Abstract
Pigs were infected with a Bolivian strain of Trypanosoma cruzi (genotype I) and evaluated up to 150 days postinoculation (dpi) to determine the use of pigs as an animal model of Chagas disease. Parasitemia was observed in the infected pigs during the acute phase (15–40 dpi). Anti-T.cruzi immunoglobulin M was detected during 15–75 dpi; high levels of anti-T.cruzi immunoglobulin G were detected in all infected pigs from 75 to 150 dpi. Parasitic DNA was observed by western blot (58%, 28/48) and polymerase chain reaction (27%, 13/48) in urine samples, and in the brain (75%, 3/4), spleen (50%, 2/4), and duodenum (25%, 1/4), but no parasitic DNA was found in the heart, colon, and kidney. Parasites were not observed microscopically in tissues samples, but mild inflammation, vasculitis, and congestion was observed in heart, brain, kidney, and spleen. This pig model was useful for the standardization of the urine test because of the higher volume that can be obtained as compared with other small animal models. However, further experiments are required to observe pathological changes characteristic of Chagas disease in humans.
Introduction
Chagas disease is caused by the parasite Trypanosoma cruzi and is mainly transmitted by blood-feeding insects of the family Reduviidae, subfamily Triatominae (kissing bugs).1 The disease is spread from north of Mexico to south of Argentina. It is an important cause of morbidity and mortality among humans from Latin American countries, and it is becoming a public health problem in many developed nations. The World Health Organization has estimated that 8–10 million people are infected, and 100 million more are at risk of infection.2
In humans, the disease has three different stages: an acute, indeterminate, and chronic phase. In the acute phase, parasites are found in the blood and this is generally accompanied by nonspecific signs and symptoms.3 The early chronic phase or so-called indeterminate phase bears no clinical signs and symptoms and has no detectable parasitemia1; and the chronic phase which accounts for 30% of the infected individuals and represents the most detrimental consequence of the disease. In this phase, the parasites can be found in various tissues but are primarily in the heart, colon, and esophagus.4,5 Pathologies of the heart include myocarditis, cardiomegaly, cardiac failure, conduction disturbances, and alterations in the repolarization phase of the electrocardiogram.4,6 Histologically, one can observe the following changes: degeneration and necrosis of cardiac muscle fibers, focal or diffuse inflammatory infiltrate, and interstitial fibrosis.7,8 Gastrointestinal pathologies include megaesophagus and megacolon, which involve the gross dilatation of the hollow viscera and the wall thickening, causing the loss of coordination and a decrease in motility of parts of the digestive system.1
The life cycle of this parasite involves humans, the arthropod vector, and a large number of mammals that serve as reservoirs. Animals play an important role due to their close relationship with both humans and triatomines, the arthropod vector. Dogs and cats are the most important domestic reservoirs,9 while in Peru and Bolivia, the main reservoir can also include guinea pigs (Cavia porcellus).10 Several studies to investigate and understand the immunology, pathogenesis, transmission, and other factors associated with Chagas disease have been carried out in mice, dogs, and monkeys. The mouse is the most common animal model for Chagas disease, with several T. cruzi genotypes successfully reproduced in specific murine strains.11,12 However, the mouse cardiovascular system and immune response are significantly different compared with humans.13 Dogs and monkeys, on the other hand, have served as better animal models because they develop the chronic phase of the disease and are good electrocardiographic models. However, one of the drawbacks of using these animals as experimental disease models is that they need a long period of time to develop the chronic phase.14,15
Recently, the porcine animal model has gained importance in biomedical research.16,17 Pigs share many physiological and anatomical similarities with humans, making them an optimal animal species for research. For instance, humans and pigs share a similar cardiovascular system. The porcine coronary artery distribution is more similar to humans than other large animals18 and therefore have enabled pigs to be the preferred animal model of heart failure.19 Another advantage of using pigs as an animal model for disease is the large volume of blood and urine samples that can be obtained. This serves as a great advantage in cases when it is necessary to purify analytes that are present at physiologically low concentrations. In addition, pigs may serve as potential animal reservoir for T. cruzi as naturally infected pigs have been reported in Mexico, Paraguay, and Brazil.20–22 Pigs are considered one of the most important sources of blood meal for vectors in some regions of Brazil,23 Argentina,24 Bolivia,25 and Colombia.26
There have been two previous studies of experimental infection of pigs with different strains of T. cruzi (Peruvian and North American strains). In both studies, a few parasites were observed in blood and/or tissues, but histopathological changes were rarely found.27,28 Our goal, therefore, was to experimentally infect domestic pigs with trypomastigotes of Trypanosoma cruzi (Bolivian strain, genotype I) and assess the presence of the parasite in blood, tissues, and urine; in addition we aimed to evaluate the immunological response and histopathological changes after the infection.
Materials and Methods
Parasites.
Trypomastigotes of the Bolivian strain were harvested from LLC-MK2 (rhesus monkey kidney epithelial cells) cell cultures in Roswell Park Memorial Institute media. Parasites were collected from the supernatant and then counted using a Neubauer chamber. The strain was isolated from a patient from Santa Cruz (Bolivia) who was diagnosed with cardiomyopathy and tested positive for Chagas disease (information obtained from Japanese Hospital, Santa Cruz, Bolivia). This strain was maintained in vitro in the Laboratory of Infectious Diseases at Universidad Cayetano Heredia, Lima, Peru. The strain was classified as genotype Tc1 by Dr. Alejandro Schijman in the Instituto de Investigaciones en Ingeniería Genética y Biología Molecular, Buenos Aires, Argentina.
Animals.
Five mix-breed 60-day-old female pigs were used for this experiment. The animals were purchased in one of the commercial farms in Lima, an area of no endemic transmission of T. cruzi. Blood samples were taken from the animals to confirm they were not infected with T. cruzi by serological tests and polymerase chain reaction (PCR). The animals were maintained at the facilities of the Veterinary School of San Marcos University, Lima, Peru. They were fed with commercial pig food and water ad libitum. The protocol was approved by the Ethics Committee of Animal Welfare of the Veterinary School, San Marcos University, Lima, Peru.
Experimental infections.
Pigs were anesthetized prior to inoculation of the parasites with a combination of ketamine (20 mg/kg) and xylazine (2 mg/kg) by an intramuscular injection. Blood samples were taken from each animal from the jugular vein prior to the infection, using the vacutainer system. The central ear vein was used for the intravenous (IV) inoculation and the medial side of the right leg was used for the intradermal (ID) infection. Previous studies with T. cruzi have demonstrated that pigs show a mild infection with undetectable parasitemia, for this reason we decided to evaluate two doses and different routes of inoculation. Pigs were randomly assigned to receive one of the four inoculum: 1) 1 × 106 trypomastigotes/kg body weight (bw) IV; 2) 1 × 106 trypomastigotes/kg bw ID, 3) 5 × 106 trypomastigotes/kg bw IV, and 4) 5 × 106 trypomastigotes/kg bw ID. These two doses are higher than ones used in other studies in pigs (106 compared with 104) to ensure the infection. The fifth pig served as the control and was inoculated IV with RPMI-1640 media. After inoculation, blood samples were collected every 5 days for the first 3 months, and then every 14 days for the next 2 months for evaluation of parasitemia and collection of serum and clot. Urine samples were also collected in a sterile bottle after intramuscular administration of furosemide (5 mg/kg bw). All animals were necropsied 5 months after infection and tissue samples were taken from lung, esophagus, pancreas, small intestine (duodenum), large intestine, liver, uterus, mesenteric nodes, bronchi nodes, tongue, muscle (rectus abdominis), skin, cerebellum, bladder, heart, colon, brain, kidney, and spleen. These tissues were fixed in 10% phosphate-buffered saline (PBS)–formalin and ethanol, and stored for histopathology and PCR analysis, respectively. We took heart tissue samples from the wall of both right and left ventricle, right and left atrium, and also from the right and left apex; three samples were taken from each anatomical point.
Parasitemia evaluation.
Blood samples were collected every 5 days from the beginning of the experiment using four heparinized microhematocrit capillary tubes and parasitemia was determined by microconcentration technique.29
Treatment of urine samples.
Approximately 20 minutes after the injection of furosemide, the operator collected the urine by waiting and following the animals until they urinated. Samples were stored at 4°C until processed. Approximately 10–50 mL of urine was collected from each animal at 15, 25, 35, 45, 55, 65, 75, 80, 85, 90, 95, and 150 days postinfection (a total of 12 urine samples per pig). Urine samples were immediately centrifuged at 800 × g for 20 minutes, and then the supernatant was stored at −20°C until use. Before concentration, urine samples were again centrifuged and the supernatant was concentrated at 80× by ultrafiltration using Miniplus (Millipore, Bedford, MA), cut-off of 15 kDa. The concentrated samples were stored at −20°C until tested.
Production of the trypomastigote excretory–secretory antigen of T. cruzi (TESA).
TESA was obtained as described previously.30 It has been reported that serum from patients with Chagas disease produce two band patterns: 1) six bands in a ladder at 130–160 kDa designated as shed acute-phase antigen, which is a diagnostic indicator of acute infection; and 2) a broad antigen band at 150–160 kDa, which is an indicator of chronic infection.31
Production of antibodies anti-TESA.
The 150–160 kDa band from the TESA antigen was visualized by western blot and cut from the nitrocellulose membrane. Four ID immunizations at 2-week intervals were performed in two rabbits with the 150–160 kDa band (protein concentration 100 mg/mL) in Freund's adjuvant (Sigma-Aldrich, St. Louis, MO).
Enzyme-linked immunosorbent assay (ELISA) test for the detection of T. cruzi immunoglobulin M (IgM) and immunoglobulin (IgG).
The kinetics for the production of IgM and IgG to T. cruzi was determined by epimastigote alkaline extract (EAE)-ELISA, as described previously with some modifications.32 Briefly, ELISA 96-well plates (Immulon 2; Thermo Labsystems, Franklin, MA) were coated with 2 μg/mL EAE antigen in 1 M bicarbonate buffer (pH 9.6) overnight and blocked with 5% skim milk (Nestle) in PBS (pH 7.2) and 0.05% Tween 20 (PBST). The ELISA plates were incubated with pig serum diluted 1:100 (IgG detection) or 1/50 (IgM detection) in 1% dried skim milk/PBST for 1 h at 37°C. After repeated six washing steps, the plates were incubated at 37°C for 1 hour with a 1:10,000 dilution of horseradish peroxidase–conjugated goat anti-pig IgG (KPL Laboratories, Gaithersburg, MD) or a 1/2,500 dilution of goat anti-pig IgM (KPL Laboratories). Then the plates were incubated with 0.1 mg/well o-phenylenediaminedihydrochloride (Sigma-Aldrich) and 0.05% H2O2 in 0.1 M sodium citrate buffer for 5 minutes. The reaction was stopped using 2 M H2SO4. The optical density (OD) was read at 495 nm using a VersaMax microplate spectrophotometer (Molecular Devices Corporation, Sunnyvale, CA). The cut-off was established as the mean OD of the negative controls plus three standard deviations. Each serum sample was analyzed in duplicates.
DNA extraction and PCR.
DNA was purified by Proteinase K digestion (Invitrogen, Carlsbad, CA) and was extracted following a standard phenol–chloroform extraction protocol as previously described.32,33 We used 500 μL of clot or 25 mg of tissue stored in ethanol. The extracted DNA was resuspended in 100 μL of 10 mM TrisHCl and 1 mM ethylenediaminetetraacetic acid. PCR was performed as previously described using primers 121/122 (kinetoplast DNA, 330 bp)34 and TcZ1/TcZ2 (nuclear DNA, 188 bp).35 DNA extraction from urine samples was developed according to published protocols with some modifications.32 Briefly, 25 μL of concentrated urine samples were incubated with 10 mmol/L TrisHCl, pH 7.6, 10 mmol/L NaCl, and mixed gently for 5 minutes. Sodium dodecyl sulfate (SDS) and Proteinase K (Invitrogen) were added to reach concentrations of 0.25% and 0.50 mg/mL, respectively, and the specimens were incubated for 1 hour at 56°C. DNA was extracted following a standard phenol–chloroform extraction protocol and ethanol precipitation. PCR was performed as previously described using primers TcZ1/TcZ2 (nuclear DNA, 188 bp).35 The PCR was considered negative after three DNA extractions, and two amplifications of each extraction were found to be negative.
Histology.
Tissues samples were collected at necropsy (5 months after infection); they were fixed in 10% formalin–PBS embedded in paraffin and 20 sections of 5 μm were obtained from all tissue samples. Each slide was then cut in additional six sections and stained with hematoxylin and eosin (H&E). Microscopic evaluation was performed by reading 50 fields of each cut at 40×. Histological analysis was performed to determine the level of tissue damage and identify the presence of amastigote nests. This entire process was performed blinded.
Urine antigen detection by western blot.
Antigens in urine samples were detected as previously described.36 Briefly, concentrated urine samples were treated with 10% SDS, 5% dithiotreitol, 10% glycerol, and 0.01% bromophenol blue and heated to 95°C for 5 minutes. The antigens were separated on polyacrylamide gel 10% and transferred to nitrocellulose membranes (BioRad Laboratories, Berkeley, CA). The membranes were incubated with 5% nonfat milk in PBS with 0.3% Tween 20 for 1 hour and then with 1/50 rabbit polyclonal antibody anti-TESA. The antigens were incubated with 1/2,000 peroxidase conjugated goat anti-rabbit IgG (KPL Laboratories) for 90 minutes. The antigen–antibody complexes were detected after incubation with 0.5 μg/mL of 3,5-diaminobenzidine (Sigma-Aldrich) and 0.03% H2O2. The molecular weight was determined using Broad Range Standards (BioRad Laboratories).
Results
During the whole study period all pigs consumed food and water in normal quantities, appeared to be healthy, and showed no clinical signs and symptoms of Chagas disease or any other infectious disease.
Parasitemia evaluation.
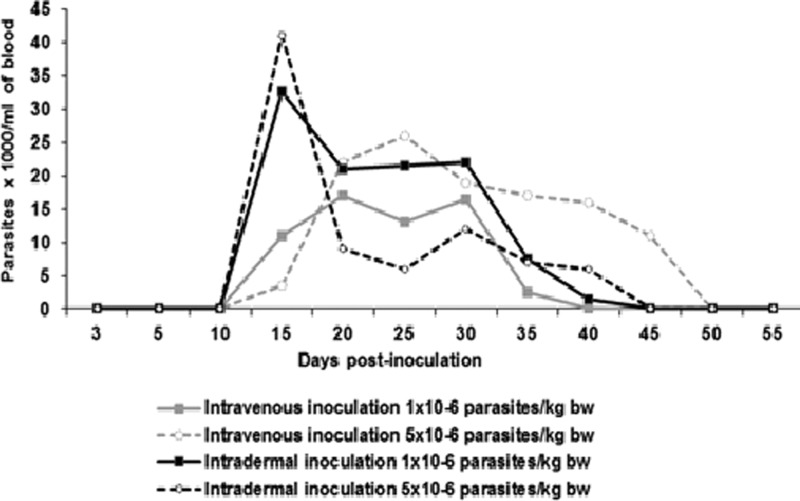
Microscopic observations of the buffy coat of the infected animals were negative during the first 2 weeks after the inoculation. However, from day 15, 100% (N = 4/4) of the inoculated animals were positive. At day 40 postinoculation, 75% (N = 3/4) remained positive, and at day 45 only the pig inoculated with higher dose IV remained positive until day 50. The animals infected ID showed the highest number of parasites on day 15 postinoculation (up to 40,000 parasites/mL of blood) (Figure 1 ).
Figure 1.
Dynamics of parasitemia in experimentally infected pigs with trypomastigotes of Trypanosoma cruzi Bolivia strain (genotype Tc1).
Evaluation of the immune response against the parasite.
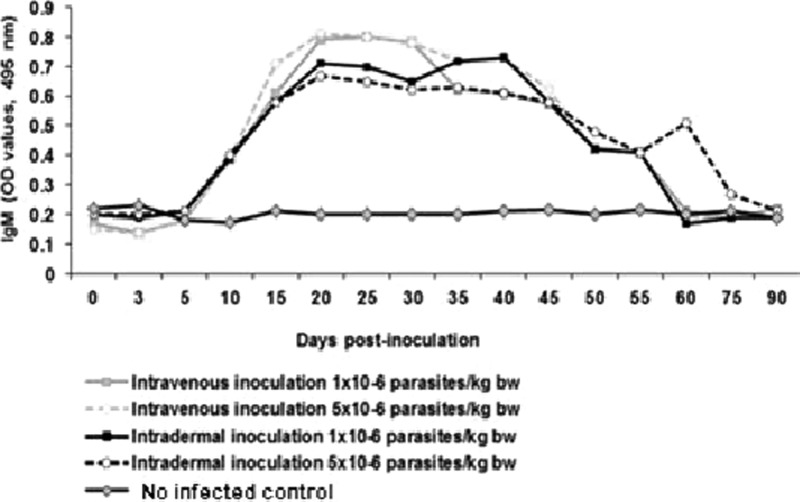
The EAE-ELISA test showed that the anti-T. cruzi IgM values began to increase at day 10 postinoculation. Anti-T.cruzi IgM production was maintained until day 55 postinoculation, with the exception of the pig inoculated ID with 5 × 106 trypomastigotes, which showed a secondary peak on day 60 postinoculation. The peak production of antibodies was observed between day 20 and 25 postinoculation. After day 25, the anti-T.cruzi IgM values begin to decline and 100% of infected animals were negative from day 75 postinoculation up to the end of the study period (150 days postinoculation [dpi]) (Figure 2 ).
Figure 2.
Dynamics of anti-Trypanosoma cruzi immunoglobulin M (IgM) antibody production in pigs experimentally infected with T. cruzi.
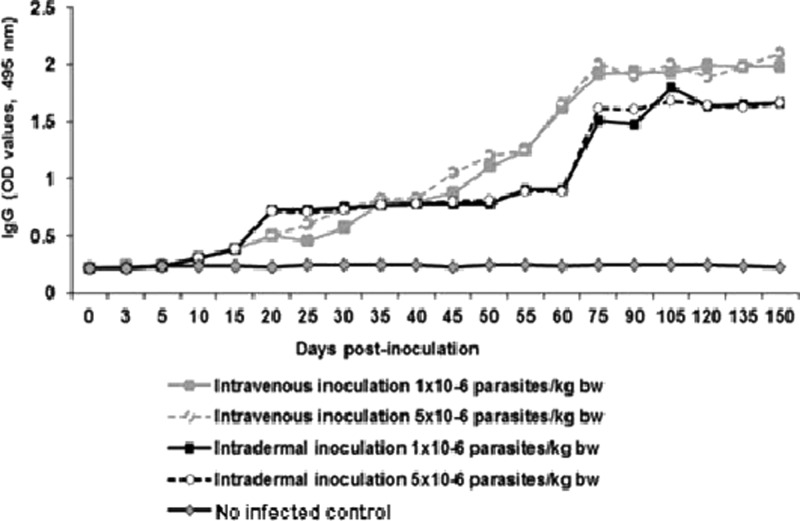
EAE-ELISA test was also used for the detection of anti-T. cruzi IgG antibodies. In Figure 3 , it shows that all of the infected pigs had elevated levels of anti-T. cruzi IgG starting from day 20 postinoculation. The peak production of IgG antibodies was detected on day 75 postinoculation in all of the infected pigs. The anti-T.cruzi IgG values remained high until the end of the experiment (Figure 3).
Figure 3.
Dynamics of anti-Trypanosoma cruzi immunoglobulin G (IgG) antibody production in pigs experimentally infected with T. cruzi.
Parasitic DNA in blood and tissues.
The results of the PCR in blood demonstrated that all of the infected animals tested positive from day 3 to day 60 postinoculation. However, from day 75 postinoculation until the end of the experiment, the detection of the parasitic DNA in blood was intermittent and was not related to the doses of parasite or route of inoculation (Table 1). The control pig was negative during the entire follow-up period. Regarding to the detection of T. cruzi DNA in tissues, it was detected in the brain in three out of four infected pigs; of which the one without any parasites detected in the brain was the one challenged with 5 × 106 parasites ID. Similarly, two out of four infected pigs (infected with 1 × 106 parasites, ID and 5 × 106 parasites, IV) showed T. cruzi DNA in spleen, and finally only one infected pig (5 × 106 parasites, ID) showed T. cruzi DNA in the small intestine. No parasitic DNA was detected in the heart, colon, kidney, or the other tissue samples collected.
Table 1.
Detection of Trypanosoma cruzi DNA in blood samples of experimentally infected pigs with T. cruzi (Bolivia strain, Tc1)
| Days postinoculation | Intravenous inoculation | Intradermal inoculation | Positive animals, n (%) | ||
|---|---|---|---|---|---|
| Doses of parasites (parasites/kg bw) | |||||
| 1 × 106 | 5 × 106 | 1 × 106 | 5 × 106 | ||
| 0 | − | − | − | − | 0 (0) |
| 3 | + | + | + | + | 4/4 (100) |
| 5 | + | + | + | + | 4/4 (100) |
| 10 | + | + | + | + | 4/4 (100) |
| 15 | + | + | + | + | 4/4 (100) |
| 20 | + | + | + | + | 4/4 (100) |
| 25 | + | + | + | + | 4 (100) |
| 30 | + | + | + | + | 4/4 (100) |
| 35 | + | + | + | + | 4 (100) |
| 40 | + | + | + | + | 4/4 (100) |
| 45 | + | + | + | + | 4/4 (100) |
| 50 | + | + | + | + | 4/4 (100) |
| 55 | + | + | + | + | 4/4 (100) |
| 60 | + | + | + | + | 4/4 (100) |
| 75 | + | + | − | − | 2/4 (50) |
| 90 | − | − | + | − | 1/4 (25) |
| 105 | − | − | + | + | 2/4 (50) |
| 120 | + | + | − | − | 2/4 (50) |
| 135 | − | + | − | + | 2/4 (50) |
| 150 | − | − | − | + | 1/4 (25) |
Necropsy and histology.
All of the infected pigs showed no macroscopic alterations in any of the organs. We did not find any nest of amastigotes in the sample tissues. However, the most common lesion observed in heart, brain, and kidney was light to mild perivasculitis in approximately 10–25% of the tissue samples. There were three out of four pigs with perivasculitis in the heart, one out of four in the brain and kidney. The perivascultitis was composed of mainly lymphocytes and minor congestion. The same type of inflammation was also observed in the pericardium and meninges. The spleen capsule exhibited signs of swelling in one of the infected pigs (5 × 106 trypomastigotes/kg bw IV). The connective tissue of the spleen capsule was thickened as compared with the control pig. Other pathological manifestations were also observed such as increased lymphoid nests (hyperplasia) with focus of necrosis in both white and red pulp (necrosis of lymphocytes and red cells); we also found congestion and perivasculitis with mononuclear cells, predominantly lymphocytes. Kidney tissue had a mild focal glomerulonephritis and perivasculitis, both of them with lymphocyte infiltration.
Detection of the parasite in urine by western blot and PCR.
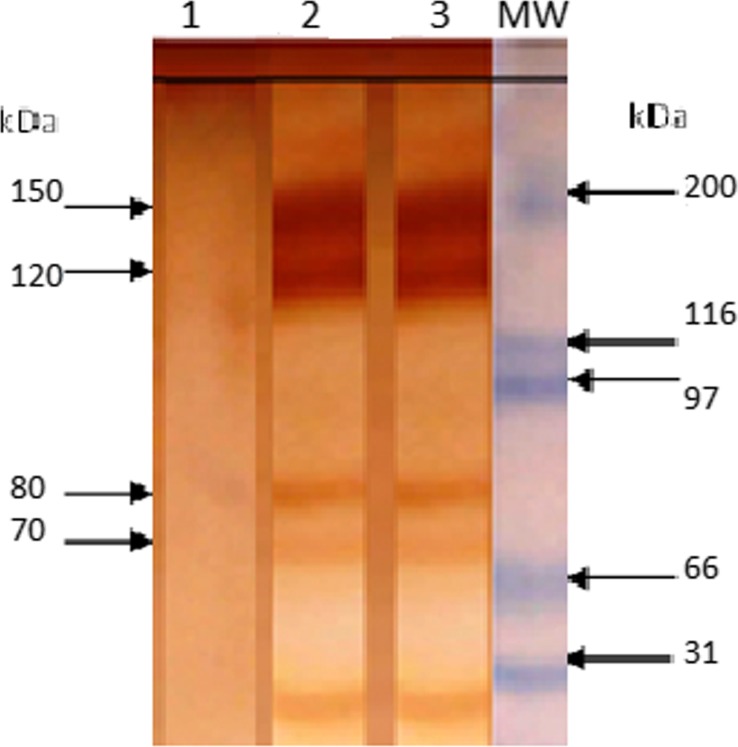
We detected four specific bands of 75, 80, 120, and 150 kDa that were recognized only in urine samples of infected pigs (Figure 4 ). The proportion of pigs with positive results to each of the diagnostic bands in urine samples are shown in Table 2. The bands of 75 and 80 kDa were detected during all the course of observation (15–150 dpi), but the bands of 120 and 150 kDa were detected only after the first month of infection (35–150 dpi). The percentage of animals with positive results for antigen detection changed during the course of observation, with highest percentages during the acute phase (75%, 4/5) and lowest percentages during the early chronic phase (50%, 2/4), suggesting a relationship between levels of parasitemia and excretion of T. cruzi antigens in urine.37
Figure 4.
Antigenic bands in urine samples of pigs infected with Trypanosoma cruzi. Bands were detected by western blot using a polyclonal antibody against excretory–secretory trypomastigote T. cruzi. Bands under 70 kDa were not specific. (1) Urine sample from no infected pig. (2 and 3) Urine samples from infected pig at 55 and 150 days postinoculation, respectively. MW = molecular weight marker in kilodalton (kDa).
Table 2.
Number and percentages of positive pigs with Trypanosoma cruzi antigens in urine after experimental infection with trypomastigotes during 150 dpi*
| dpi | Band (molecular weight in kDa)‡ | Total number of positive animals‡ (%) | |||
|---|---|---|---|---|---|
| 75 | 80 | 120 | 150 | ||
| 0 (Before infection)† | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0) |
| 15 | 3 (75%) | 3 (75%) | 0 (0%) | 0 (0%) | 3/4 (75) |
| 25 | 3 (75%) | 3 (75%) | 0 (0%) | 0 (0%) | 3/4 (75) |
| 35 | 3 (75%) | 3 (75%) | 1 (25%) | 1 (25%) | 3/4 (75) |
| 45 | 3 (75%) | 3 (75%) | 3 (75%) | 3 (75%) | 3/4 (75) |
| 55 | 2 (50%) | 2 (50%) | 2 (50%) | 2 (50%) | 2/4 (50) |
| 65 | 3 (75%) | 3 (75%) | 1 (25%) | 1 (25%) | 3 (75) |
| 75 | 2 (50%) | 2 (50%) | 1 (25%) | 1 (25%) | 2 (50) |
| 80 | 3 (75%) | 3 (75%) | 1 (25%) | 1 (25%) | 3 (75) |
| 85 | 1 (25%) | 1 (25%) | 1 (25%) | 1 (25%) | 1/4 (25) |
| 90 | 2 (50%) | 2 (50%) | 2 (50%) | 2 (50%) | 2/4 (50) |
| 95 | 1 (25%) | 1 (25%) | 1 (25%) | 1 (25%) | 1/4 (25) |
| 150 | 2 (50%) | 2 (50%) | 2 (50%) | 2 (50%) | 2/4 (50) |
dpi = days postinoculation; kDa = kilodalton.
Urine samples from the noninoculated pig (negative control) were negative at all time points of urine collection.
Urine samples collected before infection were negative.
The presence of any of the bands of 75, 80, 120, and 150 kDa was considered to give a positive result.
In addition, T. cruzi DNA (nuclear DNA, 188 bp) was also detected in urine samples of the infected pigs; however, the proportion of animals with positive results (27%, 13/48) was less than the detection of antigens in urine (58%, 28/48) and detection of T. cruzi DNA in blood (71%, 34/48) (Table 3).
Table 3.
Detection of DNA of Trypanosoma cruzi in blood and urine samples by PCR and relationship with antigenuria in experimentally infected Sus scrofa
| Days postinfection | n | DNA clot | DNA urine | Antigenuria* |
|---|---|---|---|---|
| Positives (%) | Positives (%) | Positives (%) | ||
| 15 | 4 | 4 (100) | 2 (50) | 3 (75) |
| 25 | 4 | 4 (100) | 3 (75) | 3 (75) |
| 35 | 4 | 4 (100) | 2 (50) | 3 (75) |
| 45 | 4 | 4 (100) | 2 (50) | 3 (75) |
| 55 | 4 | 4 (100) | 2 (50) | 2 (50) |
| 60 | 4 | 4 (100) | 1 (25) | 3 (75) |
| 75 | 4 | 2 (50) | 0 (0) | 2 (50) |
| 80 | 4 | 2 (50) | 0 (0) | 3 (75) |
| 85 | 4 | 1 (25) | 0 (0) | 1 (25) |
| 90 | 4 | 1 (25) | 1 (25) | 2 (50) |
| 95 | 4 | 2 (50) | 0 (0) | 1 (25) |
| 150 | 4 | 1 (25) | 0 (0) | 2 (50) |
| Total | 48 | 34 (71) | 13 (27) | 28 (58) |
PCR = polymerase chain reaction.
The presence of any of the bands of 75, 80, 120, and 150 kDa was considered as a positive result.
Discussion
This study evaluates the domestic pig as an animal model of Chagas disease. The results show that all pigs inoculated with the parasite were successfully infected; the infection was characterized by an acute phase with detectable parasitemia and anti-T.cruzi IgM response; and an early chronic phase with high levels of anti-T.cruzi IgG, mild histopathological changes, and no detectable parasitemia. Furthermore, T. cruzi DNA was detected in blood and urine by PCR. The detectable parasitemia and immune response suggest that pigs could be a useful animal model of Chagas disease; however, similar to humans, pigs may develop the chronic phase years after T. cruzi infection.
Overall the number of parasites found in the blood by microscopy (maximum 40 × 103 parasites/mL blood in pig with ID inoculation) was relatively low as compared with previous studies. Nargis and others38 and Roffê and others39 found 54 × 104 parasites/mL in guinea pigs after ID inoculation and 3 × 106 parasites/mL in mice after intraperitoneal inoculation, respectively. However, the parasitemia in this study was similar to the dog model, which demonstrated 50 × 103 parasites/mL.40 Although, these differences could be attributed to the parasite strain, the host (differences in immune response), and the methodologies used for experimental infection, these results could also indicate that the infection with T. cruzi in the pig model is not fulminant, which is comparable to humans.
Interestingly, parasitemia levels immediately after inoculation were higher in pigs with ID inoculation compared with IV inoculation (Figure 1). This observation could be explained by the parasite amplification process that occurs at the site of inoculation. When metacyclic trypomastigotes are inoculated in the skin, polymorphonuclear leucocytes are rapidly attracted to the site of inoculation to prevent infection. However, T. cruzi is capable of surviving in different types of cells (including macrophages, fibroblasts, and muscle cells) where the parasite differentiates into amastigote forms. In these cells, amastigotes multiply by binary fission and differentiate into trypomastigote forms.41 In mice, for example, removing the skin at the inoculation site even a few minutes after inoculation (5 minutes) results in lower levels of parasitemia.42
There have been previous attempts to infect pigs with T. cruzi. However, those studies have not been as successful. For instance, in the study carried out by Marsden in 1970,27 none of the experimentally infected pigs with T. cruzi Peruvian strain showed parasitemia, whereas in the study by Diamond and Rubin in 1958,28 parasites in blood were observed only in one out of the four pigs infected with the North American strain of T. cruzi, and just one parasite was observed on blood smears. These differences could be explained by the diversity of T. cruzi strain virulence and by the high number of parasites inoculated in our study. However, our goal was to demonstrate the feasibility of using domestic pigs as an animal model for Chagas disease; therefore, we attempted the most common route used in other studies (IV and ID) with a high dose of infection to ensure the parasite would reproduce and invade the cells and tissues.
One of the limitations of this study was that we could not demonstrate serious lesions in the target organs. This study, as all of the published pig model papers, showed minimal pathological changes. For instance, Marsden reported a marked, diffuse, and focal lymphocytic infiltration in the pericardium and myocardium of the atrium in one pig, but the ventricle was free of lymphocytes. They also observed perivascular and interstitial edema in this organ and some muscle fibers showed loss of striations.27 On the other hand, Diamond and Rubin28 found lack of clinical signs, an absence of pathologic alteration in the pig model. This study showed a light to mild perivasculitis composed of mainly lymphocytes and minor congestion in the heart, brain, and kidney. Evaluation for a longer period of time after inoculation and a more detailed and systematic study of heart tissue may be needed to see cardiac changes similar to humans. Another limitation of our study is that we did not collect other tissues such as tonsil, bone marrow, nerves and myenteric plexus, adrenal glands, thyroid, and spinal cord, where T. cruzi might be observed by histopathology. Given that the heart is one of the target organs of the parasite, this study should have taken standard serial sections seeking the parasite as it might be possible that T. cruzi was located in different areas from the anatomical points collected. More systematic studies are needed to better characterize the histopathological changes in the heart of this animal model. Other molecular techniques such as immunohistochemistry and in situ PCR should also be considered to evaluate experimental infections. Similarly, hematology and serum chemistry may be useful in future studies to evaluate experimentally infected animals.
The detection of high levels of anti-T.cruzi IgM and anti-T.cruzi IgG during the infection compared with the no infected pig demonstrated that the pigs activated an immune response against the parasite. The disappearance of circulating anti-T.cruzi IgM from 75 dpi suggests the ending of the acute phase of the infection. This is supported by the fact that no circulating parasites were detected from 50 dpi onward. Furthermore, the increasing levels of anti-T.cruzi IgG from 75 dpi may indicate the beginning of the early chronic phase.
The results from using PCR on clot samples showed that 100% of animals were positive for parasite presence from day 3 to day 60 postinoculation. This confirms the results obtained by Veloso and others43 in blood samples, which found increased sensitivity of this technique in the acute phase. The number of blood samples positive for PCR decreased during the chronic phase, and similar results were observed in the chronic model of T. cruzi infection in guinea pigs.32
PCR was also used in the evaluation of different tissues collected at 150 dpi. Unfortunately, none of the infected pigs were positive for the presence of DNA in the heart, which is different from other animal models studied such as guinea pig in which the heart was the most frequent tissue with parasite kinetoplast DNA as compared with other organs at three different stages of infection: acute phase (5–55 days), early chronic phase (115–165 days), and chronic phase (365 days).32 Interestingly, our study found histopathological alterations in the heart without the detection of the parasite. This is similar to a study with chronically infected dogs that demonstrated no detectable parasites in the heart either by microscopic observation or by PCR.40 The histopathological changes observed in the heart of pigs such as mild inflammation, vasculitis, and congestion at 150 dpi could correspond to an early chronic phase or indeterminate phase in humans as human hearts show mild or no pathological changes and rarely parasites are observed. The inability to detect the parasite in the pig hearts in our study pigs could be explained by the small amount of tissue evaluated by PCR and microscopy as compared with the size of these organs in pigs. Nevertheless, to identify the parasite in the heart may be difficult as documented by Diamond28 who found only one positive animal using H&E stain after an exhaustive search.
Another possible explanation for why no parasites were found in the heart could be the parasite tropism, as the DNA of the parasite was detected in other organs of our infected pigs, such as the brain, the spleen, and the small intestine but not in the heart. In the pathogenesis of Chagas disease, it is still unknown why some patients develop heart disease while others present megaesophagus or megacolon, or why T. cruzi does not attack organs such as lungs and kidneys where it circulates during the acute and chronic phase. It might be possible that the parasite tissue invasion is not only correlated with genetic variations of the parasite but also with some histotropic receptors for different strains and clones of the parasite.44 In this context, there has been a series of experiments that have demonstrated the genetic variability of the parasites as one of the major components determining tissue tropism and the pathogenesis in experimentally infected mice.45 Furthermore, it has been reported that genetic aspects of the host play an important role in immune response. Major histocompatibility complex variability has an important role in influencing the differential tissue distribution pattern of T. cruzi infection, and this may be the primary determinant for the clinical form of Chagas disease in humans.45,46 Finally, we should note that the evaluation of different tissues was carried out during the chronic phase of the infection (150 dpi). In this phase, the number of parasites in target tissues decrease32 and it is difficult to detect the parasites. Performing necropsy at different time points of infection might increase the possibility of demonstrating the presence of amastigotes in the tissues.
Splenomegaly was not observed in the infected pigs although some histopathological alterations and the presence of parasitic DNA were detected in these animals. The detection of T. cruzi in the spleen was observed previously in infected mice with abundant parasitism of macrophages in red and white pulps, hyperplasia of lymphoid follicles, and necrosis.47 Splenomegaly is also an important characteristic of T. cruzi infection in dogs during the acute and chronic phase and it was also reported in patients with Chagas disease,48,49 but our model did not show this macroscopic alteration.
One of the significant advantages of our pig model is that it allowed for the purification of T. cruzi parasite proteins found in urine. Specific proteins and DNA of T. cruzi were detected in urine samples during the course of infection with more frequency in the acute phase than in the chronic phase. The presence of T. cruzi antigens in urine was previously reported by other research groups with sensitivity of 60–100%.36,50,51 The molecular weight of the antigenic bands detected in the urine samples of the infected pigs are similar to those detected by our group in other animal models and in humans.37 The presence of soluble fragments of parasitic DNA (188 bp) in urine can be explained by small fragments of DNA of parasites that have died by apoptosis and subsequently crossed the glomerulus barrier in the kidney.52 Nevertheless, the presence of antigens and DNA of the parasite in urine was not associated with the presence of the parasites in the kidney, indicating that these molecules come from the systemic circulation. The pig model allowed us to obtain higher volume of urine than in other laboratory animal models (e.g., mice, guinea pigs), facilitating the concentration and detection of antigen and DNA of T. cruzi. The urine collection in pigs is also relatively easy to perform and it does not require complete sedation as is necessary with small animal models.
The disappearance of the parasite and the detection of high levels of anti-T. cruzi IgG after 45 days of infection might indicate that the pig model can successfully control the parasite burden during the acute phase. This is also supported by the presence of parasitic DNA in other tissues and the observation of pathological alterations and signs of inflammation in the heart, brain, kidney, and spleen. In conclusion, our pig model is a useful animal model for T. cruzi infection, but further experiments are required to observe the histopathological changes of T. cruzi infection as seen in humans.
ACKNOWLEDGMENTS
The authors thank Yessenia Loza Gordillo, Cesar Quispe Asto, and Henry Torres for technical assistance. We also thank Katherine Fu for revising the English of this manuscript.
Footnotes
Financial support: This project was funded by Fogarty International Center (5 R24 TW007988, D43 TW006581) and National Institute of Allergy and Infectious Diseases (R01 AI087776) at the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: The protocol was approved by the Ethics Committee of Animal Welfare of the Veterinary School, San Marcos University, Lima, Peru.
Authors' addresses: VerónicaYauri, Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru, E-mail: vyauri@gmail.com. Yagahira E. Castro-Sesquen, Manuela Verastegui, and Noelia Angulo, Laboratorio de Investigación en Enfermedades Infecciosas, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: yagahiraelizabeth@hotmail.com, manuela.verastegui@upch.pe, and shaki2700@yahoo.es. Fernando Recuenco and Ines Cabello, Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru, E-mails: fer_recuenco@hotmail.com and itacab18@hotmail.com. Edith Málaga, Laboratorio de Investigación en Enfermedades Infecciosas, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mail: edith.malaga@gmail.com. Caryn Bern, Global Health Sciences, Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA; E-mail: Caryn.Bern2@ucsf.edu. Cesar M. Gavidia, Facultad de Medicina Veterinaria, Universidad Nacional Mayor de San Marcos, Lima, Peru, E-mail: cmgavidia@yahoo.com. Robert H. Gilman, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, E-mail: gilmanbob@gmail.com.
References
- 1.Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA. Chagas disease. Postgrad Med J. 2006;82:788–798. doi: 10.1136/pgmj.2006.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Control of Chagas disease. World Health Organ Tech Rep Ser. 2002;905:1–109. i–vi. [PubMed] [Google Scholar]
- 3.Rassi A, Jr, Rassi A, Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Infect Dis Clin North Am. 2012;26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Andrade ZA. Immunopathology of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94((Suppl 1)):71–80. doi: 10.1590/s0074-02761999000700007. [DOI] [PubMed] [Google Scholar]
- 5.Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum MB. Chagasic myocardiopathy. Prog Cardiovasc Dis. 1964;7:199–225. doi: 10.1016/s0033-0620(64)80020-7. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco Guerra HA, Palacios-Pru E, Dagert de Scorza C, Molina C, Inglessis G, Mendoza RV. Clinical, histochemical, and ultrastructural correlation in septal endomyocardial biopsies from chronic chagasic patients: detection of early myocardial damage. Am Heart J. 1987;113:716–724. doi: 10.1016/0002-8703(87)90712-5. [DOI] [PubMed] [Google Scholar]
- 8.Dias E, Laranja FS, Miranda A, Nobrega G. Chagas' disease; a clinical, epidemiologic, and pathologic study. Circulation. 1956;14:1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 9.Coura JR, Dias JC. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz. 2009;104((Suppl 1)):31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- 10.Levy MZ, Bowman NM, Kawai V, Waller LA, Cornejo del Carpio JG, Cordova Benzaquen E, Gilman RH, Bern C. Periurban Trypanosoma cruzi-infected Triatoma infestans, Arequipa, Peru. Emerg Infect Dis. 2006;12:1345–1352. doi: 10.3201/eid1209.051662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade SG, Andrade V, Brodskyn C, Magalhaes JB, Netto MB. Immunological response of Swiss mice to infection with three different strains of Trypanosoma cruzi. Ann Trop Med Parasitol. 1985;79:397–407. doi: 10.1080/00034983.1985.11811938. [DOI] [PubMed] [Google Scholar]
- 12.Melo RC, Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978;64:475–482. [PubMed] [Google Scholar]
- 13.Suzuki Y, Yeung AC, Ikeno F. The representative porcine model for human cardiovascular disease. J Biomed Biotechnol. 2011;2011:195483. doi: 10.1155/2011/195483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pung OJ, Hulsebos LH, Kuhn RE. Experimental Chagas' disease (Trypanosoma cruzi) in the Brazilian squirrel monkey (Saimiri sciureus): hematology, cardiology, cellular and humoral immune responses. Int J Parasitol. 1988;18:115–120. doi: 10.1016/0020-7519(88)90045-8. [DOI] [PubMed] [Google Scholar]
- 15.Guedes PM, Veloso VM, Talvani A, Diniz LF, Caldas IS, Do-Valle-Matta MA, Santiago-Silva J, Chiari E, Galvao LM, Silva JS, Bahia MT. Increased type 1 chemokine expression in experimental Chagas disease correlates with cardiac pathology in beagle dogs. Vet Immunol Immunopathol. 2010;138:106–113. doi: 10.1016/j.vetimm.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Vodicka P, Smetana K, Jr, Dvorankova B, Emerick T, Xu YZ, Ourednik J, Ourednik V, Motlik J. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci. 2005;1049:161–171. doi: 10.1196/annals.1334.015. [DOI] [PubMed] [Google Scholar]
- 17.Cooper JE. The use of the pig as an animal model to study problems associated with low birthweight. Lab Anim. 1975;9:329–336. doi: 10.1258/002367775780957188. [DOI] [PubMed] [Google Scholar]
- 18.Hiebl B, Müller C, Hünigen H, Gemeinhardt O, Plendl J, Jung F, Hamm B, Niehues SM. Gross anatomical variants of the vasculature of theGöttingenTM minipig. Appl Cardiopulm Pathophysiol. 2010;14:236–243. [Google Scholar]
- 19.Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J. Animal models of cardiovascular diseases. J Biomed Biotechnol. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazar-Schettino PM, Bucio MI, Cabrera M, Bautista J. First case of natural infection in pigs. Review of Trypanosoma cruzi reservoirs in Mexico. Mem Inst Oswaldo Cruz. 1997;92:499–502. doi: 10.1590/s0074-02761997000400010. [DOI] [PubMed] [Google Scholar]
- 21.Valente VC, Valente SA, Noireau F, Carrasco HJ, Miles MA. Chagas disease in the Amazon Basin: association of Panstrongylus geniculatus (Hemiptera: Reduviidae) with domestic pigs. J Med Entomol. 1998;35:99–103. doi: 10.1093/jmedent/35.2.99. [DOI] [PubMed] [Google Scholar]
- 22.Fujita O, Sanabria L, Inchaustti A, De Arias AR, Tomizawa Y, Oku Y. Animal reservoirs for Trypanosoma cruzi infection in an endemic area in Paraguay. J Vet Med Sci. 1994;56:305–308. doi: 10.1292/jvms.56.305. [DOI] [PubMed] [Google Scholar]
- 23.Goncalves TC, Rocha DS, Cunha RA. Feeding patterns of Triatoma vitticeps in the State of Rio de Janeiro, Brazil. Rev Saude Publica. 2000;34:348–352. doi: 10.1590/s0034-89102000000400006. [DOI] [PubMed] [Google Scholar]
- 24.Cecere MC, Vazquez-Prokopec GM, Gurtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am J Trop Med Hyg. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- 25.Pizarro JC, Stevens L. A new method for forensic DNA analysis of the blood meal in chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca, Bolivia. PLoS One. 2008;3:e3585. doi: 10.1371/journal.pone.0003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farfan AE, Gutierrez R, Angulo VM. Using ELISA for identifying Triatominae feeding behaviour in Colombia. Rev Salud Publica (Bogota) 2007;9:602–608. doi: 10.1590/s0124-00642007000400013. [DOI] [PubMed] [Google Scholar]
- 27.Marsden PD, Blackie EJ, Rosenberg ME, Ridley DS, Hagstrom JW. Experimental Trypanosoma cruzi infections in domestic pigs (Sus scrofa domestica) Trans R Soc Trop Med Hyg. 1970;64:156–158. [PubMed] [Google Scholar]
- 28.Diamond LS, Rubin R. Experimental infection of certain farm mammals with a North American strain of Trypanosoma cruzi from the raccoon. Exp Parasitol. 1958;7:383–390. doi: 10.1016/0014-4894(58)90034-1. [DOI] [PubMed] [Google Scholar]
- 29.MINSA . Enfermedad de Chagas. In: Salud Md, Epidemiologia OGd, Salud INd., editors. Lima, Peru: Centro de Documentación del INS/OGE; 2001. p. 43. [Google Scholar]
- 30.Jazin EE, Luquetti AO, Rassi A, Frasch AC. Shift of excretory-secretory immunogens of Trypanosoma cruzi during human Chagas' disease. Infect Immun. 1991;59:2189–2191. doi: 10.1128/iai.59.6.2189-2191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umezawa ES, Nascimento MS, Kesper N, Jr, Coura JR, Borges-Pereira J, Junqueira AC, Camargo ME. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J Clin Microbiol. 1996;34:2143–2147. doi: 10.1128/jcm.34.9.2143-2147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro-Sesquen YE, Gilman RH, Yauri V, Angulo N, Verastegui M, Velasquez DE, Sterling CR, Martin D, Bern C. Cavia porcellus as a model for experimental infection by Trypanosoma cruzi. Am J Pathol. 2011;179:281–288. doi: 10.1016/j.ajpath.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzwater S, Calderon M, Lafuente C, Galdos-Cardenas G, Ferrufino L, Verastegui M, Gilman RH, Bern C. Polymerase chain reaction for chronic Trypanosoma cruzi infection yields higher sensitivity in blood clot than buffy coat or whole blood specimens. Am J Trop Med Hyg. 2008;79:768–770. [PubMed] [Google Scholar]
- 34.Wincker P, Bosseno MF, Britto C, Yaksic N, Cardoso MA, Morel CM, Breniere SF. High correlation between Chagas' disease serology and PCR-based detection of Trypanosoma cruzi kinetoplast DNA in Bolivian children living in an endemic area. FEMS Microbiol Lett. 1994;124:419–423. doi: 10.1111/j.1574-6968.1994.tb07318.x. [DOI] [PubMed] [Google Scholar]
- 35.Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, Carlier Y, Svoboda M. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am J Trop Med Hyg. 2003;68:574–582. doi: 10.4269/ajtmh.2003.68.574. [DOI] [PubMed] [Google Scholar]
- 36.Umezawa ES, Shikanai-Yasuda MA, da Silveira JF, Cotrim PC, Paranhos G, Katzin AM. Trypanosoma cruzi: detection of a circulating antigen in urine of chagasic patients sharing common epitopes with an immunodominant repetitive antigen. Exp Parasitol. 1993;76:352–357. doi: 10.1006/expr.1993.1043. [DOI] [PubMed] [Google Scholar]
- 37.Castro-Sesquen YE, Gilman RH, Yauri V, Cok J, Angulo N, Escalante H, Bern C. Detection of soluble antigen and DNA of Trypanosoma cruzi in urine is independent of renal injury in the guinea pig model. PLoS One. 2013;8:e58480. doi: 10.1371/journal.pone.0058480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nargis M, Chisty MM, Ihama Y, Sato H, Inaba T, Kamiya H. Kinetics of Trypanosoma cruzi infection in guinea-pigs, with special reference to the involvement of epidermal Langerhans' cells in the induction of immunity. Parasitology. 2001;123:373–380. doi: 10.1017/s0031182001008551. [DOI] [PubMed] [Google Scholar]
- 39.Roffê E, Souza AL, Machado PP, Barcelos LS, Romanha AJ, Mariano FS, Silva JS, Machado CR, Tanowitz HB, Teixeira MM. Endothelin-1 receptors play a minor role in the protection against acute Trypanosoma cruzi infection in mice. Braz J Med Biol Res. 2007;40:391–399. doi: 10.1590/s0100-879x2007000300015. [DOI] [PubMed] [Google Scholar]
- 40.Machado EM, Fernandes AJ, Murta SM, Vitor RW, Camilo DJ, Jr, Pinheiro SW, Lopes ER, Adad SJ, Romanha AJ, Pinto Dias JC. A study of experimental reinfection by Trypanosoma cruzi in dogs. Am J Trop Med Hyg. 2001;65:958–965. doi: 10.4269/ajtmh.2001.65.958. [DOI] [PubMed] [Google Scholar]
- 41.Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 42.Schuster JP, Schaub GA. Trypanosoma cruzi: skin-penetration kinetics of vector-derived metacyclic trypomastigotes. Int J Parasitol. 2000;30:1475–1479. doi: 10.1016/s0020-7519(00)00119-3. [DOI] [PubMed] [Google Scholar]
- 43.Veloso VM, Guedes PM, Andrade IM, Caldas IS, Martins HR, Carneiro CM, Machado-Coelho GL, de Lana M, Galvao LM, Bahia MT, Chiari E. Trypanosoma cruzi: blood parasitism kinetics and their correlation with heart parasitism intensity during long-term infection of Beagle dogs. Mem Inst Oswaldo Cruz. 2008;103:528–534. doi: 10.1590/s0074-02762008000600003. [DOI] [PubMed] [Google Scholar]
- 44.Coura JR, Borges-Pereira J. Chagas disease. What is known and what should be improved: a systemic review. Rev Soc Bras Med Trop. 2012;45:286–296. doi: 10.1590/s0037-86822012000300002. [DOI] [PubMed] [Google Scholar]
- 45.Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Trypanosoma cruzi: role of host genetic background in the differential tissue distribution of parasite clonal populations. Exp Parasitol. 2002;100:269–275. doi: 10.1016/s0014-4894(02)00024-3. [DOI] [PubMed] [Google Scholar]
- 46.Freitas JM, Andrade LO, Pires SF, Lima R, Chiari E, Santos RR, Soares M, Machado CR, Franco GR, Pena SD, Macedo AM. The MHC gene region of murine hosts influences the differential tissue tropism of infecting Trypanosoma cruzi strains. PLoS One. 2009;4:e5113. doi: 10.1371/journal.pone.0005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima ES, Andrade ZA, Andrade SG. TNF-alpha is expressed at sites of parasite and tissue destruction in the spleen of mice acutely infected with Trypanosoma cruzi. Int J Exp Pathol. 2001;82:327–336. doi: 10.1046/j.1365-2613.2001.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guedes PM, Veloso VM, Afonso LC, Caliari MV, Carneiro CM, Diniz LF, Marques-da-Silva EA, Caldas IS, Do Valle Matta MA, Souza SM, Lana M, Chiari E, Galvao LM, Bahia MT. Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-gamma, TNF-alpha, and low IL-10 production during the acute infection phase. Vet Immunol Immunopathol. 2009;130:43–52. doi: 10.1016/j.vetimm.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Zaidenberg M, Segovia A. Congenital Chagas disease in the city of Salta, Argentina. Rev Inst Med Trop Sao Paulo. 1993;35:35–43. [PubMed] [Google Scholar]
- 50.Katzin A, Alves MJ, Abuin G, Colli W. Antigenuria in chronic chagasic patients detected by a monoclonal antibody raised against Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1989;83:341–343. doi: 10.1016/0035-9203(89)90497-5. [DOI] [PubMed] [Google Scholar]
- 51.Corral RS, Altcheh J, Alexandre SR, Grinstein S, Freilij H, Katzin AM. Detection and characterization of antigens in urine of patients with acute, congenital, and chronic Chagas' disease. J Clin Microbiol. 1996;34:1957–1962. doi: 10.1128/jcm.34.8.1957-1962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Umansky SR, Tomei LD. Transrenal DNA testing: progress and perspectives. Expert Rev Mol Diagn. 2006;6:153–163. doi: 10.1586/14737159.6.2.153. [DOI] [PubMed] [Google Scholar]