Abstract
There is a significant heterogeneity in reported performance of serological assays for Chagas disease diagnosis. The conventional serology testing in laboratory diagnosis and in blood banks is unsatisfactory because of a high number of inconclusive and misclassified results. We aimed to assess the quality of four commercially available enzyme-linked immunosorbent assay tests for their ability to detect Trypanosoma cruzi antibodies in 685 sera samples. Cross-reactivity was assessed by using 748 sera from patients with unrelated diseases. Initially, we found that the reactivity index against T. cruzi antigen was statistically higher in sera from Chagas disease patients compared with those from non-chagasic patients, supporting the notion that all evaluated tests have a good discriminatory ability toward the diagnosis of T. cruzi infection in patients in the chronic phase of the disease. Although all tests were similarly sensitive for diagnosing T. cruzi infection, there were significant variations in terms of specificity and cross-reactivity among them. Indeed, we obtained divergent results when testing sera from patient with unrelated diseases, particularly leishmaniasis, with the levels of cross-reactivity being higher in tests using whole T. cruzi extracts compared with those using recombinant proteins. Our data suggest that all four tests may be used for the laboratory diagnosis and routine blood screening diagnose for Chagas disease. We also emphasize that, despite their general good performance, caution is needed when analyzing the results when these tests are performed in areas where other diseases, particularly leishmaniasis, are endemic.
Introduction
Chagas disease is a complex zoonosis caused by the protozoan hemoflagellate Trypanosoma cruzi. The parasite is transmitted to humans by domestic or sylvatic hematophagous triatomine bugs through the insect's infected feces while it is feeding on blood. Parasites within the triatomine excreta may enter the human body through either broken skin or mucous membranes, including the conjunctiva or oral/digestive mucosa. Secondary and less frequent routes of transmission include blood transfusion, congenital transmission, organ transplantation, and accidental laboratory contamination.1 Outbreaks caused by contaminated food and beverages have also been described.2 Infection with T. cruzi causes mortality in 14,000 people3 and morbidity in up to 10 million people in the continental Western Hemisphere, resulting in a substantial disease burden in the 22 endemic countries.4 In recent years, intensification of the migratory flow due to socioeconomic factors has increased T. cruzi infection beyond the borders of Latin America, becoming a worldwide health concern, particularly in the United States, Canada, Spain, Italy, Germany, and Japan.5,6 As a result, blood transfusion is now a risk for transmission in many areas of the world.
Chronic Chagas disease presents itself with intermittent or low parasitemia and lack of symptoms, thus reducing the sensitivity (Se) of direct parasitological assays. Therefore, immunological methods are the elective procedure to detect the presence of specific anti-T. cruzi antibodies in a patient's blood during the chronic phase.7,8 However, immunological methods present some divergences because of the different protocols of antigen preparations and genetic variations of circulating strains, yielding differences in performance among available commercial tests. This is particularly worrying with regard to blood donor screening. In 1995, Wendel Neto9 had already given attention to the risk of blood transmission, emphasizing that the prevalence of infected donors ranges from 0.01% to as high as 60% in some cities from Latin America, and the risk of infection via transfusion of a contaminated whole blood unit is in the range of 12–25%. More recent work has revealed that 1.91% of the blood donors screened for Chagas disease living on the island of Majorca, Spain, and coming from Chagas-endemic areas were seropositive, particularly from Argentina and Bolivia.10 Since no test has been found to be sufficiently sensitive and specific to be designated the sole screening assay for Chagas disease, World Health Organization advises that, to ensure a reliable diagnosis, screening must be carried out by at least two assays based on distinct techniques and used concomitantly. In Brazil, the Health Ministry recommends the use of a test with high Se (with total antigen or semi-purified fractions of the parasite) in combination with a high specificity (Sp) test in parallel (using T. cruzi-specific, recombinant antigens).11
The most widely used immunological methods are indirect immunofluorescence (IFA), indirect hemagglutination and enzyme-linked immunosorbent assays (EIA). Among these, EIA is the most used because of its high automation level and flexibility to using different antigen preparations.12 Antigens for the EIA assays are provided by total or semi-purified homogenate of epimastigote forms of T. cruzi. As it could be expected, there is considerable variation in the reproducibility, feasibility, and reliability of such positive control samples.13 Most worrisome is the reported cross-reactivity with related protozoan parasites, particularly Leishmania spp. and Trypanosoma rangeli. Other preparations use recombinant proteins or a collection of short recombinant peptides containing specific T. cruzi epitopes, increasing the Sp of the assays.14–16 More recently, chimeric recombinant proteins designed with selected amino acid sequences increased the Se and Sp in diagnostic assays.17,18 In this scenario, because of the heterogeneity of the performance reported by the tests for Chagas disease diagnosis, we decided to assess the reported quality of four EIA tests for their ability to specifically detect T. cruzi antibodies.
Materials and Methods
Clinical specimens.
All serum samples used in this study were obtained from the serum bank at the Reference Laboratory for Chagas Disease (Oswaldo Cruz Foundation, Pernambuco, Brazil). Samples were from 186 chagasic (Ch) patients diagnosed with chronic Chagas disease with confirmed clinical, epidemiological, and serological diagnosis living in endemic areas in the State of Pernambuco, Brazil, which have been monitored at the Chagas Disease and Heart Failure Outpatient Clinic (PROCAPE) from the University of Pernambuco, Brazil. The patient selection was based on the positivity in two positive serological tests for Chagas disease and clinical tests (radiologic examination, electrocardiogram, chest X-ray, and echocardiogram) for characterization of the clinical forms. According to their clinical status, patients were classified as follows: mild cardiac (N = 49), who presented cardiac alterations but no heart dilatation; severe cardiac (N = 46), who presented clinical signs of severe cardiomyopathy with heart enlargement; digestive (N = 16); cardio-digestive (N = 29); and indeterminated patients (N = 46) that were asymptomatic.11 Negative serum samples (non-chagasic [NCh]) were obtained from healthy blood donors (N = 499) (Pernambuco Blood Bank, Hemope Foundation, Brazil) from a Chagas-endemic area. Beside the positive and negative sera, 748 patients with unrelated diseases, as defined by their serological diagnoses, composed the serum collection to evaluate cross-reaction: dengue (N = 50), filariasis (N = 50), hepatitis B virus (N = 87), hepatitis C virus (HCV; N = 55), human immunodeficiency virus (N = 115), human T-cell lymphotropic virus (N = 62), Leishmania ssp. (N = 35), leptospirosis (N = 98), rubella (N = 15), measles (N = 23), schistosomiasis (N = 43), and syphilis (N = 115). Serum samples were stored in sealed well-labeled microtubes at −20°C until enzyme immunoassays were performed.
Laboratory evaluation.
The selection of tests used in this work was based on Technical Note N°03/06 from the Brazilian Health Ministry,19 which evaluated the performance to detect the presence of specific anti-T. cruzi antibodies in 12 commercially available diagnostic tests in Brazil. The Technical Note recommends the use of tests with Se and Sp greater than or equal to 99% and 97%, respectively. Currently, only five of the 12 analyzed tests presented a valid registration in the Brazilian Health Surveillance Agency, but only four of them fulfilled the above criteria. Thus, the following commercial Chagas disease-specific enzyme immunoassays tests were selected for the present study: Imuno-ELISA Chagas (batch 14D061; Wama Diagnóstica, São Paulo, Brazil) and Pathozyme® Chagas (batch 7042779; Omega Diagnostics, Scotland, United Kingdom), which are based on recombinant antigens; ELISA Chagas III (batch 1F130525; BIOSChile, Ingeniería Genética S.A., Santiago, Chile), which uses whole extracts of T. cruzi strains Mn and Tulahuen as antigens; and Gold ELISA Chagas (batch CHA132A; Rem, São Paulo, Brazil), which uses both recombinant antigens and purified lysates from Brazilian strains of T. cruzi epimastigotes. All commercial tests were performed under strict adherence to the manufacturer's specifications. Additional positive and negative control sera, previously assayed for Chagas disease by Western blot (TESA blot; Biomérieux, Rio de Janeiro, Brazil) and IFA (Immunocruzi; Biomérieux) methods, were included in parallel. The cutoff values as well as the gray zone were calculated for each plate by using the following calculation: ([mean absorbance of low positive control]/1.5) for Imuno-ELISA Chagas and Pathozyme Chagas tests; ([mean absorbance reading of positive controls + mean absorbance reading of negative controls] × 0.35) for Chagas ELISA III; and ([mean absorbance reading of negative controls] + 0.180) for Gold ELISA Chagas. Enzyme immunoassays results, recorded as measurements of optical density (OD) at 450 nm, were expressed by plotting an index that represents the ratio between the OD of the samples and the OD of the cutoff. This index is referred to as reactivity index (RI) and all results < 1.00 were considered negative. However, samples were deemed inconclusive (or in the gray zone) if the RI values fell into the undetermined zone, which was defined as RI values of 1.0 ± 10% to Gold ELISA Chagas and ELISA Chagas III; and 1.0–1.5% to Imuno-ELISA Chagas and Pathozyme Chagas.
Statistical analysis.
Data were encoded and analyzed using scatter computer graphic software (Prism version 6; GraphPad, San Diego, CA). Descriptive statistics were presented as geometric mean ± standard deviation. To test the normality of data sets, the Shapiro–Wilk test followed by Student's t test was used, and when homogeneity presumption was not confirmed, the Wilcoxon signed-rank test was used. All analyses were two-tailed, and a P value of less than 5% was considered significant (P value < 0.05). The enzyme immunoassays tests performance was computed using a dichotomous approach and compared in terms of Se, Sp, accuracy, and Youden index (J).20,21 Confidence interval (CI) was used to address precision of the proportion estimates, and the magnitude of confidence was set to 95%. The strength of agreement with EIA tests was assessed by the Cohen's kappa coefficient (κ),22 which accounts for agreement taking place only by chance beyond simple percent agreement calculations. Its values are interpreted as poor (κ ≤ 0), slight (0 < κ ≤ 0.20), fair (0.21 < κ ≤ 0.40), moderate (0.41 < κ ≤ 0.60), substantial (0.61 < κ ≤ 0.80), and almost perfect agreement (0.81 < κ ≤ 1.0).
Ethical considerations.
This investigation was performed after approval by the Ethical Committee for Human Research from Aggeu Magalhães Research Center, Oswaldo Cruz Foundation, Recife, Brazil (CAEE: 15812213.8.0000.5190), following the principles expressed in the Declaration of Helsinki.
Results
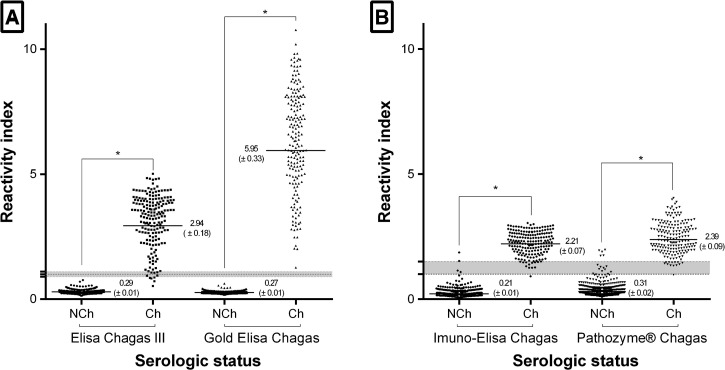
A total of 1,433 serum samples were evaluated by all four commercial IgG T. cruzi enzyme immunoassays. IgG survey in the serum samples of 186 Ch patients showed varied values of positivity, ranging from 95.2% for Pathozyme Chagas and 96.2% for ELISA Chagas III to 99.5% for Imuno-ELISA Chagas and 100% for Gold ELISA Chagas. Figure 1 shows that, as expected, antibody levels against T. cruzi antigens were higher in sera from Chagas disease patients compared with those from NCh individuals (P < 0.0001). The positive Ch serum samples displayed reactivity when assayed with every commercial test. However, different distributions of the RIs were observed, rendering discrimination efficiency values of 5.95, 2.94, 2.39, and 2.21 for Gold ELISA Chagas, ELISA Chagas III, Pathozyme Chagas, and Imuno-ELISA Chagas, respectively.
Figure 1.
Anti-Trypanosoma cruzi IgG level in serum samples from chagasic (Ch) and non-chagasic (NCh) individuals. Samples were tested using ELISA Chagas III or Gold ELISA Chagas (Panel A), and Imuno-ELISA Chagas or Pathozyme® Chagas (Panel B). The cutoff value is 1.0 and shadowed area represents the gray zone. Horizontal lines and numbers for each group of results represent the geometric means (±standard deviation). * Significant different mean values (P < 0.0001) between positive and negative samples.
On the basis of the total number of samples testing positive or negative, Ses and Sps of each EIA test were calculated (Table 1). The serological analysis of 186 Ch and 499 NCh patients by Gold ELISA Chagas test provided correct results for both positive and negative samples with 100% sensibility, Sp, and accuracy, and no inconclusive reactions were observed. ELISA Chagas III test showed 100% Sp, followed by Imuno-ELISA Chagas with 99.2% and Pathozyme Chagas with 97.0%. ELISA Chagas III showed higher false-negative rates than the others. Differently from the Gold ELISA Chagas, which was found to be 100% accurate, the three other tests presented inconclusive results (see the shadowed area in Figure 1). The strength of agreement between expected results obtained with Ch and NCh serum samples varied from 100% for the Gold ELISA Chagas to 94.2% for the Pathozyme Chagas. Intermediate values were found for ELISA Chagas III and Imuno-ELISA Chagas (Table 1). There were no statistically significant differences among the different clinical presentations when the presence of specific antibodies against crude and/or recombinant antigens was assessed (data not shown).
Table 1.
Diagnostic performance and strength of agreement of enzyme immunoassay kits for Trypanosoma cruzi IgG detection
| Kit | Se % (95% CI) | Sp % (95% CI) | Accuracy % (95% CI) | J index (%) | κ % (95% CI) | Agreement |
|---|---|---|---|---|---|---|
| ELISA Chagas III* | 97.3 (93.8–98.8) | 100 (99.2–100) | 99.0 (97.9–99.5) | 96.9 | 97.4 (95.5–99.3) | Almost perfect |
| Imuno-ELISA Chagas† | 99.5 (97.0–99.9) | 99.2 (98.0–99.7) | 99.3 (98.3–99.7) | 98.7 | 98.2 (96.6–99.8) | Almost perfect |
| Gold ELISA Chagas*† | 100 (98.0–100) | 100 (99.2–100) | 100 (99.4–100) | 100 | 100 | Perfect |
| Pathozyme® Chagas† | 99.5 (97.0–99.9) | 97.0 (95.1–98.2) | 97.7 (96.2–98.6) | 96.5 | 94.2 (91.4–97.0) | Almost perfect |
CI = confidence interval; ELISA = enzyme-linked immunosorbent assay; κ = Cohen's kappa coefficient; Se = sensitivity; Sp = specificity.
Crude antigen.
Recombinant antigen.
Next, we assessed the Chagas-specific diagnostic products to unrelated diseases, in a cross-reactivity test. Table 2 points to an overall 2.4% cross-reaction for the ELISA Chagas III, particularly to Leishmania ssp. (42.9%). Gold ELISA Chagas results showed 1.2% cross-reaction, particularly to Leishmania ssp. (17.1%). Imuno-ELISA Chagas returned 5.5% cross-reaction, especially to rubella (20.0%), measles (13.0%), Leishmania ssp. (8.6%), and syphilis (7.8%). Pathozyme Chagas displayed 2.1% cross-reaction, more prominently to HCV (9.1%), Leishmania ssp. (8.6%), and rubella (6.7%). Inconclusive results are also shown in the Table 2.
Table 2.
Anti-Trypanosoma cruzi cross-reactive antibodies detected by enzyme immunoassay kits for T. cruzi IgG detection
| Disease | ELISA Chagas III* (%) | Imuno-ELISA Chagas† (%) | Gold ELISA Chagas*† (%) | Pathozyme® Chagas† (%) |
|---|---|---|---|---|
| Dengue (N = 50) | 0 | 0 | 0 | R: 1 (2.0) |
| I: 1 (2.0) | ||||
| Filariasis (N = 50) | 0 | 0 | 0 | 0 |
| HBV (N = 87) | 0 | R: 6 (6.9) | 0 | 0 |
| I: 4 (4.6) | ||||
| HCV (N = 55) | 0 | R: 1 (1.8) | 0 | R: 5 (9.1) |
| I: 1 (1.8) | I: 5 (9.1) | |||
| HIV (N = 115) | R: 1 (0.9) | R: 6 (5.2) | R: 1 (0.9) | 0 |
| I: 6 (5.2) | ||||
| HTLV (N = 62) | 0 | R: 4 (6.5) | 0 | R: 2 (3.2) |
| I: 3 (4.8) | I: 1 (1.6) | |||
| Leishmania ssp. (N = 35) | R: 15 (42.9) | R: 3 (8.6) | R: 6 (17.1) | R: 3 (8.6) |
| I: 3 (8.6) | I: 2 (5.7) | I: 1 (2.9) | I: 3 (8.6) | |
| Leptospirosis (N = 98) | I: 1 (1.0) | R: 6 (6.1) | R: 1 (1.0) | 0 |
| I: 1.4 (4.1) | ||||
| Rubella (N = 15) | 0 | R: 3 (20.0) | 0 | R: 1 (6.7) |
| I: 2 (13.3) | I: 1 (6.7) | |||
| Measles (N = 23) | 0 | R: 3 (13.0) | 0 | 0 |
| I: 3 (13.0) | ||||
| Schistosomiasis (N = 43) | R: 1 (2.3) | 0 | 0 | R: 1 (2.3) |
| I: 1 (2.3) | ||||
| Syphilis (N = 115) | R: 1 (0.9) | R: 9 (7.8) | R: 1 (0.9) | R: 3 (2.6) |
| I: 6 (5.2) | I: 3 (2.6) | |||
| Total (N = 748) | R: 18 (2.4) | R: 41 (5.5) | R: 9 (1.2) | R: 16 (2.1) |
| I: 4 (0.5) | I: 31 (4.1) | I: 1 (0.1) | I: 15 (2.0) |
HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; HTLV = human T-cell lymphotropic virus; I = inconclusive results; R = reactivity.
Crude antigen.
Recombinant antigen.
Discussion
Our study assessed the accuracy of four commercially available T. cruzi tests based on EIA principles. In the past years, it has been repeatedly reported that chronic Chagas disease is commonly diagnosed by a wide variety of serologic assays that can lead to false-positive results or, perhaps more importantly, failure to detect true positives.23–25 Total or semi-purified homogenate of T. cruzi as well as recombinant or chimeric proteins were used as antigen, introducing a source of variability in the final product that can lead to controversial results. For this reason, it is evident the need to identify an optimal antigenic reagent for obtaining an accurate diagnosis. In this study, we compared the performance of the ELISA Chagas III, the Imuno-ELISA Chagas, the Gold ELISA Chagas, and the Pathozyme Chagas. The main results are that all four EIA tests gave satisfactory results, with sensibility and Sp ranging from 97.3% to 100% and from 97.0% to 100%, respectively. The Gold ELISA Chagas was the only one found to be 100% accurate. Differences in the performances among them are not statistically significant as judging by the overlapping CI. All tests are easily performed, with controls, conjugate, and substrate supplied in color-coded bottles as ready-to-use solutions, and results could be obtained in within 2 hours. All tests are 8-well strip plates totalizing 96 tests for Imuno-ELISA Chagas and Pathozyme Chagas and 192 tests for ELISA Chagas III. The Gold ELISA Chagas provides 480 tests, making it difficult to use in laboratories with low demand for Chagas disease serology. Visual reading was possible only by using Gold ELISA Chagas due to lack of background. Cost analysis was not performed in this study.
RI against T. cruzi antigen was statistically higher in sera from Chagas disease patients compared with those from NCh patients, supporting the notion that all evaluated tests have a good discriminatory ability toward the diagnosis of T. cruzi infection in patients in the chronic phase of Chagas disease. The index of the positive specimens obtained by using Gold ELISA Chagas was better (twice) than with other tests. Concerning the NCh specimens, the lowest RI was achieved by using Imuno-ELISA Chagas. According to Duarte and others,8 differences in RI values can be explained by differences in composition and mixtures of synthetic peptides or recombinant T. cruzi proteins.
The ELISA Chagas III was found to be 97.3% sensitive and 100% specific, which is in perfect agreement with other published results. As shown by the Technical Note N°03/06 from the Brazilian Health Ministry,19 the ELISA Chagas III showed 99.0% sensibility and 98.0% Sp. Otani and others25 deduced the same conclusion by showing 99.4% sensibility and 99.6% Sp. Remesar and others26 used latent class analysis to reach 95.6% Se and 99.8% Sp. Analysis of the sera negative for Chagas disease but positive for unrelated diseases showed a cross-reactivity of 42.9% for patients with leishmaniasis. These cross-reactions may be because the ELISA Chagas III employs whole extracts of T. cruzi Mn and Tulahuen strains. The observed cross-reaction against anti-Leishmania antibodies can be attributed to molecular mimicry between antigenic structures.14 Indeed, an Argentine study in a co-endemic area for Chagas disease and leishmaniasis showed 81.8% (9/11) cross-reactivity by using conventional ELISA27 and a relative large number of false-positive or inconclusive results have been reported using conventional serologic testing.13,28,29
Imuno-ELISA Chagas and Pathozyme Chagas use recombinant proteins as antigen. As has already been shown, this approach proved to be successful, with improvement of Sp and Se.13,15,16 Herein, both tests were found to display 99.5% Se. Similar results were obtained by Peralta and others,14 who showed that recombinant antigens were 91–93% sensitive. These data are in good agreement with others.13,14,25,30 In our hands, 5.5% of the unrelated diseases specimens were incorrectly classified as Chagas disease (false positive) and 4.1% were deemed inconclusive. As expected for a test that uses recombinant antigens, the cross-reactivity to leishmaniasis was less frequent with the Imuno-ELISA Chagas. Similar outcomes were found with the Pathozyme Chagas, which showed 8.6% cross-reactivity for leishmaniasis. Although these products have shown high performance, special attention should be given to the excessive cross-reaction results to non-leishmaniasis samples. Interestingly, Imuno-ELISA Chagas test displayed cross-reactivity also to rubella and measles. In fact, regarding unrelated diseases, cross-reaction was more frequently observed with the Imuno-ELISA Chagas test than with any other kit tested, which was probably due to the amino acid content or antigenic conformation. This happens when the similarity between two different epitopes are shared by different pathogens. However, it might be solved by excising the peptide fragments responsible for cross-reaction.31 Our findings are in agreement with Saba and others data,32 which reported 47.9% and 46.3% cross-reactivity to measles and rubella, respectively, when a recombinant peptide was used in EIA tests on French serum samples (from residents of non-endemic area).
Gold ELISA Chagas, which employs both recombinant proteins and purified lysates from T. cruzi epimastigotes of Brazilian strains as antigen, showed to be 100% sensitive, specific, and accurate. Similar results were reported by the Brazilian Health Ministry.19 Concerning the cross-reactivity, we observed that 1.2% of unrelated diseases cross-react against T. cruzi antigens, and this cross-reactivity occurred only for leishmaniasis samples. These findings were expected since the test employs purified lysates from the T. cruzi aside from recombinant proteins.
The characteristics of a serological test for Chagas disease depend on circumstances of its application. Silveira and others16 argued that, for laboratory diagnosis, diagnostic tests should have high Sp to avoid false-positive results, which could lead to psychological suffering, social discrimination, and unnecessary treatment. With that in mind, the main findings in this work allowed us to conclude that all assessed tests may be used for laboratory diagnosis. However, a special attention should be given to the Pathozyme Chagas test, which has shown 97% Sp, statistically lower than ELISA Chagas III and Gold ELISA Chagas. Conversely, if the intended application is blood screening, a highly sensitive test is advised, since a false negative may transmit the parasite and the consequences are catastrophic. We observed that all tests are highly sensitive and may be used for blood screening, except ELISA Chagas III that presented 2.7% false-negative results. Despite high performance and reproducibility, all tests should be used with caution in endemic areas for unrelated diseases, particularly to leishmaniasis. The data presented herein suggest the need for further research for new diagnostic markers, with no cross-reactivity with other diseases.
ACKNOWLEDGMENTS
We would like to address a special thanks to Maria Betania do Amaral Pinto and staff at Pernambuco Blood Bank, Hemope Foundation (Recife, Brazil), for their assistance in collection of samples. We are grateful to Alexandre D. Costa (Carlos Chagas Institute, Fiocruz-Paraná, Brazil) for the critical reading of the manuscript.
Disclaimer: The findings, interpretations and conclusions expressed here are entirely those of the authors and do not necessarily represent the views of the institutions they work for or are affiliated with.
Footnotes
Financial support: The funding support was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Proc. 404242/2012-0) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco-FACEPE (Proc. APQ-1257-2.11/12). Yara de Miranda Gomes, Wayner Vieira de Sousa, and Marco Aurélio Krieger are research fellows of CNPq Proc. No. 304543/2012-8, 306222/2013-2, and 590032/2011-9, respectively.
Authors' addresses: Fred Luciano Neves Santos, Michelle da Silva Barros, Mineo Nakazawa, and Yara de Miranda Gomes, Reference Laboratory for Chagas Disease, Aggeu Magalhães Research Center, Oswaldo Cruz Foundation, Recife, Brazil, E-mails: fred.santos@cpqam.fiocruz.br, michelle.barros@cpqam.fiocruz.br, nakazawa@cpqam.fiocruz.br, and yara@cpqam.fiocruz.br. Wayner Vieira de Souza, Public Health Department, Aggeu Magalhães Research Center, Oswaldo Cruz Foundation, Recife, Brazil, E-mail: wayner@cpqam.fiocruz.br. Marco Aurélio Krieger, Carlos Chagas Institute–Molecular Biology Institute of Paraná, Curitiba, Brazil, E-mail: kriegeribmp@gmail.com.
References
- 1.Amato Neto V, Lopes M, Umezawa ES, Aveiro Ruocco M, Dias JC. Outras formas de transmissão do Trypanosoma cruzi. Rev Patol Trop. 2000;29:115–129. [Google Scholar]
- 2.Steindel M, Kramer Pacheco L, Scholl D, Soares M, de Moraes MH, Eger I, Kosmann C, Sincero TCM, Stoco PH, Murta SM, de Carvalho-Pinto CJ, Grisard EC. Characterization of Trypanosoma cruzi isolated from humans, vectors, and animal reservoirs following an outbreak of acute human Chagas disease in Santa Catarina State, Brazil. Diagn Microbiol Infect Dis. 2008;60:25–32. doi: 10.1016/j.diagmicrobio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . The World Health Report. 2002. Annex Table 2—Deaths by cause, sex and mortality stratum in WHO regions, estimates for 2002.http://www.who.int/whr/2004/annex/topic/en/annex_2_en.pdf Available at. Accessed January 10, 2015. [Google Scholar]
- 4.Hotez PJ, Dumonteil E, Woc-Colburn L, Serpa JA, Bezek S, Edwards MS, Hallmark CJ, Musselwhite LW, Flink BJ, Bottazzi ME. Chagas disease: “the new HIV/AIDS of the Americas. PLoS Negl Trop Dis. 2012;6:e1498. doi: 10.1371/journal.pntd.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coura JR, Viñas PA. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Manne-Goehler J, Reich MR, Wirtz VJ. Access to care for Chagas disease in the United States: a health systems analysis. Am J Trop Med Hyg. 2015;93:108–113. doi: 10.4269/ajtmh.14-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rassi A, Rassi A, Marcondes de Rezende J. American trypanosomiasis (Chagas disease) Infect Dis Clin North Am. 2012;26:275–291. doi: 10.1016/j.idc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Duarte LF, Flórez O, Rincón G, González CI. Comparison of seven diagnostic tests to detect Trypanosoma cruzi infection in patients in chronic phase of Chagas disease. Colomb Med. 2014;45:61–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Wendel Neto S. Current concepts on the transmission of bacteria and parasites by blood components. Sao Paulo Med J. 1995;113:1036–1052. doi: 10.1590/s1516-31801995000600007. [DOI] [PubMed] [Google Scholar]
- 10.Cancino-Faure B, Fisa R, Riera C, Bula I, Girona-Llobera E, Jimenez-Marco T. Evidence of meaningful levels of Trypanosoma cruzi in platelet concentrates from seropositive blood donors. Transfusion. 2015;55:1249–1255. doi: 10.1111/trf.12989. [DOI] [PubMed] [Google Scholar]
- 11.Brazilian Health Ministry Brazilian consensus on Chagas disease [in Portuguese] Rev Soc Bras Med Trop. 2005;38:7–29. [PubMed] [Google Scholar]
- 12.Gadelha AA, Vercosa AF, Lorena VM, Nakazawa M, Carvalho AB, Souza WV, Ferreira AG, Silva ED, Krieger MA, Goldenberg S, Gomes YM. Chagas' disease diagnosis: comparative analysis of recombinant ELISA with conventional ELISA and the haemagglutination test. Vox Sang. 2003;85:165–170. doi: 10.1046/j.1423-0410.2003.00340.x. [DOI] [PubMed] [Google Scholar]
- 13.Gomes YM, Pereira VR, Nakazawa M, Rosa DS, Barros MD, Ferreira AG, Silva ED, Ogatta SF, Krieger MA, Goldenberg S. Serodiagnosis of chronic Chagas infection by using EIE-Recombinant-Chagas-Biomanguinhos kit. Mem Inst Oswaldo Cruz. 2001;96:497–501. doi: 10.1590/s0074-02762001000400009. [DOI] [PubMed] [Google Scholar]
- 14.Peralta JM, da GM Teixeira M, Shreffler WG, Pereira JB, Burns JM, Sleath PR, Reed SG. Serodiagnosis of Chagas' disease by enzyme-linked immunosorbent assay using two synthetic peptides as antigens. J Clin Microbiol. 1994;32:971–974. doi: 10.1128/jcm.32.4.971-974.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umezawa ES, Bastos SF, Coura JR, Levin MJ, Gonzalez A, Rangel-Aldao R, Zingales B, Luquetti AO, da Silveira JF. An improved serodiagnostic test for Chagas' disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion. 2003;43:91–97. doi: 10.1046/j.1537-2995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 16.Da Silveira JF, Umezawa ES, Luquetti AO. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 2001;17:286–291. doi: 10.1016/s1471-4922(01)01897-9. [DOI] [PubMed] [Google Scholar]
- 17.Camussone C, Gonzalez V, Belluzo MS, Pujato N, Ribone ME, Lagier CM, Marcipar IS. Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clin Vaccine Immunol. 2009;16:899–905. doi: 10.1128/CVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández P, Heimann M, Riera C, Solano M, Santalla J, Luquetti AO, Beck E. Highly effective serodiagnosis for Chagas' disease. Clin Vaccine Immunol. 2010;17:1598–1604. doi: 10.1128/CVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brazilian Health Ministry Nota Técnica N° 03/06, CGLAB/CGDT/DEVEP/SVS/MS, Brasília, Brazil. Resultado da Avaliação dos “kits” para diagnóstico de doença de Chagas. 2006. http://www.chagas.cl/3_evaluaciones/est_comp_msb.pdf Available at. Accessed March 1, 2015.
- 20.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Ouchchane L, Rabilloud M, Boire J-Y. Sensibilité, spécificité et valeurs prédictives. In: Beuscart R, Bénichou J, Roy P, Quantin C, editors. Évaluation des méthodes d'analyse appliquées aux sciences de la vie et de la santé—Biostatistique. Paris, France: Omniscience; 2009. pp. 49–78. [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 23.Pirard M, Iihoshi N, Boelaert M, Basanta P, López F, Van der Stuyft P. The validity of serologic tests for Trypanosoma cruzi and the effectiveness of transfusional screening strategies in a hyperendemic region. Transfusion. 2005;45:554–561. doi: 10.1111/j.0041-1132.2005.04214.x. [DOI] [PubMed] [Google Scholar]
- 24.Furucho CR, Umezawa ES, Almeida I, Freitas VL, Bezerra R, Nunes EV, Sanches MC, Guastini CM, Teixeira AR, Shikanai-Yasuda MA. Inconclusive results in conventional serological screening for Chagas' disease in blood banks: evaluation of cellular and humoral response. Trop Med Int Health. 2008;13:1527–1533. doi: 10.1111/j.1365-3156.2008.02172.x. [DOI] [PubMed] [Google Scholar]
- 25.Otani MM, Vinelli E, Kirchhoff LV, del Pozo A, Sands A, Vercauteren G, Sabino EC. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion. 2009;49:1076–1082. doi: 10.1111/j.1537-2995.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 26.Remesar MC, Gamba C, Colaianni IF, Puppo M, Sartor PA, Murphy EL, Neilands TB, Ridolfi MA, Leguizamón MS, Kuperman S, Del Pozo AE. Estimation of sensitivity and specificity of several Trypanosoma cruzi antibody assays in blood donors in Argentina. Transfusion. 2009;49:2352–2358. doi: 10.1111/j.1537-2995.2009.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vega Benedetti AF, Cimino RO, Cajal PS, Juarez MD, Villalpando CA, Gil JF, Marcipar IS, Krolewiecki AJ, Nasser JR. Performance of different Trypanosoma cruzi antigens in the diagnosis of Chagas disease in patients with American cutaneous leishmaniasis from a co-endemic region in Argentina. Trop Med Int Health. 2013;18:1103–1109. doi: 10.1111/tmi.12144. [DOI] [PubMed] [Google Scholar]
- 28.Almeida IC, Covas DT, Soussumi LM, Travassos LR. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion. 1997;37:850–857. doi: 10.1046/j.1537-2995.1997.37897424410.x. [DOI] [PubMed] [Google Scholar]
- 29.Longhi SA, Brandariz SB, Lafon SO, Niborski LL, Luquetti AO, Schijman AG, Levin MJ, Gómez KA. Evaluation of in-house ELISA using Trypanosoma cruzi lysate and recombinant antigens for diagnosis of Chagas disease and discrimination of its clinical forms. Am J Trop Med Hyg. 2012;87:267–271. doi: 10.4269/ajtmh.2012.11-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flores-Chávez M, Cruz I, Rodríguez M, Nieto J, Franco E, Gárate T, Cañavate C. Comparación de técnicas serológicas convencionales y no convencionales para el diagnóstico de la enfermedad de Chagas importada en España. Enferm Infecc Microbiol Clin. 2010;28:284–293. doi: 10.1016/j.eimc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Garcia VS, Gonzalez VD, Marcipar IS, Gugliotta LM. Immunoagglutination test to diagnose Chagas disease: comparison of different latex-antigen complexes. Trop Med Int Health. 2014;19:1346–1354. doi: 10.1111/tmi.12379. [DOI] [PubMed] [Google Scholar]
- 32.Saba ES, Gueyffier L, Dichtel-Danjoy ML, Pozzetto B, Bourlet T, Gueyffier F, Mekki Y, Pottel H, Sabino EC, Vanhems P, Zrein MA. Anti-Trypanosoma cruzi cross-reactive antibodies detected at high rate in non-exposed individuals living in non-endemic regions: seroprevalence and association to other viral serologies. PLoS One. 2013;8:e74493. doi: 10.1371/journal.pone.0074493. [DOI] [PMC free article] [PubMed] [Google Scholar]