Summary
Efficient nuclear reprogramming of somatic cells to pluripotency requires activation of innate immunity. Because innate immune activation triggers reactive oxygen species (ROS) signaling, we sought to determine if there was a role of ROS signaling in nuclear reprogramming. We examined ROS production during the reprogramming of doxycycline (Dox) inducible mouse embryonic fibroblasts (MEFs) carrying the Yamanaka Factors (OSKM; Oct4, Sox2, Klf4, and c-Myc) into induced pluripotent stem cells (iPSCs). ROS generation was substantially increased with the onset of reprogramming. Depletion of ROS using antioxidants or Nox inhibitors substantially decreased reprogramming efficiency. Similarly, both knockdown and knockout of p22phox — a critical subunit of the Nox (1-4) complex, decreased reprogramming efficiency. However, excessive ROS generation using genetic and pharmacological approaches also impaired reprogramming. Overall, our data indicate that ROS signaling is activated early with nuclear reprogramming, and optimal levels of ROS signaling are essential to induce pluripotency.
Zhou et al. show that early generation of reactive oxygen species (ROS) is required for nuclear reprogramming of somatic cells to pluripotency. Genetic knockdown and knockout of the oxidative enzyme Nox (1-4), or addition of antioxidants, suppresses reprogramming. The findings provide insight into mechanisms by which pluripotent stem cells may be generated.
Keywords: nuclear reprogramming, reactive oxygen species, NADPH oxidase, iPSCs, Nrf2, CRISPR/Cas9

Introduction
The generation of induced pluripotent cells (iPSCs) is associated with a metabolic switch from oxidative phosphorylation (in the somatic cells) to glycolysis (in the pluripotent cells) (Folmes et al., 2011; Xu et al., 2013). By comparison to somatic cells, iPSCs have smaller and fewer mitochondria, synthesize less ATP, and preferentially generate energy by glycolysis (Folmes et al., 2011; Prigione et al., 2010). Furthermore, iPSCs propagate better in low oxygen conditions (Haneline, 2008), generate less reactive oxygen species (ROS) and are sensitive to ROS-induced apoptosis (Wu et al., 2013). Mitochondrial activity is restrained in iPSCs (Armstrong et al., 2010; Folmes et al., 2011; Prigione et al., 2010), so iPSCs utilize the pentose phosphate shunt to generate energy and materials for synthesis of nucleotides (Zhang et al., 2012).
Paradoxically, in the current paper, we show evidence that the effective generation of iPSCs begins with an early increase in reactive oxygen species (ROS) in the reprogramming of somatic cells. This oxidative activity is tamed in later stages of nuclear reprogramming by an upregulation of antioxidant enzymes. These surprising findings are nevertheless consistent with our previous observation that activation of innate immunity is required for efficient nuclear reprogramming (Lee et al., 2012). Innate immune signaling during reprogramming induces NF-κB- and IRF3-mediated changes in the expression of epigenetic modifiers that favor an open probability state of the chromatin (Lee et al., 2012).
It is generally accepted that activation of innate immunity in somatic cells is associated with a substantial increase in ROS signaling (Nathan and Cunningham-Bussel, 2013; Panday et al., 2014; Yang et al., 2013b). However, whether ROS signaling plays a role in the process of nuclear reprogramming from somatic cells (e.g. fibroblasts) to iPSCs was heretofore uncharted territory. In this study, we delineate the role of ROS signaling in reprogramming. Our observations reveal that both the intensity and kinetics of ROS signaling are critical for efficient nuclear reprogramming.
Results
iPSCs maintain low ROS status
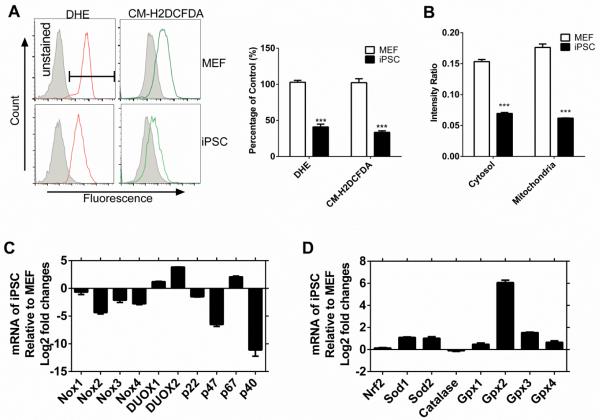
We generated iPSCs using secondary dox-inducible MEFs at passage 3. The mature iPSC colonies were characterized by positive staining for Oct4, Sox2 and stage-specific embryonic antigen 1 (SSEA1) (Figure S1A). These colonies expressed high levels of Sox2, Nanog and Oct4 (Figure S1B). Intracellular levels of ROS (by flow cytometry using DHE or CM-H2DCFDA) were 50% lower in iPSCs by comparison to parental MEFs (Figure 1 A). We confirmed these results using a redox sensitive green fluorescent protein (roGFP; (Waypa et al., 2010) (Figure 1B). These data indicate that iPSCs maintain low levels of intracellular ROS.
Figure 1. iPSCs have low ROS level in comparison to MEFs.
(A) Flow cytometry of DHE and CM-H2DCFDA staining for ROS in iPSCs and MEFs — left. Quantitation of flow cytometry data — right. Data are represented as mean ± SD, n=3. See also Figure S1. (B) Ratiometric measurements of redox status by roGFP2. Data are represented as mean ± SE, n=4. *: P<0.05; **: P<0.01; ***: P<0.001. (C) mRNA levels of “oxidant” genes in iPSCs relative to MEFs. Data are represented as mean ± SD, n=3. (D) mRNA levels of “anti-oxidant” genes as indicated in iPSCs relative to MEFs. Data are represented as mean ± SD, n=3.
Next, we examined the expression of key ROS regulators. The NADPH oxidase (Nox) family members were downregulated in iPSCs, with the exception of DUOX1/2 and p67phox (Fig 1C). Interestingly, p67phox is known to inhibit Nox2 activity (Krishnaiah et al., 2013). We then examined antioxidant genes and found that SOD1/2 and Gpx (Figure 1D) were upregulated. These findings are consistent with a low level of intracellular ROS in iPSCs.
Nuclear reprogramming is associated with increased ROS levels, and NFκB-mediated Nox2 upregulation
We examined intracellular ROS levels during reprogramming, and observed an early increase in ROS that gradually decreased over time (Figure 2C). We found that Nox1 was upregulated about 10-fold at day 2, returning to basal values by day 6 (Figure 2A). Notably, Nox2, an enzyme reported to be differentially expressed in phagocytic cells for immune defense (Nauseef, 2008), was upregulated by about 70-fold during the early stage of reprogramming (Figure 2A). The increased expression of Nox2 was inhibited about 70% by BAY117085 (20 µM), an irreversible inhibitor of IκBα phosphorylation (Figure 2D). This observation is consistent with previous observations that NF-κB signaling induces Nox2 expression (Anrather et al., 2006). In addition, the inhibition of NF-κB decreased reprogramming efficiency by about 50% (Figure 2E). Other components of Nox2 complex, p22phox and p67phox, were also upregulated during reprogramming (Figure 2B). Since ROS level is determined by both generation and elimination, we also examined the expression of genes encoding antioxidant proteins. Major antioxidant genes were upregulated later during reprogramming (Figure S2A and B), in association with the expression of Nrf2, a sensor of ROS (Figure S2C).
Figure 2. NF-κB upregulates Nox2 during nuclear reprogramming.
(A) mRNA levels of Nox isoforms during nuclear reprogramming process. Data are represented as mean ± SD, n=3. (B) mRNA levels of Nox complex components during nuclear reprogramming. Data are represented as mean ± SD, n=3. (C) Time course of ROS status during nuclear reprogramming. DHE staining for ROS was performed every other day as indicated. Detailed methods were described in Methods section. Data are represented as mean ± SD, n=3. (D) NF-κB inhibitor inhibits Nox2 expression. Reprogramming was initiated by adding ES media containing 2µg/ml of Dox in the absence or presence of BAY117085 (20 µM). Nox2 message was examined at day 1 after reprogramming. Data are represented as mean ± SD, n=3. (E) NF-κB inhibitor impairs reprogramming efficiency. Reprogramming was performed in the absence or presence of BAY117085 (20 µM) for the first 4 days. AP positive colonies were counted at day 21. Data are represented as mean ± SD, n=6. *: P<0.05.
ROS signaling is required in early phase of nuclear reprogramming
We next assessed the effect of manipulating ROS levels during reprogramming. Pharmacological manipulation was performed either at early (day 1-7) or at later (day 8-14) phases of reprogramming (Figure 3A&B; Figure S3C&D) using selective ROS scavengers (EUK134, Ebselen and Mito-TEMPO) and Nox inhibitors (DPI and Apocynin). Each of these well-characterized antioxidants were observed to reduce ROS levels in fibroblasts (Fig S4). Early depletion of ROS substantially decreased AP positive colony yield (Figure 3A&B). However, no effect was observed with late administration of antioxidants (Figure S3C&D). We further defined the dynamics of ROS signaling by administering antioxidants at different points during reprogramming (Figure S3B). ROS signaling was most critical in the first two days of reprogramming (Figure S3E, F&G).
Figure 3. Early ROS repression impairs nuclear reprogramming efficiency.
(A) Selective scavengers (25µM EUK134, 50 µM Ebselen and 100 µM Mito-TEMPO) and (B) Nox inhibitors (50 nM DPI and 10 µM Apocynin) decrease AP positive iPSC colonies. Data are represented as mean ± SD, n=6. **: P<0.01; ***: P<0.001; ****: P<0.0001. See also Figure S3. (C) Knockdown of p22phox. The mRNA level was examined by qRT-PCR at day 3 and 6 of reprogramming. Data are represented as mean ± SD, n=3. p22phox (D) knockdown and (E) knockout decrease AP positive iPSC colonies. Data are represented as mean ± SD, n=6. See also Figure S3H, I & J.
To further confirm the role of ROS signaling in nuclear reprogramming, we performed knockdown (KD) and knockout (KO) studies of p22phox as it is the essential subunit for Nox complexes 1-4. A 90% KD of expression was achieved and maintained at day 3 and 6 of reprogramming (Figure 3C). We observed that p22phox KD reduced AP positive colony yields (Figure 3D). Next, we generated p22phox KO 3T3 cell line using CRISPR/Cas9 technology. By polyacrylamide gel electrophoresis (Figure S3H) and gDNA sequencing (Figure S3I), we validated that there was a 453bp deletion between intron 2 and exon 4 of p22phox gene (Figure 3J). Consistent with p22phox KD studies, p22phox KO 3T3 cells have impaired ability to reprogram (Figure 3E).
Excessive ROS impairs nuclear reprogramming efficiency
To determine if increased ROS levels could increase or accelerate nuclear reprogramming, we examined the effect of genetic or pharmacological measures to increase ROS levels. Overexpression of Nox2 (Figs S4 and S5) increased ROS levels as shown by DHE. Intriguingly, the overexpression of Nox2 actually decreased reprogramming efficiency (Figure 4A). GSH synthesis inhibitor—Buthionine sulfoximine (BSO) decreased the generation of AP positive colonies by 50% at 50 µM (Figure 4B). By the addition of exogenous hydrogen peroxide, we observed a biphasic pattern with a tendency for an increase in iPSC yield at a low dose of H2O2, with impaired reprogramming efficiency at higher doses (Figure 4C).
Figure 4. Accumulation of ROS impairs nuclear reprogramming.
(A) Overexpression of Nox2 decreased AP positive colony yield. To overexpress Nox2 in secondary Dox-inducible MEFs, cells were plated in a 6-well plate at 3 × 105 cells/well. After 12 hours serum starvation, Nox2 (Cybb, from Origene) plasmids were introduced via FuGENE 6 according to manufacturer’s instruction. Two days after Nox2 overexpression, reprogramming was initiated and AP positive colony numbers were counted at day 21. Data are represented as mean ± SD, n=6. See also Figure S4. ***: P<0.001. (B) Buthionine sulphoximine (BSO) decreased AP positive colony yield. Nuclear reprogramming was initiated in the presence of 50 µM BSO until day 12. Data are represented as mean ± SD, n=6. *: P<0.05. (C) H2O2 decreased AP positive colony yield in a dose-dependent manner. Nuclear reprogramming was initiated in the presence of H2O2 until day 12. Data are represented as mean ± SD, n=6. **: P<0.01.
Discussion
Innate immunity and generation of iPSCs
We previously found that activation of innate immunity is required for efficient nuclear reprogramming of somatic cells to pluripotency (Lee et al., 2012). The retroviral vectors carrying the Yamanaka factors activate pattern recognition receptors (PRRs) such as Toll-like receptor 3 (TLR3), which triggers innate immune signaling. This signaling pathway causes global changes in epigenetic modifiers, such as downregulation of histone deacetylase (HDAC) family members and upregulation of histone acetylases (HAT). Mediated by NF-κB and IRF-3, these changes result in greater epigenetic plasticity, facilitating the action of the Yamanaka transcription factors (Lee et al., 2012).
Innate immunity activates ROS signaling
Reactive oxygen species (ROS) signaling is triggered by activation of innate immunity (Yang et al., 2013a). Furthermore, NF-κB is activated by ROS intermediates (Bonizzi et al., 1999; Escobar et al., 2012). Interestingly, NF-κB also induces the expression of Nox2 (gp91phox) in MEFs (Anrather et al., 2006). Thus, activation of innate immunity may induce a positive feedback cycle of ROS signaling. Because ROS signaling also modulates differentiation, senescence, apoptosis, and proliferation (Droge, 2002), ROS generation and elimination is tightly regulated.
Whereas oxidative phosphorylation is predominant in somatic cells, iPSCs exist in a glycolytic state through up-regulation of glycolytic enzymes and down-regulation of electron transport chain (ETC) subunits (Folmes et al., 2011). Furthermore, antioxidant genes (e.g. UCP2 (Zhang et al., 2011), SOD2, and Gpx2) are upregulated in iPSC and downregulated during differentiation (Saretzki et al., 2008). As a result, stem cells generate low levels of ROS during proliferation and maintenance (Haneline, 2008). However, it is not known if ROS signaling plays a role in the nuclear reprogramming to pluripotency.
To address this question, we focused on the NADPH oxidases (Nox1-4) and Dual oxidase (Duox1/2) family of proteins because these are the major sources of non-mitochondrial ROS in mammalian cells (Lambeth et al., 2007). Among the Nox family members, Nox2 is a key member of the family that complexes with various subunits including p22phox, p40phox, p47phox, p67phox and Rac to form a functional oxidase. The cellular levels of ROS are counter-regulated by scavengers and antioxidants, whose expression is determined by transcription factors such as Nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Nrf2 is a member of the “cap-and-collar” (Cnc) transcription factors that are involved in the regulation of many antioxidants (Kobayashi and Yamamoto, 2005) and in metabolic control of proliferating cells (DeNicola et al., 2011; Hayes and Ashford, 2012; Hochmuth et al., 2011). Recently, it has been reported that Nrf2 controls self-renewal and pluripotency in human embryonic stem cells (Jang et al., 2014).
ROS levels in iPSCs
By comparison to parental murine embryonic fibroblasts, we show that murine iPSCs have low ROS levels consistent with the known metabolic switch in iPSCs (Zhu et al., 2010). This reduction in ROS levels is associated with downregulation of members of the Nox family and upregulation of genes encoding antioxidant proteins. We find Gpx2 is highly induced particularly in the later stages of nuclear reprogramming and its expression is then maintained at a high level in iPSCs. The increased expression of antioxidant enzymes maintains intracellular levels of ROS at a low level in iPSCs (Chen et al., 2008). By maintaining a low level of ROS generation, iPSCs limit the risk of cellular and genomic damage during self-renewal, and prevent differentiation (Naka et al., 2008; Saretzki et al., 2008).
Early ROS signaling is required for nuclear reprogramming
Notably, we find that an early increase in ROS signaling is required during the initial stage of nuclear reprogramming. We observe marked upregulation of Nox2 during reprogramming, associated with an increase in ROS levels. Pharmacological and genetic attenuation of the Nox (1-4) complex in the early phase of reprogramming impairs reprogramming efficiency. Inhibition of Nox family members reduces oxidative stress and increases genomic stability (Pazhanisamy et al., 2011). By contrast, activation of ROS signaling in lung epithelial cells is known to reduce HDAC and to increase HAT activity (Rahman et al., 2004). Furthermore, activation of tightly regulated Nox complexes produces hydrogen peroxide (H2O2) from superoxide (O2−). Hydrogen peroxide, a signaling molecule (Winterbourn, 2008), modifies specific cysteine residues in target proteins to influence the fate of cells (Hall et al., 2009). Indeed, cysteine oxidation of transcriptional factors or epigenetic modifiers may affect pluripotency (Boland et al., 2014; Ramakrishna et al., 2014).
The ROS-induced reprogramming to pluripotency seems to be largely due to cytosolic sources (eg. Nox2) as EUK and ebselen are most effective at reducing the generation of iPSCs. However, there is also a modest reduction of iPSC yield in the presence of the mitochondrially-directed antioxidant, mito-TEMPO. These data indicate that ROS derived from mitochondrial respiration are also involved in nuclear reprogramming. Of note, there appears to be a critical period during which these antioxidants are effective. Specifically, when added from the start of reprogramming, the antioxidants can substantially block iPSC generation (with EUK inhibiting iPSC yield by over 90%). When added after 2 days, the antioxidants have little or no effect. These data are consistent with the observations using the antagonists of Nox activity. Both DPI or apocyanin can substantially reduce iPSC yield when added during the first 7 days, but have no effect when added during the last 7 days, of the reprogramming process.
Excessive ROS signaling impairs reprogramming
We were surprised that genetic or pharmacological measures to increase ROS generation actually impaired reprogramming to pluripotency. A cell line overexpressing Nox 2 generated fewer iPSCs. Furthermore, the addition of BSO (for the first 12 days of reprogramming) to inhibit the synthesis of glutathione, a major endogenous antioxidant, was also associated with impaired iPSC generation. Finally, treating the reprogramming cells with exogenous H2O2 (for the first 12 days of reprogramming) revealed a biphasic response, with a tendency for low doses of H2O2 to facilitate, whereas higher doses of H2O2 impaired, the generation of iPSCs. Although these observations were initially unexpected, they are consistent with the fact that senescent somatic cells, which typically exhibit higher ROS levels, are more difficult to reprogram (Banito et al., 2009). Our studies indicate that an optimal level of ROS signaling is required for effective reprogramming.
Furthermore, these data are also consistent with the upregulation of antioxidant enzymes late in the reprogramming process, orchestrated in part by Nrf2 (Niture et al., 2014). Normally, Nrf2 is sequestered in the cytosol by Keap1, and released by ROS signaling (DeNicola et al., 2011). Keap1 has multiple reactive cysteine residues, which makes it a target of ROS and electrophiles (Dinkova-Kostova et al., 2002). In addition to activation by ROS, Nrf2 also can be activated by Keap1-independent phosphorylation (Li et al., 2012; Rojo et al., 2012). We find that the expression of Nrf2 is increased gradually and then plateaus during late reprogramming. Upregulation of antioxidant enzymes accompanies this activation of Nrf2. The temporal course of ROS generation that we observe during reprogramming is in part explained by the early upregulation of Nox2, followed later by an upregulation of antioxidant enzymes. Thus, there appears to be a careful orchestration of ROS levels during reprogramming, with an early phase characterized by increased ROS levels that is required for effective generation of iPSCs. In later stages, ROS signaling does not seem to be required, and in fact may be deleterious.
ROS biology in adult stem cells
Recently, the importance of ROS signaling in the regulation of stem cell fate has been appreciated (Le Belle et al., 2011; Morimoto et al., 2013; Paul et al., 2014d; Wang et al., 2013). ROS signaling plays an important role in adult stem cell proliferation and differentiation (Hamanaka et al., 2013; Hom et al., 2011; Jang et al., 2014; Malinska et al., 2012; Owusu-Ansah and Banerjee, 2009; Paul et al., 2014a; Tormos et al., 2011; van Galen et al., 2014; Wang et al., 2013). The importance of ROS levels in proliferation of adult stem cells is tissue-specific (Naka et al., 2008; Wang et al., 2013). Adult stem cells maintaining high levels of ROS have been found to be more proliferative (Paul et al., 2014a). By contrast, ROS levels are maintained at lower levels in hematopoietic stem cells and mammary epithelial stem cells than their mature progeny, so as to facilitate self-renewal (Juntilla et al., 2010; Naka et al., 2008; Toyokuni, 2006; Zhang et al., 2008). Paul et al. showed that low-to-moderate ROS level is required for self-renewal and proliferation of mouse and human airway basal stem cells (Paul et al., 2014a).
In conclusion, our study shows that ROS signaling is required in the early stages of nuclear reprogramming to pluripotency. In the later phase of reprogramming, upregulation of antioxidant mechanisms is observed, and mature iPSC colonies exist in a cellular environment with low levels of intracellular ROS.
Experimental Procedures
Reagents and Cells
The reagents and cells used in this study, and their sources, are more fully described in the Supplementary section. For isolation of secondary dox-inducible MEFs, chimeric embryos were obtained from transgenic R26rtTA; Col1a12lox-4F2A mice expressing the loxP-flanked, dox-inducible polycistronic 4F2A cassette (Oct4, Sox2, Klf4, c-Myc). Secondary MEFs were isolated as previously described (Wernig et al., 2008), and expanded for two passages before freezing. Passage 3 cells were used in all the experiments unless indicated otherwise. Culture plates were coated with 0.1% gelatin solution for 30 min before use. All cells were cultured in ES medium under standard condition (5% CO2, 37°C) unless stated otherwise. Alkaline phosphatase staining for enumeration of colonies is described in the supplementary section. For generation of RNAi and transduction, see Supplementary section. For generation of iPSCs with piggyBac transposon system we used plasmids PB-CAG-rtTA and PB-TET-MKOS (c-Myc, Klf4, Oct4 and Sox2 ORFs linked with 2A peptide sequences) provided by Dr. Andras Nagy. The pCyL43 PB transposase plasmid was from Wellcome Trust Sanger Institute.
Molecular and biochemical assays
Predesigned Taqman probes were purchased from Life Technologies. RNA isolated from cells was reverse-transcribed by qScript cDNA SuperMix. Quantitative PCR was performed using QuantStudioTM 12k Flex Real-Time PCR System. Normalized 2−ΔΔCt was calculated and compared with control. To assess ROS levels dihydroethidium (DHE) was dissolved in DMSO and diluted into cell imaging medium to 5 µM final concentration. Incubation was performed at 37 °C for 30 min in dark. Data were quantified by measuring fluorescence intensity at 518 nm excitation and 606 nm emission. In some studies cells were transfected by adenovirus expressing cyto-roGFP2 or mito-roGFP2 (Waypa et al., 2010) to assess intracellular ROS levels by fluorescence intensity. All experiments were repeated at least three times. Student’s t test or a nonparametric Mann-Whitney U test was employed to compare differences between two groups. P value < 0.05 was considered statistically significant.
Supplementary Material
Highlights.
□ Optimal ROS signaling is required in the early stages of nuclear reprogramming
□ Antagonism of ROS signaling in early reprogramming suppresses iPSC generation
□ Anti-oxidant enzymes increase in the late phase of reprogramming
Acknowledgments
We thank Frank Ospino for isolation of Dox-inducible MEFs. We are grateful to Dr. Andras Nagy (University of Toronto, Canada) for kindly providing the plasmids PB-CAG-rtTA and PB-TET-MKOS.
Disclosure of potential conflicts of interest
Dr. Cooke is an inventor on patents owned by Stanford University related to innate immune signaling for nuclear reprogramming. This work was supported by grants from the NIH (U01HL100397 to JPC; 5K01HL118683 to YTG); from the Cancer Prevention and Research Institute of Texas (CPRIT; # RP150611 to JPC) and from HMRI (project ID 25150001 to YTG).
Abbreviations and Acronyms
- ROS
reactive oxygen species
- Dox
doxycycline
- MEFs
mouse embryonic fibroblasts
- iPSCs
induced pluripotent stem cells
- Nox
nicotinamide adenine dinucleotide phosphate Oxidase
- DUOX
Dual Oxidase
- OSKM
Oct4, Sox2, Klf4, and c-Myc respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Conception and design (GZ; YTG and JPC); collection and assembly of data (GZ; SM and YL); data analysis and interpretation (GZ; YTG and JPC); manuscript drafting (GZ); revisions and final approval (SM; YL; and JPC)
References
- Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. The Journal of biological chemistry. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Armstrong L, Tilgner K, Saretzki G, Atkinson SP, Stojkovic M, Moreno R, Przyborski S, Lako M. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem cells. 2010;28:661–673. doi: 10.1002/stem.307. [DOI] [PubMed] [Google Scholar]
- Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes & development. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland MJ, Nazor KL, Loring JF. Epigenetic regulation of pluripotency and differentiation. Circulation research. 2014;115:311–324. doi: 10.1161/CIRCRESAHA.115.301517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V. Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Molecular and cellular biology. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem cells. 2008;26:960–968. doi: 10.1634/stemcells.2007-0509. [DOI] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiological reviews. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Escobar J, Pereda J, Lopez-Rodas G, Sastre J. Redox signaling and histone acetylation in acute pancreatitis. Free radical biology & medicine. 2012;52:819–837. doi: 10.1016/j.freeradbiomed.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell metabolism. 2011;14:264–271. doi: 10.1016/j.cmet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins--structures, mechanisms and functions. The FEBS journal. 2009;276:2469–2477. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Science signaling. 2013:ra8. doi: 10.1126/scisignal.2003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneline LS. Redox regulation of stem and progenitor cells. Antioxidants & redox signaling. 2008;10:1849–1852. doi: 10.1089/ars.2008.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Ashford ML. Nrf2 orchestrates fuel partitioning for cell proliferation. Cell metabolism. 2012;16:139–141. doi: 10.1016/j.cmet.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell stem cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS, Porter GA., Jr. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Developmental cell. 2011;21:469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J, Wang Y, Kim HS, Lalli MA, Kosik KS. Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem cells. 2014;32:2616–2625. doi: 10.1002/stem.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants & redox signaling. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Krishnaiah SY, Dodia C, Feinstein SI, Fisher AB. p67(phox) terminates the phospholipase A(2)-derived signal for activation of NADPH oxidase (NOX2) FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:2066–2073. doi: 10.1096/fj.12-222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free radical biology & medicine. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell stem cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012;151:547–558. doi: 10.1016/j.cell.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Paonessa JD, Zhang Y. Mechanism of chemical activation of Nrf2. PloS one. 2012;7:e35122. doi: 10.1371/journal.pone.0035122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinska D, Kudin AP, Bejtka M, Kunz WS. Changes in mitochondrial reactive oxygen species synthesis during differentiation of skeletal muscle cells. Mitochondrion. 2012;12:144–148. doi: 10.1016/j.mito.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell stem cell. 2013;12:774–786. doi: 10.1016/j.stem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxidants & redox signaling. 2008;10:1883–1894. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nature reviews. Immunology. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM. Biological roles for the NOX family NADPH oxidases. The Journal of biological chemistry. 2008;283:16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free radical biology & medicine. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cellular & molecular immunology. 2014 doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al. Dynamic Changes in Intracellular ROS Levels Regulate Airway Basal Stem Cell Homeostasis through Nrf2-Dependent Notch Signaling. Cell stem cell. 2014a doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, et al. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell stem cell. 2014d;15:199–214. doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazhanisamy SK, Li H, Wang Y, Batinic-Haberle I, Zhou D. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis. 2011;26:431–435. doi: 10.1093/mutage/ger001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigione A, Fauler B, Lurz R, Lehrach H, Adjaye J. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem cells. 2010;28:721–733. doi: 10.1002/stem.404. [DOI] [PubMed] [Google Scholar]
- Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochemical pharmacology. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S, Kim KS, Baek KH. Posttranslational modifications of defined embryonic reprogramming transcription factors. Cellular reprogramming. 2014;16:108–120. doi: 10.1089/cell.2013.0077. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Medina-Campos ON, Rada P, Zuniga-Toala A, Lopez-Gazcon A, Espada S, Pedraza-Chaverri J, Cuadrado A. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: Role of glycogen synthase kinase-3. Free radical biology & medicine. 2012;52:473–487. doi: 10.1016/j.freeradbiomed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, Stewart R, Hoare S, Stojkovic M, Armstrong L, et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell metabolism. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokuni S. Novel aspects of oxidative stress-associated carcinogenesis. Antioxidants & redox signaling. 2006;8:1373–1377. doi: 10.1089/ars.2006.8.1373. [DOI] [PubMed] [Google Scholar]
- van Galen P, Kreso A, Mbong N, Kent DG, Fitzmaurice T, Chambers JE, Xie S, Laurenti E, Hermans K, Eppert K, et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature. 2014;510:268–272. doi: 10.1038/nature13228. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhang T, Dong Q, Nice EC, Huang C, Wei Y. Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell death & disease. 2013;4:e537. doi: 10.1038/cddis.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circulation research. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nature biotechnology. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nature chemical biology. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang X, Kang X, Li N, Wang R, Hu T, Xiang M, Wang X, Yuan W, Chen A, et al. Oxidative stress inhibits adhesion and transendothelial migration, and induces apoptosis and senescence of induced pluripotent stem cells. Clinical and experimental pharmacology & physiology. 2013;40:626–634. doi: 10.1111/1440-1681.12141. [DOI] [PubMed] [Google Scholar]
- Xu X, Duan S, Yi F, Ocampo A, Liu GH, Izpisua Belmonte JC. Mitochondrial regulation in pluripotent stem cells. Cell metabolism. 2013;18:325–332. doi: 10.1016/j.cmet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Yang CS, Kim JJ, Lee SJ, Hwang JH, Lee CH, Lee MS, Jo EK. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1. Journal of immunology. 2013a;190:6368–6377. doi: 10.4049/jimmunol.1202574. [DOI] [PubMed] [Google Scholar]
- Yang Y, Bazhin AV, Werner J, Karakhanova S. Reactive oxygen species in the immune system. International reviews of immunology. 2013b;32:249–270. doi: 10.3109/08830185.2012.755176. [DOI] [PubMed] [Google Scholar]
- Zhang H, Trachootham D, Lu W, Carew J, Giles FJ, Keating MJ, Arlinghaus RB, Huang P. Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia. 2008;22:1191–1199. doi: 10.1038/leu.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Khvorostov I, Hong JS, Oktay Y, Vergnes L, Nuebel E, Wahjudi PN, Setoguchi K, Wang G, Do A, et al. UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. The EMBO journal. 2011;30:4860–4873. doi: 10.1038/emboj.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell stem cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell stem cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.