Abstract
Heterozygous hemoglobin S (HbAS), or sickle trait, protects children from life-threatening falciparum malaria, potentially by attenuating binding of Plasmodium-infected red blood cells (iRBCs) to extracellular ligands. Such binding is central to the pathogenesis of placental malaria (PM). We hypothesized that HbAS would be associated with reduced risks of PM and low birth weight (LBW). We tested this hypothesis in 850 delivering women in southern Malawi. Parasites were detected by polymerase chain reaction in placental and peripheral blood, and placentae were scored histologically for PM. The prevalence of HbAS was 3.7%, and 11.2% of infants were LBW (< 2,500 g). The prevalence of Plasmodium falciparum was 12.7% in placental and 8.5% in peripheral blood; 24.4% of placentae demonstrated histological evidence of P. falciparum. HbAS was not associated with reduced prevalence of P. falciparum in placental (odds ratio [OR]: 1.27, 95% confidence interval [CI]: 0.50–3.23, P = 0.61) or peripheral blood (OR: 2.53, 95% CI: 1.08–2.54, P = 0.03), prevalence of histological PM (OR: 0.97, 95% CI: 0.40–2.34, P = 0.95), or prevalence of LBW (OR: 0.82, 95% CI: 0.24–2.73, P = 0.74). Mean (standard deviation) birth weights of infants born to HbAS (2,947 g [563]) and, homozygous hemoglobin A (2,991 g [465]) mothers were similar. Across a range of parasitologic, clinical, and histologic outcomes, HbAS did not confer protection from PM or its adverse effects.
Introduction
Heterozygous hemoglobin S (HbAS), or sickle trait, consistently protects African children from clinical manifestations of falciparum malaria.1–4 Across ethnic groups and transmission settings, HbAS reduces the risk of severe malaria by 91% and the risk of uncomplicated malaria by 31% but offers no protection against asymptomatic parasitemia.2 Despite this consistent biological effect, the underlying mechanisms of protection are incompletely understood. Several hypotheses have been investigated in vitro, including 1) a restriction of parasite growth within HbAS red blood cells (RBCs) owing to increased oxidative damage stress to RBCs5; 2) more rapid clearance and destruction of parasitized HbAS RBCs6; and 3) abnormal or reduced display of the parasite's major cytoadherence ligand and virulence factor, Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1), resulting in attenuated binding of the infected RBCs (iRBCs) to extracellular ligands.2,5,7 Although HbAS offers a model of malaria protection with which to identify mechanisms of parasite pathogenesis, there is a paucity of mechanistic investigations in vivo.
Placental malaria offers a distinct molecular and cellular in vivo model of P. falciparum pathogenesis. In malaria-endemic areas, antenatal P. falciparum infections can ultimately manifest as placental malaria, in which iRBCs sequester in the intervillous space.8,9 This placental sequestration results from the binding of iRBCs to receptors on the placenta such as placental chondroitin sulfate A (CSA). This interaction with CSA is mediated by the expression on the iRBC surface of the conserved PfEMP1 variant VAR2CSA. Expression of VAR2CSA is upregulated in iRBCs selected for adherence to CSA, and attenuation of var2csa expression abrogates adherence to CSA.10–12 Therefore, placental malaria serves as an in vivo model of P. falciparum sequestration.
The aim of this study was to investigate the association between HbAS and placental malaria. Because adherence of iRBCs is critical for placental sequestration in vivo and is attenuated by HbAS in vitro, we hypothesized that HbAS would reduce placental sequestration and therefore the prevalence of placental malaria. In addition, because placental malaria reduces birth weight, we further hypothesized that HbAS would be associated with increased birth weight.
Methods
Study design and population.
A cross-sectional survey of consecutive delivering women was conducted between November 2009 and January 2011 at three health facilities near Blantyre, Malawi. Malaria transmission is perennial in these districts with peaks observed during the monsoon season (November–March). Plasmodium falciparum causes over > 90% of all malaria infections, and Plasmodium malariae and Plasmodium ovale infections have also been reported. Eligible women were those with singleton pregnancies who could provide written informed consent. Women were excluded if they did not reside in the study area. National treatment guidelines were followed to treat both women at delivery, who tested positive for malaria parasites in peripheral blood and infants with parasites in cord blood. The study protocol and consent procedures were approved by the ethical review boards of the University of Malawi College of Medicine and the Liverpool School of Tropical Medicine; testing of de-identified specimens was approved by the University of North Carolina.
After enrollment, demographics, human immunodeficiency virus (HIV) status, and receipt of intermittent preventive treatment in pregnancy (IPTp) with sulphadoxine–pyrimethamine (SP) were collected from antenatal care (ANC) records. A fingerprick blood sample was collected for storage on filter paper as a dried blood spot (DBS). Placental tissue samples were collected for histologic examination of P. falciparum infection and inflammation. At delivery, infant birth weight was recorded to the nearest gram within 24 hours using a calibrated digital scale.
Laboratory procedures.
At delivery, an incision was made on the maternal side of the placenta to collect placental blood, which was then stored as a DBS. Genomic DNA (gDNA) was extracted from placental and peripheral blood DBS using 20% Chelex (BioRad, Hercules, CA) as previously described.13 For histologic analysis, placental tissue was collected by excising from the maternal side a 2 × 2 × 1-cm specimen, which was then placed in formalin and prepared and stained with Gurr's modified Giemsa or hematoxylin and eosin. All placental tissue samples were examined by a trained technician using a standard 5-point scale for placental malaria and inflammation.14
Molecular tests.
All samples were tested for Plasmodium spp. parasites using two real-time polymerase chain reaction (PCR) assays targeting the parasite 18S ribosomal DNA as previously described.15 In brief, samples were first tested by a genus-specific real-time PCR, and all samples testing positive in the pan-species assay were then amplified in a speciation assay testing for P. falciparum. A positive control consisting of P. falciparum strain 3D7 gDNA and a negative control consisting of water was included on each reaction plate. All reactions were run in duplicate on the ABI 7300 Real-Time PCR machine (Applied Biosystems, Foster City, CA). Fluorescent threshold lines were manually set based on the controls to eliminate background noise.
To estimate relative P. falciparum densities, all placental and peripheral blood samples collected from women that tested positive for P. falciparum in either specimen were then tested in a duplex real-time PCR assay targeting the P. falciparum lactate dehydrogenase gene (pfldh) and the human β-tubulin gene. For this assay, we used previously described primers and a FAM-TAMRA probe for pfldh,16 β-tubulin primers (forward: AAG GAG GC GAT GAG CAG AT, reverse: GCT GTC TTG ACA TTG TTG GG) at 400 nM, probe (VIC-TTA ACG TGC AGA ACA AGA ACA GCA GCT-TAMRA) at 600 nM, and 2 μL of gDNA template in a 12 μL reaction; samples were amplified for 40 cycles on a BioRad CFX384 platform on plates. In this assay, samples were tested on plates that included a series of 13 standards tested in duplicate, which contained known quantities of parasites and white blood cells. From these standards, a standard curve was computed for each plate by fitting a regression line to a plot of the known parasite density against the ratio of the cycle quantity (Cq) value for pfldh to that of human β-tubulin. Using these standard curves for each plate, we estimated a parasite density for each peripheral or placental blood sample that was indexed to the amount of human DNA present. With these estimates of parasite densities in placental and peripheral blood, we defined the placental sequestration index as the ratio of the density of parasites in placental blood to that in peripheral blood.
Human β-globin gene (HBB) was typed in all women by amplification and Sanger sequencing. The 5′ end of HBB was amplified from gDNA using a touchdown PCR with previously published primers.17 The PCR reaction mix contained: 5 μL of 5× KAPA HiFi Buffer (KAPA Biosystems, Wilmington, MA), 20 μM of each primer, 10 μM of dNTPs, 0.5 μL of KAPA HiFi HotStart DNA Polymerase (KAPA Biosystems), and 5 μL of template DNA in a 25-μL reaction. The touchdown PCR cycling conditions were as follows: 95°C for 5 minutes, 40 cycles of 98°C for 20 seconds, 75–60°C for 15 seconds (−1°C per cycle during first 15 cycles, then 60°C for 25 cycles), and 72°C for 30 seconds, and a final extension of 72°C for 3 minutes. PCR products were visualized on a 2% agarose gel and bidirectionally Sanger sequenced. Samples that were negative were re-amplified and sequenced. Nucleotide sequences and chromatograms were aligned in Sequencher (version 4.8; Gene Codes Corp., Ann Arbor, MI) and scored manually for the A → T substitution in codon 6 that encodes HbS.
Data analysis.
We examined associations between sickle trait and placental malaria and adverse pregnancy outcomes such as low birth weight (LBW) in our cross-sectional cohort. We calculated odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) between HbAS and outcomes, which included placental and peripheral malaria and birth weight. Continuous variables (birth weight and parasite density) were compared using Mann–Whitney test if they were not normally distributed. Otherwise, for normally distributed variables, Student's t tests were used. For categorical variables (placental malaria, peripheral malaria, histological evidence of malaria, LBW, and gravidity), in addition to ORs, differences in proportions were tested using a χ2 test. An α of 0.05 was determined a priori to test for significant associations.
Since the exposure of interest in this study is a product of Mendelian randomization, crude prevalence ORs would sufficiently approximate the causal effect of the exposure on the outcome. In this case, no measured covariates (maternal age, gravidity, and HIV status) confound the relationship between the exposure and outcome; hence, bivariate analysis would estimate the association of interest. We also examined known associations between pregnancy and malaria. Using multivariable logistic regression to control for known confounders, we estimated the association between birth weight and malaria after controlling for maternal age, HIV status, and gravidity along with association between gravidity and malaria after controlling for maternal age and HIV status. All data were first entered into Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) and ultimately analyzed in SAS (v9.2.2; SAS Institute Inc., Cary, NC) or R (v3.1; The R Foundation, Vienna, Austria).
Results
Study population.
Of 1,206 enrolled women who delivered in the parent IPTp study (Table 1), 854 were successfully genotyped for HBB; and for 850 of these women, peripheral and placental blood parasite testing and infant birth weight were available. These women constituted the analytical population; among these, complete placental histology readings were available for 753 women. Among the 850 women, 3.7% (N = 32) were HbAS and 0.5% (N = 4) were homozygous hemoglobin S (HbSS). Overall, 28.8% (N = 245) were primigravid and 9.3% (N = 79) were HIV positive. Of the 245 primigravid women, 4% (N = 10) were HbAS, and of 79 HIV-positive women, 5% were HbAS.
Table 1.
Description of the study population (N = 850)
| Age, years, mean (SD) | 24.7 (5.7) |
| Gravidity, n (%) | |
| Primigravid | 245 (28.8) |
| Secundigravid | 182 (21.4) |
| Multigravid | 423 (49.8) |
| HIV-positive, n (%) | 79 (9.3) |
| Birth weight, mean (SD) | 2,989.9 (469.2) |
| Low birth weight,* n (%) | 95 (11.2) |
| Plasmodium falciparum prevalence, n (%) | |
| Peripheral blood | 71 (8.35) |
| Placental blood | 108 (12.7) |
| Any histological evidence of placental malaria (N = 753), n (%) | 207 (27.5) |
SD = standard deviation.
Defined as < 2,500 g.
Malaria, birth weight, and gravidity.
The overall prevalence detected by the presence of P. falciparum via real-time PCR was 8.3% (N = 71) in peripheral blood and 12.7% (N = 108) in placental blood. Placental malaria, defined as any histological evidence of P. falciparum, was present in 27.5% (N = 207) (Table 1). The mean birth weight of newborns was 2,990 g (standard deviation [SD] = 469.15); 11.2% (N = 95) of newborns were LBW (< 2,500 g) (Table 2).
Table 2.
Associations between HbAS and delivery outcomes
| HbAS (N = 32) | HbAA (N = 814) | OR* (95% CI) | P value† | |
|---|---|---|---|---|
| Real-time PCR testing | ||||
| Plasmodium falciparum positive, n (%) | ||||
| Peripheral blood | 6 (18.8) | 65 (7.7) | 2.53 (1.08–5.94) | 0.03 |
| Placental blood | 5 (15.6) | 103 (12.6) | 1.27 (0.50–3.23) | 0.61 |
| P. falciparum density (N = 76), parasites/μL, median (IQR)‡ | ||||
| Peripheral blood | 2,467 (552–7,736) | 6,118 (2,195–34,241) | NA | 0.14 |
| Placental blood | 5,683 (5,492–10,850) | 7,008 (1,863–22,133) | NA | 0.85 |
| Placental sequestration index§ | 1.21 | 0.99 | NA | 0.37 |
| Histology, n (%) (N = 753) | ||||
| Any evidence of infection | 7 (26.9) | 200 (27.5) | 0.97 (0.40–2.34) | 0.95 |
| Past infection | 3 (11.5) | 90 (12.4) | ||
| Chronic infection | 4 (15.4) | 58 (8) | ||
| Acute infection | 0 | 52 (7.15) | ||
| No infection | 19 (73.1) | 527 (72.5) | ||
| Birth outcomes | ||||
| Low birth weight, n (%) | 3 (9.4) | 92 (11.3) | 0.82 (0.24–2.73) | 0.74 |
| Birth weight, g, mean (SD) | 2,947 (563) | 2,991 (465) | NA | 0.67 |
CI = confidence interval; HbAA = homozygous hemoglobin A; HbAS = heterozygous hemoglobin S; IQR = interquartile range; NA = not applicable; OR = odds ratio; PCR = polymerase chain reaction; SD = standard deviation.
Unadjusted OR; reference group for each comparison was women with HbAA.
Computed using the Student's t test or Mann–Whitney test for continuous outcomes and χ2 test for categorical outcomes.
Estimated using real-time PCR on only women with concurrent peripheral and placental infection.
Defined as the ratio of the density of parasites in placental blood to that in peripheral blood.
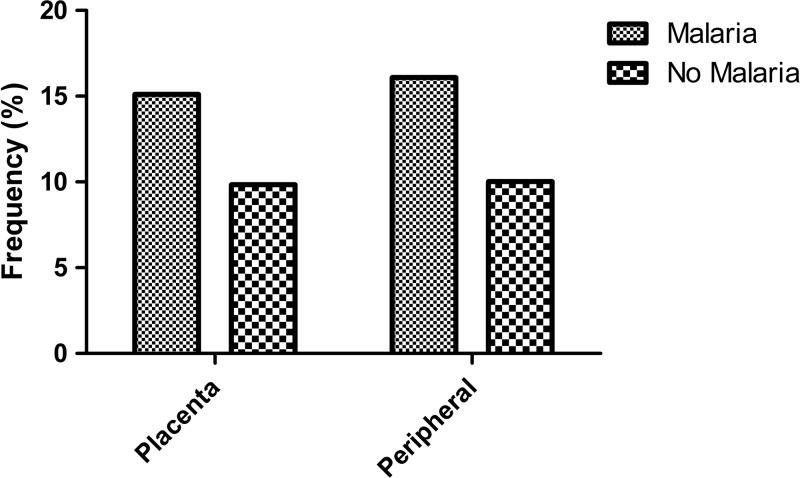
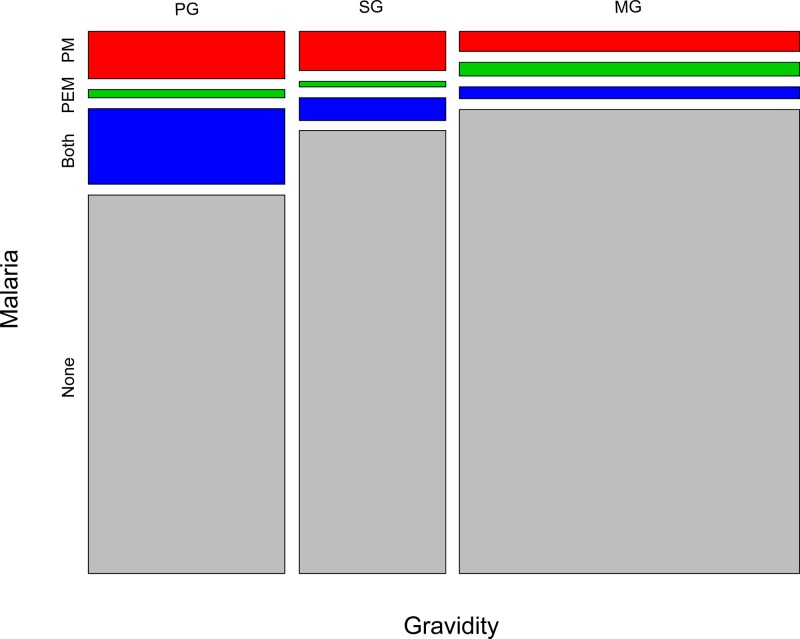
We first examined relationships between P. falciparum, LBW, and gravidity. As expected, P. falciparum parasitemia at delivery was associated with LBW. In analyses adjusted for gravidity, maternal age, and HIV infection compared with aparasitemic women, the prevalence of LBW was higher among infants born to women with P. falciparum in either peripheral blood (OR: 1.54, 95% CI: 0.79–2.99, P = 0.20) or placental blood (OR: 1.37, 95% CI: 0.77–2.45, P = 0.29) (Figure 1 ). In addition, compared with multigravidae and after adjusting for maternal age and HIV infection, P. falciparum infection in the placenta was more common in both primigravidae (OR: 1.64, 95% CI: 0.98–2.75, P = 0.06) and secundigravidae (OR: 1.20, 95% CI: 0.59–2.48, P = 0.61) (Figure 2 ). Associations of similar magnitude and direction were observed between gravidity and P. falciparum infection in the peripheral blood.
Figure 1.
Distribution of low birth weight according to maternal malaria infection in the placental and peripheral compartments via polymerase chain reaction.
Figure 2.
Distribution of maternal malaria infection in the placental (PM) and peripheral (PEM) compartments via polymerase chain reaction according to gravidity: primigravidae (PG) (N = 245) vs. secundigravidae (SG) (N = 182) vs. multigravidae (MG) (N = 423).
Effect of HbAS on placental malaria and birth outcomes.
We next quantified the associations between HbAS and placental malaria or LBW. The prevalence of P. falciparum in placental blood detected by real-time PCR was similar in HbAS (15.6%) and homozygous hemoglobin A (HbAA; 12.6%) women (OR: 1.27, 95% CI: 0.50–3.23, P = 0.61). Similarly, the prevalence of any histological evidence of acute, chronic, or past placental malaria was similar between HbAS (26.9%) and HbAA (27.5%) women (OR: 0.97, 95% CI: 0.40–2.34, P = 0.95) (Table 2).
There was a significantly higher prevalence by real-time PCR of peripheral P. falciparum in HbAS (20%) than HbAA (8%) women (OR: 2.53, 95% CI: 1.08–5.94, P = 0.03) (Table 2). We hypothesized that HbAS may permit peripheral parasite propagation while specifically preventing placental sequestration, and we tested this by quantifying the ratio of parasite densities in the placental and peripheral blood in women who were concurrently infected in both compartments. After quantifying parasite densities in matched blood samples from 71 HbAA and five HbAS women and computing placental density ratios, there was no evidence of reduced placental sequestration in HbAS women (Table 2).
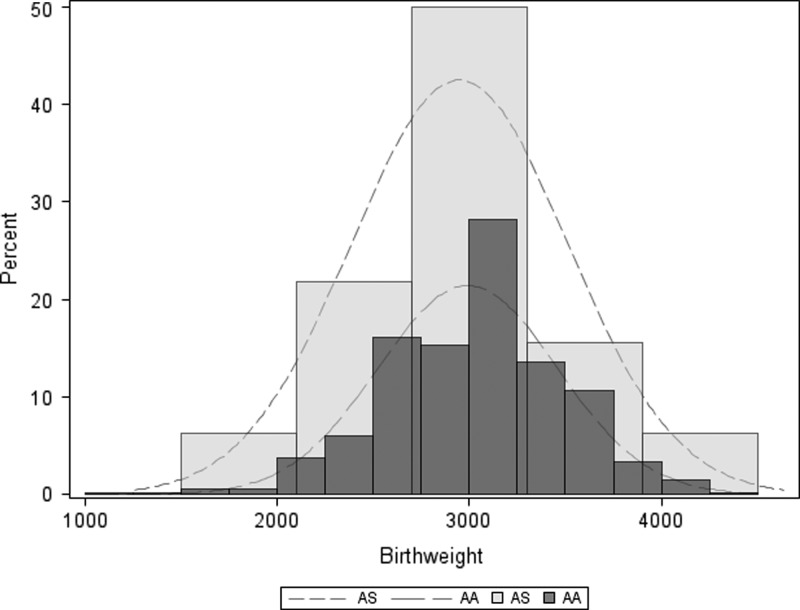
The prevalence of LBW was similar among newborns born to HbAS (9.4%) and HbAA (11.3%) women (OR: 0.82, 95% CI: 0.24–2.73, P = 0.74) (Table 2). Similarly, mean (SD) birth weights were similar in newborns born to HbAS (2,947 g [563]) and HbAA (2,991 g [465]) women, and distributions of birth weight did not appear to differ by the mother's HBB genotype (Figure 3 ).
Figure 3.
Kernel density plots of infant birth weight by sickle cell trait (heterozygous hemoglobin S [AS] vs. homozygous hemoglobin A [AA]).
Discussion
In this cross-sectional study of delivering women in Malawi, HbAS did not confer protection from placental malaria or from its consequences. There were no significant differences between women with and without HbAS on placental or peripheral P. falciparum prevalence, placental parasite density, histological evidence of placental infection or inflammation, or birth weight. Although the low prevalence of HbAS undoubtedly limited our ability to detect differences, the absence of any apparent effect across a broad range of parasitological, histological, and clinical outcomes suggests that HbAS likely does not affect the ability of P. falciparum-infected RBCs to accumulate in the placenta.
We hypothesized that HbAS would reduce the risk of placental malaria. This hypothesis was predicated on in vitro evidence that HbAS attenuates the binding of infected RBCs to microvascular endothelium, possibly by quantitatively or qualitatively affecting the expression of PfEMP1 on the iRBC surface.18–20 Because the conserved PfEMP1 protein VAR2CSA is the principal parasite ligand that enables placental sequestration of iRBCs in vivo,8,10 HbAS could be expected to also disrupt iRBC binding to CSA in the syncytiotrophoblast. However, we found no evidence of these effects.
Why did HbAS fail to prevent placental malaria? Aberrant expression of PfEMP1 on the HbAS iRBC surface might still permit CSA binding owing to the lower flow conditions of the intervillous space (relative to the microvasculature)21 or to the high affinity of VAR2CSA for CSA.22 HbAS may restrict the expression only of specific PfEMP1 variants on the iRBC surface while permitting VAR2CSA expression, although this is not supported by recent evidence that a shared parasite protein export machinery traffics VAR2CSA as well as other surface antigens to the erythrocyte membrane.23 Alternatively, aberrant PfEMP1 expression may be less disruptive to the specific VAR2CSA–CSA interaction than it is to the binding of PfEMP1 to a myriad of endothelial receptors. Finally, there may broadly exist differences in quality between iRBC binding to CSA and to endothelium, with the latter characterized by varieties of receptors and parasite ligands as well as an active dialogue between iRBC and activated endothelium.
There was no clear association between maternal HbAS and birth weight (Table 2 and Figure 3). Although the prevalence of LBW was slightly lower among newborns born to women with HbAS (9.4%) than with HbAA (11.3%) (Table 2), this difference was not statistically significant, and mean birth weight overall was 44 g lower in births to women with HbAS (Figure 3). Prior studies of the association between HbAS and birth weight have yielded mixed results: in some studies, reduced birth weight is more frequent in newborns born to HbAS women,24–26 while in others there was no difference between women with HbAS and HbAA,27–31 including in two Nigerian studies in malaria-endemic settings.32,33 Taken together with our data, HbAS does not appear to consistently impact newborn birth weight, and nor does this relationship appear to be modified by malaria endemicity.
Consistent with prior studies, placental malaria was associated with first pregnancy and with lower birth weight (Figure 2). The higher susceptibility of primigravidae to placental malaria is well described,34 as is the association between placental parasite infection and LBW (Figure 1).
Our study has several potential limitations. The low prevalence of the HbS allele limited our ability to detect small differences in outcomes; to mitigate the risk of a type II error, we used a large patient cohort and analyzed a broad range of outcomes. We could not assess ex vivo phenotypes of placental or CSA binding of the placental parasites, and therefore we cannot directly measure the effect of HbAS on placental sequestration. Similarly, we cannot confirm that the concentration of CSA in the syncytiotrophoblast is unaffected by HbAS. Finally, our analyses of birth weight may have been confounded by newborn genotype, because newborn HbSS is associated with LBW; however, the low HbS allele prevalence suggests that few offspring harbored HbSS.
In our cross-sectional study of delivering women in a high-transmission setting in Malawi, HbAS was not associated with a reduced risk of placental malaria or LBW. Although HbAS confers substantial protection from severe malaria in children and reduces cytoadherence in vitro, these data suggest that HbAS does not attenuate the ability of infected RBCs to accumulate in the placenta.
ACKNOWLEDGMENTS
We thank Rick Fairhurst (NIAID) and Stephanie Doctor (University of North Carolina) for helpful discussion and laboratory assistance. Ultimately, we are indebted to the women who participated in the study.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by the Malaria in Pregnancy Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine (to Feiko O. ter Kuile), and by the National Institute of Allergy and Infectious Diseases under award no. K08AI100924 (to Steve M. Taylor).
Authors' addresses: Jaymin C. Patel, Kyaw L. Thwai, and Steven R. Meshnick, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, E-mails: jaymin.patel@unc.edu, thwai@email.unc.edu, and meshnick@unc.edu. Victor Mwapasa, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Blantyre, Malawi, and Department of Community Medicine, College of Medicine, Blantyre, Malawi, E-mail: vmwapasa69@gmail.com. Linda Kalilani, Department of Community Medicine, College of Medicine, Blantyre, Malawi, E-mail: lkalilani@hotmail.com. Feiko O. ter Kuile and Carole Khairallah, Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, United Kingdom, E-mails: feiko.terkuile@lstmed.ac.uk and carole.khairallah@liverpool.ac.uk. Steve M. Taylor, Division of Infectious Diseases and International Health and Duke Global Health Institute, Duke University Medical Center, Durham, NC, and Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, E-mail: steve.taylor@duke.edu.
References
- 1.Taylor SM, Cerami C, Fairhurst RM. Hemoglobinopathies: slicing the Gordian knot of Plasmodium falciparum malaria pathogenesis. PLoS Pathog. 2013;9:e1003327. doi: 10.1371/journal.ppat.1003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:457–468. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams TN, Obaro SK. Sickle cell disease and malaria morbidity: a tale with two tails. Trends Parasitol. 2011;27:315–320. doi: 10.1016/j.pt.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Aidoo M, Terlouw DJ, Kolczak MS, McElroy PD, ter Kuile FO, Kariuki S, Nahlen BL, Lal AA, Udhayakumar V. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–1312. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 5.Bunn HF. The triumph of good over evil: protection by the sickle gene against malaria. Blood. 2013;121:20–25. doi: 10.1182/blood-2012-08-449397. [DOI] [PubMed] [Google Scholar]
- 6.Ayi K, Turrini F, Piga A, Arese P. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood. 2004;104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 7.Cabrera G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJ. The sickle cell trait is associated with enhanced immunoglobulin G antibody responses to Plasmodium falciparum variant surface antigens. J Infect Dis. 2005;191:1631–1638. doi: 10.1086/429832. [DOI] [PubMed] [Google Scholar]
- 8.Brabin BJ, Romagosa C, Abdelgalil S, Menéndez C, Verhoeff FH, McGready R, Fletcher KA, Owens S, d'Alessandro U, Nosten F, Fischer PR, Ordi J. The sick placenta—the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 10.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 11.Sander AF, Salanti A, Lavstsen T, Nielsen MA, Theander TG, Leke RG, Lo YY, Bobbili N, Arnot DE, Taylor DW. Positive selection of Plasmodium falciparum parasites with multiple var2csa-type PfEMP1 genes during the course of infection in pregnant women. J Infect Dis. 2011;203:1679–1685. doi: 10.1093/infdis/jir168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogerson SJ, Mwapasa V, Meshnick SR. Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am J Trop Med Hyg. 2007;77:14–22. [PubMed] [Google Scholar]
- 13.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 14.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 15.Taylor SM, Messina JP, Hand CC, Juliano JJ, Muwonga J, Tshefu AK, Atua B, Emch M, Meshnick SR. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One. 2011;6:e16420. doi: 10.1371/journal.pone.0016420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rantala AM, Taylor SM, Trottman PA, Luntamo M, Mbewe B, Maleta K, Kulmala T, Ashorn P, Meshnick SR. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J. 2010;9:269. doi: 10.1186/1475-2875-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modiano D, Luoni G, Sirima BS, Simpore J, Verra F, Konate A, Rastrelli E, Olivieri A, Calissano C, Paganotti GM, D'Urbano L, Sanou I, Sawadogo A, Modiano G, Coluzzi M. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–308. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 18.Fairhurst RM, Fujioka H, Hayton K, Collins KF, Wellems TE. Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: implications for the malaria protective effect of the homozygous state. Blood. 2003;101:3309–3315. doi: 10.1182/blood-2002-10-3105. [DOI] [PubMed] [Google Scholar]
- 19.Fairhurst RM, Baruch DI, Brittain NJ, Ostera GR, Wallach JS, Hoang HL, Hayton K, Guindo A, Makobongo MO, Schwartz OM, Tounkara A, Doumbo OK, Diallo DA, Fujioka H, Ho M, Wellems TE. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature. 2005;435:1117–1121. doi: 10.1038/nature03631. [DOI] [PubMed] [Google Scholar]
- 20.Cholera R, Brittain NJ, Gillrie MR, Lopera-Mesa TM, Diakite SA, Arie T, Krause MA, Guindo A, Tubman A, Fujioka H, Diallo DA, Doumbo OK, Ho M, Wellems TE, Fairhurst RM. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci USA. 2008;105:991–996. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhao S. Vascular Biology of the Placenta. Integrated Systems Physiology: From Molecules to Function to Disease. San Rafael, CA: Morgan and Claypool Life Sciences; 2010. [PubMed] [Google Scholar]
- 22.Srivastava A, Gangnard S, Round A, Dechavanne S, Juillerat A, Raynal B, Faure G, Baron B, Ramboarina S, Singh SK, Belrhali H, England P, Lewit-Bentley A, Scherf A, Bentley GA, Gamain B. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci USA. 2010;107:4884–4889. doi: 10.1073/pnas.1000951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elsworth B, Matthews K, Nie CQ, Kalanon M, Charnaud SC, Sanders PR, Chisholm SA, Counihan NA, Shaw PJ, Pino P, Chan JA, Azevedo MF, Rogerson SJ, Beeson JG, Crabb BS, Gilson PR, de Koning-Ward TF. PTEX is an essential nexus for protein export in malaria parasites. Nature. 2014;511:587–591. doi: 10.1038/nature13555. [DOI] [PubMed] [Google Scholar]
- 24.Larrabee KD, Monga M. Women with sickle cell trait are at increased risk for preeclampsia. Am J Obstet Gynecol. 1997;177:425–428. doi: 10.1016/s0002-9378(97)70209-6. [DOI] [PubMed] [Google Scholar]
- 25.Roopnarinesingh S, Ramsewak S. Decreased birth weight and femur length in fetuses of patients with the sickle-cell trait. Obstet Gynecol. 1986;68:46–48. [PubMed] [Google Scholar]
- 26.Brown S, Merkow A, Wiener M, Khajezadeh J. Low birth weight in babies born to mothers with sickle cell trait. JAMA. 1972;221:1404–1405. [PubMed] [Google Scholar]
- 27.Baill IC, Witter FR. Sickle trait and its association with birthweight and urinary tract infections in pregnancy. Int J Gynaecol Obstet. 1990;33:19–21. doi: 10.1016/0020-7292(90)90649-6. [DOI] [PubMed] [Google Scholar]
- 28.Blattner P, Dar H, Nitowsky HM. Pregnancy outcome in women with sickle cell trait. JAMA. 1977;238:1392–1394. [PubMed] [Google Scholar]
- 29.Stamilio DM, Sehdev HM, Macones GA. Pregnant women with the sickle cell trait are not at increased risk for developing preeclampsia. Am J Perinatol. 2003;20:41–48. doi: 10.1055/s-2003-37953. [DOI] [PubMed] [Google Scholar]
- 30.Tan TL, Seed P, Oteng-Ntim E. Birthweights in sickle cell trait pregnancies. BJOG. 2008;115:1116–1121. doi: 10.1111/j.1471-0528.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- 31.Tuck SM, Studd JW, White JM. Pregnancy in women with sickle cell trait. Br J Obstet Gynaecol. 1983;90:108–111. doi: 10.1111/j.1471-0528.1983.tb08892.x. [DOI] [PubMed] [Google Scholar]
- 32.Adeyemi AB, Adediran IA, Kuti O, Owolabi AT, Durosimi MA. Outcome of pregnancy in a population of Nigerian women with sickle cell trait. J Obstet Gynaecol. 2006;26:133–137. doi: 10.1080/01443610500443428. [DOI] [PubMed] [Google Scholar]
- 33.Okonofua FE, Odutayo R, Onwudiegwu U. Maternal sickle cell trait is not a cause of low birthweight in Nigerian neonates. Int J Gynaecol Obstet. 1990;32:331–333. doi: 10.1016/0020-7292(90)90110-7. [DOI] [PubMed] [Google Scholar]
- 34.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]