Abstract
Data on the burden of dengue and its economic costs can help guide health policy decisions. However, little reliable information is available for Colombia. We therefore calculated the burden of the disease, expressed in disability-adjusted life years (DALYs), for two scenarios: endemic years (average number of cases in non-epidemic years 2011 and 2012) and an epidemic year (2010, when the highest number of dengue cases was reported in the study period). We also estimated the total economic cost of the disease (U.S. dollars at the average exchange rate for 2012), including indirect costs to households derived from expenses such as preventing entry of mosquitos into the home and costs to government arising from direct, indirect, and prevention and monitoring activities, as well as the direct medical and non-medical costs. In the epidemic year 2010, 1,198.73 DALYs were lost per million inhabitants versus 83.88 in endemic years. The total financial cost of the disease in Colombia from a societal perspective was US$167.8 million for 2010, US$129.9 million for 2011, and US$131.7 million for 2012. The cost of mosquito prevention borne by households was a major cost driver (accounting for 46% of the overall cost in 2010, 62% in 2011, and 64% in 2012).
Introduction
The dengue virus, responsible for dengue fever (DF) and dengue hemorrhagic fever (DHF), is the most geographically widespread vector-borne infection in countries of the southern hemisphere. In recent years, a resurgence of the disease has occurred in Latin America.1 In 2010, Colombia had a major outbreak with more than 150,000 cases and 289 deaths, while more than a million reported cases occurred throughout Latin America in 2012, with 187,647 of these cases in the Andean subregion (Bolivia, Colombia, Ecuador, Peru, and Venezuela).2 In the first half of 2013, 24,116 cases of DF were reported in the Andean subregion, with 990 deaths, yielding a mortality rate of 0.5%.3
The high incidence of dengue disease and the associated burden4 represent a prominent public health problem, which is further compounded by the complexity of disease control and the factors associated with transmission (demographic, ecological, entomological, environmental, and social).5 Inhabitants of endemic areas may be particularly aware of the disease if they have experienced serious consequences, such as the death of a family member or neighbor.6,7
In Colombia, legislation passed in 1990 (and subsequently in 1993 and 2001) initiated a decentralization process, whereby the responsibility for top-down vector control programs devolved by the Ministry of Health and Social Protection to the states (known as departments) and municipalities. In the current setup, the ministry directly allocates funds for the programs to the departments and districts, which also use their own resources for these activities. Individuals also personally pay for certain protective measures such as repellents or mosquito nets. All these actions together incur direct and indirect costs to society and individuals. Evaluation of these costs is important to enable informed decision making and appropriate allocation of resources to the most cost-effective strategies for controlling dengue.8–10
We have previously calculated the cost per case to the health system and to the individual of DF and DHF.11 The cost to the health system was derived from official data sources, whereas the cost to the individual and households was based on an extensive survey of a population-based sample. However, the true cost of DF and DHF to society includes other components.12 In this article, we present estimates from 2010 to 2012 for the burden of the disease and the overall cost, calculated as the sum of medical costs, income lost owing to premature death, loss of productivity, and expenditure on direct, indirect, and prevention and monitoring activities for dengue infection in Colombia.
Methods
Epidemiological data.
Data on the incidence of DF and DHF, age of onset, and sex distribution used for calculation and modeling of the burden of disease were taken from cases notified to the National Public Health Surveillance System (Sistema Nacional de Vigilancia en Salud Pública [SIVIGILA]) for the period 2010–2012. SIVIGILA is a nationwide platform that systematically collects information of relevance to public health in Colombia at a municipal level; notification is mandatory for certain transmissible diseases, such as dengue, as well as other nontransmissible diseases, such as cancer.13 Deaths due to dengue infection were identified from the databases of the Colombian National Statistics Administrative Department (Departamento Administrativo Nacional de Estadística [DANE], http://www.dane.gov.co/index.php/poblacion-y-registros-vitales/nacimientos-y-defunciones/nacimientos-y-defunciones) as of 2013. Two scenarios were defined: endemic years (2011 and 2012 in the study period, where the average number of symptomatic dengue infections was calculated) and an epidemic year (referring to 2010, the year in the study period with the highest number of cases).
Burden of disease.
We estimated the burden of disease by calculating the disability-adjusted life years (DALYs) according to the model first described by Murray.14 This model uses the formula shown below:
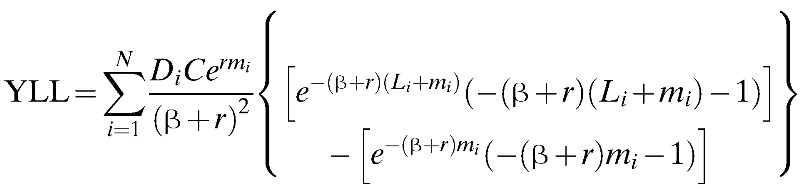 |
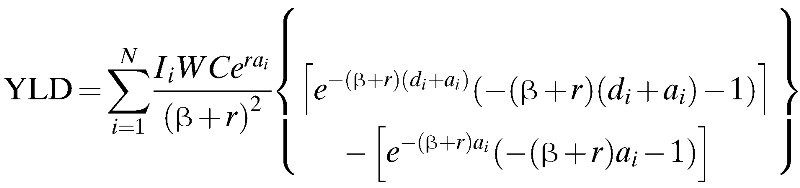 |
where D is a weighting for disability, r is the discount rate, C and β are age-weighting adjustments, a is the age of onset, m is for death, W is the life expectancy for each age, I is disease incidence, d is the years lost due to disability (YLD) and L is the years of life lost (YLL) to premature death. The subindices i indicate age group. The disability weighting for DF of 0.81 (0.6–0.92) was derived from a study by Meltzer and others.15
Using data from the 2005 DANE statistics, an exponential population growth model was derived to identify the trends in the average size and distribution of the population of Colombia in the endemic and epidemic years by age and sex and thus to estimate the YLL. The duration of disease was defined as 15 days (range = 10–21 days) based on the data from Meltzer and others.15
The above information was entered into the DISMOD II program, a computer application distributed free of charge by the World Health Organization (WHO).16 The goal of using DISMOD II is to verify that the calculated DALYs are comparable to the WHO theoretical models. DISMOD uses the downhill simplex method, which is a method of estimation of a multifactorial quantity obtained by varying factors using geometrical concepts. This allows uncertainty intervals to be calculated.17 We used this to obtain consistent estimators for the behavior of the disease and data on specific mortality due to dengue, the accumulated incidence and risk of dengue, together with the starting age and the average duration of the disease, separated by sex and age groups.
We obtained information on treated cases from the SIVIGILA database (individual record of health care provided). For the final calculations, a social discount rate of r = 0.03, an age-weighting coefficient of β = 0.04, and an age-weighting constant of C = 0.1648 were used.18
Sensitivity analyses for K, which is a parameter used to remove non-uniform age weights, were performed as indicated below:
where R is the social value of age and a is age. When K takes the value of the weighting function of age, K is equal to the value used by Murray in his study14; when K is 0, all ages receive equal weight. Thus, for the analysis values, β = 0.04, C is assigned a value of 0.1648 and K a value of 0 and 1. When K = 0, the effect of the parameter is abolished. K = 1 was the value proposed by Murray and has been used in previous studies of disease burden.
A sensitivity analysis was performed using only the population at risk in an urban area living below 1,800 m above sea level (DANE data). Additional sensitivity analyses were performed with variations in age of presentation (< 1 year, 1–4 years, and then 5-year intervals up to > 80 years), duration of disease (10–21 days), and disability weighting (values between 0.60 and 0.92). In addition, a sensitivity analysis was performed using Japanese life expectancies (life expectancy of 73.81 years for men and 76.94 years for women), but otherwise DANE (http://www.dane.gov.co/index.php/estadisticas-por-tema/demografia-y-poblacion) data were used for Colombia (to investigate the impact of longer life expectancy). Finally, a sensitivity analysis was performed assuming underreporting of DF and DHF, using the underreporting factors estimated by Shepard and others19 (eight for DF and 1.3 for DHF).
Costs of dengue.
The total costs related to dengue infection in Colombia were estimated as the sum of 1) treatment costs (direct medical and non-medical costs); 2) indirect costs (loss of productivity and absenteeism of both the patient and carer in the case of nonfatal episodes); 3) costs arising from lost income resulting from premature death; and 4) costs of prevention and monitoring campaigns (both by government and households, including awareness campaigns, control, and surveillance) for each period, that is, 2010, 2011, or 2012. The number of cases was taken from the SIVIGILA database for the corresponding year. All costs were calculated in pesos and converted to U.S. dollars at the average exchange rate for 2012 (1,798.23 pesos per U.S. dollars).
Total treatment costs included direct medical costs to the health system (medical appointments, treatments, and other services) and households (including co-payment of medical expenses), non-medical costs, and indirect costs to the patient and their households. For calculation of direct medical costs to the health system, information was extracted from the Individual Registries for Health Service Provision (Registro Individual de Prestación de Servicios de Salud [RIPS]). Direct and indirect medical costs to households were derived from the results of a survey of 1,483 households conducted by the Center of Economic Development Studies (Centro de Estudios sobre Desarrollo Económico) of the Universidad de los Andes, Bogotá, Colombia, in 2012 (see Castro and others11 for full methodological details of the survey). The estimate of the total cost under this heading corresponds to the average values per case for the period 2010–2012 multiplied by the number of cases reported by SIVIGILA in the same period (Table 1).
Table 1.
Cases of dengue in Colombia, 2010–2012
| Disease classification | Setting | No. of cases (%) | ||
|---|---|---|---|---|
| 2010 | 2011 | 2012 | ||
| Dengue fever | Outpatient | 107,016 (69.9) | 19,366 (59.3) | 33,054 (57.7) |
| Hospitalized | 36,404 (23.8) | 11,970 (36.7) | 22,719 (39.7) | |
| Dengue hemorrhagic fever | Intensive care | 9,745 (6.4) | 1,303 (4.0) | 1,465 (2.6) |
| Total | 153,165 | 32,639 | 57,238 | |
Data from Sistema Nacional de Vigilancia en Salud Pública, Bogotá, Colombia.
The estimate for the average loss of income owing to premature death had also been calculated previously.11 In the calculations presented here, total cost due to loss of income was calculated using the number of deaths recorded in each year of the study period (using the SIVIGILA database and the Extensive Integrated Household Survey [Gran Encuesta Integrada de Hogares conducted by the DANE] to determine the mean salary).
The costs of prevention and monitoring activities takes into account spending by national and local government bodies and households. The figure for government expenditure for these campaigns and control of the vector includes allocations made by central government to regional bodies (departments and districts) for activities concerning vector-borne diseases, and dengue in particular, according to the particular needs of each regional body. For allocations to vector-borne diseases in general, a weighting was assigned to the part corresponding to dengue based on examination of actual expenditure in each department (44.75% in 2010, 45.58% in 2011, and 44.75% in 2012). The figure also includes contracts from the Colombian Ministry of Health and Social Protection for the purchase of materials and supplies specifically for dengue and expenditure that local bodies have incurred under the terms of the Collective Action Plans (activities performed by districts to prevent, monitor, and raise awareness about dengue). The latter component was estimated by calculating an average allocation per person from a sample of municipalities and departments. The total value was then calculated by extrapolation to give a figure for the overall population at risk, which, as explained previously,11 was limited to approximately 24 million inhabitants of municipalities at an altitude below 1,800 m, where transmission is thought to occur.20
The resources allocated by the government for epidemiological surveillance, under the auspices of the Colombian National Health Institute (Instituto Nacional de Salud [INS]), comprised fees paid to staff and expenditure on technical support (transport, travel costs, and support for dengue-related events) for each of the years analyzed.
Finally, the expenditure incurred by households comprised expenditure on elements such as shutters, nets, repellents, screens, and insecticides to reduce the presence and/or restrict the intrusion of mosquitos into the household. The total cost to households for prevention was calculated by extrapolating the average costs obtained to the total population at risk, with a weighting factor assumed to be the same as used above for government spending to account for the fact that not all the above costs would apply exclusively to control the mosquito vector of the dengue virus.
To assess the uncertainty in the cost calculations, a bootstrapping analysis was performed with 10,000 repetitions, with variation in the elements recorded in the home survey and the RIPS. In addition, the impact of potential underreporting was investigated by running the calculations with underreporting factors detailed previously (eight for DF and 1.3 for DHF).19
Results
In the endemic years 2011 and 2012, 3,989.7 DALYs were lost annually (83.88 per million inhabitants). In 2010 epidemic year, the total number of DALYs lost was more than 10 times greater (57,017 DALYs in total; 1,198.73 per million inhabitants) (Table 2).
Table 2.
DALYS lost per million inhabitants in the endemic and epidemic scenarios, by sex, in Colombia
| Age (years) | Men | Women | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Population size | DALYs* (endemic) | DALYs* (epidemic) | Population size | DALYs* (endemic) | DALYs* (epidemic) | Population size | DALYs* (endemic) | DALYs* (epidemic) | |
| 0–4 | 1,815,577 | 340.94 | 2,150.57 | 1,707,616 | 363.08 | 1,650.82 | 3,523,193 | 351.67 | 1,908.35 |
| 5–14 | 3,604,510 | 102.75 | 2,308.24 | 3,396,185 | 153.22 | 1,773.47 | 7,000,695 | 127.23 | 2,048.81 |
| 15–29 | 7,222,646 | 81.49 | 1,345.59 | 6,960,513 | 55.39 | 1,013.06 | 14,183,159 | 68.68 | 1,182.39 |
| 30–44 | 5,703,263 | 71.40 | 1,325.24 | 5,946,071 | 25.91 | 1,085.65 | 11,649,334 | 48.18 | 1,202.95 |
| 45–59 | 3,319,696 | 18.38 | 768.81 | 3,539,977 | 48.50 | 660.88 | 6,859,673 | 33.93 | 726.66 |
| 60–69 | 1,161,649 | 36.33 | 85.15 | 1,279,273 | 13.47 | 14.93 | 2,440,922 | 34.35 | 48.35 |
| 70–79 | 667,696 | 7.74 | 47.07 | 798,468 | 29.02 | 29.44 | 1,466,164 | 19.33 | 37.47 |
| ≥ 80 | 192,682 | 0.09 | 21.28 | 248,923 | 2.14 | 12.56 | 441,605 | 6.88 | 16.36 |
| Total | 23,687,719 | 88.42 | 1,362.78 | 23,877,026 | 79.37 | 1,035.97 | 47,564,745 | 83.88 | 1,198.73 |
DALY = disability-adjusted life-year.
In-house calculations are based on mortality information from Departamento Administrativo Nacional de Estadística. Cases occurring are from Sistema Nacional de Vigilancia en Salud Pública, Bogotá, Colombia.
No. of DALYs per million inhabitants.
In the sensitivity analysis of the population at risk in an urban area and living below 1,800 m, the burden of disease ranged from 765.4 to 1,376.5 DALYs per million inhabitants in the endemic scenario and from 9,845.9 to 13,584.2 DALYs per million inhabitants in the epidemic scenario. When the life expectancy data for Japan were used, the DALYs ranged from 201.3 to 4,120.5 per million inhabitants in the endemic scenario and from 12,347.6 to 46,781.0 per million inhabitants in the epidemic scenario. Finally, in the sensitivity analysis for underreporting, the burden of disease was 108.12 DALYs per million inhabitants in the endemic scenario and 1,222.28 DALYs per million inhabitants in the epidemic scenario.
The total treatment cost (Table 3 based on the cost per case analysis presented by Castro and others11) incurred by the health system for the period 2010–2012 was much higher in the epidemic year 2010 compared with the endemic years 2011 and 2012 (for 2010 versus 2011: 3.3-fold higher for outpatient DF, 1.8-fold higher for hospitalized DF, and 6-fold higher for DHF settings). Similarly, the estimated direct medical and non-medical costs incurred by households were much higher in the epidemic year compared with the endemic years. Indirect costs (arising from loss of income for both patients and caregivers) were six times greater in the epidemic year (2010) compared with the endemic years (2011 and 2012). Table 3 also shows the estimated values for loss of income through premature death. This figure, like indirect costs arising from loss of income, was more than six times greater in 2010 compared with the other two years of the study. Table 3 also shows the 95% confidence intervals calculated using the bootstrap approach described in the Methods section. To investigate the impact of underreporting, the above costs were recalculated (along with 95% confidence intervals from the bootstrap analysis) using the underreporting factors described by Shepard and others19 (eight for DF and 1.3 for DHF). Using these factors led to approximately a 3.5-fold increase in cost compared with the calculations with no underreporting (Table 4).
Table 3.
Total cost to the health system and households between 2010 and 2012 and cost of loss of income due to premature death (with no correction for underreporting)
| Type of cost | Cost (US$) (95% CI*) | ||
|---|---|---|---|
| 2010 | 2011 | 2012 | |
| Cost to health system† | |||
| Outpatient DF | 5,650,192 (5,586,556–5,713,360) | 1,804,802 (1,753,591–1,851,990) | 1,569,644 (1,438,477–1,703,801) |
| Hospitalized DF | 8,585,571 (8,013,977–9,145,049) | 4,601,103 (3,912,993–5,104,008) | 5,160,365 (4,320,472–5,810,157) |
| DHF | 14,736,394 (8,939,089–24,885,807) | 2,412,136 (1,983,166–2,789,072) | 2,907,075 (2,076,198–3,898,951) |
| Total | 28,972,157 (22,539,621–39,744,215) | 8,818,041 (7,649,750–9,745,069) | 9,637,084 (7,835,147–11,412,910) |
| Cost to household | |||
| Direct medical costs‡ | |||
| Outpatient DF | 1,421,718 (1,176,112–1,692,248) | 257,279 (212,833–306,235) | 439,126 (363,265–522,684) |
| Hospitalized DF | 1,268,639 (1,101,221–1,468,579) | 417,141 (362,092–482,883) | 791,732 (687,250–916,510) |
| DHF | 558,397 (442,114–715,721) | 74,663 (59,115–95,699) | 83,946 (66,465–107,597) |
| Total | 3,248,754 (2,719,447–3,876,548) | 749,084 (634,041–884,818) | 1,314,804 (1,116,980–1,546,791) |
| Direct non-medical costs§ | |||
| Outpatient DF | 3,177,839 (2,903,072–4,466,444) | 575,073 (525,350–627,300) | 981,538 (896,671–1,070,679) |
| Hospitalized DF | 1,698,782 (1,569,223–1,836,728) | 558,576 (515,976–603,935) | 1,060,175 (979,320–1,146,265) |
| DHF | 610,148 (540,237–686,268) | 81,583 (72,235–91,761) | 91,726 (81,216–103,169) |
| Total | 5,486,769 (5,012,532–5,989,440) | 1,215,232 (1,113,561–1,322,995) | 2,133,439 (1,957,207–2,320,113) |
| Indirect costs | |||
| Loss of productivity (patient) | 4,667,680 (3,669,908–5,821,233) | 1,003,138 (778,103–1,266,680) | 1,762,657 (1,364,210–2,230,539) |
| Loss of productivity (caregiver) | 3,462,388 (2,839,031–4,192,188) | 743,476 (614,549–893,959) | 1,284,073 (1,063,016–1,542,116) |
| Total | 8,130,068 (6,508,939–10,013,421) | 1,746,614 (1,392,651–2,160,639) | 3,046,730 (2,427,227–3,772,655) |
| Loss of income due to premature death | 17,680,602 | 2,847,140 | 2,908,688 |
| Total costs | 63,518,350 (54,461,141–77,304,226) | 15,376,111 (13,637,144–16,960,661) | 19,040,745 (16,245,249–21,961,157) |
CI = confidence interval; DF = dengue fever; DHF = dengue hemorrhagic fever.
Costs are derived from the survey of Centro de Estudios sobre Desarrollo Económico, prepared from Ministry of Health and Social Protection and survey of households, Registro Individual de Prestación de Servicios de Salud (RIPS; 2010–2012), RIPS sample of towns (2010–2012), and Sistema Nacional de Vigilancia en Salud Pública (2010–2012) and are based on cost per case analysis presented by Castro and others.11
Calculated using a bootstrap method (see text for details).
Includes medical appointments, treatments, and other services.
Co-payment and medicines.
Transport, nurses' and carers' fees, lodging, food, child carers' fees, changes to living quarters, post-disease expenses, and other expenses.
Table 4.
Total cost to the health system and households between 2010 and 2012 and cost of loss of income due to premature death (with correction for underreporting)
| Type of cost | Cost (US$) (95% CI*) | ||
|---|---|---|---|
| 2010 | 2011 | 2012 | |
| Cost to health system† | 104,492,248 (89,271,056–129,691,203) | 32,373,664 (29,343,487–34,821,992) | 32,681,911 (27,658,635–37,665,162) |
| Cost to household | |||
| Direct medical costs‡ | 16,997,645 (14,134,680–20,254,125) | 3,446,663 (2,884,278–4,086,857) | 5,966,190 (5,002,931–7,059,605) |
| Direct non-medical costs§ | 33,911,095 (30,979,409–37,000,886) | 6,648,026 (6,081,039–7,245,800) | 11,483,218 (10,509,272–12,509,813) |
| Indirect costs¶ | 52,814,507 (42,562,718–64,632,220) | 10,086,761 (8,196,274–12,497,799) | 17,427,649 (14,105,003–21,526,503) |
| Loss of income due to premature death | 17,680,602 | 2,847,140 | 2,908,688 |
| Total costs | 225,896,097 (194,628,463–269,259,035) | 55,402,254 (49,352,219–61,499,589) | 70,467,656 (60,184,530–81,669,772) |
CI = confidence interval.
Costs are derived from the survey of Centro de Estudios sobre Desarrollo Económico, prepared from Ministry of Health and Social Protection and survey of households, Registro Individual de Prestación de Servicios de Salud (RIPS; 2010–2012), RIPS sample of towns (2010–2012) and Sistema Nacional de Vigilancia en Salud Pública (2010–2012) and are based on cost per case analysis presented by Castro and others.11
Calculated using a bootstrap method (see text for details).
Includes medical appointments, treatments, and other services.
Co-payment and medicines.
Transport, nurses' and carers' fees, lodging, food, child carers' fees, changes to living quarters, post-disease expenses, and other expenses.
Loss of productivity.
The total costs for prevention and monitoring activities did not show sharp year-on-year variations compared with other dengue-associated costs (Table 5). These costs were mainly borne by households (73% on average). Of note is that the cost of epidemiological surveillance undertaken by central government through the INS is almost insignificant (4%) compared with the total cost.
Table 5.
Total costs for prevention, awareness campaigns, control, and surveillance (prevention and monitoring activities)
| Type of cost | Cost (US$) (95% CI*) | ||
|---|---|---|---|
| 2010 | 2011 | 2012 | |
| Costs of prevention for households | 77,303,117 (72,330,832–82,275,403) | 80,941,711 (75,712,167–86,171,258) | 84,921,809 (78,978,777–90,864,844) |
| Costs of prevention, awareness campaigns, and control for government | 26,923,049 | 33,518,468 | 27,645,552 |
| Transfers from the Ministry of Health and Social Protection | 9,177,877 | 13,042,424 | 9,337,393† |
| Contracts | 0 | 1,278,987 | 0‡ |
| Expenditure on collective action plans | 17,745,172 | 19,197,057 | 18,308,160 |
| Cost of epidemiological surveillance | 41,712 | 42,610 | 44,488 |
| Total costs | 104,267,878 (99,295,594–109,240,164) | 114,502,790 (109,273,245–119,732,336) | 112,611,849 (106,668,818–118,554,884) |
CI = confidence interval.
In-house calculations are based on Registro Individual de Prestación de Servicios de Salud, survey of households, Ministry of Public Health and Social Protection, Sistema Nacional de Vigilancia en Salud Pública, Health Secretariats, Instituto Nacional de Salud, and Departamento Administrativo Nacional de Estadística.
Calculated using a bootstrap method (see text for details).
Figures calculated in pesos for December 2012 (Resolutions 404/2012 and 461; 5233/11) and converted to U.S. dollars.
Information available as of September 2012.
The total cost of DF (managed either in outpatient and hospital settings) and DHF, calculated by summing all the individual cost components above, is US$167.8 million for 2010, US$129.8 million for 2011, and US$131.7 million for 2012. The summary is shown in Table 6, including figures adjusted for underreported cases.
Table 6.
Total cost of dengue fever and dengue hemorrhagic fever
| Type of cost | Cost (millions of US$) |
||
|---|---|---|---|
| 2010 (million) | 2011 (million) | 2012 (million) | |
| Total without correction | 167.8 | 129.8 | 131.7 |
| With correction for underreporting | 330.1 | 169.9 | 183.1 |
In-house calculations are based on Registro Individual de Prestación de Servicios de Salud, survey of households, Ministry of Public Health and Social Protection, Sistema Nacional de Vigilancia en Salud Pública, Health Secretariats, Instituto Nacional de Salud, Departamento Administrativo Nacional de Estadística, and Shepard and others.19
Discussion
Information on the burden of disease and economic costs is important to allow informed policy decisions to be made and to allocate finite resources to minimize suffering due to ill health.14 In the case of dengue infection, the burden of disease is recognized to be high,19 and there are some indications that this burden may be underestimated.17,21 Moreover, the epidemiology of the disease may be changing, with factors such as globalization, climate change, and urbanization potentially contributing to a risk of more widespread epidemics.22 The economic burden of dengue infection is also high, although many estimates focus on the cost of medical care and neglect other cost components, such as awareness campaigns and prevention measures.23
In Colombia, where the disease is endemic, specific data on burden of disease on a national level are not available and general extrapolations may be subject to error in the case of dengue infection.24 In the case of economic costs, we recently published data on medical costs per case of DF and DHF in Colombia.11 Here, we extend that analysis to overall costs of the disease and, as before, our analysis included a breakdown by epidemic and endemic years.
For the burden of disease, our estimate of 83.88 DALYs per million inhabitants for endemic years (2011 and 2012) is much lower than the estimate of 1,198.73 DALYs per million inhabitants for the epidemic year (2010). Other studies have also noted large year-on-year variations generally in line with our estimates. For example, Meltzer and others15 found on average 658 DALYs per million inhabitants between 1984 and 1994 in Puerto Rico, with a minimum value of 145 DALYs (in 1984) and a maximum value of 1,492 DALYs (in 1994). The number of reported cases of dengue in Puerto Rico was 10-fold higher in 1994 than in 1984. In our study, more than 150,000 cases were reported in 2010 versus 33,000 cases in 2011 and 57,000 cases in 2012. In an analysis in Nicaragua, of the years 1996–2010, the annual DALYs per million inhabitants ranged from 99 in 2004, the year with lowest incidence of cases in the study period, to 805 in 2010, the year with highest incidence of cases.25 The figures for Latin American countries appear to be similar to those reported in other regions; for example, a recent study reported a range from 240.3 to 1,006 DALYs in Cambodia.26 In general, most of the burden of disease is attributed to DF rather than DHF,25,26 presumably reflecting the much higher incidence of DF. In the sensitivity analyses performed for individuals in urban areas below 1,800 m, the burden of disease was found to be much higher and in line with that reported in studies in Brazil27 and Thailand.28
One of the advantages of estimating burden of disease through DALYs is that it is possible to compare the impact of different diseases. For example, Meltzer and others15 concluded that the burden of dengue in Puerto Rico was similar to the burden attributed in Latin American countries to diseases such as meningitis, childhood infectious diseases (polio, mumps, pertussis, diphtheria, and tetanus), hepatitis, and malaria. Another study of neglected tropical diseases in Latin America and the Caribbean placed dengue infection fifth in terms of DALYs, behind hookworm infection, ascariasis, trichuriasis, and Chagas disease.4 Moreover, in the case of dengue infection, the authors recognized there are some indications that the burden may be underestimated given the potential for underreporting of the disease.17,21 Some comparisons of estimates of DALYS in Colombia are shown in Table 7.29
Table 7.
Comparison of DALYs found in the burden of disease study at the Javeriana University 201029
| Disease | DALYs per million inhabitants | |
|---|---|---|
| Our study | Burden of disease in Colombia | |
| Dengue | ||
| Endemic scenario | 4,000 | 20* |
| Epidemic scenario | 57,017 | 20,000* |
| Cysticercosis | NA | 3,000 |
| AIDS | NA | 21,000 |
| Hypertensive heart disease | NA | 131,000 |
AIDS = acquired immunodeficiency syndrome; DALY = disability-adjusted life-year; NA = not available.
2010: point estimate with 2008 data for number of deaths and number of cases from Sistema Nacional de Vigilancia en Salud Pública for 2010.
In the economic analysis shown in Tables 3 and 4, interesting findings are highlighted. Table 3 shows estimated costs of treatment and management of the disease, which are 6-fold greater for households and 3-fold greater for the health system in the epidemic year (2010) versus the non-epidemic years (2011 and 2012). A different pattern is seen in Table 5, which shows that costs relating to prevention and monitoring activities accounted for between 62% (2010) and 88% (2011) of the total cost of DF (Table 6). Our results for costs relating to prevention and monitoring activities are high compared with figures of 43% in Panama,30 49% in Puerto Rico,12 and 39% in Thailand,31 and may reflect differences in the weighting given to dengue for measures aimed at mosquito control. They may also reflect the high proportion of this expense borne by households (74%). Interestingly, although overall cost increased in epidemic years as expected and in line with other studies, costs for prevention and monitoring activities show an increasing trend in the household expenses, indicating awareness in the population about dengue infection in Colombia. Moreover, government costs increased after the epidemic year but returned to the initial level the following year (2012). This may reflect the way budget allocation occurs in Colombia; when the epidemic occurred in 2010, an increased budget for prevention and control was allocated to the following fiscal year.
Finally, in the macroeconomic context of Colombia, the cost of dengue in an endemic period (2012) represented 107.61% of the budget for the Expanded Program on Immunization, 0.14% of the total national budget, and 0.036% of the gross domestic product, reflecting the considerable economic impact on a country with scant resources.
Potential limitations of the study include underestimation of both the burden of disease and costs. Our incidence data are based on entries in the SIVIGILA database where notification of cases of dengue is mandatory and so patients who seek medical care for their disease are likely to be registered in the system, assuming that DF and DHF are correctly diagnosed. This is in contrast to the study in Nicaragua reported by Wettstein and others,25 in which expansion factors were applied because the Ministry of Health database used in that study captured considerably fewer cases than those estimated from cohort studies.32 Patients who do not seek medical attention are not included in our estimate, but it can also be supposed that the burden of diseases and costs in these patients will be minimal. Although, for these reasons, the impact of underreporting on the results for costs is debatable, we nevertheless performed the cost calculations assuming the underreporting factors (Table 6) derived from a recent work that attempted to quantify underreporting.19 In the epidemic year, the total costs increased approximately 2-fold, whereas the increases were smaller in the endemic years, presumably mainly reflecting higher treatment costs. No sensitivity analysis was performed for possible underreporting of deaths. Although it might be expected that such events would not be underreported, recent evidence suggests that this may indeed be the case, even in places such as Puerto Rico with well-funded surveillance systems.33
The extent to which the household survey used for calculating costs can be considered representative was discussed in our previous analysis of medical costs per case.11 Overall, we considered our sample as representative of the population of patients with dengue infection in Colombia but biases could not be ruled out.
In conclusion, the high burden of disease for dengue, as measured by DALYs, is confirmed in Colombia. Similarly, the economic costs of the disease are high and are largely borne by households. This highlights the importance of considering such expenditures when studying the economic impact of the disease.
ACKNOWLEDGMENTS
We thank Leonardo García, Juan Pablo Pimentel, and José Israel Galindo for their part in obtaining the primary information. We also acknowledge the editorial assistance with the preparation of the manuscript provided by the professional medical writer, Greg Morley of MedSense.
Footnotes
Financial support: The funding of this project was provided by Sanofi Pasteur.
Disclosure: Juan Guillermo Lopez Yescas is an employee of Sanofi Pasteur.
Authors' addresses: Raúl Castro Rodríguez, Katia Galera-Gelvez, and Jorge A. Rueda-Gallardo, Department of Economics, Facultad de Economía, Universidad de los Andes, Bogotá, Colombia, E-mails: rcastro@uniandes.edu.co, k-galera@uniandes.edu.co, and ja.rueda929@uniandes.edu.co. Gabriel Carrasquilla, Centro de Estudios e Investigación en Salud (CEIS), Fundación Santa Fe de Bogotá, Bogotá, Colombia, E-mail: gabriel.carrasquilla@fsfb.org.co. Alexandra Porras, Fundación Santa Fe de Bogotá, Universidad El Bosque, Bogotá, Colombia, E-mail: porras.alexandra@gmail.com. Juan Guillermo Lopez Yescas, Sanofi Pasteur Latin America, Mexico City, Mexico, E-mail: juanguillermo.lopez@sanofi.com.
References
- 1.San Martín JL, Brathwaite O, Zambrano B, Solórzano JO, Bouckenooghe A, Dayan GH, Guzmán MG. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan American Health Organization Health Situation in the Americas: Basic Indicators. 2013. http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=23535&Itemid=270&lang=en Available at. Accessed October 7, 2015.
- 3.San Martín JL. Situación Epidemiológica del Dengue en las Américas. Bucaramanga, Colombia: Pan American Health Organization; 2014. http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=&gid=26803&lang=en Available at. Accessed October 7, 2015. [Google Scholar]
- 4.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapia-Conyer R, Méndez-Galván JF, Gallardo-Rincón H. The growing burden of dengue in Latin America. J Clin Virol. 2009;46((Suppl 2)):S3–S6. doi: 10.1016/S1386-6532(09)70286-0. [DOI] [PubMed] [Google Scholar]
- 6.Quintero J, Carrasquilla G, Suárez R, González C, Olano VA. An ecosystemic approach to evaluating ecological, socioeconomic and group dynamics affecting the prevalence of Aedes aegypti in two Colombian towns. Cad Saude Publica. 2009;25((Suppl 1)):S93–S103. doi: 10.1590/s0102-311x2009001300009. [DOI] [PubMed] [Google Scholar]
- 7.Suárez R, González C, Carrasquilla G, Quintero J. An ecosystem perspective in the socio-cultural evaluation of dengue in two Colombian towns. Cad Saude Publica. 2009;25((Suppl 1)):S104–S114. doi: 10.1590/s0102-311x2009001300010. [DOI] [PubMed] [Google Scholar]
- 8.Borrero E, Carrasquilla G, Alexander N. Decentralization and health system reform: what is their impact on malaria incidence in Colombian municipalities [in Spanish]? Bioméd Rev Inst Nac Salud. 2012;32((Suppl 1)):68–78. doi: 10.1590/S0120-41572012000500009. [DOI] [PubMed] [Google Scholar]
- 9.Carrasquilla G. Descentralización Y Gestión Del Control de Las Enfermedades Transmisibles En América Latina. Buenos Aires, Argentina: Organización Panamericana de la Salud; 2006. Descentralización, reforma sectorial y control de la malaria en Colombia. [Google Scholar]
- 10.Pinto D, Carrasquilla G, Gil F, Collazos C, Rincón J. La Certificación Como Indicador de Descentralización En Salud. Una Mirada a La Luz Del Cumplimiento de Requisitos N Municipios Colombianos. Gerencia Y Políticas de Salud, Bogotá: Pontificia Universidad Javeriana; 2005. [Google Scholar]
- 11.Castro R, Galera K, López Yescas JG, Rueda JA. Costs of dengue fever to the health system and individuals in Colombia from 2010 to 2012. Am J Trop Med Hyg. 2015;92:709–714. doi: 10.4269/ajtmh.14-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halasa YA, Shepard DS, Zeng W. Economic cost of dengue in Puerto Rico. Am J Trop Med Hyg. 2012;86:745–752. doi: 10.4269/ajtmh.2012.11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zea D, Osorio L. The status of the dengue surveillance system in a Colombian municipality [in Spanish] Rev Salud Publica (Bogota) 2011;13:785–795. doi: 10.1590/s0124-00642011000500007. [DOI] [PubMed] [Google Scholar]
- 14.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- 15.Meltzer MI, Rigau-Pérez JG, Clark GG, Reiter P, Gubler DJ. Using disability-adjusted life years to assess the economic impact of dengue in Puerto Rico: 1984–1994. Am J Trop Med Hyg. 1998;59:265–271. doi: 10.4269/ajtmh.1998.59.265. [DOI] [PubMed] [Google Scholar]
- 16.Mathers CD, Vos T, Lopez AD, Salomon J, Ezzati M, editors. National Burden of Disease Studies: A Practical Guide. 2nd edition. Geneva, Switzerland: World Health Organization; 2001. WHO Global Program on Evidence for Health Policy. [Google Scholar]
- 17.Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr. 2003;1:4. doi: 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray CJL, Lopez AD. The Global Burden of Disease and Injury Series, Volume 1: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Geneva, Switzerland: World Health Organization; 1996. [Google Scholar]
- 19.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg. 2011;84:200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuno G. Review of the factors modulating dengue transmission. Epidemiol Rev. 1995;17:321–335. doi: 10.1093/oxfordjournals.epirev.a036196. [DOI] [PubMed] [Google Scholar]
- 21.Torres JR, Castro J. The health and economic impact of dengue in Latin America. Cad Saude Publica. 2007;23((Suppl 1)):S23–S31. doi: 10.1590/s0102-311x2007001300004. [DOI] [PubMed] [Google Scholar]
- 22.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 23.Gubler DJ. The economic burden of dengue. Am J Trop Med Hyg. 2012;86:743–744. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT-A, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 25.Wettstein ZS, Fleming M, Chang AY, Copenhaver DJ, Wateska AR, Bartsch SM, Lee BY, Kulkarni RP. Total economic cost and burden of dengue in Nicaragua: 1996–2010. Am J Trop Med Hyg. 2012;87:616–622. doi: 10.4269/ajtmh.2012.12-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beauté J, Vong S. Cost and disease burden of dengue in Cambodia. BMC Public Health. 2010;10:521. doi: 10.1186/1471-2458-10-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luz PM, Grinsztejn B, Galvani AP. Disability adjusted life years lost to dengue in Brazil. Trop Med Int Health. 2009;14:237–246. doi: 10.1111/j.1365-3156.2008.02203.x. [DOI] [PubMed] [Google Scholar]
- 28.Clark DV, Mammen MP, Nisalak A, Puthimethee V, Endy TP. Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg. 2005;72:786–791. [PubMed] [Google Scholar]
- 29.Peñaloza R, Salamanca N, Rodríguez JM, Rodríguez J, Beltrán A. Estimación de la carga de enfermedad para Colombia 2010. Bogotá, Colombia: Javeriana University; 2014. [Google Scholar]
- 30.Armien B, Suaya JA, Quiroz E, Sah BK, Bayard V, Marchena L, Campos C, Shepard DS. Clinical characteristics and national economic cost of the 2005 dengue epidemic in Panama. Am J Trop Med Hyg. 2008;79:364–371. [PubMed] [Google Scholar]
- 31.Shepard DS, Undurraga EA, Halasa YA. Economic and disease burden of dengue in southeast Asia. PLoS Negl Trop Dis. 2013;7:e2055. doi: 10.1371/journal.pntd.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Standish K, Kuan G, Avilés W, Balmaseda A, Harris E. High dengue case capture rate in four years of a cohort study in Nicaragua compared to national surveillance data. PLoS Negl Trop Dis. 2010;4:e633. doi: 10.1371/journal.pntd.0000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomashek KM, Gregory CJ, Rivera Sánchez A, Bartek MA, Garcia Rivera EJ, Hunsperger E, Muñoz-Jordán JL, Sun W. Dengue deaths in Puerto Rico: lessons learned from the 2007 epidemic. PLoS Negl Trop Dis. 2012;6:e1614. doi: 10.1371/journal.pntd.0001614. [DOI] [PMC free article] [PubMed] [Google Scholar]


