Abstract
Background
Diagnostic nasal endoscopy is a routine measure of sinonasal inflammation in patients with chronic rhinosinusitis (CRS). Although multiple staging systems have been proposed and evaluated, evidence of association between concurrent symptoms and endoscopic findings remains discordant. The goal of this study is to identify the relevant endoscopic attributes associated with symptom burden, and to systematically derive a weighted endoscopic scale that optimizes prediction of concurrent symptoms.
Methods
Reported baseline symptom (Sinonasal Outcomes Test-22 {SNOT-22}) and endoscopic evaluation scores (Lund-Kennedy {LK}) were obtained from patients with CRS enrolled in a prospective cohort study. Canonical correlation analysis of the SNOT-22 subdomains and LK variables was completed.
Results
A total of 629 patients were included in analysis including 343 with prior endoscopic sinus surgery. Significant canonical correlations outperformed aggregate correlations in explaining variance of the data (33% vs. 3%, respectively). The first canonical correlation was dominated by the Rhinologic symptom domain and the endoscopic polyp score (r=0.54; p<0.05) while additional significant canonical correlation was found between the Extra-rhinologic symptom subdomain and the edema score in patients without prior ESS (r=0.21; p<0.05), and discharge in patients with prior ESS (r=0.22; p<0.05). All other domains and endoscopic variables did not significantly contribute to the canonical correlation.
Conclusions
Although aggregate symptoms and endoscopic scores demonstrate minimal correlation, a weighted combination of symptom domains and endoscopic attributes greatly improves this correlation. A simple approximation of the weights of each of the endoscopic variables of polyps, edema, discharge, scarring, and crusting, is an approximate ratio of 4:2:1:0:0, respectively.
MeSH Key Words: Endoscopy, statistics as topic, sinusitis, epidemiologic measurements, chronic disease
INTRODUCTION
Diagnostic nasal endoscopy is an examination routinely used to evaluate burden of sinonasal inflammation in subjects with chronic rhinosinusitis (CRS). Multiple grading systems have been described in an effort to standardize evaluations of sinonasal cavities to facilitate longitudinal monitoring and research studies.1–7 Although there are a variety of scoring symptoms, they all operationalize some number of sinonasal characteristics by summing an ordinal score assigned to a variety of sinonasal cavity characteristics (specifically, degree of mucosal inflammation, discharge, purulents, scarring, crusting and middle turbinate positioning) generating a composite score. Theoretically, there is some correlation between the burden of sinonasal inflammation and burden of patient symptoms. Efforts to investigate the correlation of endoscopy scoring systems and disease-specific quality of life (QOL) measures, however, have found either no correlation or, at best, weak correlations.8–11
A clinical sense that these correlations underrepresent the ability of nasal endoscopy to predict symptoms has lead to efforts to refine endoscopic scoring systems in an effort to more closely correlate with QOL measures.5,6 Marginal improvements in correlation of endoscopic scores and QOL measures is, in effect, achieved by changing the weights of each sinonasal endoscopic characteristic either through elimination of attributes (effectively weighting this attribute zero)5 or amplifying one of the variables.6 The methods employed to change the weights associated with a given attribute is for the most part based on either an agnostic approach with even weighting throughout,1 weights perceived by clinical judgment,6 correlation of scores to QOL measures, and selective exclusion of the SNOT-22.5 Each of these methodologies has limitations by either potentially ignoring important endoscopic findings in the agnostic approach or over emphasizing the significance of a given variable on the endoscopic exam when relying on the clinical judgment of the investigators. Canonical correlation analysis (CCA) represents a more accurate and systematic method for interpreting the significance of the endoscopic findings and would minimize potential biases while helping clinicians and researchers to focus on the endoscopic findings that correlate to clinically meaningful burden of disease.
The present study seeks to perform a CCA to elucidate which variables of a commonly used endoscopic scoring system (Lund-Kennedy {LK})1 are most effective at predicting contemporaneous QOL in patients with CRS, and to build a model that more accurately predicts QOL by systematically weighting sinonasal endoscopic characteristics.
MATERIALS & METHODS
Patient Population and Inclusion Criteria
Adult (≥18 years) study participants were enrolled across five academic, tertiary care rhinology practices including the Oregon Health & Science University (OHSU, Portland, OR), the Medical University of South Carolina (Charleston, SC), Stanford University (Palo Alto, CA), University of Calgary (Calgary, Alberta, Canada) and University of Utah (Salt Lake City, UT). All subjects had a diagnosis of CRS based on the 2007 Rhinosinusitis Task force criteria12 and were enrolled after failing either broad-spectrum and/or culture-directed antibiotics and a trial of oral and topical steroid therapy. Study participants were allowed to elect continued medical management or to elect endoscopic sinus surgery. All subjects were required to be fluent in both spoken and written English and able to complete all necessary baseline evaluations in addition to giving informed consent. Study participants diagnosed with recurrent acute sinus, a ciliary dyskinesia or cystic fibrosis were excluded from the analysis. The Institutional Review Board (IRB) at each enrollment location provided oversight and annual review of the informed consent process and all investigational protocols, while central review and coordination services were conducted at OSHU (IRB #7198). This data is part of an on-going, multi-institutional, prospective, observational cohort study that has been previously reported.13–16
Outcome Measures
All study participants completed the 22-item Sinonasal Outcome Test (SNOT-22) upon enrollment. The SNOT-22 is a validated CRS-specific outcome measure consisting of 22 items that captures sinus-specific and general health-related impact of the disease process (©2006, Washington University, St. Louis, MO).17 Factor analysis of the SNOT-22 has revealed that the instrument measures 5 distinct health domains.13 The rhinologic domain consists of the questions addressing all of the cardinal symptoms of CRS except for facial pain/pressure.
Diagnostic rigid nasal endoscopy was also performed by the operating surgeon at each site and graded according to the LK Endoscopy Score.1 The LK Endoscopy Score consists of five terms (polyposis, discharge, edema, scarring and crusting) graded on an ordinal scale from 0–2 for each side. Higher scores indicate worse observed disease (Table 1).
Table 1.
Lund-Kennedy endoscopic grading system
| Characteristics: | Score definition |
|---|---|
| Nasal polyps | 0 = none; 1=confined to middle meatus; 2=beyond middle meatus |
| Discharge | 0 = none; 1=clear and thin; 2=thick and purulent |
| Edema | 0=absent; 1=mild; 2=severe |
| Scarring | 0=absent; 1=mild; 2=severe |
| Crusting | 0=absent; 1=mild; 2=severe |
Statistical Analysis
Analysis of the subjects was stratified by history of prior surgery and whether or not the subjects elected a surgical intervention or continued medical therapy alone. This stratification was based on the concern that un-operated sinuses look fundamentally different than patients that have undergone prior surgery. Treatment selection was used for stratification as well given that prior study has identified differences in the domains and aggregate SNOT-22 scores in patients electing surgical therapy over medical therapy alone.18 Differences between subgroups of SNOT-22 and LK scores were compared using one-way analysis of variance (ANOVA). When the overall ANOVA tests were significant (p<0.05), Tukey’s tests and confidence intervals were calculated (with family-wise error rate of 5%) for differences in means between pairs of groups.
Canonical Correlation Analysis
Canonical correlation analysis (CCA) is a general statistical tool to explore and test for relationships between two sets of variables. Indeed, common statistical methods such as multiple regression analysis, the analysis of variance (ANOVA), and multiple analysis of variance (MANOVA) are all special cases of CCA.19 In multiple regression analysis, for example, a set of predictor variables is related to a set of outcome variables where the later set has only one variable. The unstandardized regression coefficients provide a prediction equation that maximizes the linear relationship between these two sets, captures by the multiple correlation coefficient R. These unstandardized regression coefficients also provide clues of the relative importance of each predictor variable in predicting the outcome. CCA generalizes this approach to where there can be several variables in each set. The analog to the multiple correlation R in CCA is called the canonical correlation and the analog to the regression weights in CCA is the function coefficients or weights. In the following analyses, the five endoscopic scores are in one set and the five SNOT-22 domains are in the other set. Below a brief summary of CCA is provided; further information is available elsewhere.19–22
CCA is a multivariate procedure where the data are reduced into a set of orthogonal dimensions and where variables load onto those dimensions. These dimensions are called canonical variates. As there are five variables in each set (5 domains of the SNOT-22 and 5 variables of the LKES), there will be five orthogonal canonical variates in each set. A canonical correlation is the correlation between a canonical variate in one set with the allied canonical variate in the other set. Thus, there will be at most five canonical correlations.
CCA is best used with reasonably large sample sizes.20 The focus of the CCA analysis is therefore the two patient groups electing sinus surgery, one with prior sinus surgery and one without prior sinus surgery.
RESULTS
Study Population and Baseline Characteristics
A total of 629 participants were enrolled between February 2011 and December 2014. Table 2 contains descriptive characteristics of the subjects available for analysis.
Table 2.
Demographics and clinical comorbidities of study subjects (n=629)
| Patient characteristics: | Mean [SD] | N (%) |
|---|---|---|
| Age (years) | 51.0 [14.8] | |
| Males | 299 (48%) | |
| Asthma | 242 (39%) | |
| Nasal polyposis | 242 (39%) | |
| Prior sinus surgery | 343 (55%) | |
| Septal deviation | 223 (36%) | |
| Depression | 89 (14%) | |
| Allergy (mRAST/skin prick confirmed) | 286 (46%) | |
| Aspirin sensitivity | 56 (9%) | |
| Current smoker/tobacco use | 37 (6%) | |
| Diabetes mellitus (Type I/II) | 46 (7%) | |
| Corticosteroid dependency | 51 (8%) |
Notes: SD=standard deviation; mRAST=modified radioallergosorbent testing
Correlational Analysis of SNOT-22 and LKES
Table 3 provides the correlations between LK total endoscopy scores and SNOT-22 total scores and SNOT-22 Rhinologic Domain scores at baseline for the total sample and for four patient subgroups (i.e., those with and without prior sinus surgery and those whose current treatment is either surgical or continued medical therapy). For the total sample, the baseline correlation between endoscopy scores and the SNOT-22 total score is positive, statistically significant, but modest in size; the correlation between endoscopy scores and Rhinologic symptom domain scores is larger in size. For patients whose current treatment is surgery, these two correlations are similar in size for those with and without prior history of sinus surgery. For patients whose current treatment is continued medical therapy, these two correlations are smaller in size and not statistically significant for those with and without prior history of sinus surgery.
Table 3.
Correlations between Baseline Lund-Kennedy Endoscopy Scores with Baseline SNOT-22 Total Scores and Rhinology Domain Scores
| Sample | SNOT-22 Total Scores | SNOT-22 Rhinologic Domain Scores |
|---|---|---|
| Total Sample (N = 629) | .138* | .368* |
| Surgical Patients without Prior History of ESS (N = 238) | .170* | .417* |
| Surgical Patients with Prior History of ESS (N = 277) | .152* | .389* |
| Continued Medical Therapy Patients without Prior History of ESS (N = 48) | −.047 | .195 |
| Continued Medical Therapy Patients with Prior History of ESS (N = 66) | −.050 | .143 |
Notes:
p < .05;
SNOT-22=22-item SinoNasal Outcome Test; ESS=endoscopic sinus surgery
Table 4 provides the results of one-way ANOVA tests for differences in baseline LK endoscopy scores and SNOT-22 total and Rhinologic Domain scores across the four patient types. The one-way ANOVAs revealed significant mean differences across patient types for each of these three baseline variables. Post-hoc Tukey tests were conducted to explore the nature of these mean differences and to find homogenous subsets of patient types. The following homogeneous subsets were observed. For the baseline endoscopy scores, patients without prior surgery (irrespective of current treatment) had significantly lower mean scores than patients with prior surgery. For baseline SNOT-22 total scores and Rhinologic Domain scores, current medical treatment patients without prior sinus surgery had significantly lower mean scores than the other three patient types.
Table 4.
Baseline Mean Differences for Lund-Kennedy Endoscopy Scores, SNOT-22 Total Scores, and SNOT-22 Rhinology Domain Scores at Baseline for Surgical and Continued Medical Therapy Patients with and without Prior Sinus Surgery
| Lund-Kennedy Total Endoscopy Scores | SNOT-22 Total Scores | SNOT-22 Rhinologic Domain Scores | |
|---|---|---|---|
| Surgical Patients without Prior History of ESS (N = 238) | 4.97 | 52.21 | 16.05 |
| Surgical Patients with Prior History of ESS (N = 277) | 7.00 | 54.94 | 16.93 |
| Continued Medical Therapy Patients without Prior History of ESS (N = 48) | 4.90 | 41.52 | 13.42 |
| Continued Medical Therapy Patients with Prior History of ESS (N = 66) | 6.94 | 48.94 | 16.50 |
| F (df=3,625) | 15.535* | 6.632* | 4.315* |
Notes:
p < .05;
SNOT-22=22-item SinoNasal Outcome Test; ESS=endoscopic sinus surgery; F=F-test statistic; df=degrees of freedom
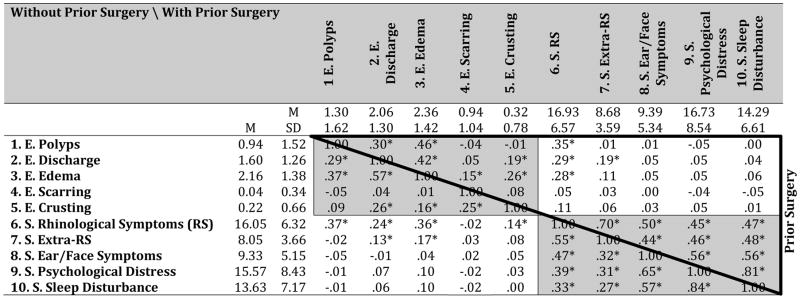
Table 5 provides descriptive statistics for the ten study variables, reported separately for patients without prior surgery (below main diagonal, in lower left) and patients with prior surgery (above main diagonal, in upper right). In both patient groups, all correlations among the five SNOT-22 domains are positive and statistically significant; the correlations among the five endoscopic scores are more modest in size and variable in terms of statistical significance.
Table 5.
Descriptive Statistics for Endoscopy and SNOT-22 Domain Scores at Baseline for Surgical Patients without and with Prior Surgery.
Notes: SNOT-22=22-item SinoNasal Outcome Test; E = Endoscopy Domain; S = SNOT-22 Domain. Shaded blocks contain within-set correlations; unshaded blocks contain across-set correlations.
p < .050.
Patients without prior surgery below main diagonal (N=238). Patients with prior surgery above main diagonal (N = 277).
Of primary interest are the correlations among variables across the two sets (unshaded blocks). In both patient groups, SNOT-22 Rhinologic Symptom domain scores correlated positively and significantly with endoscopic polyp, edema, and discharge scores; furthermore, SNOT-22 Extra-Rhinologic Symptom domain scores correlated positively and significantly with endoscopic discharge scores. With patients without prior surgery, SNOT-22 Extra-Rhinologic Symptom domain scores also correlated positively and significantly with endoscopic edema scores and SNOT-22 Rhinologic Symptom domain scores also correlated positively and significantly with endoscopic crusting scores. No other correlations were significant.
Canonical Correlational Analysis of SNOT-22 and LKES
Table 6 provides the five canonical correlations for the two patient groups. For each patient group, only the first two canonical correlations are sizable and statistically significant. Given that the last three canonical correlations are trivial in size and not statistically significant, the remainder of the focus is on the first two canonical correlations. Together the first two canonical correlations in each patient group account for about 33% of the variance of the variables in each set. This is a marked improvement of the shared variance based on the total scores in each measure (i.e., r2 = .172 = 3%) and for total endoscopic scores with the SNOT-22 Rhinological Symptoms domain (i.e., r2 = .4172 = 17%).
Table 6.
Canonical Correlations and Percent Variance Explained for Endoscopic and SNOT-22 Domain Scores for Patients without and with Prior Surgery.
| Patients Without Prior Surgery
|
Patients With Prior Surgery
|
|||||
|---|---|---|---|---|---|---|
| r | r2 | Cumulative | r | r2 | Cumulative | |
| Function I Pair | .54* | .30 | .30 | .53* | .28 | .28 |
| Function II Pair | .21* | .05 | .34 | .22* | .05 | .33 |
| Function III Pair | .10 | .01 | .35 | .09 | .01 | .34 |
| Function IV Pair | .07 | .00 | .36 | .06 | .00 | .34 |
| Function V Pair | .04 | .00 | .36 | .01 | .00 | .34 |
Notes:
p < .05.
Patients without prior surgery, N = 238; Patients with prior surgery, N = 277.
Each canonical correlation is a weighted combination of the variables in each set. Table 7 provides these weights along with other statistics useful for interpreting the canonical variates. In Table 7, the weights are similar to unstandardized regression coefficients where the outcome is the canonical variate and the statistic rs is the correlation between that variable and the canonical variate. Both statistics are important for interpreting the canonical variate, particularly when the variables within a set are correlated.19 The former provides information on the unique association between a variable and the canonical variate after account for the relationships among the variables; the latter provides information on the overall association between a variable and a canonical variate. The h2 statistic (last column in Table 7) captures how well the first two canonical variates explain variance in the five SNOT-22 and Endoscopy scores.
Table 7.
Canonical Function Coefficients and Correlations between Endoscopy and SNOT-22 Domain Scores and the First Two Canonical Variates for Surgical Patients without and with Prior Surgery.
| Patients Without Prior Surgery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sets | Variable | Function I Pair
|
Function II Pair
|
h2 | ||||
| Weight | rs | rs2 | Weight | rs | rs2 | |||
| SNOT | Rhinologic Symptoms | 0.20 | .80 | .64 | −0.02 | .46 | .21 | .85 |
| Extra-Rhinologic Symptoms | −0.12 | .09 | .01 | 0.26 | .95 | .91 | .92 | |
| Ear / Facial Symptoms | −0.10 | −.03 | .00 | −0.03 | .33 | .11 | .11 | |
| Psychological Distress | −0.00 | .07 | .00 | 0.02 | .50 | .25 | .26 | |
| Sleep Disturbances | 0.01 | .06 | .00 | 0.03 | .49 | .24 | .24 | |
|
| ||||||||
| Endoscopy | Polyps | 0.52 | .94 | .87 | −0.48 | −.34 | .11 | .99 |
| Discharge | 0.02 | .47 | .22 | 0.24 | .57 | .32 | .54 | |
| Edema | 0.24 | .66 | .43 | 0.57 | .71 | .50 | .94 | |
| Scarring | −0.24 | −.09 | .01 | 0.10 | .11 | .01 | .02 | |
| Crusting | 0.15 | .21 | .04 | 0.14 | .25 | .06 | .10 | |
| Patients With Prior Surgery | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sets | Variable | Function I Pair
|
Function II Pair
|
h2 | ||||
| Weight | rs | rs2 | Weight | rs | rs2 | |||
| SNOT | Rhinologic Symptoms | 0.22 | .74 | .55 | −0.02 | .54 | .29 | .84 |
| Extra-Rhinologic Symptoms | −0.19 | .12 | .01 | 0.30 | .91 | .82 | .83 | |
| Ear / Facial Symptoms | −0.04 | .05 | .00 | −0.02 | .27 | .07 | .07 | |
| Psychological Distress | −0.03 | −.05 | .00 | 0.07 | .42 | .17 | .18 | |
| Sleep Disturbances | −0.00 | .01 | .00 | −0.10 | .20 | .04 | .04 | |
|
| ||||||||
| Endoscopy | Polyps | 0.55 | .95 | .90 | −0.39 | −.26 | .07 | .97 |
| Discharge | 0.14 | .50 | .25 | 0.68 | .81 | .65 | .90 | |
| Edema | 0.03 | .58 | .34 | 0.16 | .34 | .11 | .45 | |
| Scarring | 0.12 | .13 | .02 | 0.08 | .20 | .04 | .05 | |
| Crusting | 0.20 | .20 | .04 | 0.09 | .31 | .10 | .14 | |
Notes: Weights = Coefficients that each variable score is multiplied by to generate a patient’s score on the underlying canonical variate (similar to an unstandardized regression weight in multiple regression). rs = correlation between the variable and the canonical variate. rs2 = rs squared (i.e., proportion of variable explained by the canonical variate). h2 = the sum of the two rs2 values
For both patient groups, the canonical variates underlying the first canonical correlation have similar interpretations. Rhinologic Symptoms scores dominate the first canonical variate for the SNOT-22; the endoscopic polyps scores dominate the first canonical variate for the endoscopy measure. Although these variables dominate, the other variables play lesser but important roles as can be observed comparing the first canonical correlations with the correlations between the SNOT-22 Rhinologic Symptoms scores and endoscopic polyps scores in Table 5.
The canonical variate for the SNOT-22 underlying the second canonical correlation has a similar interpretation across the two patient groups; Extra-Rhinologic Symptoms scores dominate this second SNOT-22 canonical variate. The interpretation for the second canonical variate for the endoscopy scores differ slightly across the two patient groups. For patients without prior surgery, endoscopic edema scores are more dominant and the most influential variable followed secondarily by endoscopic discharge scores; for patients with prior surgery, the order of dominance and influence is reversed.
In summary for patients without prior surgery, moderately large multivariate associations are observed between endoscopy scores and the SNOT-22 domain scores at baseline. These associations can be summarized by the correlation between two pairs of canonical variates. The first canonical variate pair can be describes as the correlation between two variables, U1 and V1, where:
The correlation coefficent between U1 and V1 is .54. Thus, for a unit increase in each SNOT-22 domain score, holding the other domain scores constant, the Rhinological Symptom domain has the largest regression weight; for a unit increase in each endoscopy score, holding the other endoscopy scores constant, the polyps score has the largest regression weight. The second canonical variate pair can be described at the correlation between two variables, U2 and V2, where:
The correlation coerfficent between U2 and V2 is .21. Thus, for a unit increase in each SNOT-22 domain score, holding the other domain scores constant, the Extra-Rhinological Symptom domain has the largest regression weight; for a unit increase in each endoscopy score, holding the other endoscopy scores constant, the edema score has the largest regression weight. Similar weighted correlation equations can be derived for patients with prior surgery with the values from Table 6.
DISCUSSION
The present study employed CCA to investigate the most clinically important endoscopic variables for patients with CRS. Outcomes revealed that the endoscopic variables account for a much larger amount of the variance in symptoms than just straight correlation of aggregate scores (33% vs. 3%, respectively). Specifically, nasal polyposis, edema and discharge are most closely correlated with sinonasal specific symptoms (i.e., Rhinologic and Extra-Rhinologic symptoms). Furthermore, each of these endoscopic variables has a differentially weighted correlation with the symptom domains of CRS with nasal polyposis and edema dominating the correlation with the Rhinologic domain, and edema and discharge dominating the Extra-rhinologic domain (see Table 7). Furthermore, the endoscopic variables of scarring and crusting were not significantly correlated with the health domains, and none of the endoscopic variables correlated with the Sleep and Psychologic domains.
Appropriate weighting of an endoscopic scoring system is valuable for both clinical and research purposes. For clinicians who follow and manage patients with CRS with nasal endoscopy, a clearer understanding of the impact of each endoscopic variable on patient symptoms can help dictate management. Similarly, research focused on predicting symptoms and/or optimizing sinonasal cavities to better improve patient outcomes will allow for more clinically significant endpoints. Based on the present analysis and data, the total endoscopic variable score would better predict symptoms (specifically, the Rhinologic and Extra-rhinologic domains) if the potential score for each of the endoscopic variables of polyps, edema, discharge, scar and crust were in an approximate ratio of 4:2:1:0:0, respectively. That is, the scale of the polyp score should be 4 times that of the discharge score, the scale of the edema score should be twice that of the discharge score, and the scarring and crusting score can be abandoned in an effort to best predict sinonasal symptoms. This ratio would be an alternative to the current LK score, which gives equal weighting to each variable, and would refine the clinicians and researchers estimate of clinically significant endoscopic findings.
Past studies on nasal endoscopy scoring systems are either agnostic on the importance of each endoscopic variable (i.e., the unit-weighting in the LK scoring system) or effectively weight each variable based on clinical judgment.3,5,6 However, taken collectively, the clinical intuition and wisdom of past authors parallels the findings of our systematic analysis. For example, the Modified LK scoring system evaluation dropped many of the questions in the Sleep and Psychologic domains5 of the SNOT-22 when seeking to optimize correlation between the sinonasal symptoms and nasal endoscopy. The Modified Lund-Mackay Postoperative score weights edema/polyp formation, discharge, scarring, and crusting in a ratio of 6:4:0:0, respectively.6 Similarly, the Peri-operative Sinus Endoscopy (POSE) score3 increases the clinical significance of polyp and edema by including a potential a mucosal edema, polypoid change and polyposis category for the ethmoid bed, as well as an additional potential score for frontal sinus and/or sphenoid sinus involvement of edema and/or polyps. The present analysis mirrors the collective wisdom of these prior studies, but also validates and refines past studies on weighting of the endoscopic variables by using a systematic statistical methodology.
The present study has some limitations worthy of discussion. Although the present prospective cohort is a relatively large cohort in the CRS literature, it still constitutes a relatively small sample size for CCA. Prior research on CCA indicates a positive small-sample bias in these correlations in the same way that a sample R2 in multiple regression analysis tends to overstate the population R2 and various adjustments have been proposed.20 These adjustments are not used here because 1) the two patient groups provide an opportunity to cross-validate these canonical correlations and 2) the sample size in each patient group is larger than what may be considered small. Given the similarity of these canonical correlations across patient groups, we conclude that these are not likely attributable solely to an over fitting of the data.
This study also does not control for potential comorbid confounding factors which may contribute to the weighted relationship between SNOT-22 scores and sinonasal characteristics identified by the endoscopy staging system. Potentially, there could be differential associations within various comorbid criteria (e.g., depression, fibromyalgia, allergy, asthma, etc.) that may be disproportionately contributing to the reported correlations coefficients. Patients with CRS typically have high rates of comorbid conditions so these analytical findings are more reflective of a ‘real world’ milieu found in an academic, tertiary referral centers.
Correlational analysis also suggests that scores from an endoscopic exam are associated with SNOT-22 total and Rhinological Domain scores only for those patients with conditions serious enough to warrant surgical treatment. Descriptively within the continued medical therapy patient group, the correlation between endoscopy scores and SNOT-22 Rhinological Domain scores is much stronger than correlations with SNOT-22 total scores. This is consistent with prior analysis of the medical and surgical cohort SNOT-22 domains that demonstrates a disproportionate burden of the non-Rhinologic domains in subjects electing surgical therapy with relative parity of the Rhinologic domains between the cohorts.18
Future study examining the correlation of endoscopic and patient-reported disease measures is warranted. The present study was designed to optimize concurrent symptoms with endoscopic sinonasal scores. This optimization helps to clarify the role and value of nasal endoscopy at characterizing the burden of patient-centered disease, but it fails to address the ability of diagnostic nasal endoscopy to forecast treatment outcomes. A clinician that uses nasal endoscopy to evaluate the sinonasal cavity incorporates this endoscopic data into a management decision. Ideally, an endoscopic scoring system could, in part, forecast treatment responses to medical and/or surgical interventions. CCA would serve as an effective means to optimize the correlation between baseline endoscopic scores and post-treatment symptom domains to better clarify our understanding of the significance of nasal endoscopy findings.
CONCLUSION
Historically, correlations between endoscopic findings and patient reported symptoms are low. Canonical correlation analysis reveals that this discrepancy results in part from use of aggregate scores (on both SNOT-22 and LK scales) that dilute the underlying meaningful correlations. Based on the results from this study, the statistically derived weighting of the endoscopic variables to maximize correlation with the symptoms of CRS would have an approximate ratio of 4:2:1:0:0 variables of polyps, edema, discharge, scarring, and crusting, respectively.
Footnotes
Financial Disclosures: Timothy L. Smith, Jess C. Mace, and Jeremiah A. Alt are supported by a grant for this investigation from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD., USA (R01 DC005805; PI/PD: TL Smith). Public clinical trial registration (www.clinicaltrials.gov) ID# NCT01332136. This funding organization did not contribute to the design or conduct of this study; collection, management, analysis, or interpretation of the data; preparation, review, approval or decision to submit this manuscript for publication. Timothy L. Smith and Adam S. Deconde are consultants for IntersectENT, (Menlo Park, CA, USA) which is not affiliated with this investigation. Todd E. Bodner, PhD is supported by grants from the National Heart, Lung, and Blood Institute/Kaiser Permanente, the National Institute for Occupational Safety and Health, and the U.S. Department of Defense, none of which are associated with funding for this study. There are no financial disclosures for Luke Rudmik.
Potential Conflicts of Interest: None to report
The abstract for this manuscript was accepted for podium presentation to the American Rhinologic Society during the American Academy of Otolaryngology-Head and Neck Surgery annual meeting in Dallas, Texas, September, 25-26th, 2015.
References
- 1.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 2.Tsuzuki K, Hinohira Y, Takebayashi H, Kojima Y, Yukitatsu Y, Daimon T, et al. Novel endoscopic scoring system after sinus surgery. Auris Nasus Larynx. 2014;41(5):450–454. doi: 10.1016/j.anl.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Wright ED, Agrawal S. Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: evaluation with the novel Perioperative Sinus Endoscopy (POSE) scoring system. Laryngoscope. 2007;117(11 Pt 2 Suppl 115):1–28. doi: 10.1097/MLG.0b013e31814842f8. [DOI] [PubMed] [Google Scholar]
- 4.Philpott CM, Javer AR, Clark A. Allergic fungal rhinosinusitis - a new staging system. Rhinology. 2011;49(3):318–323. doi: 10.4193/Rhino10.121. [DOI] [PubMed] [Google Scholar]
- 5.Psaltis AJ, Li G, Vaezeafshar R, Cho K-S, Hwang PH. Modification of the lund-kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope. 2014;124(10):2216–2223. doi: 10.1002/lary.24654. [DOI] [PubMed] [Google Scholar]
- 6.Snidvongs K, Dalgorf D, Kalish L, Sacks R, Pratt E, Harvey RJ. Modified Lund Mackay Postoperative Endoscopy Score for defining inflammatory burden in chronic rhinosinusitis. Rhinology. 2014;52(1):53–59. doi: 10.4193/Rhino13.056. [DOI] [PubMed] [Google Scholar]
- 7.Durr ML, Pletcher SD, Goldberg AN, Murr AH. A novel sinonasal endoscopy scoring system: the discharge, inflammation, and polyps/edema (DIP) score. Int Forum Allergy Rhinol. 2013;3(1):66–72. doi: 10.1002/alr.21074. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan BA, Kountakis SE. Role of nasal endoscopy in patients undergoing endoscopic sinus surgery. Am J Rhinol. 2004;18(3):161–164. [PubMed] [Google Scholar]
- 9.Mace JC, Michael YL, Carlson NE, Litvack JR, Smith TL. Correlations Between Endoscopy Score and Quality-of-Life Changes After Sinus Surgery. Arch Otolaryngol Head Neck Surg. 2010;136(4):340–346. doi: 10.1001/archoto.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan WR, Ramachandra T, Hwang PH. Correlations between symptoms, nasal endoscopy, and in-office computed tomography in post-surgical chronic rhinosinusitis patients. Laryngoscope. 2011;121(3):674–678. doi: 10.1002/lary.21394. [DOI] [PubMed] [Google Scholar]
- 11.Smith TL, Rhee JS, Loehrl TA, Burzynski ML, Laud PW, Nattinger AB. Objective Testing and Quality-ofLife Evaluation in Surgical Candidates with Chronic Rhinosinusitis. Am J Rhinol. 2003;17(6):351–356. [PubMed] [Google Scholar]
- 12.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 13.DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014;140(8):712–719. doi: 10.1001/jamaoto.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeConde AS, Mace JC, Alt JA, Schlosser RJ, Smith TL, Soler ZM. Comparative effectiveness of medical and surgical therapy on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2014;4(9):725–733. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeConde AS, Suh JD, Mace JC, Alt JA, Smith TL. Outcomes of complete vs targeted approaches to endoscopic sinus surgery. Int Forum Allergy Rhinol. 2015;5(8):691–700. doi: 10.1002/alr.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeConde AS, Mace JC, Alt JA, Soler ZM, Orlandi RR, Smith TL. Investigation of change in cardinal symptoms of chronic rhinosinusitis after surgical or ongoing medical management. Int Forum Allergy Rhinol. 2015 Jan;5(1):36–45. doi: 10.1002/alr.21410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009 Oct;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 18.DeConde AS, Mace JC, Bodner T, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):972–979. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson B. Canonical correlation analysis. In: Grimm L, Yarnold P, editors. Reading and understanding more multivariate statistics. Washington, DC: American Psychological Association; 2000. pp. 285–316. [Google Scholar]
- 20.Cohen J, Cohen P, West S, Aiken L. Applied multiple regression/correlation analysis for the behavioral sciences. 3. Mahwah, NJ: Lawrence Erlbaum; 2003. [Google Scholar]
- 21.Johnson R, Wichern D. Applied multivariate statistical analysis. 6. Upper Saddle River, NJ: Pearson Prentice Hall; 2007. [Google Scholar]
- 22.Tabachnick B, Fidell L. Using multivariate statistics. 5. New York: NY: Pearson Allyn & Bacon; 2007. [Google Scholar]