Abstract
The high frequency of relapse of epithelial ovarian tumors treated with standard chemotherapy has highlighted the necessity to identify targeted therapies that can improve patient outcomes. The dynamic relationship between Cyclin E and PKCiota frequent overexpression in high-grade ovarian tumors poses a novel pathway for therapeutic investigation. We hypothesized that a PI3K dependent signaling pathway activating PKCiota perpetuates cyclin E deregulation during ovarian tumorigenesis. We observed a positive correlation between PKCiota and cyclin E in a panel of 19 ovarian cancer cell lines. Modulation of cyclin E had no effect on PKCiota knockdown/overexpression however PKCiota differentially regulated cyclin E expression. In the serous ovarian cancer cells (IGROV, OVCAR-3), shPKCiota decreased proliferation, caused a G1 arrest, and significantly prolonged overall survival in xenograft mouse models. In vitro shPKCiota decreased the ability of IGROV cells to grow under anchorage independent conditions and form aberrant acini, which was dependent upon Ad-cyclin E or Ad-LMW-E expression. RPPA analysis of PKCiota wild-type, catalytic active, dominant negative protein isoforms strengthened the association between phospho-PKCiota levels and PI3K pathway activation. Inhibitors of PI3K coordinately decreased phospho-PKCiota and Cyclin E protein levels. In conclusion, we have identified a PI3K/PKCiota/Cyclin E signaling pathway as a therapeutic target during ovarian tumorigenesis.
Keywords: PKC, cyclin E, ovarian cancer
INTRODUCTION
High-grade serous ovarian cancer (HGSOC) accounts for over 70% of ovarian cancer cases with a 5-year survival rate of approximately 15%, making HGSOC a highly aggressive and deadly form of ovarian cancer 29. The emergence and affordability of next generation sequencing in the clinic has slowly demystified the molecular origins of ovarian cancer progression highlighting defects within key homologous recombination (HR) genes (e.g. BRCA1, BRCA2, RAD51, PALB2) giving rise to ovarian cancer 40. Aside from defects within HR DNA repair genes and tumor suppressors (e.g. PTEN, TP53), Cyclin E amplification/overexpression is the most frequently associated genetic alteration observed in ovarian tumors (14–21%), that correlates with decreased overall and progression free survival in ovarian cancer patients 20. However, a complete understanding of the molecular initiators of cyclin E expression in ovarian tumorigensis is severely lacking and requires further investigation. Cyclin E overexpression correlated with poor overall survival in several tumor types including breast, ovarian, lung and pancreatic cancer 20, 27. The Low Molecular Weight isoforms of cyclin E (LMW-E) specifically correlate with advanced stages and grade of ovarian cancer 10, 14. Additionally, tumors that typically overexpress LMW-E exhibit higher CDK2 activity and poor overall survival in ovarian tumors compared to full length expression of cyclin E alone, highlighting the need to understand the mechanism of cyclin E deregulation in ovarian cancer 10, 11.
PKCiota has emerged as a bonafide oncogene in several types of cancers, including ovarian cancer 44. In addition to being genomically amplified in ovarian cancer as part of the 3q26.2 amplicon, PKCiota is induced by several potent oncogenic regulators (e.g. PI3K, Kras, EGFR, AKT & mTOR) and is thus differentially expressed between ovarian cancer and normal epithelial ovarian tissue 42 and represents the only PKC isoform that has been designated as an oncogene in ovarian cancer 44. PKCiota induction signals oncogenic transformation in lung, pancreatic and ovarian tumor types 18, 21. The oncogenic transformation of lung squamous cell carcinoma is highly dependent upon co-induction of PKCiota and SOX2, signaling the transformative potential of PKCiota 23. In ovarian tumors, the expression of PKCiota exhibits a significant correlation with a reduced median survival time as well as increased expression of cyclin E 42. Cyclin E deregulation correlates with decreased survival in several tumor types suggesting that in ovarian cancer, PKCiota plays a pivotal role in the deregulation of Cyclin E as a molecular driver of ovarian cancer progression.
Therefore we hypothesized that cyclin E is a downstream oncogenic target of PKCiota and further that activation of PKCiota by phosphatidylinositol 3 kinase signal would further activate the signaling between PKCiota and cyclin E. Additionally, we posit that attenuation of the PI3K/PKCiota/cyclin E pathway is an actionable target in ovarian cancer. We show that depletion of PKCiota expression in HGSOC cell lines inhibits proliferation, alters the cell cycle profile, inhibits acini filling in vitro and prolongs survival in xenograft mouse models. Inhibition of the PI3K pathway attenuates the induction of Cyclin E by PKCiota signaling an actionable target for clinical advancement.
RESULTS
Cyclin E and PKCiota Exhibit in vivo and in vitro Correlations
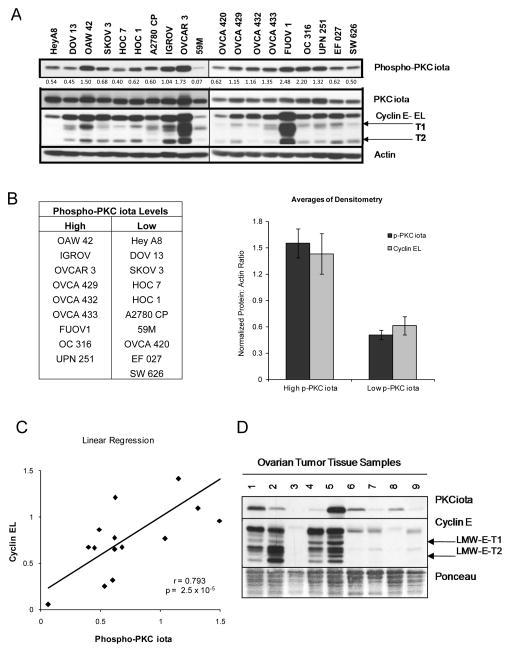
We previously established a positive correlation between PKCiota expression and cyclin E deregulation in tumor samples derived from serous ovarian cancer patients that dictates decreased survival and an overall poor prognosis 16. To determine if ovarian cancer cell lines show a similar coordinate expression of PKCiota and cyclin E, we examined a panel of nineteen ovarian cancer cell lines for PKCiota, phospho-PKCiota and cyclin E expression (Figure 1A). Phospho-PKCiota was used as the functional, activated form of PKCiota protein and the cut off of 1.0 (from densitometry values) to separate the cells into high and low phospho-PKCiota expressing cells. The FUOV 1, OVCAR3, IGROV, and OAW 42 cell lines exhibited the highest levels of phospho-PKCiota and total cyclin E (full-length and LMW-E), while 59M and OVCA 420 had low expression of both PKCiota and cyclin E. The remainder of cell lines had an intermediate amount of both PKCiota and cyclin E expression. The phospho-PKCiota levels were dichotomized into high and low phospho-PKCiota groups, and cyclin E levels were quantitated by densitometric analysis. A positive and significant correlation (p = 2.5 × 10−5; r = 0.793) was established for the relationship between phospho-PKCiota and cyclin E (Figures 1B and 1C) in the nineteen cell lines. To determine whether this positive relationship between cyclin E and phospho-PKCiota persists in tissue samples of patients with ovarian cancer, we examined the expression of these proteins in nine tumor tissue samples (Figure 1D). PKCiota and cyclin E levels exhibited similar expression patterns to the cell lines with either high PKCiota/high cyclin E (lanes 1,2, and 5) or low PKCiota/low cyclin E (lanes 3, 6–9). The consistency between cyclin E and PKCi expression in both ovarian cancer cell lines and patient samples provided an opportunity to examine the cause and effect relationship between these two proteins in transformation and oncogenesis.
Figure 1. Phospho-PKCiota and Cyclin E expression are positively correlated in ovarian cancer cell lines.
(A) Nineteen ovarian cancer cell lines of differing histological subtypes were subjected to western blot analysis with antibodies against Phospho-PKCiota, PKCiota, Cyclin E. Actin was used as a loading control. Relative levels of phospho-PKCi was determined by denistometric analysis and values (as a function of actin) are indicated below the western blot. (B) Phospho-PKCiota was used as the functional, activated form of PKCiota protein to separate the 19 cells into high and low phospho-PKCiota expressing cells with the cut off of 1.0 (from densitometry values) (Table). Relative levels of full length cyclin E (also determined by densitometric analysis of western blots) was next used to plot the mean phospho-PKCiota and cyclin EL levels (Bar graph). (C) Linear regression of Cyclin EL values as a function of phospho-PKCiota were plotted (r = 0.79; p = 2.5 × 10−5). (D) Ovarian cancer patient samples were analyzed by Western blot analysis with PKCiota and cyclin E [full-length (EL), LMW-E-T1 and LMW-E-T2] antibodies. Ponceau stain was used as a loading control.
Cyclin E overexpression does not alter PKCiota expression
While the correlation has been established between PKCiota and cyclin E, the functional significance and mechanism of the apparent codependency is not known. The oncogenic transformative properties of cyclin E have been well documented in ovarian cancer 10, 26; therefore we initially investigated if overexpression of either full length or LMW-E leads to modulation of PCKiota levels or activity. To test this hypothesis, IGROV and OVCA420 cells were chosen for further analysis as they represent opposing sides of the cyclin E expression profile of the serous epithelial ovarian cancer subtype (Figure 1A). IGROV cells express high levels of PKCiota and cyclin E while OVCA420 cells have relatively low endogenous levels of PKCiota and cyclin E. Both cell lines were infected with adenovirus containing full length (Ad-cyclin EL) or LMW-E forms of cyclin E (Ad-LMW-E) to further examine whether cyclin E could alter PKCiota levels (Supplemental Figure 1).
Cyclin E was overexpressed at the expected molecular weights after both adenoviral and pcDNA vector mediated expression (Supplemental Figure 1A and 1B). However, after 48, 72 and 144 hours of full-length cyclin E (EL) or LMW-E expression in IGROV cells, PKCiota levels and phosphorylation remained unaffected (Supplemental Figure 1A). Similarly, infection or transfection of OVCA420 with EL or LMW-E did not alter the level or phosphorylation of endogenous PKCiota (Supplemental Figure 1B). Collectively our results show that the expression of cyclin E or LMW-E does not affect PKCiota protein level or activity.
PKCiota expression regulates cyclin E
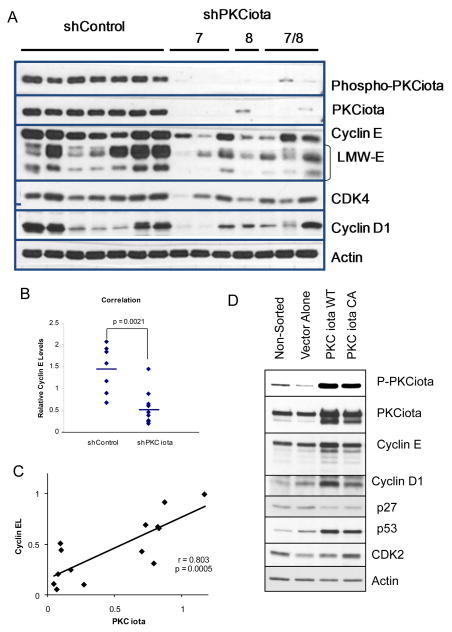
Our analysis of cyclin E overexpression studies suggest that the positive correlation observed between these two proteins is likely to be a direct result of PKCiota regulation of cyclin E and not due to the cyclin E mediated regulation of PKCiota. To test this hypothesis, cyclin E expression was examined in IGROV cells with stable knockdown of PKCiota via shRNA (Figure 2A). Two different PKCiota shRNAs were used (i.e. construct 7 and 8), targeting two different exons of PKCiota (exons 13 and 14) which resulted in the downregulation of PKCiota and decreased levels of phospho-PKCiota (active form of PKCiota), cyclin E, CDK4, and cyclin D1 (Figure 2A). Overall, there was a statistically significant reduction in full length cyclin E (cyclin EL) as well as LMW-E expression in the shPKCiota versus the shControl clones (p = 0.0021, Figure 2B, and supplemental figure 2A). Additionally, linear regression analysis revealed that decreased PKCiota expression was proportional to that of cyclin E protein expression (r = 0.803; p = 0.0005) (Figure 2C). To further support our hypothesis and to rule out clonal variation among the shControl and shPKCiota clones (Figure 2A), PKCiota was overexpressed in its wild type (WT) or constitutively active (CA) form along with GFP in IGROV cells (Figure 2D). Following transfection the cells were sorted for GFP + cells. As expected, the GFP+ cells cotransfected with the WT or CA PKCiota constructs demonstrated overexpression of PKCiota and phospho-PKCiota (Figure 2D). Both PKCiota WT and CA cells also show increased expression of cyclin E (EL and LMW-E) and cyclin D1, CDK2 and p53 levels and a decrease in expression of p27 protein. These studies suggest that PKCiota is an upstream mediator of cyclin E and several other G1 checkpoint proteins.
Figure 2. Downregulation of PKCiota decreases the expression cyclin E.
(A) shControl (CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG) and shPKCiota clones (constructs 7, 8, and 7/8) (construct 7-sequence TGACCAGAACACAGAGGATTATCTCTTCC targeting exon 14; construct 8 CAGGAGATACAACCAGCACTTTCTGTGGT targeting exon 13), of IGROV cells were subjected to Western blot analysis using phospho-PKCiota, PKCiota, cyclin E, CDK4, cyclin D1, and actin (as loading control). (B) Western blots from panel A were subjected to densitometric analysis and the resultant values for cyclin E (full length and LMW-E) were plotted for each of the shControl and shPKCiota clones. p=0.0021 (C) Linear regression of cyclin EL densitometric values as a function of PKCiota for each of the clones in panel A were plotted. (r=0.803; p= 0.0005). (D) IGROV ovarian cancer cells were transfected with shControl, shPKCiota PKCiota (Wt-wildtype, Ca-constitutively active (A129E), or DN-dominant negative (K122STOP), and GFP (negative control) and harvested at 24 and 48 hours post transfection and subjected to cell sorting for GFP positive cells. Immediately following cell sorting the GFP+ cells were lysed and subjected to western blot analysis using antibodies to phospho-PKCiota, PKCiota, cyclin E, cyclin D1, CDK2, and p53. Actin was used as a loading control.
Depletion of PKCiota increases doubling time of cells
Since loss of PKCiota alters the expression of several G1/S phase transition proteins (Figure 2D), we examined the effect of PKCiota mediated cyclin E expression on proliferation, in IGROV shControl and shPKCiota transfected cells (Supplemental Figure 2A). Growth curve analysis showed that downregulation of PKCiota resulted in an increase in doubling time from 27.5 hours to 30.0 hours (p = 0.043, Supplemental Figure 2B). Additionally, downregulation of PKCiota did not result in the induction of apoptosis (i.e. no change in TUNEL positive or sub-G1 cells-Supplemental Figure 2C). However, cell cycle analysis revealed that shPCKiota cells accumulated in the G1 phase with concomitant decrease in S phase (Supplemental Figure 2D and 2E). Such a cell cycle alteration is often suggestive of a more regulated cell cycle transition from G1 to S phase that could affect biological functions leading to transformation.
PKCiota expression alters acini formation in ovarian cancer cells
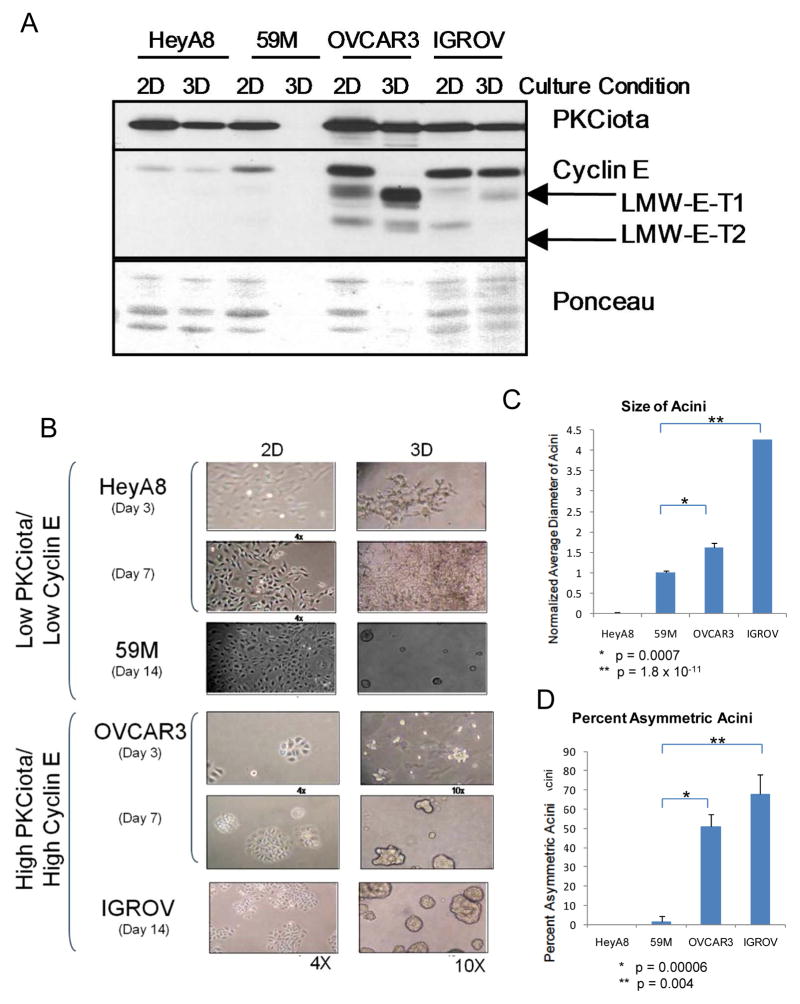
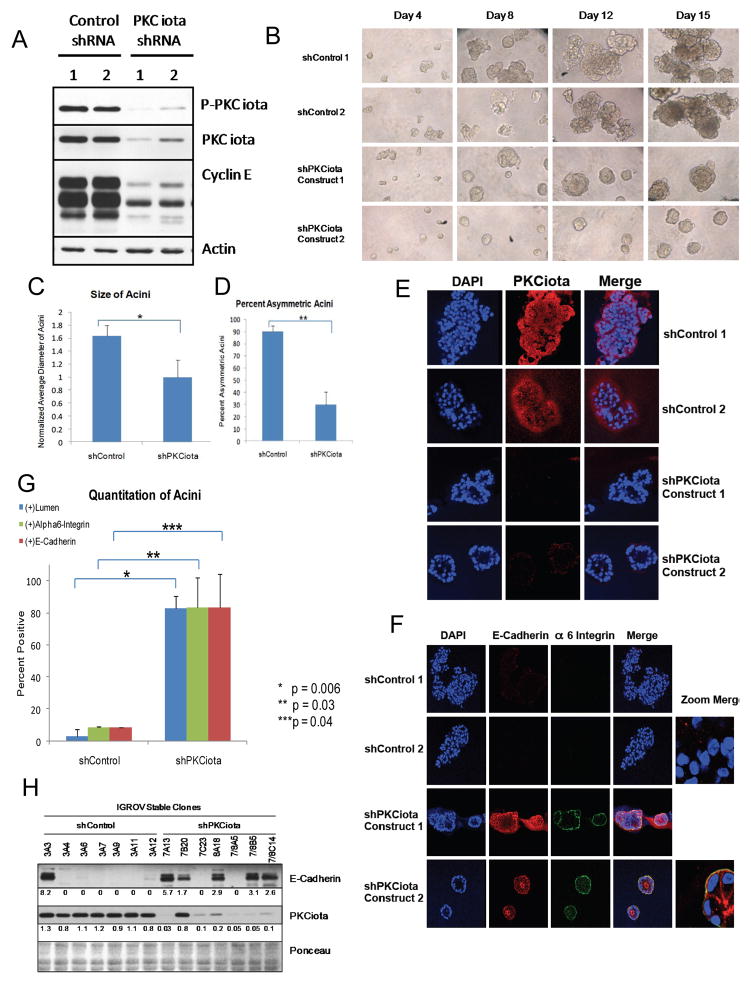
Since PKCiota, is mislocalized or overexpressed in serous epithelial cancer 16, its co-overexpression with cyclin E could lead to deregulation of acinar development, one of the hallmarks of oncogenicity. To test this hypothesis, ovarian cancer cells with either low (HeyA8 and 59M) or high (OVCAR3 and IGROV) PKCiota and cyclin E levels as determined by western blot (Figure 3A: 2D cultures) were subjected to culture on matrigel. The HeyA8 cells did not form acini and the 59M cells formed very small acini (Figure 3B). However, the OVCAR3 and IGROV cells formed acini that were significantly larger (P <0.001) (Figure 3C) and of asymmetric size (P<0.005) (Figure 3D) compared to those formed by the 59M cells. Western blot analysis using cell lysates prepared from 2D cultured cells and 3D acini showed that the levels of both cyclin E and PKCiota did not change significantly from 2D cultures compared to 3D acini in OVCAR3 and IGROV cells (Fig 3A) suggesting that both proteins are likely to be important for the larger and asymmetrical acini formation. To interrogate whether the increased acini size could be attributed to the increased PKCiota in these cell lines, PKCiota gene expression was silenced in the IGROV cell line with shRNA and subjected to 3D cultures (Figure 4). Two different IGROV shPKCiota expressing cells were generated (using two different shRNA constructs against PKCiota) and both exhibited stable knockdown of PKCiota with a concomitant decrease in full length and LMW cyclin E, compared to the control shRNA cells (Figure 4A). When PKCiota was stably silenced in IGROV cells, the acini were significantly smaller (p = 0.00003, Figures 4B and 4C) and were more symmetrical (p = 0.002, Figures 4B and 4D) than the shControl cells. Additionally, confocal microscopy of DAPI and PKCiota stained acini revealed that cells lacking PCKiota had a cleared lumen with apical-basal polarity of cells (Figure 4E). To interrogate if cells forming the acini were arranged with appropriate apical-basal polarity following PKCiota knockdown, expression levels of E-cadherin and alpha 6 integrin were assessed and quantitated in the 3D cultures (Figures 4F–H). Subsequent to PKCiota knockdown, levels of both E-cadherin and alpha 6 integrin increased as the lumen cleared (Figure 4F). In the shControl cells, the lumen was mostly undetectable and there was minimal expression of both E-cadherin (western blot analysis and confocal microscopy) and Alpha 6 integrin. Thus, our data suggest that polarity of acini forming cells is inhibited by PKCiota expression and upon its downregulation, the polarity is restored.
Figure 3. Endogenous overexpression of cyclin E and PKCiota results in deregulated acini formation in 3D cultures.
Cell lines with low PKCiota/cyclin E expression (HEYA8 and 59M) versus those with high PKCiota/cyclin E (OVCAR3 and IGROV) were plated in 2D and 3D cultures and subjected to (A) western blot analysis and (B) microscopy. (A) For western blot, analysis, expression of PKCiota and cyclin E were examined in 2D and 3D conditions in all four cell lines using antibodies against PKCiota and cyclin E. Ponceau staining of the immune blot depicts loading. (B) Cells in 2D and 3D matrigel cultures were subjected to microscopy-images for the 2D cultures are 4X and those for 3D cultures are at 10X. Quantitation and statistical analysis of the (C) size (diameter of acini formed) in each cell line and (D) percent asymmetry of the acini in each cell line are presented in bar graphs.
Figure 4. Downregulation of PKCiota restores acini formation and polarity.
(A) PCKiota was downregualted in IGROV cells using two different shRNA constructs to PKCiota. Two different scrambled shRNA constructs were used as negative controls. Stably expressing shPKCiota and shControl cells were subjected to western blot analysis with antibodies against phospho-PKCiota, PKCiota and cyclin E. Actin was used as a loading control. (B) Two IGROV shControl and 2 shPKCiota clones were plated in matrigel and imaged at days 4, 8, 12, and 15 post seeding. Images are at 10X zoom. (C) Diameter (size) and (D) percent asymmetric acini for day 15 were quantitated and plotted, n (shControl)= 66 and n (shPKCiota)= 36. Experiment was replicated three times. *p=0.002 and **p=0.00003. (E) IGROV shControl and shPKCiota clones were plated in matrigel for 2 weeks and stained with PKCiota antibody. Images were visualized using confocal microscopy and reveals that PKCiota is mislocalized to the basal membrane and is predominantly nuclear. DAPI is used to stain the nuclei of cells. (F) Alpha 6 integrin and E-cadherin were used to determine polarity of the acini and (G) positively stained acini were counted and graphed for shControl (n= 154) and shPKCiota (N=172) acini. P-values are indicated. (H) Western blot analysis was used to examine the levels of E-cadherin in the IGROV shControl and shPKCiota clones
PKCiota facilitates anchorage independent growth and tumor formation through cyclin E
Next we examined the role of both PKCiota and cyclin E in anchorage independent growth in a soft agar assay using the IGROV panel of shPKCiota and shControl cells (Figure 4A). All four cell lines were subjected to soft agar colony formation over a 14 day period at which point colonies were enumerated. Results revealed that downregulation of PKCiota resulted in a significant decrease in the number of soft agar colonies formed as compared to shControl cells (Figure 5A). Since the downregulation of PKCiota also results in concomitant downregulation of total cyclin E (EL and LMW-E), we next asked if cyclin E expression is necessary for growth advantage of IGROV cells in soft agar conferred by PKCiota. To this end, IGROV shPKCiota clones were infected with adenovirus harboring the cyclin E (EL and LMW-E) transgenes and subjected to soft agar colony formation assay. The results revealed that overexpression of cyclin E (EL and each of the LMW-E forms) increased the number of soft agar colonies in the shPKCiota clones to that of shControl clones (Figure 5B). The reversion of the shPKCiota phenotype by reintroduction of cyclin E is statistically significant and consistent with cyclin E expression rescuing the cells from the inhibitory effects of shPKCiota on soft agar colony formation. Western blot analysis confirmed that cyclin E was expressed in these cells at the expected molecular weights and that overexpression of any form of cyclin E did not alter the expression of PKCiota (Figure 5C). Thus, decreasing cyclin E levels by depleting PKCiota is sufficient to decrease anchorage-independent growth of IGROV cells.
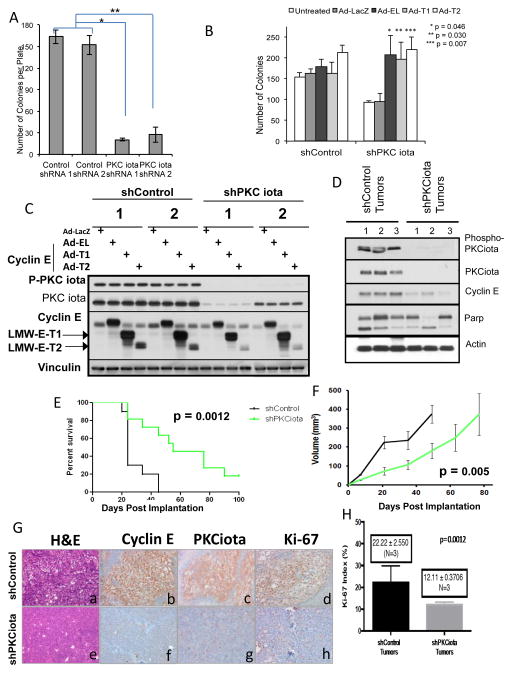
Figure 5. PKCiota knockdown inhibits soft agar colony and tumor formation through inhibition of cyclin E.
(A) The number of colonies formed in soft agar for shControl and shPKCiota clones after 15 days was determined. * p = 2.7 ×10−7; ** p = 4.4 × 10−5. (B) IGROV shControl and shPKCiota stable clones were infected with Ad-LacZ, Ad-Cyclin EL, Ad-LMW-E-T1 or –T2 and were plated in soft agar for 10 days at which point the colonies were enumerated. Number of colonies were statistically different between LacZ, shControl versus shPKCiota (p = 0.001). (C) Western blot analysis confirming the infection of the IGROV stable shRNA clones with Ad-cyclin E. Phospho-PKCiota and PKCiota antibodies. Vinculin was used as a loading control. (D–H) 1 × 106 shControl IGROV and shPKCiota IGROV cells were injected into 2 groups of 5 nude mice and tumor volume was monitored over time. Mice were euthanized when tumor volume reached 1.5 cm. The experiment was repeated 3 times, each time with two groups of 5 mice in each injection arm. (D) Western blots were performed on the tumor tissue dissected from mice, upon sacrifice to assess PKCiota, phospho-PKCiota, cyclin E, and cleaved Parp expression. Actin was used as a loading control. (E) Kaplan-Meier estimates of surviving mice (15 in each group) was generated. (F) Tumor volume of mice (15 in each group) are plotted (G) Representative sections of invasive breast tumors from shControl and shPKCiota examined histology by H & E (a, e) expression of cyclin E (b,f), PKCι (c,g) and Ki-67 (d,h) antibodies. Representative section are at ×200 magnification H) Ki67 index was expressed as percentage of positive cells in 600 tumor cells. N=3 for each condition (tumors from ShControl and shPKCiota mice).
To examine whether PKCiota depletion alters tumor formation in mice, ShControl and ShPKCiota IGROV cells were introduced as xenografts into nude mice (Figure 5D–5H). By 45 days post inoculation, all 15 of the shControl mice were sacrificed due to tumor burden; however, the survival of the shPKCiota mice was significantly increased (due to smaller tumors) to where 2 mice lived past 100 days (data not shown). This significantly increased survival (p = 0.0012) as shown in a Kaplan-Meier curve (Figure 5E). The tumor volume of the shControl mice were significantly larger than the shPKCiota mice (Figure 5F). The tumors formed in mice inoculated with the shPKCiota cells had reduced PKCiota, phosho-PKCiota, cyclin E and cleaved Parp compared to the tumors from the shControl mice (Figure 5D). Additionally, hematoxylin and eosin (H & E) staining of the tumors revealed that when PCKiota was knocked down, the tumors had a lower grade and better differentiation characteristics compared to the shControl tumors (Figure 5G). Consistently, immunohistochemical staining of the shcontrol and shPKCiota tumors with PKCiota, cyclin E and Ki67 showed lower expression of all three proteins, suggesting that when PKCiota is downregulated it results with reduced proliferation (Figure 5G and 5H). Therefore, downregulation of PKCiota resulting in cyclin E downregulation can prolong survival in mice bearing ovarian tumors.
PKCiota regulates cyclin E stability
Cyclin D has been previously reported to be upregulated in response to PKCiota, through transcriptional activation of the cyclin D promoter. We next interrogated if the regulation of cyclin E expression by PKCiota is also through transcriptional activation. We used two approaches to interrogate this hypothesis. First a luciferase reporter assay was used with two different cyclin E reporter constructs, one with the wild-type cyclin E promoter (−207 to +79; termed pE) and one with a mutant cyclin E promoter harboring two mutations in the E2F binding sites (termed pME). These two constructs were used to test the luciferase activity in shControl compared to shPKCiota IGROV cells. Results revealed no difference in luciferase activity, between the shControl or shPKCiota cells for either promoter (Data not shown). Next we quantitated cyclin E mRNA levels in IGROV cells transfected with PKCiota siRNA post downregulation of PKCiota. Western blot analysis show that PKCiota, phospho- PKCiota and cyclin E are all downregulated at 24 hours post siPKCiota transfection (Fig 6A). However, qRT-PCR analysis of cyclin E and PKCiota transcripts show that while the PKCiota transcript decreased within 24 hours of PKCiota siRNA transfection, cyclin E mRNA levels remained unchanged at all time points examined (Figure 6B). These results suggest that the regulation of cyclin E by PKCiota is most likely not via transcription.
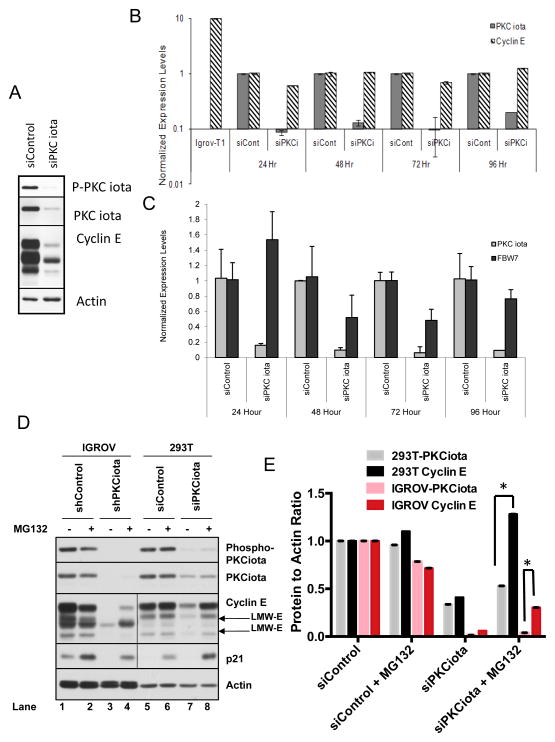
Figure 6. PKCiota regulates cyclin E through its degradation rather than transcription.
IGROV cells were transfected with siControl or siPKCiota and subjected to (A) western blot analysis with the indicated antibodies and (B, C) qRT-PCR. To this end upon transient knockdown of PKCiota for 24, 48, 72 and 96 hours RNA was harvested at each time point and used for measurement of (B)cyclin E and PKCiota levels or (C) total FBW-7 levels. IGROV cells infected with cyclin E-T1 (IGROV-T1) was used as control for cyclin E primers. GAPDH was used as a loading control in the qRT-PCR assay and the results for cyclin E and PKCiota for each condition was normalized with GAPDH. Experiment was repeated 3 times. (D) IGROV stable shPKCiota cells and 293T transient siPKCiota cells were treated with MG132, and western blot analysis was performed using phospho-PKCiota, PKCiota, cyclin E, p21 (control for MG132 response), and actin (loading control). Although all samples were run on the same gel, the exposure time for cyclin E and p21 Western blots were much longer in the 293T cells than the IGROV cells due to the low endogenous levels of these two proteins in 293T cells. (E) Cyclin E and PKCiota levels were quantitated by densitometric analysis for (D) 293 T and IGROV cells.
Next we addressed if PKCiota alters the transcriptional regulation of FBW-7, the ubiquitin ligase responsible for the degradation of cyclin 28. To determine the effect of PKCiota knockdown on FBW7 expression, IGROV cells transiently silenced with siRNA against PKCiota were examined for changes in FBW7 expression, as a function of time, by qRT-PCR analysis (Figure 6C). The results revealed that in response to silencing of PKCiota, there was no change in the transcriptional regulation of FBW-7 at any of the time intervals (0–96 hours) we examined. Thus, changes in FBW7 mRNA levels are likely not the cause of PKCiota knockdown-induced cyclin E degradation
To determine if changes in cyclin E protein levels are a result of proteosomal degradation following PKCiota downregulation, IGROV and 293T cells with PKCiota knocked down either stably or transiently, respectively, were treated with the proteasome inhibitor MG132 (Figure 6D, 6E). Silencing PKCiota either stably in IGROV cells or transiently in 293T cells caused a decrease in cyclin E levels as seen in lane 3 (IGROV shPKCiota) and lane 7 (293T siPKCiota) compared to controls (lanes 1 and 5). However, upon treatment of cells with MG132, cyclin E levels were markedly increased in both cell lines (Figure 6D, 6E). Addition of MG132 to IGROV stable PKCiota knockdown cells (left panel) resulted in a modest accumulation of cyclin E as compared to 293T cells (right panel), which exhibited levels of cyclin E similar to siControl cells. Collectively, these results suggest that that the PKCiota mediated downregulation of cyclin E occurs postranslationally.
PI3K regulates cyclin E through activation of PKCiota
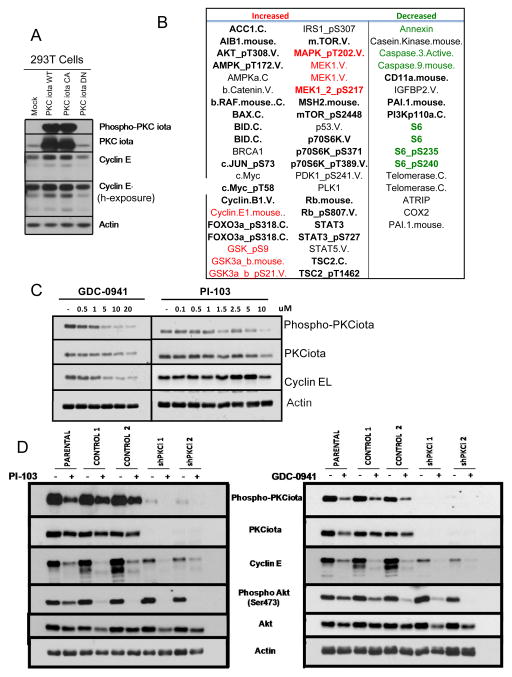
Next, we set out to identify other proteins in the PKCiota/cyclin E pathway that could be involved in the process of tumor progression. To this end 293T cells transiently expressing wild-type (WT), dominant negative (DN) or constituitively active (CA) PKCiota were subjected to an RPPA (Reverse Phase Protein Array, Figure 7A). As expected, cyclin E and PKCiota are increased in the WT and CA, but not DN or Mock (i.e. GFP cells Figure 7A). Several proteins and pathways were identified as being differentially expressed between WT and CA compared to DN or control cells (Figure 7B). Specifically, phosphorylation of AKT, GSK3, PDK1, TSC2, p70S6K, mTOR and Foxo3A were increased consistent with activation of the PI3K pathway whereas phosphorylation of MEKT and MAPK were increased consistent with activation of the RAS/MAPK pathway (Figure 7B, highlighted in red). Conversely, pathways leading to translation (decreased phosphoS6) and apoptosis (decreased Caspase 9, highlighted in green) were inhibited, while anchorage-independent growth (increased cyclin E) and proliferation (increased c-myc, STAT3, and cyclin E) pathways are activated. These results suggest that MEK and PI3K pathways, which are known factors, that either influence or are influenced by PKCiota 1, 12, 25, 39 may also regulate cyclin E expression. We validated these findings in IGROV cell line with PKCiota stably silenced as compared to the shControl cells. Using the lysates of these cells, we were able to validate the RPPA data by Western blot analysis (Supplementary Figure 3A). As expected, cyclin E, phsopho Akt (S473 and T308), Fox03A and Phospho S6 are decreased in response to PKCiota knockdown. However, in this cell line, GSK3 is minimally altered. Therefore, we hypothesized that inhibition of PI3K could also inhibit the phosphorylation of PKCiota which could then downregulate cyclin E. To test this hypothesis, we treated and IGROV parental (Fiugre 7C) cells and 293T cells (Supplementary Figure 3B) with increasing concentrations of two different PI3K inhibitors (GDC-0841 and PI-103) and examined the expression of PKCiota (total and phosphorylated forms). The levels of total PKCiota protein slightly decreased in IGROV treated cells (Figure 7C) and remained unchanged in 293T cells (Supplementary Figure 3B). However, the levels of phospho-PKCi, were reduced following treatment of cells with 5μM GDC-0941 or 1.5 μM PI-103 (Fiugre 7C and Supplementary Figure 3B). Next, 293T cells were treated with aforementioned concentrations of each agent and levels of cyclin E and AKT (total and phospho-AKT) were monitored by western blot analysis (Supplementary Figure 3C). Results revealed that not only was the active form of PKCiota (phospho-PKCiota) decreased in response to drug treatment, but also the levels of cyclin E (cyclin EL and LMW-E) and phospho-AKT were also decreased.
Figure 7. RPPA Analysis of 293T Cells with PKCiota Overexpression.
(A) 293T cells were transiently transfected with wildtype (Wt), constitutively active (CA), or dominant negative (DN) PKCiota were subjected to (A) Western blot analysis with the indicated antibodies and RPPA analysis. (B) List of proteins whose expression levels either increased or decreased in the 293T-PKCiota Wt and CA samples but not the control or PKCiota DN samples. Bold letters indicate proteins with greatest correlation. (C) IGROV parentl cells were treated PI3K inhibitors, GDC-0941 and PI-103, at increasing concentrations. Western blot analysis was used to examine changes in phospho-PKCiot, PKCiota and cyclin E levels. Actin was used as a loading control (D) IGROV parental, shControl (two independent clones) and ShPKCiota (two independent clones) were treated with 5 μM GDC-0941 and 1.5 μM PI-103 for 24 hours and subjected to western blot analysis with the indicated antibodies. Each experiment was performed in triplicate.
Next we interrogated if PI3K can directly regulate cyclin E expression independent of PKCiota in ovarian cancer cell lines. To this end, we treated the IGROV shControl and IGROV shPKCiota cell lines with two different PI3K inhibitors, PI-103 and GDC-0941 and examined cyclin E and phospho-AKT expression (Figure 7D). In the parental and shControl IGROV cell lines, PI3K inhibition resulted in a marked decrease in cyclin E expression. However, shPKCiota cell lines treated with PI3K inhibitors also resulted in a decrease in the levels of cyclin E. Collectively, these results suggest that PI3K or PKCiota inhibitors could provide a target for inhibition of several oncogenic pathways including cyclin E, PKCiota and PI3K, simultaneously in serous ovarian cancer tumors.
DISCUSSION
The role of cyclin E in ovarian cancer tumorigenesis has been extensively characterized but has provided few answers regarding the specific upstream activators that maintain a hierarchal role in influencing deregulation of the cell cycle via cyclin E. Thus the observation that PKCiota expression correlates with cyclin E deregulation in ovarian cancer patient samples provides a plausible understanding of the tumorigensis pathway via a PKCiota→cyclin E activation pathway. In this study we have examined the relationship between PKCiota and cyclin E that highlights PI3K as an upstream mediator of PKCiota activation/phosphorylation and have concluded that cyclin E plays a significant role in the activation of ovarian cancer tumorigenesis via a PKCiota regulated mechanism.
As a member of the Protein Kinase C family of proteins, PKCiota has emerged as an oncogene in several tumor types including, ovarian, glioblastoma, lung cancer and pancreatic cancer 8, 17, 18, 21. Several oncogenes have demonstrated a capacity to activate PKCiota, including KRAS, PI3K, Brc-Abl. Src, EGFR and HER2/neu 31, Both Cyclin E and PKCiota individually have been implicated in anchorage-independent growth and transformation, one of the initial events in ovarian tumorigenesis 13, 19, 24, 30. Thus, we hypothesized that with PKCiota and cyclin E having seemingly overlapping roles during tumor transformation requiring PKCiota overexpressing cells to maintain cyclin E expression during transformation. The downstream activation of cyclin E via PKCiota suggests that deregulation of the cell cycle lies at a central node of several oncogenic signaling pathways, which may be a crucial checkpoint of ovarian tumorigensis. Loss of PKCiota correlates with loss of cyclin E expression both in vivo and in vitro leading to inhibition of tumor growth, aberrant acini formation, activation of subsequent PI3K feedback loop and prolonged overall survival of xenografted mice. The ability to attenuate the PKCiota→cyclin E pathway via PI3K inhibitors demonstrates a possible therapeutic target for PKCiota/cyclin E over expressing ovarian tumors.
Cyclin E and the Low Molecular Weight (LMW-E) isoforms play a potent role during oncogenic transformation of breast tissue 15, 33. Research in our lab has also determined that LMW-E is a viable target in breast cancer, specifically in the triple negative subset of breast cancer 32. Due to lack of ER, PR, and Her2 receptors, which provide functional targets for treatment, triple negative breast cancer tends to be more aggressive and more difficult to treat. PKCiota, which is an oncogene, is also overexpressed in breast cancer 5, 34 and recently, several publications have emerged that establish PKCiota as an essential driving force in triple negative breast cancer 35,38. These studies suggest that PKCiota mediates its oncogenic activity in breast cancer through suppression of senescence and colocalization with MT1-MMP, which is responsible for invasion through degradation of the extracellular matrix. Because of the ability of oncogenic LMW-E to bypass normal cell cycle checkpoints 2, 7, 36, 43 and upregulation of LMW-E in invasive tumors (this study and 9), we reason that the oncogenic role of PKCiota in triple negative breast cancer could at least in part be carried.
In ovarian cancer however, we show that neither EL nor the LMW-E isoforms demonstrated any regulatory capacity over PKCiota protein expression or phosphorylation. Additionally, induced expression of EL and LMW-E isoforms abolished the inhibition of colony formations observed in PKCiota shRNA knockdown cells. This data strengthens the model of downstream activation of cyclin E by PKC iota, however it also signifies the influential role cyclin E plays in ovarian tumorigenesis. We have also observed a proteosomal dependent degradation mechanism of cyclin E/LMW-E regulation by PKCiota. The specific mediators of this aspect of the pathway remain unknown.
Our efforts to elucidate this novel PI3K, PKCiota, and cyclin E pathway provide a novel target for therapeutic intervention for ovarian tumors. As stated previously, phospho-PKCiota and cyclin E are possible biomarkers that could be used to evaluate PI3K inhibitors clinically. The observation that cyclin E overexpression abrogates PKCiota driven colony formation suggests that if cyclin E remains elevated due to other mechanisms, it may abrogate the effects of inhibition of PI3K or PKCiota and may also serve as a biomarker of efficacy of PI3K or PKCiota inhibition. At present clinical inhibition of cyclin E directly is not feasible however CDK2 is the preferential binding partner of cyclin E and CDK2 inhibition has been shown to abrogate cyclin E driven breast tumorigensis in a CDK2−/− genetic mouse model and with CDK2 inhibitor Roscovitine 3, 4, providing additional rational for investigation of CDK inhibitors in this subset of PKCiota driven ovarian tumors. Most notably, there are no specific PKCiota inhibitors presently available for clinical evaluation thus providing rationale for the investigation of small molecule inhibitors that target either PKCiota activity or phosphorylation.
Lastly, in serous epithelial ovarian cancer, because PKCiota is consistently mislocalized or overexpressed at least in part due to amplification of PKCiota and potentially PIK3CA, the gene that encodes the catalytic subunit of PI3K at 3q26.2 16, ovarian cancer patients with this cancer subtype would be most likely to benefit from PKCiota inhibition.
MATERIALS AND METHODS
Detailed description of methods on tissue culture conditions, growth curve analysis, flow cytometry analysis, Cell Sorting using GFP, apoptosis assays, 3D culture conditions and assay, soft agar colony formation, confocal microscopy, western blot analysis, MG132 treatment, luciferase assay and immunohistochemical analysis of tumor tissues is provided in the Supplementary Methods.
Generation of PKCiota constructs
pCMV6-XL5 PKCiota wildtype (Wt) cDNA was purchased from origene (SC118455) and mutated by site-directed mutagenesis using the QuikChange Site-Directed Mutagenesis kit (Agilent). The constitutively active (CA) mutation (A129E) and the dominant negative (DN) mutation (K122STOP) were performed using primer described and validated previously 22, 37. Confirmation of the targeted mutation was through sequencing using the T7 primer for transfection into 293T cells.
Infection with Ad-Cyclin E/Ad-E2F1
Cyclin E (EL, LMW-E-T1, and LMW-E-T2), E2F1, and LacZ adenoviruses were constructed using the AdEasy XL adenoviral vector system kit (Agilent, Santa Clara, CA). IGROV cells (1 × 106 cells/10 cm plate) were infected with 1 000 MOI of either Ad-Cyclin EL, Ad-LMW-E-T1, or Ad-LMW-E-T2 as published 6, 7. Cells were then harvested and examined by Western blot analysis with indicated antibodies.
Transfections
siRNA to PKCiota in OVCAR3, IGROV, and SKOV3 was performed using siPORT amine reagent (Ambion, Grand Island, NY) using the reverse transfection protocol according to the manufacturer’s instructions. 2.3 × 105 cells were used per well of 6 well plates. 20nM PKCiota siRNA (gene code 309 and 311) and Silencer Negative Control Number 1 (cat# 4611), both from Ambion were transfected with X-tremeGENE (Genentech, San Francisco, CA), according to the manufacturer’s protocol. To select stable IGROV cells, they were transfected with pRS-shPKCiota (Origene, Rockville, MD #TR320472) using GeneJuice (Millipore) according to the manufacturer’s protocol. shPKCiota 7 sequence TGACCAGAACACAGAGGATTATCTCTTCC targeting exon 14 and and shPKCiota 8 sequence CAGGAGATACAACCAGCACTTTCTGTGGT targeting exon 13 were determined to target only a single sequence. shControl (shRNA 3; CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG) was the control, not specific for PKCiota. Stable clones were selected using 1 μg/mL Puromycin. OVCA420 cells were transfected with pcDNA3.1 constructs containing sequences coding for full-length cyclin E (EL) or the LMW-E forms (T1 and T2) using fuGENE 6 (Roche) according to the manufacturer’s instructions.
Xenograft studies
1×106 cells were injected into the mammary fat pad of 4- to 6-week-old female Balb/c Nu/nu mice (Taconic, Germantown, NY). To observe effects of PKCiota knockdown on tumor growth, 30 nude mice were randomized into two groups and injected with shControl (n=15) or shPKCiota (n=15) cloned cells in the mammary fat pads. The tumor volume was calculated every other day. Mice were euthanized when tumors were greater than 1.5 cm in diameter at the widest dimension of the tumor. Mice were housed five per cage in sterilized micro-isolator cages (Lab Products, Seaford, DE) furnished with corncob bedding. The investigator who injected the mice with the shControl and shPKCiota cells was blinded to this groupings. Once the tumor measurement and survival data were captured, the identity of mice were revealed. Mice received care in accordance with the Animal Welfare Act, the National Institutes of Health “Guide for the Care and Use of Laboratory Animals,” and the institutional guidelines of MD Anderson Cancer Center.
RPPA Analysis
RPPA (Reverse Phase Protein Array), a high-throughput technique of quantitated proteomic analysis, was performed as previously published 41. 293T cells were transfected with either vector alone, PKCiota Wt, CA, or DN, and harvested 48 hours later. Lysates were printed onto nitrocellulose-coated glass slides using an automated robotic arrayer (GeneTac, BST scientific, Singapore). Primary antibody treatment was followed by washes and a biotinylated secondary antibody incubation. The amplified signal was detected by a readout device and quantitated using an automated RPPA module (MicroVigene- VigeneTech, Carlisle, MA). Analysis of the quantitated RPPA data was performed using Microsoft Excel.
PI3K Inhibition
293T and IGROV cells were plated at 5 × 105 cells per 10 cm plate, and were treated with PI3K inhibitors GDC-0941 and PI-103 (Selleck Chemicals, Houston, TX) 24 hours post plating. Following 24 hours of treatment, cells were harvested as above and subjected to western blot analysis.
Statistical Consideration
All experiments were performed at least in triplicate. The results of each experiment are reported as the mean of experimental replicates. Error bars represent the standard deviation from the mean. If not otherwise indicated, pairwise comparisons were analyzed using the unpaired 2-sided t-test (GraphPad Prism). For all tests, p<0.05 was considered significant.
Linear regression curves were plotted on Microsoft Excel using the x-y scatter graph function. A linear trendline was established for each, and the correlation coefficient was determined by Pearson test for the correlation coefficient (r). The p-value was determined using a significance calculator examining the r-value and the number of trials. The student t-test (two-tailed, equal variance) was employed to derive the p-value of the experiments with a normal distribution.
Supplementary Material
Acknowledgments
This research was supported by NIH grants CA87458 and CA1522228 to KK and by NCI CCSG grant CA16672 to M.D. Anderson Cancer Center.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
References
- 1.Akimoto K, Nakaya M, Yamanaka T, Tanaka J, Matsuda S, Weng QP, et al. Atypical protein kinase Clambda binds and regulates p70 S6 kinase. The Biochemical journal. 1998;335(Pt 2):417–424. doi: 10.1042/bj3350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akli S, Keyomarsi K. Low-molecular-weight cyclin E: the missing link between biology and clinical outcome. Breast Cancer Res. 2004;6:188–191. doi: 10.1186/bcr905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akli S, Van Pelt CS, Bui T, Multani AS, Chang S, Johnson D, et al. Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res. 2007;67:7212–7222. doi: 10.1158/0008-5472.CAN-07-0599. [DOI] [PubMed] [Google Scholar]
- 4.Akli S, Van Pelt CS, Bui T, Meijer L, Keyomarsi K. Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res. 2011;71:3377–3386. doi: 10.1158/0008-5472.CAN-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awadelkarim KD, Callens C, Rosse C, Susini A, Vacher S, Rouleau E, et al. Quantification of PKC family genes in sporadic breast cancer by qRT-PCR: evidence that PKCiota/lambda overexpression is an independent prognostic factor. Int J Cancer. 2012;131:2852–2862. doi: 10.1002/ijc.27600. [DOI] [PubMed] [Google Scholar]
- 6.Bagheri-Yarmand R, Biernacka A, Hunt KK, Keyomarsi K. Low molecular weight cyclin E overexpression shortens mitosis, leading to chromosome missegregation and centrosome amplification. Cancer research. 2010;70:5074–5084. doi: 10.1158/0008-5472.CAN-09-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagheri-Yarmand R, Nanos-Webb A, Biernacka A, Bui T, Keyomarsi K. Cyclin E deregulation impairs mitotic progression through premature activation of Cdc25C. Cancer Res. 2010;70:5085–5095. doi: 10.1158/0008-5472.CAN-09-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin RM, Garratt-Lalonde M, Parolin DA, Krzyzanowski PM, Andrade MA, Lorimer IA. Protection of glioblastoma cells from cisplatin cytotoxicity via protein kinase Ciota-mediated attenuation of p38 MAP kinase signaling. Oncogene. 2006;25:2909–2919. doi: 10.1038/sj.onc.1209312. [DOI] [PubMed] [Google Scholar]
- 9.Bales E, Mills L, Milam N, McGahren-Murray M, Bandyopadhyay D, Chen D, et al. The low molecular weight cyclin E isoforms augment angiogenesis and metastasis of human melanoma cells in vivo. Cancer Res. 2005;65:692–697. [PubMed] [Google Scholar]
- 10.Bedrosian I, Lu KH, Verschraegen C, Keyomarsi K. Cyclin E deregulation alters the biologic properties of ovarian cancer cells. Oncogene. 2004;23:2648–2657. doi: 10.1038/sj.onc.1207408. [DOI] [PubMed] [Google Scholar]
- 11.Bedrosian I, Lee C, Tucker SL, Palla SL, Lu K, Keyomarsi K. Cyclin E-associated kinase activity predicts response to platinum-based chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4800–4806. doi: 10.1158/1078-0432.CCR-07-0142. [DOI] [PubMed] [Google Scholar]
- 12.Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Current biology : CB. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 13.Coghlan MP, Chou MM, Carpenter CL. Atypical protein kinases Clambda and -zeta associate with the GTP-binding protein Cdc42 and mediate stress fiber loss. Molecular and cellular biology. 2000;20:2880–2889. doi: 10.1128/mcb.20.8.2880-2889.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courjal F, Louason G, Speiser P, Katsaros D, Zeillinger R, Theillet C. Cyclin gene amplification and overexpression in breast and ovarian cancers: evidence for the selection of cyclin D1 in breast and cyclin E in ovarian tumors. International journal of cancer Journal international du cancer. 1996;69:247–253. doi: 10.1002/(SICI)1097-0215(19960822)69:4<247::AID-IJC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Duong MT, Akli S, Macalou S, Biernacka A, Debeb BG, Yi M, et al. Hbo1 is a cyclin E/CDK2 substrate that enriches breast cancer stem-like cells. Cancer Res. 2013;73:5556–5568. doi: 10.1158/0008-5472.CAN-13-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP, et al. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdogan E, Klee EW, Thompson EA, Fields AP. Meta-analysis of oncogenic protein kinase Ciota signaling in lung adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:1527–1533. doi: 10.1158/1078-0432.CCR-08-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans JD, Cornford PA, Dodson A, Neoptolemos JP, Foster CS. Expression patterns of protein kinase C isoenzymes are characteristically modulated in chronic pancreatitis and pancreatic cancer. American journal of clinical pathology. 2003;119:392–402. doi: 10.1309/bkpc9dx98r781b87. [DOI] [PubMed] [Google Scholar]
- 19.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 20.Farley J, Smith LM, Darcy KM, Sobel E, O’Connor D, Henderson B, et al. Cyclin E expression is a significant predictor of survival in advanced, suboptimally debulked ovarian epithelial cancers: a Gynecologic Oncology Group study. Cancer Res. 2003;63:1235–1241. [PubMed] [Google Scholar]
- 21.Fields AP, Regala RP. Protein kinase C iota: human oncogene, prognostic marker and therapeutic target. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;55:487–497. doi: 10.1016/j.phrs.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamieson L, Carpenter L, Biden TJ, Fields AP. Protein kinase Ciota activity is necessary for Bcr-Abl-mediated resistance to drug-induced apoptosis. The Journal of biological chemistry. 1999;274:3927–3930. doi: 10.1074/jbc.274.7.3927. [DOI] [PubMed] [Google Scholar]
- 23.Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer cell. 2014;25:139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang JS, Krauss RS. Ras induces anchorage-independent growth by subverting multiple adhesion-regulated cell cycle events. Molecular and cellular biology. 1996;16:3370–3380. doi: 10.1128/mcb.16.7.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanoh Y, Sajan MP, Bandyopadhyay G, Miura A, Standaert ML, Farese RV. Defective activation of atypical protein kinase C zeta and lambda by insulin and phosphatidylinositol-3,4,5-(PO4)(3) in skeletal muscle of rats following high-fat feeding and streptozotocin-induced diabetes. Endocrinology. 2003;144:947–954. doi: 10.1210/en.2002-221017. [DOI] [PubMed] [Google Scholar]
- 26.Karst AM, Jones PM, Vena N, Ligon AH, Liu JF, Hirsch MS, et al. Cyclin E1 deregulation occurs early in secretory cell transformation to promote formation of fallopian tube-derived high-grade serous ovarian cancers. Cancer research. 2014;74:1141–1152. doi: 10.1158/0008-5472.CAN-13-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, et al. Cyclin E and survival in patients with breast cancer. The New England journal of medicine. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 28.Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–177. doi: 10.1126/science.1065203. [DOI] [PubMed] [Google Scholar]
- 29.Lakhani SR, Manek S, Penault-Llorca F, Flanagan A, Arnout L, Merrett S, et al. Pathology of ovarian cancers in BRCA1 and BRCA2 carriers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:2473–2481. doi: 10.1158/1078-0432.ccr-1029-3. [DOI] [PubMed] [Google Scholar]
- 30.Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D, et al. Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. The Journal of cell biology. 2004;164:797–802. doi: 10.1083/jcb.200311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray NR, Kalari KR, Fields AP. Protein kinase Ciota expression and oncogenic signaling mechanisms in cancer. Journal of cellular physiology. 2011;226:879–887. doi: 10.1002/jcp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanos-Webb A, Jabbour NA, Multani AS, Wingate H, Oumata N, Galons H, et al. Targeting low molecular weight cyclin E (LMW-E) in breast cancer. Breast cancer research and treatment. 2012;132:575–588. doi: 10.1007/s10549-011-1638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanos-Webb A, Jabbour NA, Multani AS, Wingate H, Oumata N, Galons H, et al. Targeting low molecular weight cyclin E (LMW-E) in breast cancer. Breast cancer research and treatment. 2012;132:575–588. doi: 10.1007/s10549-011-1638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paget JA, Restall IJ, Daneshmand M, Mersereau JA, Simard MA, Parolin DA, et al. Repression of cancer cell senescence by PKCiota. Oncogene. 2012;31:3584–3596. doi: 10.1038/onc.2011.524. [DOI] [PubMed] [Google Scholar]
- 35.Paul A, Gunewardena S, Stecklein SR, Saha B, Parelkar N, Danley M, et al. PKClambda/iota signaling promotes triple-negative breast cancer growth and metastasis. Cell Death Differ. 2014;21:1469–1481. doi: 10.1038/cdd.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rath SL, Senapati S. Why are the truncated cyclin Es more effective CDK2 activators than the full-length isoforms? Biochemistry. 2014;53:4612–4624. doi: 10.1021/bi5004052. [DOI] [PubMed] [Google Scholar]
- 37.Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP. Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. The Journal of biological chemistry. 2005;280:31109–31115. doi: 10.1074/jbc.M505402200. [DOI] [PubMed] [Google Scholar]
- 38.Rosse C, Lodillinsky C, Fuhrmann L, Nourieh M, Monteiro P, Irondelle M, et al. Control of MT1-MMP transport by atypical PKC during breast-cancer progression. Proc Natl Acad Sci U S A. 2014;111:E1872–1879. doi: 10.1073/pnas.1400749111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Standaert ML, Bandyopadhyay G, Sajan MP, Cong L, Quon MJ, Farese RV. Okadaic acid activates atypical protein kinase C (zeta/lambda) in rat and 3T3/L1 adipocytes. An apparent requirement for activation of Glut4 translocation and glucose transport. The Journal of biological chemistry. 1999;274:14074–14078. doi: 10.1074/jbc.274.20.14074. [DOI] [PubMed] [Google Scholar]
- 40.Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 41.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Molecular cancer therapeutics. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 42.Weichert W, Gekeler V, Denkert C, Dietel M, Hauptmann S. Protein kinase C isoform expression in ovarian carcinoma correlates with indicators of poor prognosis. International journal of oncology. 2003;23:633–639. [PubMed] [Google Scholar]
- 43.Wingate H, Bedrosian I, Akli S, Keyomarsi K. The low molecular weight (LMW) isoforms of cyclin E deregulate the cell cycle of mammary epithelial cells. Cell Cycle. 2003;2:461–466. [PubMed] [Google Scholar]
- 44.Zhang L, Huang J, Yang N, Liang S, Barchetti A, Giannakakis A, et al. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer research. 2006;66:4627–4635. doi: 10.1158/0008-5472.CAN-05-4527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.