Abstract
Q fever is an important cause of undifferentiated fever that is rarely recognized or reported in Brazil. The objective of this study was to look for the presence of Coxiella burnetii during a dengue fever outbreak in the municipality of Itaboraí, Rio de Janeiro, Brazil, where this bacterium had previously infected humans and domesticated animals. Blood samples from clinically suspected dengue fever patients were tested by polymerase chain reaction (PCR) for C. burnetii; the DNA was detected in nine (3.3%) of 272 patients. One was coinfected with dengue virus, which was also detected in another 166 (61.3%) patients. The nucleotide sequence of PCR amplification and DNA sequencing of the IS1111 transposase elements in the genome of C. burnetii exhibited 99% identity with the sequence in GenBank. The detection of C. burnetii in patients suspected of dengue fever indicates that awareness and knowledge of Q fever should be strengthened and that this bacterium is present in Brazil. Finally, because a negative molecular result does not completely rule out the diagnosis of Q fever and the serological assay based on seroconversion was not available, the actual number of this zoonosis is likely to be much higher than that reported in this study.
Introduction
Q fever is a zoonosis caused by Coxiella burnetii, a small intracellular gram-negative bacterium that is naturally maintained in many species: mammals, birds, and arthropods. Transmitted to humans primarily by aerosol inhalation, the bacterium causes a broad-spectrum clinical manifestation including self-limiting flu-like acute forms, chronic conditions, and, more rarely, endocarditis with negative blood cultures and death.1–3 Q fever is poorly known in most countries of the world, with the exception of the European countries, Australia, and the United States.4,5 Over 4,000 cases have been identified in the Netherlands,6 and an increasing number of cases have been identified in American military bases in Afghanistan and Iraq.7,8 Coxiella burnetii is considered as a tier 2 select agent and potential bioterrorism threat by the U.S. Centers for Disease Control and Prevention and has attracted much attention from the scientific community since 2009.9
Although serological tests for C. burnetii infection have been available in Brazil since 1953,10 it was not until 2008 that the bacterium was characterized molecularly from a patient with fever, thrombocytopenia, and a history of contact with goat abortion products in Rio de Janeiro, Brazil.11,12 Since then, although sporadic cases have been confirmed13–15 including endocarditis, the most severe form of chronic disease,16 it was only in 2014 that Q fever become a notifiable disease in Brazil. Acute Q fever is difficult to diagnose because it shares similar manifestations with a large number of infectious diseases, for example, fever, headache, myalgia, and/or pneumonia. Q fever can be confused with influenza, dengue, malaria, leptospirosis, and hantavirosis, among other diseases.17
In Brazil, where dengue outbreaks are often identified (e.g., the dengue virus [DENV] 1–4 introduction in 1986, 1990, 2000, and 2011),18 it is common for people exhibiting clinical features compatible with dengue to skip laboratory confirmation of the disease. This fact, combined with the lack of awareness of Q fever, make this zoonosis an apparently nonexistent infectious disease. It has been proposed that Q fever should be included in the list of differential diagnoses for flu-like diseases in the Brazilian territory. In this study, we looked for Q fever in suspected dengue patients in a public hospital in Itaboraí, Rio de Janeiro, where the first molecularly identified Brazilian case of Q fever was found in 2008.11
Materials and Methods
Patient and sample collection.
This hospital-based cross-sectional study was conducted at the Desembargador Leal Junior Hospital (DLJH) in the municipality of Itaboraí (22°44′51′′ S, 42°51′21′′ W), Rio de Janeiro, where C. burnetii infection was detected in human and domesticated animals in 2008.11,12 From March 2013 to October 2014, all patients admitted to DLJH with a diagnosis of dengue infection and who lived in Itaboraí or the surrounding areas were considered for entry into the study. Clinical and epidemiological data were systematically collected by the hospital's surveillance group using the national dengue surveillance system (Information System for Notifiable Diseases). Serum samples were tested in the laboratory of the DLJH for the presence of DENV nonstructural protein 1 by enzyme-linked immunosorbent assay (Plateleia Bio-Rad®, Hercules, CA) and dengue-specific IgM by capture assay (Panbio®, Orlando, FL). A 1-mL aliquot of blood was sent to the Laboratory of Hantaviroses and Rickettsioses, Oswaldo Cruz Institute, FIOCRUZ, for evaluation of C. burnetii infection (see Materials and Methods, section Determination of C. burnetii infection). This study was approved by the Ethics Committee of the FIOCRUZ, under number 552/10. Because these analyses were part of routine surveillance activities in an area with outbreaks of dengue and Q fever, patients were not required to provide informed consent.
Determination of C. burnetii infection.
Blood samples were stored at −20°C in the DLJH and transported to the Laboratory of Hantaviroses and Rickettsioses for molecular analysis. Blood samples were evaluated by polymerase chain reaction (PCR) as previously described, using primers targeting the IS1111 transposase elements in the genome of C. burnetii (5′-TATGTATCCACCGTAGCCAGTC-3′ and 5′-CCCAACAACACCTCCTTATTC-3′), which produce an amplification product of 687 bp.19–21 DNA was extracted from 200 μL of the blood samples using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) in accordance with the manufacturer's instructions. Negative controls were included in each extraction to check for possible DNA contamination. Each 25-μL PCR reaction was performed with 4 μL DNA in nuclease-free water (Promega, Madison, WI) containing 0.2 μM primers (IDT/Prodimol, Belo Horizonte, Brazil), 200 μM deoxynucleotide triphosphates, 1.5 mM MgCl2, and 0.1 U of Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA). The procedure consisted of an initial denaturation at 95°C for 5 minutes, then 40 consecutive cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 1 minute, then a final extension at 72°C for 7 minutes.
The PCR reactions were subjected to agarose gel electrophoresis, and the appropriately sized fragments were purified using the BigDye Terminator® X-Purification Kit (Applied Biosystems, Foster City, CA). The fragments were sequenced using the BigDye Terminator V3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA) on an ABI PRISM 3100 Nucleic Acid Sequence Analyzer (Applied Biosystems). The obtained sequences were compared with the GenBank C. burnetii IS1111 transposase elements sequences using the BLAST® program.22
Data analysis.
A χ2 test was used to compare epidemiological variables between dengue-positive and dengue-negative cases. Fisher's exact test was used in smaller samples. Statistical analyses were performed using BioEstat 5.0® (Belém, Brasil)23 and significance was set at P < 0.05. Significance was not tested for Q fever cases because of the small sample size.
Results
Patients and serological analysis.
In total, 272 patients were admitted to the DLJH with suspected dengue infection during the study period: 246 patients in 2013 and 26 in 2014. A total of 150 (58%) patients were women and 108 (425) were men, with a mean age of 35.2 ± 18.8 years and a range of 1 month to 88 years. Dengue infection was confirmed by laboratory tests in 167 (61.3%) patients. Among the 258 patients with available clinical manifestations, 156 (60.4%) were positive for dengue. The clinical and epidemiological data are shown in Table 1.
Table 1.
Comparison of the clinical and epidemiological data between dengue positive and negative cases reported in the municipality Itaboraí, Rio de Janeiro (2013–2014), Brazil
| Variable | Dengue positive (N = 156) n (%) | Dengue negative (N = 102) n (%) | P* |
|---|---|---|---|
| Gender | |||
| Male | 64 (41) | 44 (33.1) | 0.7368 |
| Female | 92 (58.9) | 58 (56.8) | |
| Age (years) | |||
| ≤ 10 | 5 (3.2) | 8 (7.8) | 0.0239 |
| 11–20 | 33 (21.1) | 30 (29.4) | |
| 21–30 | 25 (16) | 21 (20.5) | |
| 31–40 | 36 (23) | 9 (8.8) | |
| 41–50 | 24 (15.3) | 12 (11.7) | |
| ≥ 51 | 33 (32.6) | 20 (19.6) | |
| Fever | 152 (97.4) | 89 (87.2) | 0.0013 |
| Headache | 146 (93.5) | 82 (80.3) | 0.0012 |
| Myalgia | 134 (85.9) | 81 (79.4) | 0.1717 |
| Prostration | 86 (55.1) | 69 (67.6) | 0.0447 |
| Nausea/vomiting | 98 (62.8) | 55 (53.9) | 0.1549 |
| Retro-orbital pain | 98 (62.8) | 51 (50.0) | 0.0415 |
| Arthralgia | 60 (38.4) | 43 (42.1) | 0.5535 |
| Diarrhea | 51 (32.6) | 28 (27.4) | 0.3718 |
| Petechiae | 47 (30.1) | 19 (18.6) | 0.0384 |
| Rash | 36 (23.0) | 14 (13.7) | 0.0632 |
| Hemorrhagic manifestations | 2 (1.28) | 1 (0.9) | 1.000 |
| Respiratory disorders | 1 (0.64) | 1 (0.9) | 1.000 |
Statistically-significant P values are in bold.
Sample positive PCR and sequencing.
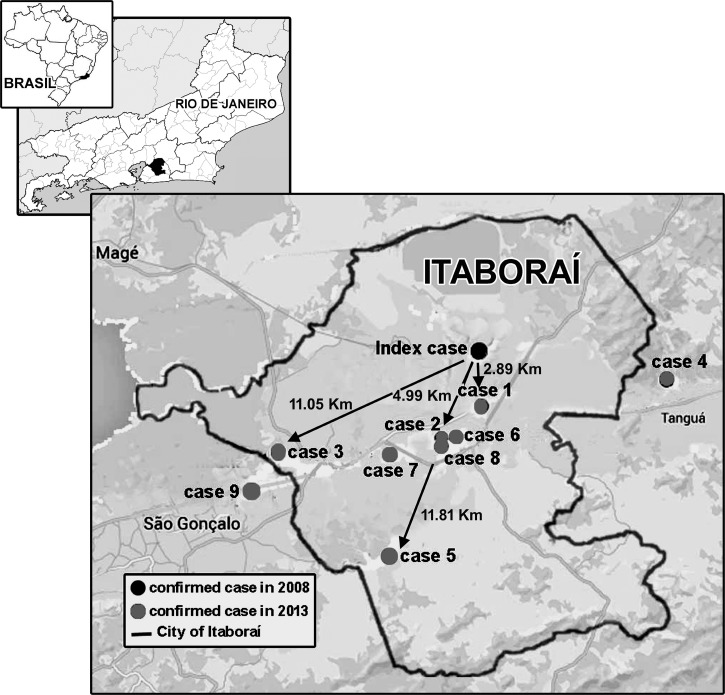
All 272 blood samples were analyzed for C. burnetii DNA by PCR. The appropriate 687-bp product was observed in nine samples, all from patients admitted during 2013. The geographic distribution of the Q fever cases in relation with index case confirmed in 2008 is displayed in Figure 1 . Cases 1, 2, 6, 7, and 8 were identified in peri-urban areas within 5 km of index area where animals that excreted C. burnetii were also detected.12 The other four cases were located more than 10 km away from the index case's house, in farm areas. One of these nine patients also had dengue infection, indicating that the patient was coinfected with both pathogens. The C. burnetii DNA fragment in the nine patients was 99% identical to the sequence in GenBank (Table 2).
Figure 1.
Spatial distribution of individuals with Q fever in Itaboraí and the surrounding areas, state of Rio de Janeiro, Brazil. The black dot represents the index Q fever cases (place of residence) and the gray dots represent the nine cases, which occurred during period of study.
Table 2.
Characteristics of the nine patients with Q fever, in Itaboraí and the surrounding areas, Rio de Janeiro, Brazil
| Patient | Age (years)/gender | Date of specimen collection (month/year) | Clinical manifestations* | GenBank accession no. |
|---|---|---|---|---|
| Patient 1† | 78/M | April/2013 | F, H, M, P, N/V, A | KP645185 |
| Patient 2 | 15/F | April/2013 | F, H, M, R, P, N/V | KP645186 |
| Patient 3 | 28/F | May/2013 | F, H, M, R, D, N/V | KP645187 |
| Patient 4 | 16/M | May/2013 | F, H, M, P, PT | KR091975 |
| Patient 5 | 13/F | September/2013 | F, ROP, AP, A | KP645188 |
| Patient 6 | 8/M | September/2013 | F, R, P | KP645189 |
| Patient 7 | 67/M | October/2013 | F, H, M, P, N/V, ROP, A | KP645190 |
| Patient 8 | 62/F | October/2013 | F, H, M, R, D, N/V, ROP, A | KP645191 |
| Patient 9 | 25/F | November/2013 | F, H, M, P, N/V, A | KR091976 |
F = female; M = male.
A = arthralgia; AP = abdominal pain; D = diarrhea; F = fever; H = headache; HM = hemorrhagic manifestations; M = myalgia; N/V = nausea/vomiting; P = prostration; PT = petechiae; R = rash; RD = respiratory disorders; ROP = retro-orbital pain.
Patient 1 contained presence of dengue virus nonstructural protein 1 by enzyme-linked immunosorbent assay (Plateleia Bio-Rad®) and dengue-specific IgM by capture assay (Panbio®), indicating coinfection with dengue virus.
Discussion
This article reports the findings of a Q fever surveillance study conducted in an area endemic for dengue in Rio de Janeiro, from March 2013 to October 2014. In Brazil, approximately 2 million dengue cases were recorded in 2013 and 587,800 dengue cases were recorded in 2014. One of the most affected areas was the state of Rio de Janeiro, where more than 220,000 dengue cases were recorded during this period, resulting from DENV-1 epidemic (Brazilian Ministry of Health, 2014)24. In the municipality of Itaboraí, where this study was performed, 5,881 and 376 dengue cases were recorded in 2013 and 2014, respectively. The relatively low number of dengue cases recorded in 2014 explains the relatively small number of cases included in this study in 2014.
Of the 272 patients we evaluated with suspected dengue fever, 61.3% were confirmed to be infected with DENV. The most common age group with dengue infection was 31–40 years. Fever, headache, prostration, petechiae, and retro-orbital pain were frequently observed in patients with dengue infection, consistent with previous studies.25,26 Seven of nine Q fever–positive patients had fever, headache, and myalgia,27 which are the most common symptoms of dengue (Table 1). The symptoms and differential diagnosis of these infectious diseases are similar, making accurate clinical diagnosis difficult without laboratory confirmation, especially during dengue outbreak.
Currently, serological testing is the most frequently used approach to identify Q fever. The definitive serological diagnosis of Q fever is based on seroconversion, performed with a blood sample collected in the early and convalescence phases in febrile cases.1 Because laboratory testing of suspected dengue cases routinely occurs in the first 5 days of illness, it was not possible during the study to obtain a second blood sample.26 For this reason, and, less often, because of an insufficient volume of the blood available, the Q feverserological assay was not included in this study.
During the study period, five Q fever cases were identified in peri-urban areas within 5 km of the index case's house whereas other cases were located more than 10 km away from the index case's house, in farm areas. Since the 1950s, several studies have demonstrated that wind plays a role in C. burnetii transmission.28–30 In this context, our results, in combination with conclusions from these studies associated with the presence of infected animals (sheep, dogs, and cats) in the index case's house,11,12 indicate that wind can be an epidemiologic factor in Q fever outbreaks occurring near sheep-rearing areas.
We identified Q fever based on the presence of the bacterium in the blood sample. The molecular analysis showed C. burnetii DNA in samples from 3.3% of the suspected dengue cases. Evidence of coinfection between DENV and C. burnetii was observed in only one of the 272 patients. Although we know of no reports of concomitant infection with C. burnetii and DENV, there are reports of patients with infectious endocarditis due to typical microorganisms (Streptococcus and Enterococcus) and C. burnetii31 and serological evidence of coinfection with C. burnetii and tick-borne agents such as Rickettsia conorii, Rickettsia slovaca, and Francisella tularensis.32 Since these infections can present overlapping clinical manifestations, Q fever should be included in the differential diagnosis of acute febrile diseases and infectious endocarditis in areas where Q fever cases have previously occurred.
Our findings, together with other data published in the last decade in Brazil—including the recent report of a Q fever outbreak in the state of São Paulo (Center for Strategic Information and Health Surveillance of São Paulo state, Brazil, 2015)33—highlight the need to include this zoonosis in febrile syndromic surveillance programs. This is especially important in cases where patients have compatible epidemiological history and have a risk factor for chronic Q fever, such as a history of valvular surgery, vascular grafting, aneurysm, kidney failure, immunosuppression, or advanced age.34
Acute Q fever is rarely recognized because it is asymptomatic or accompanied by mild clinical manifestations that do not require hospitalization. Given that all patients included in this study were admitted to the hospital and considering that serological testing for Q fever was not performed because of the lack of second blood sample and an insufficient volume of blood, the incidence of Q fever can be probably greater than the 3.3% observed here. Nevertheless, our observation is similar to a finding in Tanzania where serum antibodies against C. burnetii were detected in 5% of hospitalized patients.35 As this zoonosis was only recently (2014) classified as a notifiable disease in Brazil, its inclusion in febrile syndromic surveillance programs and a greater availability of diagnostic tests should lead to greater awareness and knowledge about this disease.
Conclusion
Although Q fever is usually a self-limiting disease, a specific antimicrobial therapy for the prevention of chronic Q fever should be used, mainly, for high-risk patients. Being more often similar to other infectious or noninfectious diseases, Q fever often goes unrecognized. This is especially true during outbreaks of other diseases with clinical manifestations similar to dengue, leptospirosis, and influenza, among others. However, given the potential for chronic Q fever development, greater attention should be given to this zoonosis, whose etiologic agent has been frequently identified in Brazil during the last decade. We conclude that Q fever is present in Brazil but can be misdiagnosed with other infectious diseases, especially dengue. We recommend that Q fever be included in the list of differential diagnoses of flu-like diseases, especially in patients with a compatible epidemiological history and having a risk factor for chronic Q fever.
Footnotes
Financial support: This study was supported by FIOCRUZ e CNPq.
Authors' addresses: Maria Angélica M. M. Mares-Guia, Tatiana Rozental, Alexandro Guterres, Michelle dos Santos Ferreira, and Elba R. S. Lemos, Laboratório de Hantavivoses e Rickettsioses, Fundação Instituto Oswaldo Cruz (FIOCRUZ), Rio de Janeiro, Brazil, E-mails: amguia@ioc.fiocruz.br, rozental@ioc.fiocruz.br, guterres@ioc.fiocruz.br, micferreira1@gmail.com, and elba.lemos@ioc.fiocruz.br. Renato De Gasperis Botticini, Laboratório de Análises Clínicas, Hospital Municipal Desembargador Leal Junior, Rio de Janeiro, Brazil, E-mail: labhmdlj@itaborai.rj.gov.br. Ana Kely Carolina Terra and Sandro Marraschi, Vigilância Epidemiológica Municipal de Itaboraí, Município de Itaboraí, Rio de Janeiro, Brazil, E-mails: anakely.terra@gmail.com and sandromarraschi@uol.com.br. Rosany Bochner, Instituto de Comunicação e Informação Científica e Tecnológica em Saúde, Fundação Instituto Oswaldo Cruz (FIOCRUZ), Rio de Janeiro, Brazil, E-mail: robochner@gmail.com.
References
- 1.Angelakis E, Raoult D. Review Q fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Fenollar F, Fournier PE, Raoult D. Molecular detection of Coxiella burnetii in the sera of patients with Q fever endocarditis or vascular infection. J Clin Microbiol. 2004;42:4919–4924. doi: 10.1128/JCM.42.11.4919-4924.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maurin M, Raoult D. Q fever. Clin Microbiol Ver. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgiev M, Afonso A, Neubauer H, Needham H, Thiéry R, Rodolakis A, Roest HJ, Stärk KD, Stegeman JA, Vellema P, van der Hoek W, More SJ. Q fever in humans and farm animals in four European countries, 1982 to 2010. Euro Surveill. 2013;18:20407. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20407 Available at. [PubMed] [Google Scholar]
- 5.Anderson AD, Kruszon-Moran D, Loftis AD, McQuillan G, Nicholson WL, Priestley RA, Candee AJ, Patterson NE, Massung RF. Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg. 2009;81:691–694. doi: 10.4269/ajtmh.2009.09-0168. [DOI] [PubMed] [Google Scholar]
- 6.Delsing CE, Kullberg BJ, Bleeker-Rovers CP. Q fever in the Netherlands from 2007 to 2010. Neth J Med. 2010;68:382–387. [PubMed] [Google Scholar]
- 7.Anderson AD, Smoak B, Shuping E, Ockenhouse C, Petruccelli B. Q fever and the U.S. military [letter] Emerg Infect Dis. 2005;11:1320–1322. doi: 10.3201/eid1108.050314. doi:10.3201/eid1108.050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronson NE, Sanders JW, Moran KA. In harm's way: infections in deployed American military forces. Clin Infect Dis. 2006;43:1045–1051. doi: 10.1086/507539. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . Bioterrorism Agents/Diseases. 2001. http://emergency.cdc.gov/agent/agentlist.asp (Page last updated February 12, 2007) Available at. [Google Scholar]
- 10.Brandão H, Valle L, Christóvão D. Investigações sobre a febre Q em São Paulo. Estudo sorológico em operários de um frigorífico. Arq Fac Hig Saude Publica Univ Sao Paulo. 1953;7:127–133. [Google Scholar]
- 11.Lemos ER, Rozental T, Mares-Guia MA, Almeida DN, Moreira N, Silva RG, Barreira JD, Lamas CC, Favacho AR, Damasco PV. Q fever as a cause of fever of unknown origin and thrombocytosis: first molecular evidence of Coxiella burnetii in Brazil. Vector Borne Zoonotic Dis. 2011;11:85–87. doi: 10.1089/vbz.2009.0261. [DOI] [PubMed] [Google Scholar]
- 12.Mares-Guia MAMM, Rozental T, Guterres A, Gomes R, Almeida DNP, Moreira NS, Barreira JD, Favacho ARM, Santana AL, Lemos ERS. Molecular identification of the agent of Q fever—Coxiella burnetii—in domestic animals in State of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2014;47:231–234. doi: 10.1590/0037-8682-0076-2013. [DOI] [PubMed] [Google Scholar]
- 13.Lamas CC, Ramos RG, Lopes GQ, Santos MS, Golebiovski WF, Weksler C, Ferraiuoli GID, Fournier P, Lepidi H, Raoult D. Bartonella and Coxiella infective endocarditis in Brazil: molecular evidence from excised valves from a cardiac surgery referral center in Rio de Janeiro, Brazil, 1998 to 2009. Int J Infect Dis. 2013;17:e65–e66. doi: 10.1016/j.ijid.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Rozental T, Mascarenhas LF, Rozenbaum R, Gomes R, Mattos GS, Magno CC, Almeida DN, Rossi MI, Favacho AR, de Lemos ER. Coxiella burnetii, the agent of Q fever in Brazil: its hidden role in seronegative arthritis and the importance of molecular diagnosis based on the repetitive element IS1111 associated with the transposase gene. Mem Inst Oswaldo Cruz. 2012;107:695–697. doi: 10.1590/s0074-02762012000500021. [DOI] [PubMed] [Google Scholar]
- 15.Lamas CR. Seroprevalence of Coxiella burnetii antibodies in human immunodeficiency virus-positive patients in Jacarepaguá, Rio de Janeiro, Brazil. Clin Microbiol Infect. 2009;15:140–141. doi: 10.1111/j.1469-0691.2008.02144.x. [DOI] [PubMed] [Google Scholar]
- 16.Siciliano RR. Endocarditis due to Coxiella burnetii (Q fever). A rare or underdiagnosed disease? Case report. Rev Soc Bras Med Trop. 2008;41:409–412. doi: 10.1590/s0037-86822008000400017. [DOI] [PubMed] [Google Scholar]
- 17.Arricau-Bouvery N, Rodolakis A. Is Q fever an emerging or re-emerging zoonosis? Vet Res. 2005;36:327–349. doi: 10.1051/vetres:2005010. [DOI] [PubMed] [Google Scholar]
- 18.Heringer M, Nogueira RM, de Filippis AM, Lima MR, Faria NR, Nunes PC, Nogueira FB, Dos Santos FB. Impact of the emergence and re-emergence of different dengue viruses' serotypes in Rio de Janeiro, Brazil, 2010 to 2012. Trans R Soc Trop Med Hyg. 2015;109:268–274. doi: 10.1093/trstmh/trv006. [DOI] [PubMed] [Google Scholar]
- 19.Willems H, Thiele D, Frolich-Ritter R, Krauss H. Detection of Coxiella burnetii in cow's milk using the polymerase chain reaction (PCR) J Vet Med. 1994;41:580–587. doi: 10.1111/j.1439-0450.1994.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 20.Hoover TA, Vodkin MH, Williams JC. A Coxiella burnetii repeated DNA element resembling a bacterial insertion sequence. J Bacteriol. 1992;174:5540–5548. doi: 10.1128/jb.174.17.5540-5548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denison AM, Thompson HA, Massung RF. IS1111 insertion sequences of Coxiella burnetii: characterization and use for repetitive element PCR-based differentiation of Coxiella burnetii isolates. BMC Microbiol. 2007;7:91. doi: 10.1186/1471-2180-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Ayres M, Ayres M, Jr, Ayres DL, Santos AS. Belém/PA, Brasil: Ong Mamirauá; 2007. BioEstat—Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas. [Google Scholar]
- 24.Secretaria de Vigilância em Saúde – Ministério da Saúde–Brasil Boletim Epidemiológico. 2014;Vol. 45(No. 17):1–6. http://portalsaude.saude.gov.br/images/pdf/2014/agosto/04/BE-2014-45--17––Dengue-SE29.pdf Available at. [Google Scholar]
- 25.Daumas RP, Passos SRL, Oliveira RVC, Nogueira RMR, Georg I, Marzochi KBF, Patrícia Brasil P. Clinical and laboratory features that discriminate dengue from other febrile illnesses: a diagnostic accuracy study in Rio de Janeiro, Brazil. BMC Infect Dis. 2013;13:77. doi: 10.1186/1471-2334-13-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins VCA, Bastos MS, Ramasawmy R, Figueiredo RP, Gimaque JBL, Braga WSM, Nogueira ML, Nozawa S, Naveca FG, Figueiredo LTM, Mourão MPG. Clinical and virological descriptive study in the 2011 outbreak of dengue in the Amazonas, Brazil. PLoS One. 2014;9:e100535. doi: 10.1371/journal.pone.0100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, Nicholson WL, Paddock CD, Sexton DJ. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR. 2013;62:1–30. http://www.cdc.gov/mmwr/pdf/rr/rr6203.pdf Available at. [PubMed] [Google Scholar]
- 28.Hawker JI, Ayres JG, Blair I, Evans MR, Smith DL, Smith EG, Burge PS, Carpenter MJ, Caul EO, Coupland B, Desselberger U, Farrell ID, Saunders PJ, Wood MJ. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187. [PubMed] [Google Scholar]
- 29.Gilsdorf A, Kroh C, Grimm S, Jensen E, Wagner-Wiening C, Alpers K. Large Q fever outbreak due to sheep farming near residential areas, Germany, 2005. Epidemiol Infect. 2008;136:1084–1087. doi: 10.1017/S0950268807009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersh GJ, Fitzpatrick KA, Self JS, Priestley RA, Kelly AJ, Lash RR, Marsden-Haug N, Nett RJ, Bjork A, Massung RF, Anderson AD. Presence and persistence of Coxiella burnetii in the environments of goat farms associated with a Q fever outbreak. Appl Environ Microbiol. 2013;79:1697–1703. doi: 10.1128/AEM.03472-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rovery C, Granel B, Casalta JP, Lepidi H, Habib G, Raoult D. Coinfection with Coxiella burnetii in infectious endocarditis. Clin Microbiol Infect. 2009;15((Suppl 2)):190–191. doi: 10.1111/j.1469-0691.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 32.Rolain JM, Gouriet F, Brouqui P, Larrey D, Janbon F, Vene S, Jarnestrom V, Raoult D. Concomitant or consecutive infection with Coxiella burnetii and tickborne diseases. Clin Infect Dis. 2005;40:82–88. doi: 10.1086/426440. [DOI] [PubMed] [Google Scholar]
- 33.Centro de Informações e Respostas Estratégicas de Vigilância em Saúde–CIEVS Informe Epidemiológico Cievs–Paraná Semana Epidemiológica 11/2015. Febre Q. 2015. http://www.saude.pr.gov.br/arquivos/File/informe_semanal_cievs_11.pdf Available at.
- 34.Kampschreur LM, Dekker S, Hagenaars JC, Lestrade PJ, Renders NH, de Jager-Leclercq MG, Hermans MH, Groot CA, Groenwold RH, Hoepelman AI, Wever PC, Oosterheert JJ. Identification of risk factors for chronic Q fever, the Netherlands. Emerg Infect Dis. 2012;18:563–570. doi: 10.3201/eid1804.111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhu M, Nicholson WL, Roche AJ, Kersh GJ, Fitzpatrick KA, Oliver LD, Massung RF, Morrissey AB, Bartlett JA, Onyango JJ, Maro VP, Kinabo GD, Saganda W, Crump JA. Q fever, spotted fever group, and typhus group rickettsioses among hospitalized febrile patients in northern Tanzania. Clin Infect Dis. 2011;53:e8–e15. doi: 10.1093/cid/cir411. [DOI] [PMC free article] [PubMed] [Google Scholar]