Summary
COP1 proteins are E3 ubiquitin ligases that regulate phototropism in plants and target transcription factors for degradation in mammals. The substrate-binding region of COP1 resides within a WD40-repeat domain that also binds to Trib proteins, which are adaptors for C/EBPα degradation. We report here structures of the human COP1 WD40 domain in isolation, and complexes of the human and Arabidopsis thaliana COP1 WD40 domains with the binding motif of Trib1. The human and Arabidopsis WD40 domains are seven-bladed β-propellers with an inserted loop on the bottom face of the first blade. The Trib1 peptide binds in an extended conformation to a highly conserved surface on the top face of the β-propeller, indicating a general mode for recognition of peptide motifs by COP1. Together, these studies identify the structural basis and key interactions for motif recognition by COP1, and hint at how Trib1 autoinhibition is overcome to target C/EBPα for degradation.
Graphical Abstract
COP1 proteins are E3 ubiquitin ligases that regulate phototropism in plants and target transcription factors for degradation in mammals. In Arabidopsis thaliana, where COP1 was first described, it represses the light response by promoting the ubiquitination and degradation of HY5/HYH family transcription factors, which contain a targeting motif with the consensus amino acid sequence ESDEExxxVP[D/E] (Ang et al., 1998; Deng et al., 1991; Deng et al., 1992; Holm and Deng, 1999; Holm et al., 2002). A. thaliana COP1 can also promote degradation of transcription factors that do not contain canonical COP1 recognition sequences, such as PIF1 and LAF1 (Maier et al., 2013; Seo et al., 2003). Emerging evidence also suggests that COP1 is regulated both by light-dependent sequestration (via the protein UVR8) and by its participation in multi-subunit cullin-based E3 ligase complexes that promote degradation of specific transcription factors (Huang et al., 2013).
In mammals, COP1 (gene name RFWD2) is essential, and has been implicated as both a tumor suppressor and a cancer promoter. Supporting a tumor suppressor function, COP1 hypomorphic mice develop tumors at much higher rates than wild-type mice (Migliorini et al., 2011). In addition, Jun and ETS transcription factors, which contain COP1 binding motifs, are direct targets of COP1-mediated degradation, and levels of COP1 inversely correlate with Jun and ETS amounts in human and murine prostate cancers (Bianchi et al., 2003; Migliorini et al., 2011; Vitari et al., 2011). In contrast, COP1 is highly expressed in several other human cancers (Dornan et al., 2004a). The tumor suppressors p53 and p27 are proposed to be degradation targets of COP1, and in the case of p27, there is evidence of direct interaction requiring a VPA motif in p27. These data suggest that COP1 promotes cancer in these contexts (Choi et al., 2015; Dornan et al., 2004b).
Another context in which COP1 promotes tumorigenesis is in a mouse model of acute myelogenous leukemia (AML). In this model, forced expression of either Trib1 or Trib2, each of which is a homologue of Drosophila tribbles, gives rise to AML with high penetrance. Tumor formation in this context relies on the integrity of a COP1 binding motif near the Trib C-terminus, recently shown to be held tethered to the N-lobe of Trib1 in the absence of COP1 (Murphy et al., 2015). Additional studies probing the molecular mechanism of leukemogenesis show that COP1 does not efficiently degrade Trib1 or Trib2. Instead, it appears that Trib1 and Trib2 serve as adaptors that bind COP1, and direct it to degrade the differentiation-promoting transcription factor C/EBPα, which lacks a canonical COP1-binding motif (Keeshan et al., 2010; Keeshan et al., 2006).
Despite the fundamental importance of the COP1 E3 ligase in both the plant and animal kingdoms, it is neither known how COP1 recognizes substrates, nor how it binds Trib1 to alter its target substrate selectivity. We report here the structure of the human COP1 WD40 domain without bound peptide, and of the human and A. thaliana COP1 WD40 domains bound to a COP1 consensus motif from Trib1, revealing a mode of substrate recognition that relies on interfacial residues that are 100% conserved. These structures, together with biochemical and cell based assays of COP1-Trib1 complexes, identify the structural basis for motif recognition by COP1, clarify the basis for substrate selectivity among various WD40-containing E3 ligases, and hint at how Trib1 autoinhibition is overcome to target C/EBPα for degradation.
Results
The COP1 WD40 domain is necessary and sufficient for Trib1 binding
In order to uncover binding partners of Trib proteins using an unbiased approach, we used tandemly tagged full-length Trib1 and Trib2 proteins as bait in a proteome-wide search for Trib interactors in HeLa cells. COP1 peptides were consistently retrieved in all experiments (Figure S1 and Table S1), confirming several previous reports that Trib proteins are in complexes with COP1 (Du et al., 2003; Vitari et al., 2011). We then performed anti-FLAG immunoprecipitations (IPs) on cells expressing HA-COP1 and Flag-Trib1 or HA-COP1 and a FLAG-Trib1 protein lacking the last 21 residues which encompass the putative COP1-binding motif (Flag-Trib1-ΔC). Flag-Trib1 recovered COP1 but the Trib1-ΔC construct did not, supporting the inference that this motif is required for COP1 binding (Figures 1 and S1).
Figure 1. The COP1 WD40 domain is necessary and sufficient for Trib1 binding.
A. Schematic illustrating forms of human COP1 tested in immunoprecipitation experiments. B. Trib1 immunoprecipitations. Flag-tagged full-length or C-terminally truncated Trib1 proteins were recovered using anti-Flag antibodies. The whole cell extracts (WCE) and the immunoprecipitates (IP) were probed with anti Flag (top), and anti-COP1 antibodies (bottom). NS indicates a non-specific band seen in all immunoprecipitates probed with anti-COP1.
In order to determine the region of human COP1 responsible for Trib1 binding, we created a series of COP1 molecules with serial truncations from the N-terminus and tested whether they co-immunoprecipitated with Flag-tagged Trib1 (Figure 1). Full-length COP1, as well as truncated COP1 molecules starting at residue 177 (immediately after the RING domain) or 386 (immediately preceding the predicted start of the WD40 domain) co-immunoprecipitate with Flag-tagged full-length Trib1 (Figure 1B, lanes 6-8), but an attempt to trim the N-terminal end of COP1 further (beyond residue 386) failed to support co-precipitation with Trib1 (Figure 1B, lane 9). The COP1-Trib1 interaction was also dependent on the presence of the 21 C-terminal residues of Trib1 in the Western blot assay, as predicted from the proteomic data (Figure 1B, lane 10). These data show that the WD40 domain (residues 386-731) of COP1 is both necessary and sufficient for Trib1 interaction, and also confirms the requirement for the presence of the C-terminal part of Trib1 containing the proposed COP1 interacting motif.
Structural basis for COP1 recognition of Trib1
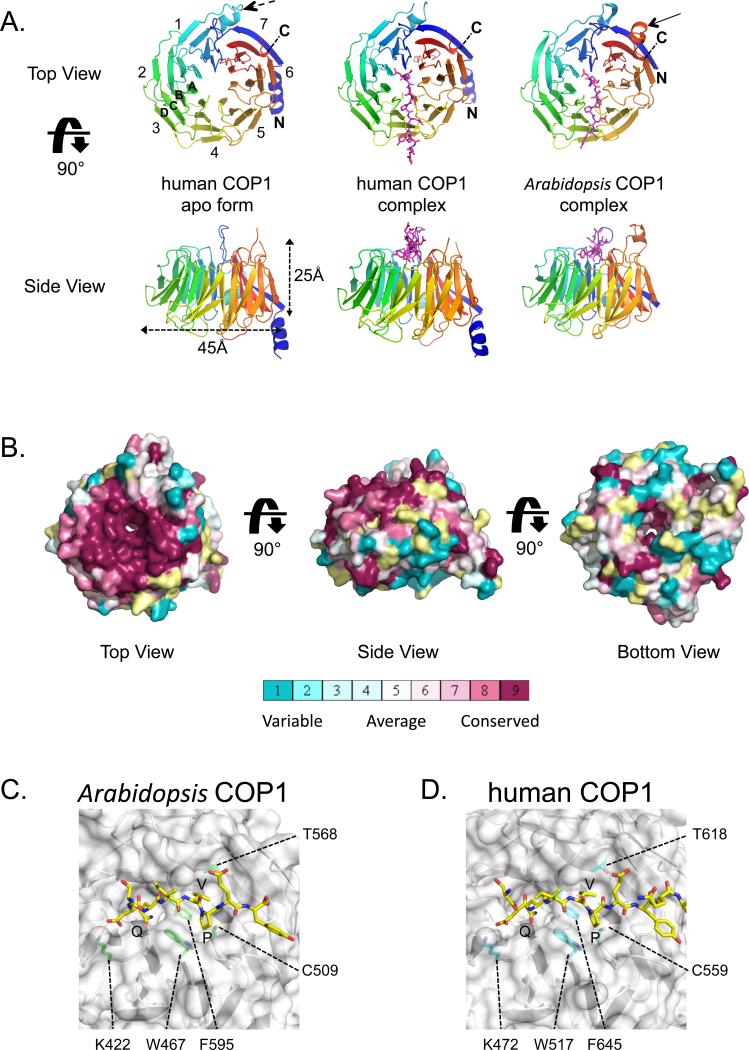
To determine how COP1 recognizes Trib1 and other proteins, we determined X-ray structures of the WD40 domain of human COP1 alone to 2.0 Å resolution, a complex of human COP1 with a Trib1 peptide spanning the COP1-recognition motif to 3.9 Å resolution, and a complex of the Arabidopsis COP1 WD40 domain with a Trib1 peptide to 1.6 Å resolution (Table 1). The WD40 domain of COP1 is a seven-bladed β-propeller (Figure 2A), in which the N-terminal beta strand (residues 403-412) forms the last strand of the 7th blade (7D), completing the fold and stabilizing the propeller on its “bottom” face (Figure 2A). In the human COP1 structures, this face is stabilized by an N-terminal helix (residues 390-401), which contacts blade six of the propeller by a salt bridge from K398 to D653, located between strands 1A and 1B. The bottom face also contains an atypical 15-residue loop between strands 1C and 1D (residues 449-463) enriched in acidic residues (Figure 2A, dashed arrow). The WD40 domain of Arabidopsis COP1 structure is very similar, with an all-atom RMSD of 1.6 Å, and has the same connectivity (Figure S2). One notable difference between the two molecules is that the Arabidopsis structure lacks the N-terminal buttressing helix seen in the human structure, as that region was not included in the cDNA used to produce the plant protein (the region immediately N-terminal to the expressed WD40 domain is predicted to be disordered and was cleaved in limited proteolysis studies; data not shown). Second, a loop enriched in acidic residues connecting β-strand 6D to 7A (A. thaliana residues 630-646) is visible on the top face of the plant structure (Figure 2A, solid arrow) but is partly disordered in the human structure. This loop does not make contact with bound peptide (see below).
Table 1.
Data collection and refinement statistics
| Human COP1 | Human COP1 with Trib1peptide | Arabadopsis COP1 with Trib1 peptide | |
|---|---|---|---|
| Data collection | |||
| Space group | P31 2 1 | C 2 2 2 | P 21 |
| Cell dimensions | |||
| a, b, c (Å) | 68.9, 68.9, 133.9 | 247.6, 249.5, 124.5 | 78.5, 87.5 94.6 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 90 | 90 92.5, 90 |
| Resolution (Å)a | 34.5-2.0 (2.075-2.0)b | 29.7-3.9 (4.04-3.9) | 64.2-1.597 (1.6-1.597) |
| Wavelength | 0.9792 | 0.97918 | 0.97931 |
| Rmergeb | 0.040 (0.436) | 0.075 (0.904) | 0.085 (1.16) |
| CC1/2 | 1 (0.95) | 1 (1.20) | 1 (0.87) |
| I / σI | 20.9 (3.29) | 8 (0.95) | 9 (0.92) |
| Completeness (%) | 100 (100) | 98 (100) | 99 (94) |
| Redundancy | 4.9 (5.0) | 2.0 (2.0) | 3.4 (3.3) |
| Refinement | |||
| Resolution (Å) | 2.0 | 3.9 | 1.6 |
| No. reflections | 25509 (2490) | 34980 (3464) | 166918 (15962) |
| Rwork / Rfree | 0.21/0.24 (0.27/0.31) | 0.25/0.29 (0.37/0.40) | 0.19/0.21 (0.41/0.42) |
| No. atoms | |||
| Protein | 2490 | 15349 | 11580 |
| Ligand/ion | 5 | 0 | 0 |
| Water | 99 | 0 | 1256 |
| B-factors | |||
| Protein | 55.32 | 186.57 | 25.15 |
| Ligand/ion | 55.56 | N/A | N/A |
| Water | 49.55 | N/A | 35.64 |
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.002 | 0.002 | 0.008 |
| Bond angles (°) | 0.6 | 0.7 | 1.0 |
Values in parentheses are for highest resolution shell.
A single crystal was used for each structure determination.
Figure 2. Structures of human and Arabidopsis COP1 WD40 domains.
A. Cartoon representation. Top and side views are shown for unbound human COP1 (left), the human COP1-peptide complex (middle) and the plant COP1-peptide complex (right). The Trib peptide is shown in magenta as sticks. Numbers denote blades from N- to C-terminus (colored on a rainbow scale from blue to red), and letters A-D indicate strands of a typical blade. A dashed arrow in the top view of human COP1 points to the helical insert between strands 1C and 1D. A second arrow in the top view of the plant COP1 indicates the 6D to 7A loop. B. Surface representation, colored according to sequence conservation on a sliding scale from maroon (highest) to cyan (lowest) using the program Consurf (Ashkenazy et al., 2010). C, D. Closeup views of the Arabidopsis COP1 (C) and human COP1 (D) complexes. The protein backbones are shown as cartoons and the surface is in white, with key interface side chains labeled and rendered as sticks. The Trib peptide is shown in yellow and residue sidechains are represented as sticks.
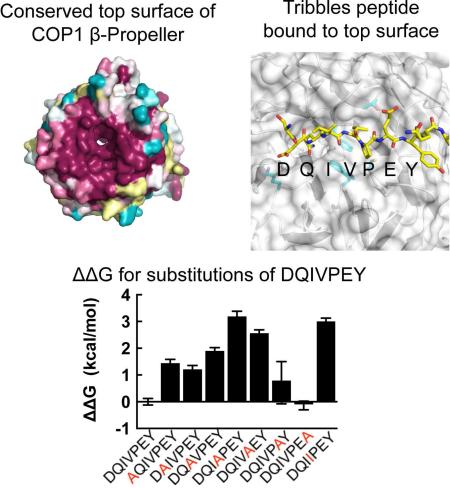
The top face of the propeller shows an extraordinarily high degree of conservation across many sequences ranging from plants to humans (Figure 2B). As residues within 4 Å of the bound peptide are identical between plants and humans (Figure S2), interactions between bound peptide and the WD40 domain in the complexes will be described in terms of both the human and plant residue numbers, with the Arabidopsis COP1 number first and the corresponding human COP1 residue in parentheses. A key feature of the motif recognition surface is the shallow binding pocket for the consensus V-P sequence of the bound peptide, residues V358-P359 of Trib1. This hydrophobic pocket is bounded by F595 (F645), T568 (T618), and C509 (C559), and the aromatic residues W467 (W517) and F595 (F645) form a ridge between the smaller V-P pocket and the larger central donut hole (Figures 2C,D and S3). The non-consensus Trib1 residue I357 points out into solvent, while Q356 sits in the central cavity, formed by COP1 residues S375 (S425), F595 (F645), and W467 (W517) (Figure 2C,D). This role for W467 is consistent with published data showing that mutation of this residue abrogates Hy5 binding in A. thaliana (Holm et al., 2001). Positively charged residues on the top face of COP1 are also important for binding. A negatively charged residue towards the N-terminus of the peptide (D/E) is part of the consensus for the COP1-binding motif. In our structure, this Trib1 residue is D355, which forms a salt bridge with K422 (K472). Surprisingly, Trib1 E360 does not form a salt bridge with the conserved K550 (K600) residue in the structure of either complex, despite the proposal that the analogous residue in HY5 (E45) is indispensible for COP1 interaction in plants (Holm et al., 2001).
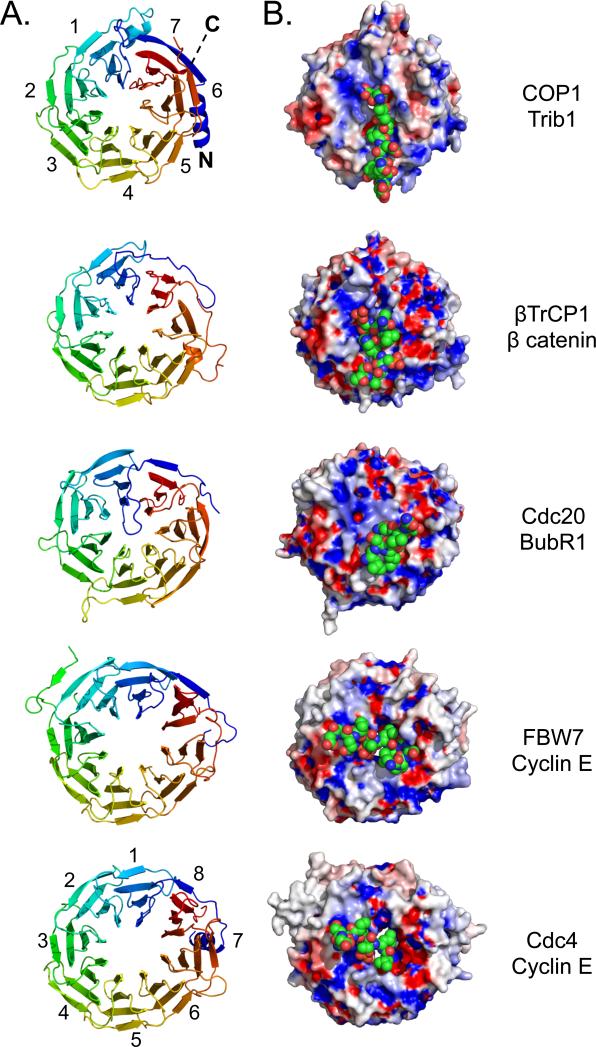
We also compared the mode of peptide binding in the COP1 complexes to substrate-recognition modes for other WD40 β-propeller domains that recognize substrates and target them for ubiquitination (Figures 3 and S3) (Hao et al., 2007; Orlicky et al., 2003; Tian et al., 2012; Wu et al., 2003). In each of these examples, the top face engages the target motif and the bottom face and/or sides of the β-propeller are available to bind other components of the respective E3 ligase complexes. However, the peptide-binding groove and its chemical characteristics vary among the different ligases, imparting distinct specificities among the ligases for their various target motifs (Figure 3B). It also appears that binding selectivity relies on residues unique to each propeller but often conserved in orthologs of the same protein, as illustrated for the top face of COP1 (Figure 2B). DDB2, which recognizes DNA molecules that are not direct degradation targets (loosely analogous to binding of the Trib adaptors by COP1), also uses the top face for binding (Fischer et al., 2011; Scrima et al., 2008).
Figure 3. Comparison of WD40 β-propellers from E3 ligases bound to peptide motifs.
A. Cartoon representation, showing a top view (clockwise from N- to C-terminus) of seven bladed WD40 β-propellers from COP1, β-TRCP1 (1P22), CDC20 (4GGA) and eight bladed propellers from CDC4 (1NEX), and FBW7 (2OVQ). B. Surface representation of these propeller domains bound to cognate peptides represented as space-filling spheres. The propeller domains are colored according to electrostatic surface potential, and the bound substrates are shown in CPK colors (carbon: green).
Relative binding contributions from Trib1 residues across the binding motif
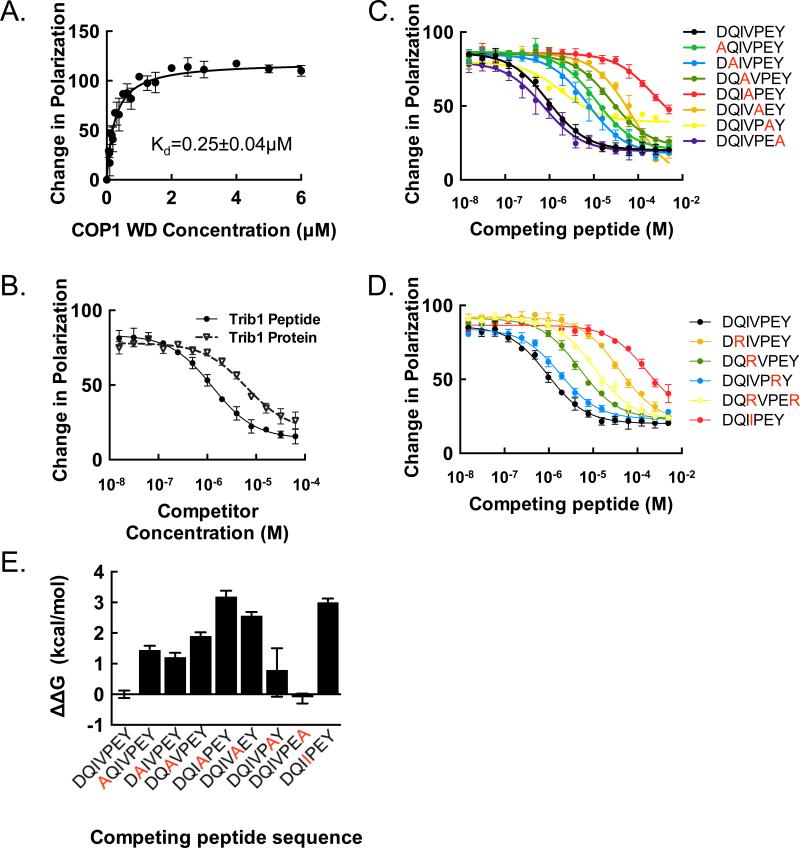
We developed a fluorescence polarization assay to measure the binding affinity of the human COP1 β-propeller for a labeled Trib1 peptide spanning the COP1 binding region. The Trib1 peptide bound with high affinity to COP1 with a Kd of 250 ± 40 nM (Figure 4A). Remarkably, the isolated Trib1 peptide has a stronger IC50 in the competition assay than a Trib1 molecule containing residues 83-372 (Figure 4B). This result is consistent with a recent structure of Trib1 in which the COP1-interacting motif engages in intramolecular interactions with the adjacent pseudokinase domain (Murphy et al., 2015).
Figure 4. Scanning mutagenesis and importance of residue position.
A. Fluorescence polarization assay measuring the binding affinity of the human COP1 β-propeller (376-731) for the Trib1 consensus motif. The change in fluorescence polarization is plotted as a function of COP1 protein concentration. B. Competition assay comparing binding of a Trib1 protein that includes the kinase-homology domain and the COP1-binding motif with the isolated C-terminal peptide. C. Alanine scanning mutagenesis. Displacement of the consensus Trib1 “wild-type” peptide FITC-SEIGTSDQIVPEYQEDSDI was monitored using a fluorescence polarization assay, and the change in polarization plotted for the indicated peptides as a function of unlabeled peptide concentration. D. Competition assay using arginine and isoleucine mutagenesis, using the same fluorescence polarization as in (A). E. Plot of ΔΔG (in kcal/mol) for the alanine scan using the formula ΔΔG = -RTln(i) where i is the ratio of the competing peptide IC50 value to that of the wild-type peptide under the same conditions. Graphs represent ΔΔG derived from triplicate measurements of IC50 values with error bars showing ± SEM calculated using standard propagation of error calculations.
In order to determine which residues from Trib1 contribute most to the affinity for human COP1, we performed alanine and arginine scans across the binding motif using the fluorescence competition assay (Figure 4C-E). The data show that the central valine and proline residues of the motif do indeed provide a large energetic contribution to binding, as anticipated. The glutamine, which is inserted into the donut hole in our structure, is more permissive to alanine substitution than arginine. However, the acidic residue that follows the VP consensus is surprisingly permissive to charge reversal when replaced by arginine (Figure 4D), suggesting that the pool of COP1-binding proteins is likely to be larger than expected based on the existing consensus.
Trib1 competes with substrates for COP1 binding
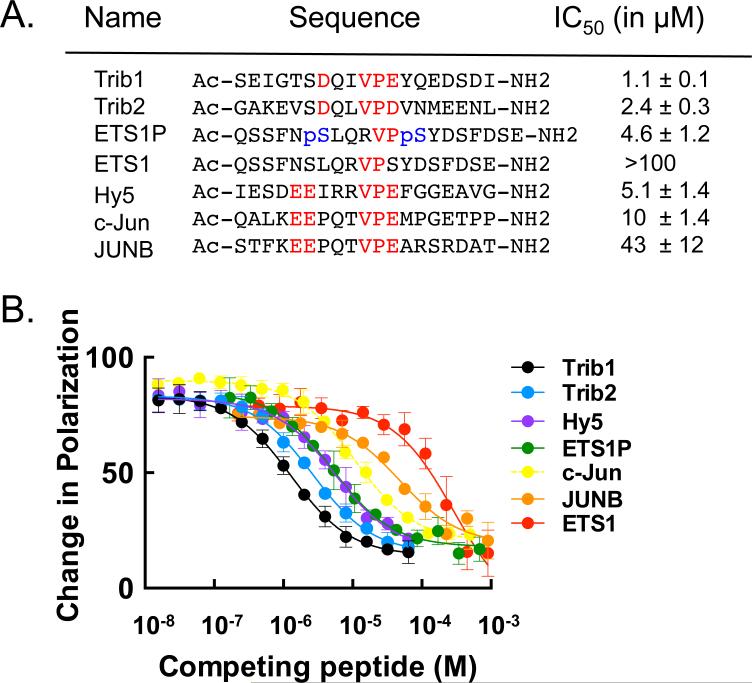
The Trib1 COP1-binding motif shares consensus residues with known ubiquitination targets of COP1 such as the Jun and ETS family transcription factors, suggesting that the Trib1 and Trib2 substrate adaptors likely compete with Jun and ETS for binding to the top face of COP1 (Figure S4). To test this idea directly, we established a fluorescence competition assay with unlabeled Trib1, and assessed whether COP1 substrates do indeed compete with Trib1 for binding to human COP1 (Figure 5). Trib1 competes with all tested substrates for COP1 binding, and the COP1 affinity for Trib1 is severalfold tighter than for Jun- and ETS-family ubiquitination substrates. These results show that the Trib1 binding site is shared by other protein substrates for ubiquitination by COP1.
Figure 5. Trib1 peptide binds human COP1 and competes with COP1 substrates.
A. Peptide sequences used in a competition experiment to compare binding affinities of reported COP1 substrates with binding of the Trib1 peptide. Red text indicates the COP1 consensus binding residues. IC50 values were calculated from the data in (B). Phosphorylated Serine residues are shown in blue in the ETS1P peptide. B. Competition experiment in which Trib2, ETS, and Jun family peptides displace FITC-Trib1 at a fixed human COP1 concentration.
Using the competition assay, we also addressed the effect of phosphorylation on the binding of an ETS family member lacking the consensus COP1-binding motif but reported to be targeted for degradation by COP1 by changes in phosphorylation (Lu et al., 2014). Indeed, a doubly phosphorylated ETS1 peptide (ETS1P, Figure 5A) competes with Trib1 for COP1 binding almost two orders of magnitude more effectively than the unphosphorylated ETS1 peptide (Figure 5B), and more effectively than a consensus-bearing c-Jun peptide. A peptide from the A. thaliana HY5, which shares the consensus motif with the mammalian sequences (Figure S4), also binds to human COP1, which is consistent with the similarities seen in our two structures.
Discussion
COP1 family E3 ubiquitin ligases play central roles in the biology of higher eukaryotes from both plant and animal kingdoms (Ang et al., 1998; Baert et al., 2010; Bianchi et al., 2003; Holm et al., 2002; Kwok et al., 1998; Wang et al., 2001; Wertz et al., 2004). The function of COP1 has been extensively studied in Arabidopsis, where it is a central hub of light response regulated by assembly into larger E3 ligase complexes (Lau and Deng, 2012). A primary activity of COP1 in these diverse species is to regulate transcription factor abundance by degrading molecules such as HY5 in Arabidopsis or ETS, c-Jun, or C/EBPα in mammals. Substrate recognition requires recognition of a binding motif directly, as for HY5, ETS, and c-Jun, or indirectly through an adaptor such as a protein of the Trib family, as for C/EBPα.
Arabidopsis COP1 is incorporated into several multiprotein complexes, including a Cul4-DDB1 E3 ligase where it appears to function in substrate recognition (Chen et al., 2010a). DDB1 serves to connect substrate recognition elements, called DDB1 and Cul4 Associated Factors (DCAFS) to Cul4. The crystal structure of the human Cul4-DDB1 in complex with the DCAF DDB2 shows the interaction to be mediated by two WD40 domains of DDB1 and a short helix-loop-helix at the N-terminus of DDB2 (Fischer et al., 2011). The structures of DDB1 in complex with HLH motifs from other known DCAFs confirm this mode of interaction (Li et al., 2010). In human cells COP1 has been found associated with DDB1 (Qi et al., 2006), raising the possibility that COP1 could function as a DCAF in humans. Structural alignment between DDB2 and the WD40 domain of human COP1 reveals that both propellers are oriented with the N-terminal helix on the opposite side from the substrate binding site, which would be permissive for concurrent binding to DDB1 and substrate. The idea that COP1 might function as a DCAF in Arabidopsis has also been proposed, along with the suggestion that a WDxR motif in Arabidopsis COP1 is involved in the interaction (Chen et al., 2010a). However, our structure shows that residues of this WDxR motif are buried and cannot be accessed without destroying the propeller. Further study will be required to determine if COP1 is a DCAF and, if so, what the function of the COP1 RING domain is in these complexes.
The work presented here reveals that COP1 binds its recognition motif on the Trib1 protein using the top face of its WD40 β-propeller domain. Comparison of the recognition interfaces for several multicomponent E3 ligases with β-propeller domains also shows that the architectural similarity among the various propellers is striking, with substrate specificity dictated by a small number of critical differences on the propeller surface. Among the various propellers, COP1 and βTrCP1 are the most alike. Both are 7-bladed propellers that bind the peptide on the top surface with a similar peptide alignment with respect to the propeller face. The charge profile of the two propellers is also similar (Figure 3B), with both molecules containing positively charged residues between blades 4 and 5, K550 (K600) in COP1 and R431 of βTrCP. These residues are positioned to exert favaroable electrostatic interactions with consensus motifs in their substrates (a conserved pSer, pSer37 in β-catenin for βTrCP, and the consensus D/E of the COP1 binding motif, E360 of human Trib1), even if direct contacts are not present. Trib1 and β-catenin share a second negatively charged residue (D355 in human Trib1 and pSer37 in β-catenin), which pair with analogous residues K422 (K472) and R285, respectively. Indeed, the affinity of the phosphorylated ETS1 peptide for COP1 (Figure 5) suggests that pSer residues readily substitute for D/E residues in COP1 binding (Lu et al., 2014). However, the shape of the binding pockets of COP1 and βTrCP1 are distinct (Figure S3). In addition to its donut hole, COP1 has a separate shallow pocket accommodating the consensus residues valine and proline. Even the addition of a single methyl group in a Val to Ile mutation is not tolerated by the COP1 surface. This constraint is particularly instructive because superimposition of the COP1 and βTrCP1 structures places the Ile of the β catenin peptide closest to the Val of the COP1 consensus site, clashing with COP1 residues S553 (S603) and T568 (T618). Thus, selectivity is achieved by both the shape of the hydrophobic pocket and the charge of the exposed peptide residues. In comparison to COP1 and βTrCP1, Cdc20 has a very shallow binding pocket where the base is formed by an insert between blade 1 and 7 (Figure S3). The insert of the propeller itself (rather than the substrate) occupies the central cavity, leaving only a shallow dimple for substrate binding. The substrate KEN box forms a helix with few contacts to the propeller, and this minimal interface allows for specificity to be determined by the combined engagement of substrates with separate binding sites on the top and the side of the propeller. By contrast, COP1 has a single known site of substrate recognition (Tian et al., 2012). Lastly, the two eight-bladed E3 ligase propeller structures available, Fbw7 and cdc4, bind substrate in an orientation perpendicular to the alignment of the peptides with the 7-bladed propellers described above (Figure 3) (Hao et al., 2007; Orlicky et al., 2003).
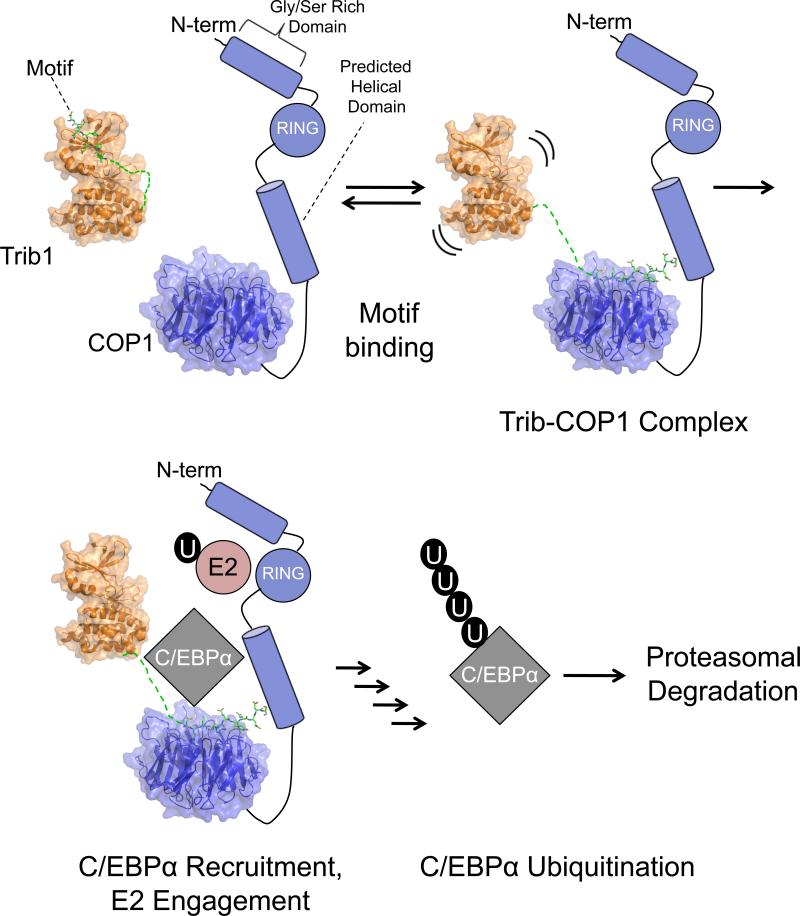
The Trib1 peptide binds to human COP1 at the substrate recognition site with nanomolar affinity (Figure 4A), but there is no evidence that Trib1 is itself a target for COP1-mediated ubiquitination. Instead, current evidence suggests that Tribbles serves to alter COP1 substrate specificity by directing the activity of COP1 toward C/EBPα (Keeshan et al., 2006). A structure of the human Trib1 pseudokinase domain containing the C-terminal COP1 binding peptide, recently published by Murphy and colleagues, shows the C-terminal COP1-binding site tethered to the N-lobe of the Trib1 pseudokinase domain, suggesting that the molecule normally adopts a conformation in which the COP1-binding motif is masked by autoinhibitory interactions with the kinase-homology domain (Murphy et al., 2015). We thus propose the following model for the molecular mechanism of degradation of and other targets by COP1-Trib1 complexes (Figure 6). The Trib1 tail is initially bound to its pseudokinase domain in the resting state, but is released from the pseudokinase domain by a regulatory event, such as phosphorylation of the tail, or by engagement of a substrate such as C/EBPα. The recruited C/EBPα substrate is then ubiquitinated and degraded by the proteosome proteasome dependent pathway. This model is consistent with the requirement for the formation of a Trib1-COP1 complex for C/EBPα ubiquitination and with our observation that the isolated Trib1 peptide has higher affinity for COP1 than does the larger Trib1 protein (Figure 4B).
Figure 6. Model for Trib-COP1 assembly.
Top left: The Trib1 COP1-binding motif is restrained by interaction with the N-lobe in autoinhibitorry interactions. Release of the Trib1 tail from the N-lobe allows it to bind to the COP1 propeller. C/EBPα is then recruited by the assembled complex, and C/EBPα is subsequently ubiquitinated and undergoes proteosome-dependent degradation. The Trib1 models were generated using coordinates with PDB ID codes 5CEK and 5CEM.
Regulation of COP1 substrate specificity by Trib family members also provides a potential explanation for how COP1 can act as a tumor suppressor or promoter depending on context. Although Trib autoinhibition may regulate the creation of Trib-COP1 complexes and subsequent targeting of C/EBPα, the high affinity of Trib1 for the COP1 WD40 domain also has the potential to displace Jun and ETS from COP1, raising the possibility that the formation of the Trib1-COP1 complex may concurrently regulate the abundance of other transcription factors. In plants, which lack Trib proteins, COP1 functions as a repressor of photomorphogenesis, but under UV-B light COP1 promotes photomorphogenesis by assembling with different protein partners (Huang et al., 2013). The extensive literature regarding COP1 in plants can guide future studies of whether and in what context Trib binding regulates the formation of cullin-based COP1 complexes in humans.
Methods
Proteomic studies (Figure S1, Table S1): Tandem-IP experiments
HeLa cell lines were transduced with retroviruses expressing the desired Trib proteins, and stable cell lines were generated by sorting for co-expressed Interleukin-2 receptor (IL2R). For proteomic studies, the resulting cell lines were grown in DMEM with 10% BGS in spinner flasks to a density of 2.5×106 cells/μL and harvested. After dounce homogenization and separation of the nuclear and cytoplasmic fractions by centrifugation, Flag immunoprecipitations were performed using Flag-conjugated agarose beads (Sigma cat #A2220) in a buffer containing 50mM Tris pH 7.5, 150mM NaCl, 1mM EDTA, 0,5% NP40, and 10% glycerol supplemented with EDTA-free protease inhibitor tablets (Roche) and activated sodium orthovanadate. The beads were washed three times and the immunoprecipitated protein was eluted with Flag peptide (Sigma #F3290). The eluate was then subjected to a second streptactin pull-down using similar conditions and eluted with biotin, respectively. Quality control gels were run on the lysates and eluates for each step. Anti-FLAG IPs (C and D). The desired Trib1 protein construct was transduced and selected with antibodies to the IL2R as above. HA-COP1 was transduced with a second retrovirus and the cells were sorted for co-expressed GFP. Anti-Flag IPs were performed as above.
Mass spectrometry analysis of Affinity Purified (TAP) Trib protein complexes
Purified protein complexes were analyzed by mass spectrometry (LC/MS-MS) as recently described (Adelmant et al., 2012) with minor modifications. Protein digestion: Proteins from the TAP samples were directly processed in solution: Cysteine residues were reduced with 10 mM DTT for 30 minutes at 56°C in the presence of 0.1% RapiGest SF (Waters , Milford, MA). Cysteines were then alkylated with 22.5 mM iodoacetamide for 20 minutes at room temperature in the dark. Proteins were digested overnight at 37°C using 5 micrograms of trypsin after adjusting the pH to 8.0 with 1M Tris. The digests were acidified by adding TFA to a final concentration of 1% and desalted by C18 solid phase extraction followed by Strong Cation Exchange (SCX), both performed in batch-mode format. Eluted peptides were concentrated in a vacuum concentrator, and reconstituted with 20 μl of 0.1% TFA. Purified peptides were loaded via autosampler injection (NanoAcquity Sample Manager, Waters, Milford, MA) onto a precolumn (4 cm POROS 10R2, Applied Biosystems, Framingham, MA) and eluted with an HPLC gradient (NanoAcquity Binary Sample Manager, Waters; 0-35% B in 45 minutes; A=0.1 M acetic acid in water, B=0.1M acetic acid in acetonitrile). Peptides were resolved on a self-packed analytical column (12 cm Monitor C18, Column Engineering, Ontario, CA) and introduced into the mass spectrometer (Orbitrap XL, Thermo, Waltham, MA) equipped with a Digital PicoView (New Objective, Woburn, MA) electrospray source platform (ESI voltage = 2.2 kV) (Ficarro et al., 2009). The mass spectrometer was operated in data dependent mode where the top 8 most abundant ions in each MS scan were subjected to CAD (35% normalized collision energy, isolation width = 2.8 Da, threshold = 20,000). Dynamic exclusion was enabled with a repeat count of 1 and exclusion duration of 30 seconds. MS spectra were recalibrated using the background ion (Si(CH3)2O)6 at m/z 445.12 +/− 0.03 and converted into a Mascot generic file format (.mgf) using multiplierz scripts (Askenazi et al., 2009; Parikh et al., 2009). Spectra were searched using Mascot (version 2.4) against three appended databases consisting of: i) human protein sequences (downloaded from RefSeq on 07/11/2011); ii) common lab contaminants and iii) a decoy database generated by reversing the sequences from these two databases. For Mascot searches, precursor tolerance was set to 20 ppm and product ion tolerance to 0.6 Da. Search parameters included trypsin specificity, up to 2 missed cleavages, fixed carbamidomethylation (C, +57 Da) and variable oxidation (M, +16 Da). Spectra matching to peptides from the reverse database were used to calculate a global false discovery rate, and were discarded. Data were further processed to remove peptide spectral matches (PSMs) to the forward database with an FDR greater than 1.0%. For each protein identified we counted only peptides that matched to a single gene. We annotated the final results to indicate the frequency with which a protein was detected on a large compendium of negative TAP controls (Rozenblatt-Rosen et al., 2012).
293T Flag-IPs (Figure 1)
293T cells were grown in standard medium in 30 cm2 dishes. Cells were transfected at 60% confluence with a pcDNA3.0 vector expressing the Flag-Trib1 proteins (Invitrogen) and a pTriEx1.1 vector for the COP1 proteins (Novagen). The cells were lysed after three days. An anti-Flag immunoprecipitation was performed as above and the resulting lysates and eluates were run on a 4-20% gradient gel blotted with anti-Flag (Sigma #A8592) or anti-COP1 (Abcam 56400) antibodies.
Structural studies (Figures 2 and 3, Table 1)
Crystallization: Human constructs: his-tagged COP1 constructs (376-731) and (386-731) were purified from Hi5 cells by metal affinity chromatography using an imidazole gradient followed by ion exchange chromatography on a MonoQ column (GE life sciences). The proteins were buffer-exchanged into crystallization buffer (20mM Tris 8.0, 5% Glycerol, 250mM NaCl, and 2mM TCEP), and concentrated to 10mg/mL. Crystals were obtained using vapor diffusion of the COP1 (376-731) construct at room temperature in 48-well hanging drops under the following conditions: Apo structure: sodium nitrate (0.4-0.6M), 10% PEG 3350, and 0.1M Bis-Tris 5.5-6.5. Cryoprotection was achieved by quick dip into 25% glycerol solution. For human co-crystals, the COP1 (386-731) construct was mixed in a 1.5:1 ratio with the peptide Ac-SEIGTSDQIVPEYQED-NH2 obtained from Lifetein technologies. The crystallization well solution was 0.1M Bis-Tris pH 5.5, 20% glycerol, 5% PEG 5000 MME. Crystals were cryoprotected by slow soaking to a final solvent composition of 30% Ethylene Glycol, 6% Glycerol, 0.1M Bis-Tris 5.9.
For Arabidopsis COP1-peptide co-crystals, a fragment encoding residues (349-675) was subcloned with a hexahistidine tag, expressed in insect Hi5 cells, and purified to homogeneity on SDS-PAGE as above. The complex with peptide (Ac-SDQIVPEY-NH2 obtained from Lifetein technologies) was crystallized in sitting drops using 96-well plate format using a 1.2:1 ratio of peptide to protein. The crystallization well solution was 0.1M Tris 8.5, 16% PEG3350, 2% Tacsimate 8.0. Crystals were cryoprotected by quickly dipping in a mixture of 50% well solution, 10% sucrose, 2% glucose, 9% glycerol, and 9% ethylene glycol, then plunged into liquid nitrogen.
Data collection and refinement
Human COP1 apoprotein: X-ray diffraction data were collected at NE-CAT (Beam ID 24C) at cryogenic temperatures (100 K). The data were indexed with the XDS package and scaled with Aimless as part of the CCP4 package (Collaborative Computational Project, 1994; Kabsch, 2010). The structure was solved by molecular replacement using Phaser with WDR5 (pdb 2H14) as the search model (McCoy et al., 2007). Following Autobuild in Phenix, the structure was refined using Phenix refine (Adams et al., 2010). Arabidopsis COP1-peptide complex: X-ray diffraction data were collected at NE-CAT (Beam ID 24C) at cryogenic temperatures (100 K). The data were indexed with the XDS package and scaled with Aimless as part of the CCP4 package (Collaborative Computational Project, 1994; Kabsch, 2010). The plant COP1-peptide structure was solved by molecular replacement using the unbound human COP1 structure as a search model using Phaser (McCoy et al., 2007). Following Autobuild in Phenix, the structure was refined using Phenix refine (Adams et al., 2010). Human COP1-peptide complex: X-ray diffraction data were collected at NE-CAT (Beam ID 24E) at cryogenic temperatures (100 K). The data were indexed with the XDS package and scaled with Aimless as part of the CCP4 package (Collaborative Computational Project, 1994; Kabsch, 2010). An initial molecular replacement solution was found using the unliganded human COP1 structure as a search model using Phaser (McCoy et al., 2007). In order to further improve the value of R-free and refine the side-chain orientation of the ligand, a second model was constructed by homology modeling using the structural information from the Arabidopsis COP1/peptide crystal. Because all residues at the binding interface are identical, the residues in the binding pocket of the working model of the human complex were manually adjusted and aligned to the Arabidopsis COP1/peptide structure. The resulting model was further refined using Phenix with secondary structure restraints and reference model restraints as well as TLS refinement. The final structure was analyzed and validated by MolProbity (Chen et al., 2010b) Statistics, structure validation and stereochemical quality are reported in Table 1. Graphics figures were rendered in Pymol (Schroedinger software). Conservation analysis was performed using the program Consurf (Ashkenazy et al., 2010).
Fluorescence polarization assay (Figures 4,5)
Fluorescence polarization assays were performed with the purified COP1 propeller (residues 376-731) in buffer containing 0.01 M HEPES pH 7.4, 0.15 M NaCl, and 0.2% v/v Surfactant P20, supplemented with 2 mM TCEP. FITC-labeled and unlabeled, competing peptides were obtained from Lifetein at a purity of >95%, as assessed by reversed-phase high pressure liquid chromatography, and used without further purification. Experiments were conducted at 30μL well volume in 384 well plates and read at 538 nM on a Spectramax M5 plate reader (Molecular Devices). Plots present data from at least three independent experiments. Error bars represent SEM.
Supplementary Material
Highlights.
X-ray structures reported for human and plant COP1 β-propeller - peptide complexes
Trib1 peptides bind to a conserved surface on the top face of the propeller
Structures reveal a universal mechanism for motif recognition by COP1
Competition binding assays confirm importance of consensus VP sequence
Acknowledgments
This work was supported by NIH grant K08 CA166227 (to S.U.), the Samuel Waxman Cancer Research Foundation (W.S.P. and S.C.B.), and a Claudia Adams Barr Award from the Dana Farber Cancer Institute (to S.C.B.). We thank Michael Eck, Eric Fischer and Andrew Kruse for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession numbers
Coordinates of the isolated COP1 β-propeller and of the Arabidopsis and human COP1 - Trib1 complexes have been deposited in the PDB with accession codes 5HQG, 5IGO, and 5IGQ respectively.
Supplemental Information
Supplemental information includes four figures and can be found online at http://zzz.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica. Section D, Biological crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelmant G, Calkins AS, Garg BK, Card JD, Askenazi M, Miron A, Sobhian B, Zhang Y, Nakatani Y, Silver PA, et al. DNA ends alter the molecular composition and localization of Ku multicomponent complexes. Molecular & cellular proteomics : MCP. 2012;11:411–421. doi: 10.1074/mcp.M111.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Molecular cell. 1998;1:213–222. doi: 10.1016/s1097-2765(00)80022-2. [DOI] [PubMed] [Google Scholar]
- Ashkenazy H, Erez E, Martz E, Pupko T, Ben-Tal N. ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic acids research. 2010;38:W529–533. doi: 10.1093/nar/gkq399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askenazi M, Parikh JR, Marto JA. mzAPI: a new strategy for efficiently sharing mass spectrometry data. Nature methods. 2009;6:240–241. doi: 10.1038/nmeth0409-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert JL, Monte D, Verreman K, Degerny C, Coutte L, de Launoit Y. The E3 ubiquitin ligase complex component COP1 regulates PEA3 group member stability and transcriptional activity. Oncogene. 2010;29:1810–1820. doi: 10.1038/onc.2009.471. [DOI] [PubMed] [Google Scholar]
- Bianchi E, Denti S, Catena R, Rossetti G, Polo S, Gasparian S, Putignano S, Rogge L, Pardi R. Characterization of human constitutive photomorphogenesis protein 1, a RING finger ubiquitin ligase that interacts with Jun transcription factors and modulates their transcriptional activity. The Journal of biological chemistry. 2003;278:19682–19690. doi: 10.1074/jbc.M212681200. [DOI] [PubMed] [Google Scholar]
- Chen H, Huang X, Gusmaroli G, Terzaghi W, Lau OS, Yanagawa Y, Zhang Y, Li J, Lee JH, Zhu D, et al. Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. The Plant cell. 2010a;22:108–123. doi: 10.1105/tpc.109.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta crystallographica. Section D, Biological crystallography. 2010b;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HH, Phan L, Chou PC, Su CH, Yeung SC, Chen JS, Lee MH. COP1 enhances ubiquitin-mediated degradation of p27Kip1 to promote cancer cell growth. Oncotarget. 2015;6:19721–19734. doi: 10.18632/oncotarget.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project N. The CCP4 suite: programs for protein crystallography. Acta crystallographica. Section D, Biological crystallography. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Deng XW, Caspar T, Quail PH. cop1: a regulatory locus involved in light- controlled development and gene expression in Arabidopsis. Genes & development. 1991;5:1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- Deng XW, Matsui M, Wei N, Wagner D, Chu AM, Feldmann KA, Quail PH. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell. 1992;71:791–801. doi: 10.1016/0092-8674(92)90555-q. [DOI] [PubMed] [Google Scholar]
- Dornan D, Bheddah S, Newton K, Ince W, Frantz GD, Dowd P, Koeppen H, Dixit VM, French DM. COP1, the negative regulator of p53, is overexpressed in breast and ovarian adenocarcinomas. Cancer research. 2004a;64:7226–7230. doi: 10.1158/0008-5472.CAN-04-2601. [DOI] [PubMed] [Google Scholar]
- Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O'Rourke K, Koeppen H, Dixit VM. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004b;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- Ficarro SB, Zhang Y, Lu Y, Moghimi AR, Askenazi M, Hyatt E, Smith ED, Boyer L, Schlaeger TM, Luckey CJ, et al. Improved electrospray ionization efficiency compensates for diminished chromatographic resolution and enables proteomics analysis of tyrosine signaling in embryonic stem cells. Analytical chemistry. 2009;81:3440–3447. doi: 10.1021/ac802720e. [DOI] [PubMed] [Google Scholar]
- Fischer ES, Scrima A, Bohm K, Matsumoto S, Lingaraju GM, Faty M, Yasuda T, Cavadini S, Wakasugi M, Hanaoka F, et al. The molecular basis of CRL4DDB2/CSA ubiquitin ligase architecture, targeting, and activation. Cell. 2011;147:1024–1039. doi: 10.1016/j.cell.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Molecular cell. 2007;26:131–143. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Holm M, Deng XW. Structural organization and interactions of COP1, a light- regulated developmental switch. Plant molecular biology. 1999;41:151–158. doi: 10.1023/a:1006324115086. [DOI] [PubMed] [Google Scholar]
- Holm M, Hardtke CS, Gaudet R, Deng XW. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. The EMBO journal. 2001;20:118–127. doi: 10.1093/emboj/20.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes & development. 2002;16:1247–1259. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Yang P, Lau OS, Chen L, Wei N, Deng XW. Conversion from CUL4-based COP1-SPA E3 apparatus to UVR8-COP1-SPA complexes underlies a distinct biochemical function of COP1 under UV-B. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16669–16674. doi: 10.1073/pnas.1316622110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. Xds. Acta crystallographica. Section D, Biological crystallography. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeshan K, Bailis W, Dedhia PH, Vega ME, Shestova O, Xu L, Toscano K, Uljon SN, Blacklow SC, Pear WS. Transformation by Tribbles homolog 2 (Trib2) requires both the Trib2 kinase domain and COP1 binding. Blood. 2010;116:4948–4957. doi: 10.1182/blood-2009-10-247361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeshan K, He Y, Wouters BJ, Shestova O, Xu L, Sai H, Rodriguez CG, Maillard I, Tobias JW, Valk P, et al. Tribbles homolog 2 inactivates C/EBPalpha and causes acute myelogenous leukemia. Cancer cell. 2006;10:401–411. doi: 10.1016/j.ccr.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok SF, Solano R, Tsuge T, Chamovitz DA, Ecker JR, Matsui M, Deng XW. Arabidopsis homologs of a c-Jun coactivator are present both in monomeric form and in the COP9 complex, and their abundance is differentially affected by the pleiotropic cop/det/fus mutations. The Plant cell. 1998;10:1779–1790. doi: 10.1105/tpc.10.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends in plant science. 2012;17:584–593. doi: 10.1016/j.tplants.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Li T, Robert EI, van Breugel PC, Strubin M, Zheng N. A promiscuous alpha- helical motif anchors viral hijackers and substrate receptors to the CUL4-DDB1 ubiquitin ligase machinery. Nature structural & molecular biology. 2010;17:105–111. doi: 10.1038/nsmb.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Zhang Q, Huang Y, Song J, Tomaino R, Ehrenberger T, Lim E, Liu W, Bronson RT, Bowden M, et al. Phosphorylation of ETS1 by Src family kinases prevents its recognition by the COP1 tumor suppressor. Cancer cell. 2014;26:222–234. doi: 10.1016/j.ccr.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Schrader A, Kokkelink L, Falke C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hulskamp M, Hoecker U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. The Plant journal : for cell and molecular biology. 2013;74:638–651. doi: 10.1111/tpj.12153. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini D, Bogaerts S, Defever D, Vyas R, Denecker G, Radaelli E, Zwolinska A, Depaepe V, Hochepied T, Skarnes WC, et al. Cop1 constitutively regulates c-Jun protein stability and functions as a tumor suppressor in mice. J Clin Invest. 2011;121:1329–1343. doi: 10.1172/JCI45784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Nakatani Y, Jamieson SA, Dai W, Lucet IS, Mace PD. Molecular Mechanism of CCAAT-Enhancer Binding Protein Recruitment by the TRIB1 Pseudokinase. Structure. 2015 doi: 10.1016/j.str.2015.08.017. [DOI] [PubMed] [Google Scholar]
- Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–256. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Parikh JR, Askenazi M, Ficarro SB, Cashorali T, Webber JT, Blank NC, Zhang Y, Marto JA. multiplierz: an extensible API based desktop environment for proteomics data analysis. BMC bioinformatics. 2009;10:364. doi: 10.1186/1471-2105-10-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science. 2006;312:1763–1766. doi: 10.1126/science.1123374. [DOI] [PubMed] [Google Scholar]
- Rozenblatt-Rosen O, Deo RC, Padi M, Adelmant G, Calderwood MA, Rolland T, Grace M, Dricot A, Askenazi M, Tavares M, et al. Interpreting cancer genomes using systematic host network perturbations by tumour virus proteins. Nature. 2012;487:491–495. doi: 10.1038/nature11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrima A, Konickova R, Czyzewski BK, Kawasaki Y, Jeffrey PD, Groisman R, Nakatani Y, Iwai S, Pavletich NP, Thoma NH. Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell. 2008;135:1213–1223. doi: 10.1016/j.cell.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, Chua NH. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature. 2003;423:995–999. doi: 10.1038/nature01696. [DOI] [PubMed] [Google Scholar]
- Tian W, Li B, Warrington R, Tomchick DR, Yu H, Luo X. Structural analysis of human Cdc20 supports multisite degron recognition by APC/C. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18419–18424. doi: 10.1073/pnas.1213438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitari AC, Leong KG, Newton K, Yee C, O'Rourke K, Liu J, Phu L, Vij R, Ferrando R, Couto SS, et al. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature. 2011;474:403–406. doi: 10.1038/nature10005. [DOI] [PubMed] [Google Scholar]
- Wang H, Ma LG, Li JM, Zhao HY, Deng XW. Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science. 2001;294:154–158. doi: 10.1126/science.1063630. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhang Z, Dornan D, Arnott D, Deshaies RJ, Dixit VM. Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science. 2004;303:1371–1374. doi: 10.1126/science.1093549. [DOI] [PubMed] [Google Scholar]
- Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Molecular cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.