Abstract
Notifications of amebiasis have been increasing in Japan. Using national surveillance data during 2000–2013, reported cases of amebiasis were analyzed. A case of amebiasis was defined as laboratory-confirmed Entamoeba histolytica infection, regardless of presence of symptoms. We described temporal trends and analyzed correlates of asymptomatic versus symptomatic cases based on odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression. Of 9,946 cases reported during 2000–2013, 7,403 were domestic cases. During this period, the proportion of domestic cases increased from 63% to 85%. Among male cases, majority were middle aged, and from 2008, the number of cases attributed to heterosexual contact surpassed that of homosexual contact. During 2010–2013, increase in notifications was associated with asymptomatic cases, colonoscopy diagnosis, and males with unknown or heterosexual route of infection. Among males, colonoscopy (OR = 31.5; 95% CI = 14.0–71.0) and cases with unknown route of infection, relative to homosexual contact (OR = 2.2; 95% CI = 1.3–3.9), were associated with asymptomatic infections in multivariate analysis. Although the recent rise may have been due to enhanced detection by colonoscopy or reporting, the large number of asymptomatic cases, with reportedly unknown or heterosexual route of infection, has led to a better understanding of amebiasis in Japan and highlights the potential public health concern.
Introduction
Amebiasis is caused by Entamoeba histolytica infection, and invasive amebiasis is the fourth leading cause of death and the third leading cause of morbidity due to protozoan infections worldwide, resulting in ∼70,000 deaths each year.1,2 Most amebiasis are asymptomatic or cause mild disease. However, 4–10% of patients develop invasive amebiasis that includes extraintestinal infection, which may result in severe and potentially fatal illness.1,3–6 Also, substantial health-care costs are necessary to care for invasive amebiasis.7 Recent studies have reported that invasive amebiasis is prevalent not only in developing countries due to contaminated food or water, but also in developed areas of east Asia (e.g., China, Japan, Korea, and Taiwan) and Australia as a sexually transmitted infection via oral–anal sexual contact, particularly among men who have sex with men (MSM).8–10
Amebiasis is a nationally notifiable disease in Japan, which is one of the few places, along with Taiwan, to conduct national surveillance for amebiasis.11 Physicians are required to report all cases of amebiasis to the National Epidemiological Surveillance of Infectious Diseases (NESID) system in Japan within 7 days of case diagnosis. Until recently, most reports of amebiasis in Japan were associated with travel to areas with poor hygiene and sanitation. However, the notification rate of domestically acquired amebiasis has been increasing according to data from NESID.12,13 And, for domestically acquired infections, while MSM have been known to be a high risk group,14–16 recently, there have been clinical reports of non-MSM, particularly mild cases, diagnosed by colonoscopy in Japan.17–19
Therefore, to assess the possible reasons for the recent increase in domestically acquired infections, our objective in this study was to describe the recent epidemiology of domestic amebiasis in Japan, with a particular focus on potential risk groups.
Materials and Methods
Study design.
This is a retrospective, exploratory, mostly descriptive epidemiologic study, based on national surveillance data and national population statistics. We assessed the temporal trend and demographic characteristics of notifications of domestic cases of amebiasis between 2000 and 2013, particularly focusing on the period between 2010 and 2013 when a large increase was observed. Furthermore, we compared the characteristics of asymptomatic and symptomatic cases during 2010–2013.
Data sources.
In compliance with the Act on Prevention of Infectious Diseases and Medical Care for Patients Suffering Infectious Diseases (the Infectious Diseases Control Law) enacted in April 1999, amebiasis was classified as a category IV notifiable infectious disease, which was then changed to a category V notifiable infectious disease by the partial amendment of the Infectious Diseases Control Law in November 2003. Since April 2006, notification of amebiasis requires clarification of the disease type, that is, intestinal and/or extraintestinal amebiasis, and since February 2011, clinicians have been able to select “colon lesion” (detected primarily by colonoscopy) from a list of clinical manifestations (previously recorded as free text by clinicians).
Notification rates were calculated using national population statistics from Population Survey Reports of the Ministry of Health, Labour and Welfare, Japan, between 2000 and 2013, where the population was estimated as of October 1 each year.20
Criteria for case reporting.
Diagnosis can be made by any of the following four detection methods in stool, colonic mucosa, or abscess fluid samples: detection of 1) trophozoites or cysts by visual detection (microscopy), 2) specific antigen by enzyme-linked immunosorbent assay (ELISA), 3) E. histolytica DNA by real-time polymerase chain reaction (PCR), and 4) serum antibodies. Standardized methods for laboratory tests are provided to local public health centers under the guidance of the National Institute of Infectious Diseases (NIID). For ELISA and serology, specific positive and negative controls are recommended to local public health centers under the guidance of NIID. For PCR, this guidance recommends the single round PCR assay for which primers were designed based on the reported E. histolytica and Entamoeba dispar small-subunit rRNA gene sequences, and have been shown to specifically differentiate each other.21 Confirmation by any of the above four methods are considered to be laboratory-confirmed E. histolytica infection in Japan,22,23 and such cases reported to NESID from 2000 to 2013 were included in this study.
The following data regarding cases were extracted from the NESID database: demographics, travel history, suspected route of infection, diagnosis method, clinical manifestations, and disease type (intestinal and/or extraintestinal). Considering the age at which colonoscopy becomes common for health screening purposes, we categorized age dichotomously at 50 years, as previously analyzed.15 Cases were defined as domestic or imported based on the judgement of the physicians who treated the cases, according to the patients' reported travel history. For the suspected route of infection, physicians could choose from the following five routes: 1) homosexual (sexual contact with someone of the same gender), 2) heterosexual (sexual contact with someone of the opposite gender), 3) sexual unspecified (indicated as sexual infection but not specified as homosexual or heterosexual), 4) nonsexual (infection attributed to contaminated food or water), and 5) unknown (none of (1) through (4) apply). In this study, cases with specific colonic mucosa lesions, such as erythema, edematous mucosa, erosion, white exudates, and ulcers,15,17 but otherwise asymptomatic and reported through NESID, were classified as asymptomatic cases.
Statistical analysis.
The number and rate of notifications and the distribution of characteristics from 2010 to 2013 were described. Using logistic regression, univariate analysis using odds ratio (OR) and 95% confidence intervals (CIs) was used to compare characteristics between asymptomatic and symptomatic cases, stratified by sex. Variables with a P value of < 0.2 in univariate analysis or hypothesized to be a priori clinically or epidemiology important were included in a multivariate model.
Statistical significance was defined at two-sided P values of < 0.05. All statistical analyses were performed with SPSS version 18 (SPSS Inc., Chicago, IL).
Ethics.
No ethical approval was necessary because this study was conducted for public health purposes using national surveillance data.
Results
Description of domestic cases between 2000 and 2013.
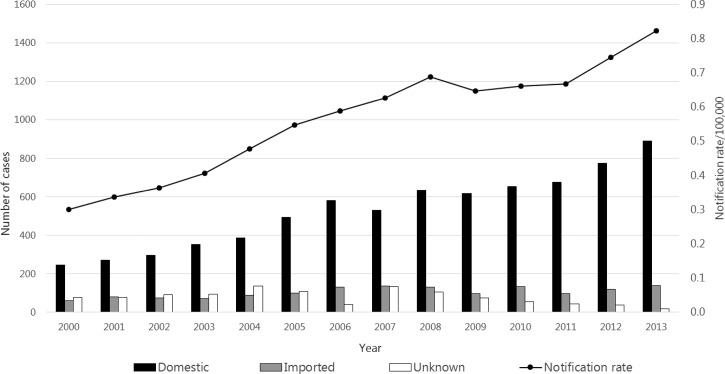
A total of 9,946 cases of amebiasis, including 7,403 domestic cases, were reported during 2000–2013. The annual notification rate rose steadily from 0.30/100,000 in 2000 (N = 381) to 0.82/100,000 in 2013 (N = 1,047) (Figure 1 ). The methods used most commonly for confirmation were detection of trophozoites or cysts (7,971/9,946 [80.1%]) and serum antibodies (2,728/9,946 [27.4%]) (includes cases confirmed by more than one diagnosis method).
Figure 1.
Number of reported domestic and imported cases of amebiasis and notification rate per 100,000 in Japan, 2000–2013 (N = 9,946). Bars express number of cases from suspected area of infection: black indicates domestic cases, gray imported cases, and white unknown cases. Line expresses notification rate/100,000 of amebiasis.
During 2000–2013, the absolute number and proportion of domestic cases increased considerably from 246 (64.5%) to 889 (84.9%). For domestic cases reported during 2000–2013, the majority were male, ranging from 85.3% in 2007 (N = 452) to 90.6% in 2003 (N = 319). During 2000–2013, among males, the proportion of cases recorded as unknown route of infection (ranging from 44.3% to 58.7%) was consistently higher than those recorded as sexually acquired (ranging from 29.5% to 41.9%). For females, the proportion of cases recorded as unknown route of infection ranged from 34.6% to 57.7% and sexually acquired cases ranged from 23.1% to 44.0%.
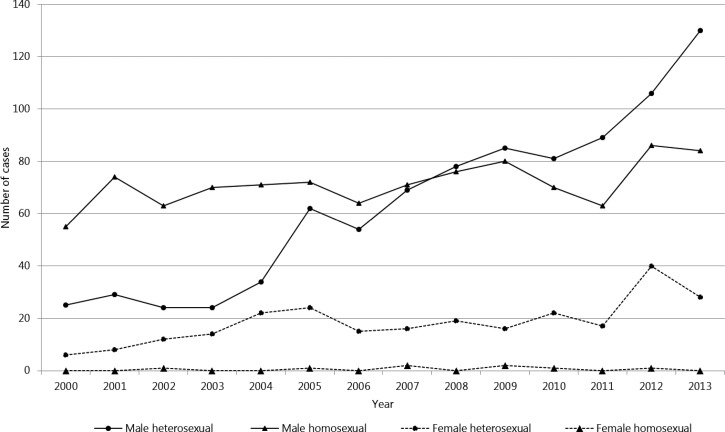
When further stratified by type of sexual contact among males, the number of cases among heterosexual males increased at a greater rate compared with homosexual males, and from 2008, the number of male cases acquired from heterosexual contact surpassed that of homosexual contact. For females, the majority of cases continued to be those associated with heterosexual contact throughout the study period (Figure 2 ).
Figure 2.
Number of reported domestically acquired amebiasis among cases with suspected sexual route of infection in Japan, 2000--2013 (N = 2,156). Solid line indicates male cases and dotted line female cases. Circle indicates heterosexual route of infection and triangle homosexual route of infection.
Description of domestic cases between 2010 and 2013.
Given the rapid increase in domestic cases in 2012 and 2013, we described the distribution of characteristics between 2010 and 2013 (Table 1). For both male and female cases, more than half of reported cases during 2010–2013 were from urban areas of Metropolitan Tokyo, Osaka, Kanagawa, Aichi, and Chiba prefectures, comprising 51.5% (1,541/2,994) of the total reported cases. The proportion of cases aged 50 years or older increased slightly from 44.0% to 49.8% during 2010–2013. The majority of cases were male (approximately 87.0%) throughout this period (Table 1).
Table 1.
Characteristics in domestic cases of amebiasis in Japan, 2010–2013
| 2010 | 2011 | 2012 | 2013 | |||||
|---|---|---|---|---|---|---|---|---|
| N = 655 | N = 675 | N = 775 | N = 889 | |||||
| n | % | n | % | n | % | n | % | |
| Age (years) | ||||||||
| < 50 | 367 | 56.0 | 342 | 50.5 | 419 | 54.1 | 446 | 50.2 |
| ≥ 50 | 288 | 44.0 | 334 | 49.5 | 356 | 45.9 | 443 | 49.8 |
| Sex | ||||||||
| Female | 80 | 12.2 | 85 | 12.6 | 106 | 13.7 | 108 | 12.1 |
| Male | 575 | 87.8 | 590 | 87.4 | 669 | 86.3 | 781 | 87.9 |
| Symptoms* | ||||||||
| No | 39 | 6.0 | 77 | 11.4 | 107 | 13.8 | 170 | 19.1 |
| Yes | 616 | 94.0 | 598 | 88.6 | 668 | 86.2 | 719 | 80.9 |
| Performed colonoscopy at diagnosis | ||||||||
| No | 229 | 35.0 | 232 | 34.4 | 263 | 33.9 | 239 | 26.9 |
| Yes | 426 | 65.0 | 453 | 65.6 | 512 | 66.1 | 650 | 73.1 |
| Amebiasis type | ||||||||
| Extraintestinal amebiasis† | 112 | 17.1 | 131 | 19.4 | 132 | 17.0 | 128 | 14.4 |
| Intestinal amebiasis | 543 | 82.9 | 544 | 80.6 | 643 | 83.0 | 761 | 85.6 |
| Route of infection among male cases | ||||||||
| Nonsexual | 108 | 18.8 | 85 | 14.4 | 100 | 14.9 | 99 | 12.7 |
| Sexual | 186 | 32.3 | 194 | 32.9 | 234 | 35.0 | 258 | 33.0 |
| Unknown | 281 | 48.9 | 311 | 52.7 | 335 | 50.1 | 424 | 54.3 |
| Route of infection among male cases attributed to sexual activity | ||||||||
| Heterosexual | 81 | 43.5 | 89 | 45.9 | 106 | 45.3 | 130 | 50.4 |
| Homosexual‡ | 70 | 37.6 | 63 | 32.5 | 86 | 36.8 | 84 | 32.6 |
| Unspecified | 35 | 18.8 | 42 | 21.6 | 42 | 17.9 | 44 | 17.1 |
| Route of infection among female cases | ||||||||
| Nonsexual | 11 | 13.8 | 17 | 20.0 | 15 | 14.2 | 19 | 17.6 |
| Sexual | 24 | 30.0 | 19 | 22.4 | 43 | 40.6 | 34 | 31.5 |
| Unknown | 45 | 56.3 | 49 | 57.6 | 48 | 45.3 | 55 | 50.9 |
| Route of infection among female cases attributed to sexual activity | ||||||||
| Heterosexual | 22 | 91.7 | 17 | 89.5 | 40 | 93.0 | 28 | 82.4 |
| Homosexual | 1 | 4.2 | 0 | 0.0 | 1 | 2.3 | 0 | 0.0 |
| Unspecified | 1 | 4.2 | 2 | 10.5 | 2 | 4.7 | 6 | 17.6 |
Cases with specific colonic mucosa lesions, such as erythema, edematous mucosa, erosion, white exudates, and ulcers, but otherwise asymptomatic and reported through National Epidemiological Surveillance of Infectious Diseases, were classified as asymptomatic cases.
Extraintestinal cases include cases diagnosed with both intestinal and extraintestinal amebiasis.
Cases marked with both heterosexual and homosexual contact were included in the homosexual category (three in 2010, four in 2011, six in 2012, and three in 2013).
During 2010–2013, the proportion of asymptomatic cases increased from 6.0% to 19.1% and the proportion of cases diagnosed by colonoscopy increased from 65.0% to 73.1% (Table 1). The proportion of male cases with unknown route of infection also increased from 48.9% in 2010 to 54.3% in 2013. Among male cases attributed to sexual activity, the proportion attributed to heterosexual contact increased over the period from 43.5% to 50.4%. The proportion of females with unknown route of infection ranged from 45.3% in 2012 to 57.6% in 2011. The proportion acquired sexually ranged from 22.4% in 2011 to 40.6% in 2012, majority of which were heterosexual (Table 1).
Analysis of asymptomatic and symptomatic cases.
A higher proportion of female cases were symptomatic (356/379 [93.9%]) compared with males (2,245/2,615 [85.8%]) and the proportion of cases < 50 years of age in females (289/379 [76.3%]) was higher than males (1,311/2,615 [50.1%]). For both males and females, comparison of asymptomatic and symptomatic domestic cases from 2010 to 2013 showed that asymptomatic status was significantly more likely to be diagnosed by colonoscopy (Table 2).
Table 2.
Comparison of characteristics between asymptomatic and symptomatic cases of amebiasis in Japan, 2010–2013
| Male (N = 2,615) | Female (N = 379) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic* (N = 370) | Symptomatic (N = 2,245) | OR (95% CI) | Adjusted OR§ (95% CI) | Asymptomatic* (N = 23) | Symptomatic (N = 356) | OR (95% CI) | Adjusted OR§ (95% CI) | |||||
| n | % | n | % | n | % | n | % | |||||
| Age (years) | ||||||||||||
| < 50 | 170 | 45.9 | 1,141 | 50.8 | Reference | Reference | 17 | 73.9 | 272 | 76.4 | Reference | Reference |
| ≥ 50 | 200 | 54.1 | 1,131 | 50.4 | 1.2 (0.9–1.4) | 1.3 (0.9–2.0) | 6 | 26.1 | 84 | 23.6 | 1.1 (0.4–3.0) | 1.3 (0.5–3.5) |
| Performed colonoscopy at diagnosis | ||||||||||||
| No | 6 | 1.6 | 806 | 35.9 | Reference | Reference | 1 | 4.3 | 150 | 42.1 | Reference | Reference |
| Yes | 364 | 98.4 | 1,439 | 64.1 | 34.0 (15.1–76.5) | 31.5 (14.0–71.0) | 22 | 95.7 | 206 | 57.9 | 16.0 (2.1–120.1) | 16.2 (2.1–123.4) |
| Route of infection among all cases† | ||||||||||||
| Sexual; homosexual‡ | 16 | 4.3 | 287 | 12.8 | Reference | Reference | 0 | 0.0 | 2 | 0.6 | – | – |
| Sexual; heterosexual | 44 | 11.9 | 362 | 16.1 | 2.2 (1.2–3.9) | 1.2 (0.7–2.3) | 1 | 4.3 | 106 | 29.8 | – | – |
| Sexual; unspecified | 15 | 4.1 | 148 | 6.6 | 1.8 (0.9–3.8) | 1.2 (0.5–2.5) | 1 | 4.3 | 10 | 2.8 | – | – |
| Nonsexual | 53 | 14.3 | 339 | 15.1 | 2.8 (1.6–5.0) | 1.5 (0.8–2.8) | 5 | 21.7 | 57 | 16.0 | – | – |
| Unknown | 242 | 65.4 | 1,109 | 49.4 | 3.9 (2.3–6.6) | 2.2 (1.3–3.9) | 16 | 69.6 | 181 | 50.8 | – | – |
OR = odds ratio; CI = confidence interval.
Cases with specific colonic mucosa lesions, such as erythema, edematous mucosa, erosion, white exudates, and ulcers, but otherwise asymptomatic and reported through National Epidemiological Surveillance of Infectious Diseases, were classified as asymptomatic cases.
Because of sparse cells, estimates for females could not be reliably estimated.
Cases marked with both heterosexual and homosexual contact were included in the homosexual category (one in asymptomatic and 15 in symptomatic).
Adjusted for each other for variables listed (age, colonoscopy, and route of infection).
In univariate analysis, among male cases, asymptomatic cases were significantly associated with heterosexual contact, nonsexual contact, and unknown route of infection compared with those with homosexual contact. Among female cases, majority of symptomatic cases were associated with heterosexual contact, with only two female cases associated with homosexual contact (Table 2). Among male cases, multivariate analysis including age, colonoscopy, and route of infection (homosexual, heterosexual, sexual unspecified, nonsexual, and unknown route of infection) showed that colonoscopy (OR = 31.5; 95% CI = 14.0–71.0) and cases with unknown route of infection, compared with those with homosexual contact (OR = 2.2; 95% CI = 1.3–3.9), remained associated with asymptomatic infections (Table 2).
Discussion
The reported number of domestically acquired cases of amebiasis increased considerably between 2000 and 2013. During 2010–2013, the proportion of reported cases from urban areas was high, as reported previously.14 Much of the recent rise in notifications was associated with increase among asymptomatic cases, those diagnosed by colonoscopy, and males associated with heterosexual or unknown route of infection. Moreover, among males, who continued to make up the majority of the cases, performing colonoscopy at diagnosis and those with unknown route of infection were significantly associated with asymptomatic status in multivariate analysis. Although MSM have been considered to be at a higher risk of being identified with amebiasis than heterosexual male cases,14,24 the large number of heterosexual male cases in recent years was not previously known and poses a potentially important public health concern. Because oral–anal sex, adjusted for MSM status, has been associated with amebiasis,24 heterosexual male cases may be acquiring infection by oral–anal sex with females.
Although few, case reports of amebiasis among females, including commercial sex workers, have been increasingly reported in Japan.12,13,15,25 In fact, symptomatic female cases associated with heterosexual contact increased during the study period (Tables 1 and 2, Figure 2). However, the absolute risk of amebiasis for females appears to be lower, as predominance of male amebiasis cases has been reported consistently from various locations that differ culturally, racially, and socioeconomically,8,10 and females appear to be less affected by amebiasis (e.g., liver abscess),26,27 which has also been found in animal studies.28 Moreover, while a higher proportion of males in their 50s are screened for colon cancer during annual health checks in Japan (41.4% for male and 34.2% for female in 2013),29 this differential screening proportion alone likely cannot explain the much larger difference consistently observed between males and females and the gender difference that has been previously reported.1–3,8–10,14,27,28 The specific reason(s) for the observed gender difference in the Japanese surveillance data requires further investigation.
Colonoscopy and biopsy can be helpful in the diagnosis of intestinal amebiasis, especially in less severe cases, but diagnosis by colonoscopy is limited because it is an invasive procedure. In Japan, colonoscopy is performed not only for diagnosis of amebiasis but also for screening of colon cancer for annual health checks and follow-up of inflammatory bowel disease.18,30 Recently, the endoscopic procedure has been cited as a potential tool for early diagnosis of amebiasis and for prevention of serious complications.17
This study, based on nationally reported surveillance data, has several limitations. First, information regarding route of infection were self-reported surveillance data, and self-reported sexual activity information may be inaccurate.31 Although 50% of cases had unknown route of infection, the “unknown” classification was selected by the reporting physician from a checkbox. The reason for such a selection may be due to the following reasons: 1) the physician did not ask the patient, 2) the patient had a suspected route in mind but did not answer the question, and 3) the patient had no idea of the potential route of infection. Because the suspected route of infection relates to sexual behavior, the high proportion of the “unknown” category may have been affected by social desirability bias. When considering the flow of clinical referrals for an asymptomatic patient detected by colonoscopy at screening settings, such bias from the physician side seems less likely. This is because such asymptomatic patients with a confirmation of amebiasis from screening would simply be referred to specialists, who would most likely be the same clinicians who see symptomatic amebiasis patients. Another possible reason for the higher proportion of unknown route of transmission for asymptomatic cases may be due to recall bias—asymptomatic cases may have more difficulty recalling a possible route of infection compared with symptomatic cases, and such bias may exist differentially between the two groups. Second, it is unknown if there have been any changes in awareness, practice, or reporting for colonoscopy during the study period. Although laboratory criteria or diagnosis method for notification have not changed,12,13 due to improved formatting of the notification form in February 2011, indicating “colon lesion” may have become easier, possibly leading to a greater chance of a case of amebiasis with only colon lesion (detected by colonoscopy) to be notified. Finally, confirmation of amebiasis based on microscopy was greater among cases diagnosed by colonoscopy (95%) relative to those diagnosed by methods other than colonoscopy (50%). This may be a potential concern as E. dispar cannot be morphologically differentiated from E. histolytica, a species that also parasitizes human intestines but is known to be nonpathogenic. In a sub-analysis restricted to 825 cases confirmed by molecular techniques (i.e., PCR, N = 44; serology, N = 770; ELISA, N = 25; ELISA and PCR, N = 2; ELISA and serology, N = 7; and PCR and serology, N = 5), where the specificity for E. histolytica is high with lower potential for false positives due to E. dispar, overall similar results were obtained. The restricted analysis showed an increase in notifications from 184 cases in 2010 to 240 in 2013, indicating 1.4-fold increase in notified cases (1.3-fold increase for total cases). In addition, the sex and age distribution were similar; the majority of cases were males (ranging from 82.7% to 89.2%) and the proportion of cases aged 50 years or older increased slightly from 44.0% to 50.0% during 2010–2013. Furthermore, while the proportion of asymptomatic cases among the restricted cases was lower than that of the total cases, there was an increase in both the number and proportion of asymptomatic cases from 2010 to 2013 (6/184 (3.3%) in 2010 to 17/240 (7.1%) in 2013). Similarly, while the proportion of restricted cases with colonoscopy was considerably lower than that of total cases, there was an increase in both the number and proportion of colonoscopy cases from 2010 to 2013 (51/184 (27.7%) in 2010 to 99/240 (41.3%) in 2013). It is expected that total cases, which included those detected by microscopy, would have a higher proportion of asymptomatic cases and those detected by colonoscopy, because most asymptomatic cases were detected by colonoscopy (Table 2), and such cases would usually be diagnosed by microscopy rather than molecular methods in the clinical setting. Similarly, males with unknown or heterosexual route of infection increased in both the number and proportion of cases from 2010 to 2013 (unknown: 72 (45.6%) in 2010 to 105 (50.2%) in 2013; heterosexual: 19 (32.8%) in 2010 to 35 (40.7%) in 2013). Furthermore, among males, colonoscopy remained significantly associated with asymptomatic infections in multivariate analysis (OR = 106.8; 95% CI = 14.6–780.0). And, when assessing routes of infection relative to homosexual contact, cases with unknown route of infection was significantly associated with asymptomatic status in univariate analysis (OR = 5.4; 95% CI = 1.3–23.0), although the association was not significant in multivariate analysis (OR = 2.7; 95% CI = 0.6–12.5). Therefore, despite smaller sample numbers, our results from the sub-analysis were similar to our main findings. Moreover, E. histolytica has been consistently found to be the predominant species isolated in Japan, with E. dispar being very rare.22,32 Such apparent lack of E. dispar in Japan appears to be a globally unique phenomenon. Thus, although majority of cases diagnosed by colonoscopy were due to microscopy, given the predominance of E. histolytica in Japan, and the similar results obtained from the sub-analysis restricting to cases diagnosed by molecular methods, misclassification due to E. dispar seems unlikely to play a role. However, studies to indicate the presence of E. histolytica and lack of E. dispar among Japanese amebiasis cases reported through surveillance may be helpful to confirm previously reported findings.
Our study is the first epidemiological study of domestically acquired amebiasis in Japan using national surveillance data. These results showed that the main increase in recent reports of amebiasis were associated with asymptomatic male cases diagnosed by colonoscopy, with either “unknown” or heterosexual route of transmission. Although the recent rise may be due to enhanced detection by colonoscopy or reporting of such cases, this study has led to a better understanding of amebiasis in Japan and highlights the potential public health concern, as indicated by the large number of asymptomatic male cases, with hitherto unreported routes of infection. It is thus important to communicate these findings to clinicians and improve the quality of surveillance data being collected, particularly regarding the suspected route of infection. Through dissemination of these epidemiological data with the clinical community, further studies, such as those focusing on identifying the potential route(s) of infection among asymptomatic male cases diagnosed by colonoscopy (e.g., sexual partnerships and specific sexual activities) and observational epidemiologic studies that focus on potential risk factors, may shed light on the current situation. With improved understanding, risk groups may be identified to effectively and efficiently control and prevent the spread of amebiasis in Japan.
ACKNOWLEDGMENTS
We thank the staff at local public health centers and prefectural and municipal public health institutes, physicians, and the Ministry of Health, Labour and Welfare. The contribution from reporting physicians and local governments through NESID is valuable for infectious disease prevention and control in Japan.
Footnotes
Financial support: This work was supported by the Research on Emerging and Re-emerging Infectious Diseases and Immunization (H27-shinkougyousei-shitei-001) of Japan.
Authors' addresses: Masahiro Ishikane, Field Epidemiology Training Program, National Institute of Infectious Diseases, Tokyo, Japan, and Division of Global Infectious Diseases, Department of Infection and Epidemiology, Graduate School of Medicine, Tohoku University, Miyagi, Japan, E-mail: ishimasa@niid.go.jp. Yuzo Arima, Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Tokyo, Japan, E-mail: arima@niid.go.jp. Atsuhiro Kanayama, Field Epidemiology Training Program, National Institute of Infectious Diseases, Tokyo, Japan, and Department of Global Infectious Diseases and Tropical Medicine, National Defense Medical College, Saitama, Japan, E-mail: kanayama@ndmc.ac.jp. Takuri Takahashi, Takuya Yamagishi, Yuichiro Yahata, Tamano Matsui, Tomimasa Sunagawa, and Kazunori Oishi, Infectious Disease Surveillance Center, National Institute of Infectious Diseases, Tokyo, Japan, E-mails: takuri@niid.go.jp, tack73@gmail.com, yahata@niid.go.jp, djyu@niid.go.jp, sunatomi@niid.go.jp, and oishik@niid.go.jp. Tomoyoshi Nozaki, Department of Parasitology, National Institute of Infectious Diseases, Tokyo, Japan, E-mail: nozaki@niid.go.jp.
Reprint requests: Yuzo Arima, Infectious Disease Surveillance Center, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162-8640, Japan, E-mail: arima@niid.go.jp, Tel: +81-3-5285-1111, Fax: +81-3-5285-1233.
References
- 1.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, García-Rivera G, Orozco E, Martínez MB, Gunatilleke SS, Barrios AM, Arkin MR, Poole LB, McKerrow JH, Reed SL. A high throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18:956–960. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade RM, Reed SL. New drug target in protozoan parasites: the role of thioredoxin reductase. Front Microbiol. 2015;30:975. doi: 10.3389/fmicb.2015.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petri WA, Haque R., Jr . Entamoeba species, including amebiasis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 8th edition. Philadelphia, PA: Churchill Livingstone Elsevier; 2015. pp. 3047–3058. [Google Scholar]
- 4.Chen KT, Chen CJ, Chiu JP. A school waterborne outbreak involving both Shigella sonnei and Entamoeba histolytica. J Environ Health. 2001;64:9–13. [PubMed] [Google Scholar]
- 5.Blessmann J, Ali IK, Nu PA, Dinh BT, Viet TQ, Van AL, Clark CG, Tannich E. Longitudinal study of intestinal Entamoeba histolytica infections in asymptomatic adult carriers. J Clin Microbiol. 2003;41:4745–4750. doi: 10.1128/JCM.41.10.4745-4750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera WL, Tachibana H, Kanbara H. Field study on the distribution of Entamoeba histolytica and Entamoeba dispar in the northern Philippines as detected by the polymerase chain reaction. Am J Trop Med Hyg. 1998;59:916–921. doi: 10.4269/ajtmh.1998.59.916. [DOI] [PubMed] [Google Scholar]
- 7.WHO/FAO Multicriteria-based Ranking for Risk Management of Food-Borne Parasites. 2014. http://apps.who.int/iris/bitstream/10665/112672/1/9789241564700_eng.pdf?ua=1 Available at. Accessed June 1, 2015.
- 8.Lo YC, Ji DD, Hung CC. Prevalent and incident HIV diagnoses among Entamoeba histolytica-infected adult males: a changing epidemiology associated with sexual transmission—Taiwan, 2006–2013. PLoS Negl Trop Dis. 2014;8:e3222. doi: 10.1371/journal.pntd.0003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi T, Okuzawa E, Nozaki T, Kobayashi S, Mizokami M, Minoshima N, Yamamoto M, Isomura S. High seropositivity of Japanese homosexual men for amebic infection. J Infect Dis. 1989;159:808. doi: 10.1093/infdis/159.4.808. [DOI] [PubMed] [Google Scholar]
- 10.James R, Barratt J, Marriott D, Harkness J, Stark D. Seroprevalence of Entamoeba histolytica infection among men who have sex with men in Sydney, Australia. Am J Trop Med Hyg. 2010;83:914–916. doi: 10.4269/ajtmh.2010.10-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung PO, Chen KH, Chen KL, Tsai YT, Liu SY, Chen KT. Epidemiological features of intestinal infection with Entamoeba histolytica in Taiwan, 2002–2010. Travel Med Infect Dis. 2014;5:673–679. doi: 10.1016/j.tmaid.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 12.NIID Amebiasis in Japan 2003–2006. Infect Agents Surveill Rep. 2007;28:103–164. [Google Scholar]
- 13.NIID Amebiasis in Japan 2010–2013. Infect Agents Surveill Rep. 2014;35:223–224. [Google Scholar]
- 14.Ohnishi K, Kato Y, Imamura A, Fukayama M, Tsunoda T, Sakaue Y, Sakamoto M, Sagara H. Present characteristics of symptomatic Entamoeba histolytica infection in the big cities of Japan. Epidemiol Infect. 2004;132:57–60. doi: 10.1017/s0950268803001389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagata N, Shimbo T, Akiyama J, Nakashima R, Nishimura S, Yada T, Watanabe K, Oka S, Uemura N. Risk factors for intestinal invasive amebiasis in Japan, 2003–2009. Emerg Infect Dis. 2012;18:717–724. doi: 10.3201/eid1805.111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe K, Gatanaga H, Escueta-de Cadiz A, Tanuma J, Nozaki T, Oka S. Amebiasis in HIV-1-infected Japanese men: clinical features and response to therapy. PLoS Negl Trop Dis. 2011;5:e1318. doi: 10.1371/journal.pntd.0001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horiki N, Furukawa K, Kitade T, Sakuno T, Katsurahara M, Harada T, Tano S, Yamada R, Hamada Y, Inoue H, Tanaka K, Gabazza EC, Ishii N, Fukuda K, Omata F, Fujita Y, Tachibana H, Takei Y. Endoscopic findings and lesion distribution in amebic colitis. J Infect Chemother. 2015;21:444–448. doi: 10.1016/j.jiac.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Kishihara T, Ishiyama A, Fumizono Y, Imada S, Ogawa T, Chino A, Uragami N, Igarashi M. Study on amebic colitis: especially differential diagnosis from IBD. Prog Dig Endosc. 2008;73:77–79. [Google Scholar]
- 19.Akazawai K, lchikawa T, Tawa Y, Oi I, Machida N, Ogiwara H, Negishi Y, Yamashiro Y, Sasaki H, Okubo S, Abe K, Fujino M. Clinical features of amebic colitis: an analysis of ten cases. Prog Dig Endosc. 2012;80:68–69. [Google Scholar]
- 20.e-Stat Portal Site of Official Statistics of Japan. 2015. http://www.e-stat.go.jp/SG1/estat/eStatTopPortalE.do Available at. Accessed June 1, 2015.
- 21.Hamzah Z, Petmitr S, Mungthin M, Leelayoova S, Chavalitshewinkoon-Petmitr P. Differential detection of Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii by a single-round PCR assay. J Clin Microbiol. 2006;44:3196–3200. doi: 10.1128/JCM.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozaki T, Kobayashi S, Takeuchi T, Haghighi A. Diversity of clinical isolates of Entamoeba histolytica in Japan. Arch Med Res. 2006;37:277–279. doi: 10.1016/j.arcmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Nozaki T, Motta SR, Takeuchi T, Kobayashi S, Sargeaunt PG. Pathogenic zymodemes of Entamoeba histolytica in Japanese male homosexual population. Trans R Soc Trop Med Hyg. 1989;83:525. doi: 10.1016/0035-9203(89)90276-9. [DOI] [PubMed] [Google Scholar]
- 24.Hung CC, Wu PY, Chang SY, Ji DD, Sun HY, Liu WC, Wu CH, Chang SC. Amebiasis among persons who sought voluntary counseling and testing for human immunodeficiency virus infection: a case-control study. Am J Trop Med Hyg. 2011;84:65–69. doi: 10.4269/ajtmh.2011.10-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagisawa N. Amebiasis. Mod Med. 2012;58:237–245. [Google Scholar]
- 26.Hoque MI, Uddin MS, Sarker AR, Uddin MK. Common presentation of amebic liver abscess—a study in a tertiary care hospital in Bangladesh. Mymensingh Med J. 2014;23:724–729. [PubMed] [Google Scholar]
- 27.Chen HL, Bair MJ, Lin IT, Wu CH, Lee YK. Clinical manifestations and risk factors of amebic liver abscess in southeast Taiwan compared with other regions of Taiwan. Am J Trop Med Hyg. 2013;89:1214–1218. doi: 10.4269/ajtmh.13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lotter H, Jacobs T, Gaworski I, Tannich E. Sexual dimorphism in the control of amebic liver abscess in a mouse model of disease. Infect Immun. 2006;74:118–124. doi: 10.1128/IAI.74.1.118-124.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ministry of Health, Labour and Welfare Comprehensive Survey of Living Conditions. 2013. http://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa13/ Available at. Accessed June 1, 2015.
- 30.Okamoto M, Kawabe T, Ohata K, Togo G, Hada T, Katamoto T, Tanno M, Matsumura M, Yamaji Y, Watabe H, Ikenoue T, Yoshida H, Omata M. Amebic colitis in asymptomatic subjects with positive fecal occult blood test results: clinical features different from symptomatic cases. Am J Trop Med Hyg. 2005;73:934–935. [PubMed] [Google Scholar]
- 31.Gallo MF, Warner L, Hobbs MM, Jamieson DJ, Hylton-Kong T, Steiner MJ. Differences in misreporting of sexual behavior over time: implications for HIV trials. Sex Transm Dis. 2015;42:160–161. doi: 10.1097/OLQ.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung CC, Chang SY, Ji DD. Entamoeba histolytica infection in men who have sex with men. Lancet Infect Dis. 2012;12:729–736. doi: 10.1016/S1473-3099(12)70147-0. [DOI] [PubMed] [Google Scholar]