was 0.35 to 980ng/mg total protein (mean 64.24ng/mg, SD 130.09). There were no significant differences comparing ECP concentrations between ethmoid and uncinate tissue, so the average ECP in ethmoid and uncinate was calculated when both were available. Using this normative data, the 95 percent threshold for ECP was 289.75ng/mg total protein. 27 (47%) were thus classified as eCRS and 30 were neCRS. 75% (n=21) of CRSwNP patients and 20.7% (n=6) of CRSsNP patients were eosinophilic. By design, patients with eCRS had a significantly higher mean ECP (1,748.42ng/mg total protein, SD 1,705.41) than neCRS (mean 110.21ng/mg total protein, SD 77.77[p<0.01]) and control patients (p<0.01). Patients with CRSwNP had significantly higher mean ECP (1,771.19ng/mg total protein, SD 726.93) than patients with CRSsNP (391.74, SD 726.93) and control patients (p<0.01). There was no significant difference in mean ECP between patients with CRSsNP and control patients.
Analysis of symptoms and QOL using a polyp-based classification
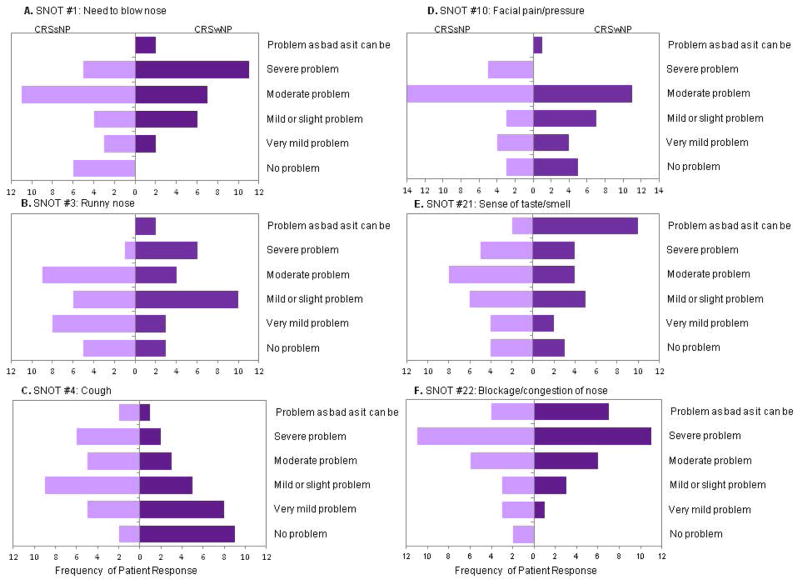
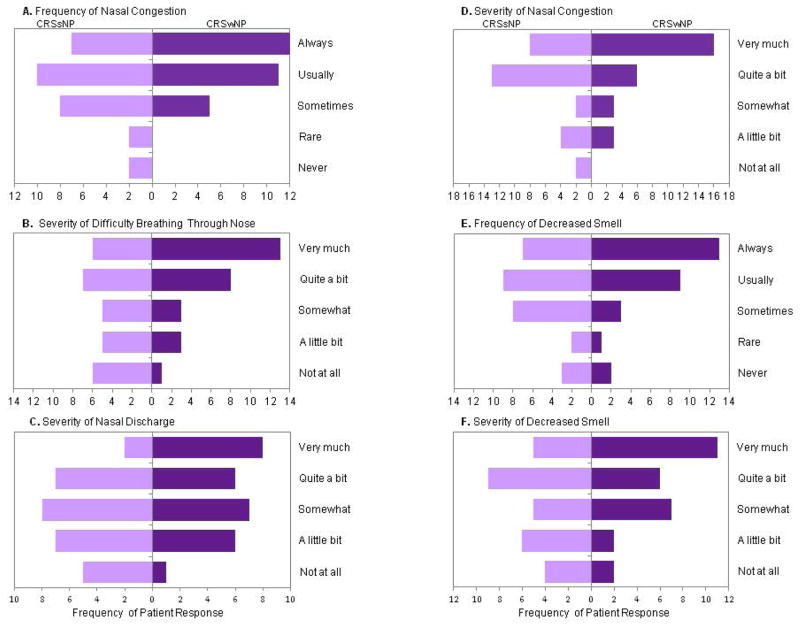
There were no significant differences between patients on the overall VAS or on the 8 validated domains of the PROMIS-29 based on polyp status. There was also no significant difference in the total SNOT-22 scores between patients with CRSwNP (mean 42.5, SD 15.3) and CRSsNP (mean 40.3, SD 15). On analysis of individual SNOT-22 items, those with CRSwNP reported significantly more bothersome need to blow nose and less cough (p<0.05) (Figure 1). When examining the effect of separating compound symptom descriptors found in the SNOT-22, patients with CRSwNP were more bothered in the frequency of nasal congestion, the severity of difficulty breathing through the nose, and the severity of nasal discharge (p<0.05) (Figure 2).
Figure 1.
On the SNOT-22, A) patients with CRSwNP had significantly more bothersome need to blow nose (p<0.05), but B) runny nose was not significantly different (0.05>p<0.1). C) Cough (p=0.005) was more bothersome for patients with CRSsNP. There were no significant differences in D) facial pain/pressure, E) difficulty with taste/smell, and F) nasal blockage/congestion (p>0.05).
Figure 2.
Patients with CRSwNP were significantly (p<0.05) more bothered with the A) frequency of nasal congestion, B) severity of difficulty breathing through nose, and C) severity of nasal discharge. D) Severity of nasal congestion (p=0.053), E) frequency of decreased smell (p=0.051), and F) severity of decreased smell were not significant (0.05>p<0.0.1).
Analysis of symptoms and QOL using an eosinophil-based classification
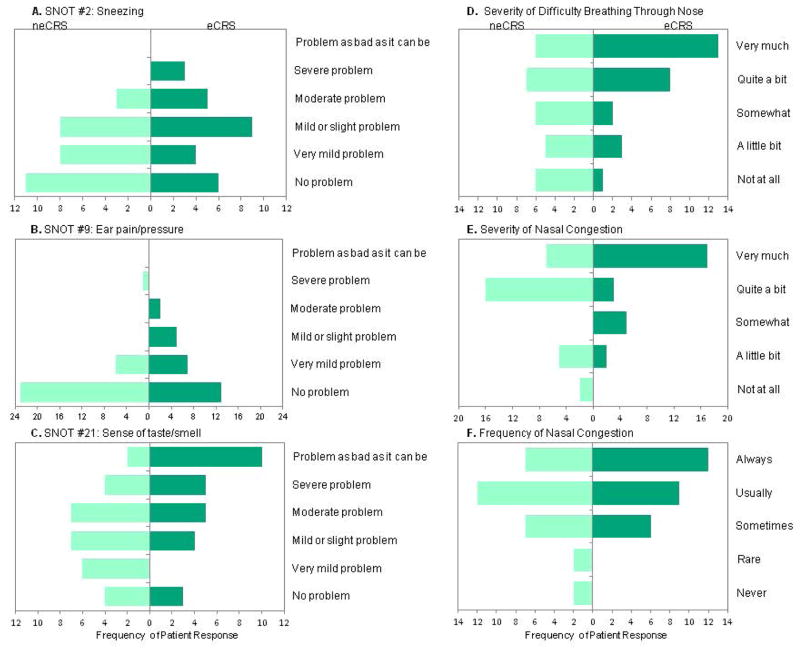
Similarly, there were no differences on the overall VAS, the 8 validated domains of the PROMIS-29, or the total SNOT-22 scores (eCRS [43.1, SD 14.9]; neCRS [mean 39.9, SD 15.3]) when analyzed by eosinophilic status. On analysis of individual SNOT-22 items, patients with eCRS complained of more bothersome disturbance in taste/smell, ear pain, and sneezing (p<0.05) (Figure 3). When examining the effect of separating compound symptom descriptors found in the SNOT-22, patients with eCRS reported more severe difficulty breathing through the nose and more severe nasal congestion (p<0.05) (Figure 3).
Figure 3.
Patients with eCRS reported more bothersome A) sneezing (p<0.05), B) ear pain (p<0.05), and C) loss of taste/smell (p<0.01). They also reported increased D) severity of difficulty breathing through nose (p<0.01) and E) severity of nasal congestion (p<0.05). F) Frequency of nasal congestion was not significant (0.05>p<0.0.1).
Discussion
Ideally, baseline symptoms could help identify CRS subtypes at presentation to help guide treatment and better predict patient prognosis. There are already some differences in treatment recommendations based on polyp status and there may be future treatment differences based on biological endotypes, such as eCRS.1,4,29 Similar to several prior studies, we found no significant differences between CRS subtypes when comparing total scores on QOL instruments.5,9,30,31 However, in this pilot study we did find differences in individual symptoms when CRS was subtyped by polyp or eosinophilic status (Figures 1–3). Although we acknowledge the potential for Type I error due to cohort size, patients in our study with CRSwNP had significantly more bothersome need to blow nose, more frequent nasal congestion, more severe difficulty breathing through nose, more severe nasal discharge, and decreased cough (Figures 1,2). Patients with eCRS complained of more bothersome sneezing, ear pain, loss of taste/smell, severe difficulty breathing through nose, and severe nasal congestion (Figure 3).
Few previous studies have used polyp status to subclassify patients with CRS and compare their baseline individual symptoms. Banjeri et al.32 utilized a modified version of the SNOT questionnaire to assess the severity and frequency of the 4 cardinal symptoms (nasal obstruction, facial pain/pressure, nasal purulence/drainage, and hysosmia/anosmia) in 126 patients with CRS. In their study, CRSwNP was associated with nasal obstruction and hyposmia/anosmia (p<0.05).32 Dietz de Loos et al.30 utilized items in the RSOM-31,33 a predecessor of the SNOT-22, to compare how bothersome symptoms are in 234 patients with CRSwNP and CRSsNP. In their study patients with CRSwNP were more bothered by nasal blockage/congestion, rhinorrhea, need to blow nose, inconvenience of having to carry tissue, and loss of smell/taste (p<0.05). Both studies also found facial pain/pressure/headache to be more bothersome in patients with CRSsNP (p<0.05).30,32 Similar to these two studies, we found items relating to nasal obstruction and discharge to be more closely associated with CRSwNP (Figures 1,2). We also found cough to be significantly associated with CRSsNP, which needs further validation.
Our study is one of only a few that have compared symptoms of patients with eCRS and neCRS using validated questionnaires, and the first study with a definition of eosinophilia based on studies with control ethmoid and uncinate tissue.31,34–37 Soler et al.31 found no differences preoperatively (n=102) in overall QOL scores and individual domain scores using the RSDI, chronic sinusitis survey (CSS), and SF-36 instruments preoperatively but did not report individual symptoms. Mori et al.34 (n=481) utilized a 6 point Likert scale and found eCRS to be significantly more associated with olfactory dysfunction, nasal obstruction, and nasal discharge, which is similar to our findings that patients with eCRS have more bothersome loss of taste/smell, severe difficulty breathing through nose, and severe nasal congestion (Figure 3). Ouyang et al.35 (n=86) found smell loss and cough to be associated with eCRS on a modified SNOT questionnaire. Both Zuo et al.36 (n=105) and Haruna et al.37 (n=84) also found eCRS to be significantly associated with smell loss utilizing a 7-point VAS scale and 10-point VAS scale, respectively. Together, these studies suggest that mucosal eosinophilia is strongly associated with smell loss. It remains unclear if mucosal eosinophilia in CRS obstructs air flow and prevents odorant molecules from reaching the olfactory cleft, or causes region specific inflammation causing direct olfactory neuroepithelial inflammatory damage.38,39 In addition to olfactory loss, our study also found increased ear pain associated with eCRS (Figure 3). Although no other prior study has confirmed this association, eosinophils in middle ear fluid have previously been associated with recalcitrant middle ear disease.40,41
A prominent eosinophilic infiltrate has been described in both CRSwNP and CRSsNP and has been associated with increased osteitis and worse scores on endoscopy, computed tomography, and smell identification tests.30,42 Matsuwaki et al.12 found mucosal infiltration to be a significant risk factor for CRS recurrence after surgery. Ishitoya et al.43 advocates for early consideration for sinus surgery and aggressive local and systemic postoperative medical treatments to maintain control in eCRS patients. Ideally, an eCRS subtype could be diagnosed before surgery. However, our data suggest that identifying patients with eCRS from those with neCRS may be challenging with symptoms alone.
eCRS is currently identified from histologic studies of tissue obtained at the time of surgery. However, the definition of eosinophilia is variable across previous studies. Soler et al.31 recommended ≥10 eosinophils/high power field (HPF) on the basis of QOL improvements after sinus surgery. Alternatively, Ikeda et al.44 recommends ≥100 eosinophils/HPF and Matsuwaki et al.12 advocates for ≥120 eosinophils/HPF as a predictor of polyp recurrence. Other groups like Cao et al.6 and Kim et. al.7 have classified eCRS using eosinophil densities of 10 and 20% of inflammatory cells, respectively. Cao et al. 6 has been the only other study to use control tissue, but their threshold was established using inferior turbinate tissue of patients undergoing septoplasty. Clearly, there is a need to establish a standardized definition for eCRS, and we are the first to use normative tissue from uncinate and ethmoid tissue of control patients without CRS to establish a threshold for eCRS. Since histologic methods are tissue and labor intensive and subject to inter-rater and intra-specimen variations in eosinophilia and diligence of interpretation, we chose to quantify eosinophilic infiltration using ECP. ECP is an eosinophilic granule protein, that has been suggested to be a more accurate reflection of eosinophilia in other eosinophilic conditions such as asthma.23 We classified eCRS using a 95th-percentile threshold for ECP established from uncinate and ethmoid tissue of 82 control patients without CRS (289.75ng/mg total protein). Additional studies with outcome data are needed to validate this threshold. Further studies are also needed to directly compare ECP concentration to number of eosinophils/HPF. Currently, our methods still required an operation to classify CRS as eosinophilic, but ECP is a soluble protein and has been measured in nasal lavage.21,45 One prior study did find a correlation between an eosinophil-specific marker, eosinophil peroxidase, from nasal lavage of patients with CRS and serum eosinophilia.46 Similar studies will also be needed to validate a correlation of ECP from nasal lavage with mucosal and serum eosinophilia to evaluate its potential as a non-tissue based biomarker.
Our study also highlights the importance of symptom wording when evaluating patient reported outcomes. Many descriptors are used interchangeably to describe the cardinal symptoms of nasal obstruction and nasal drainage, yet our study suggests that the different descriptors may hold different meanings to patients. Furthermore, our study suggests that certain SNOT-22 items may be improved upon to effectively evaluate patient responses. In our expanded battery of questions, we attempted to disambiguate compound descriptors and better define whether the severity or frequency of a particular symptom was most bothersome. For example, “blockage/congestion of the nose” on the SNOT-22 was not significantly different when classifying by polyp or eosinophil status, yet patients with CRSwNP and eCRS reported significantly more “nasal congestion” and “difficulty breathing through nose” (Figures 1,2). Likewise, the SNOT items of thick nasal discharge or post-nasal discharge were not significantly different, but patients with CRSwNP reported more severe nasal discharge (Figure 2). Our study also suggests that including taste with smell, as asked on the SNOT-22, may be more effective than asking only about the sense of smell. Many patients may not separate smell and taste even though flavor is typically a reflection of olfactory rather than gustatory function. Patients with eCRS reported significantly more bothersome loss of smell/taste, as asked on the SNOT-22, compared to patients with neCRS, but the severity and the frequency of smell loss lacked significance when asked without the descriptor of taste.
A potential limitation of our study is the size of our sample. Higher power may have resulted in more significant differences between subtypes, such as associating loss of smell/taste with CRSwNP or facial pain with CRSsNP. However, for symptoms to meaningfully dictate care, we would need symptoms that distinguish subtypes extremely well in clinical practice. Our study also correlates well with the few previously published papers that demonstrate both clinical phenotypes and endotypes are difficult for clinicians to identify without biological testing.22–24,26–29 An additional limitation is that tissue-based means of classifying eCRS still requires surgery, thus potentially selecting for a more severe group of patients. Until a more minimally invasive test to assess mucosal eosinophilia becomes available, patients complaining of a disturbance in smell/taste (p<0.005) and severe difficulty breathing through nose (p<0.01) are the best indicators at identifying patients with eCRS prior to confirming with tissue. Likewise, increased need to blow nose, frequency of nasal congestion, more severe difficulty breathing through nose, more severe nasal discharge, but less cough identify patients with CRSwNP. Unfortunately, neither definition produces clear symptomatic separation of patients with CRS.
Conclusion
CRS is a biologically heterogeneous inflammatory disorder culminating in >12 weeks of sinonasal symptoms. Using symptoms to subclassify CRS by both polyp- and eosinophilic-status may aid the practitioner in recommending treatments and predicting prognosis, especially as future treatments may directly target Type 2 inflammatory responses.29 Our study examines a wide range of symptoms and suggests that patients with CRSwNP are most bothered by the need to blow nose, the severity of difficulty breathing through nose, and decreased cough compared to those with CRSsNP. A consensus definition of eCRS has not been established, and in this study we used a non-histologic measure using an ECP threshold established on control sinus tissue from patients without CRS. Based on this quantitative measure, patients with eCRS are most likely to be bothered by the loss of taste/smell and the increased severity of difficulty breathing through the nose compared to patients with neCRS. Further validation and evaluation of prognosis following treatment is required to evaluate appropriate means of subclassifying CRS and ultimately guiding treatments.
Acknowledgments
This work was supported by NIH grants K23DC012067 and the Triological Society/American College of Surgeons (B.K.T), the Chronic Rhinosinusitis Integrative Studies Program (CRISP) U19 AI106683 (B.K.T, R.C.K, and R.P.S) R01 HL068546, R01 HL078860, R01 AI072570, (R.P.S) as well as the Ernest S. Bazley Trust (R.P.S). REDCap and the BCC are supported, in part, by NUCATS and the FSM Dean’s Office., 8UL1TR000150
Footnotes
Oral Presentation at the American Rhinologic Society, Combined Otolaryngology Spring Meetings, Boston, MA April 23–24, 2015
No conflict of interest
References
- 1.Fokkens WJ, Lund V, Mullol J. The European position paper on rhinosinusitis and nasal polyps. Rhinol Suppl. 2012;23:1–298. [PubMed] [Google Scholar]
- 2.Jiang N, Kern RC, Altman KW. Histopathological evaluation of chronic rhinosinusitis: a critical review. Am J Rhinol Allergy. 2013;27(5):396–402. doi: 10.2500/ajra.2013.27.3916. [DOI] [PubMed] [Google Scholar]
- 3.Ocampo CJ. Grammar LC. Chronic rhinosinusitis J Allergy Clin Immunol Pract. 2013;1(3):205–211. doi: 10.1016/j.jaip.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Acadecmy of Allergy and Clinical Immunology and the American Academy of Allergy. Asthma & Immunology. 2013;131(6):1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snidvongs K, Lam M, Sacks R, et al. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int Forum Allergy Rhinol. 2012;2:376–385. doi: 10.1002/alr.21032. [DOI] [PubMed] [Google Scholar]
- 6.Cao PP, Li HB, Wang BF, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124(3):478–484. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, Park JH, Rhee CS, et al. Does eosinopohilic inflammation affect the outcome of endoscopic sinus surgery in chronic rhinosinusitis in Koreans? Am J Rhinol Allergy. 2013;27(6):e166–169. doi: 10.2500/ajra.2013.27.3959. [DOI] [PubMed] [Google Scholar]
- 8.Pawliczak R, Lewandowska-Polak A, Kowalski ML. Pathogenesis of nasal polyps: an update. Curr Allergy Asthma Rep. 2005;5(6):463–471. doi: 10.1007/s11882-005-0027-7. [DOI] [PubMed] [Google Scholar]
- 9.Czerny MS, Namin A, Gratton MA, Antisdel JL. Histopathological and clinical analysis of chronic rhinosinusitis by subtype. Int Forum Allergy Rhinol. 2014;4(6):463–469. doi: 10.1002/alr.21304. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson BJ. Categorization of eosinophilic chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2004;12(3):237–242. doi: 10.1097/01.moo.0000124938.46948.c7. [DOI] [PubMed] [Google Scholar]
- 11.Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009;141(4):454–461. doi: 10.1016/j.otohns.2009.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuwaki Y, Ookushi T, Asaka D, et al. Chronic rhinosinusitis: risk factors for the recurrence of chronic rhinosinusitis based on a 5-year follow-up after endoscopic sinus surgery. Int Arch Allergy Immunol. 2008;146(Suppl 1):77–81. doi: 10.1159/000126066. [DOI] [PubMed] [Google Scholar]
- 13.Baguley C, Brownlow A, Yeung K, et al. The fate of chronic rhinosinusitis sufferers after maximal medical therapy. Int Forum Allergy Rhinol. 2014;4(7):525–532. doi: 10.1002/alr.21315. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian HN, et al. A retrospective analysis of treatment outcomes and time to relapse after intensive medical treatment for CRS. Am J Rhinol. 2002;16:303–312. [PubMed] [Google Scholar]
- 15.Young LC, et al. Efficacy of medical therapy in treatment of CRS. Allergy Rhinol. 2012;3(1):e8–e12. doi: 10.2500/ar.2012.3.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal D, Scianna JM, Stankiewicz JA. Efficacy of targeted medical therapy in CRS and predicators of failure. Am J Rhinol Allergy. 2009;23:396–400. doi: 10.2500/ajra.2009.23.3334. [DOI] [PubMed] [Google Scholar]
- 17.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;37(13):1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 18.Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;37(12):130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 20.Malm-Erjefalt M, Greiff L, Ankerst J, et al. Circulating eosinophils in asthma, allergic rhinitis, and atopic dermatitis lack morphological signs of degranulation. Clin Exp Allergy. 2005;35(10):1334–1340. doi: 10.1111/j.1365-2222.2005.02335.x. [DOI] [PubMed] [Google Scholar]
- 21.Ochkur S, Kim JD, Protheroe CA, et al. A sensitive high throughput ELISA for human eosinophil peroxidase: a specific assay to quantify eosinophil degranulation from patient-derived sources. J Immunol Methods. 2012;384(1–2):10–20. doi: 10.1016/j.jim.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groger M, Bernt A, Wolf M, et al. Eosinophils and mast cells: a comparison of nasal mucosa histology and cytology to markers in nasal discharge in patients with chronic sinonasal diseases. Eur Arch Otorhinolaryngol. 2013;270(10):2667–2676. doi: 10.1007/s00405-013-2395-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim CK, Callaway Z, Kim DW, Kita H. Eosinophilic degranulation is more important than eosinophilia in identifying asthma in chronic cough. J Asthma. 2011;48(10):994–1000. doi: 10.3109/02770903.2011.623335. [DOI] [PubMed] [Google Scholar]
- 24.Harris Paul A, Taylor Robert, Thielke Robert, Payne Jonathon, Gonzalez Nathaniel, Conde Jose G. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson S, Poposki JA, Nagarkar DR, et al. Increased expression of CC chemokine ligand 18 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;129(1):119–127. doi: 10.1016/j.jaci.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkins C, Gillett S, Slack R, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009 Oct;34(5):447–54. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 27.Morley AD, Sharp HR. A review of sinonasal outcome scoring systems—which is best? Clin Otolaryngol. 2006;31(2):103–109. doi: 10.1111/j.1749-4486.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 28.Hinchcliff M, Beaumont JL, Thavarajah K, et al. Validity of two new patient-reported outcome measures in systemic sclerosis: Patient-Reported Outcomes Measurement Information System 29-item Health Profile and Functional Assessment of Chronic Illness Therapy-Dyspnea Short Form. Arhtitis Care Res (Hoboken) 2011;63(11):1620–1628. doi: 10.1002/acr.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauwels B, Jonstam K, Bachert C. Emerging biologics for the treatment of chronic rhinosinusitis. Expert Rev Clin Immunol. 2015;11(3):349–361. doi: 10.1586/1744666X.2015.1010517. [DOI] [PubMed] [Google Scholar]
- 30.Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123(1):57–63. doi: 10.1002/lary.23671. [DOI] [PubMed] [Google Scholar]
- 31.Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010;142(1):64–71. doi: 10.1016/j.otohns.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banjeri A, Piccirillo JF, Thawley SE, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21(1):19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 33.Piccirillo JF, Edwards D, Haiduk A, et al. Psychometric and clinimetric validity of the 31-item Rhinosinusitis Outcome Measure (RSOM-31) Am J Rhinol. 1995;9:297–306. [Google Scholar]
- 34.Mori E, Matsuwak Y, Mitsuyama C, et al. Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auri Nasus Larynx. 2013;40(5):465–469. doi: 10.1016/j.anl.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Ouyang Y, Fan E, Li Y, Wang X, Zhang L. Clinical characteristics and expression of thymic stromal lympopoetin in eosinophilic and non-eosinophilic chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2013;75(1):37–45. doi: 10.1159/000346929. [DOI] [PubMed] [Google Scholar]
- 36.Zuo K, Guo J, Chen F, et al. Clinical characteristics and surrogate markers of eosinophilic chronic rhinosinusitis in southern China. Eur Arch Otorhinolaryngol. 2014;27(19):2461–2468. doi: 10.1007/s00405-014-2910-0. [DOI] [PubMed] [Google Scholar]
- 37.Haruna S, Otori N, Moriyama H, Nakanishi M. Olfactory dysfunction in sinusitis with infiltration of numerous activated eosinophils. Auris Nasus Larynx. 2006;33(1):23–30. doi: 10.1016/j.anl.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Holbrook EH, Leopold DA. Anosmia: diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 2003;11(1):54–60. doi: 10.1097/00020840-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Kern RC, Conley DB, Haines GK, 3rd, Robinson AM. Pathology of the olfactory mucosa: implications for the treatment of olfactory dysfunction. Laryngoscope. 2004;114:279–285. doi: 10.1097/00005537-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Iino Y, Tomioka-Matsutani S, Matsubara A, et al. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx. 2011;38(4):456–461. doi: 10.1016/j.anl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Childers AL, Gruen J, Sayeed S, et al. Eosinophilic otitis media. Otol Neurotol. 2014;35(6):e206–207. doi: 10.1097/MAO.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 42.Snidvongs K, McLachlan R, Chin D, et al. Osteitic bone: a surrogate marker of eosinophilia in chronic rhinosinusitis. Rhinology. 2012;50(3):299–305. doi: 10.4193/Rhino12.022. [DOI] [PubMed] [Google Scholar]
- 43.Ishitoya J, Sakuma Y, Tsukuda M. Eosinophilic chronic rhinosinusitis in Japan. Allergol Int. 2010;59(3):239–245. doi: 10.2332/allergolint.10-RAI-0231. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda K, Shiozawa A, Ono N, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope. 2013;123(11):E1–9. doi: 10.1002/lary.24154. [DOI] [PubMed] [Google Scholar]
- 45.Uhliarova B, Kopincova J, Kolomaznik M, et al. Comorbidity has no impact on eosinophil inflammation in the upper airways or on severity of the sinonasal disease in patients with nasal polyps. Clin Otolaryngol. 2015 doi: 10.1111/coa.12392. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Ochkur SI, Kim JD, Protheroe CA, et al. A sensitive high throughput ELISA for human eosinophil peroxidase: a specific assay to quantify eosinophil degranulation from patient-derived sources. doi: 10.1016/j.jim.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]