Abstract
Serum specimens from free-ranging but nonnative patas monkeys (Erythrocebus patas) and rhesus macaques (Macaca mulatta) in southwestern Puerto Rico (PR) were tested for antibodies to infection with dengue viruses (DENVs), West Nile virus (WNV), Leptospira species, and Burkholderia pseudomallei by microneutralization, plaque reduction neutralization, microscopic agglutination, and indirect hemagglutination, respectively. Of 23 animals (21 E. patas and two M. mulatta) tested, all had evidence of prior DENV infection, and of 17 animals tested for WNV, nine (53%) had evidence of prior infection. Of 24 (22 E. patas, two M. mulatta) tested for Leptospira spp., 10 (42%) had evidence of prior exposure, and one patas monkey had antibodies against B. pseudomallei. The acquisition of pathogens endemic among humans in PR by resident nonhuman primates merits further study to define modes of acquisition.
Introduction
Nonhuman primates are not native to the Caribbean island of Puerto Rico (PR). Old World patas monkeys (Erythrocebus patas) and rhesus macaques (Macaca mulatta) were imported from India and Nigeria in the 1960s and 1970s to establish La Parguera Primate Breeding Colony (LPPBC) on the islets of Guayacán and Cueva in southwestern PR.1 Before LPPBC was closed in 1982, an unknown number of monkeys of both species escaped the facility to the main island of PR. Censuses conducted in 2010 and 2012 estimated the main island population of patas monkeys at 1,460 and rhesus macaques at 1,086, respectively (Department of Natural and Environmental Resources, unpublished data). These free-ranging monkeys frequently raid local farms to forage for food, causing extensive crop damage and economic loss among farmers in southwestern PR.2 Primarily for this reason, in 2008, the U.S. Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services in cooperation with the PR Departments of Natural and Environmental Resources and Department of Agriculture, and the U.S. Fish and Wildlife Services decided to reduce this free-ranging nonhuman primate population and leave only solitary males on mainland PR by 2019.2
In PR, dengue and leptospirosis are endemic human diseases and common causes of acute febrile illness.3–5 Dengue virus (DENV) is thought to be exclusively transmitted in a human–mosquito–human cycle.6,7 However, nonhuman primates are susceptible to DENV infection, and sylvatic nonhuman primate transmission cycles have been described in Africa and Malaysia.6,8 West Nile virus (WNV) is a mosquito-borne virus that is amplified in an enzootic cycle in birds and incidentally infects humans, a dead-end host.9 Leptospirosis, a zoonotic disease of global importance, occurs following direct or indirect contact with infected animal urine, usually that of rodents. However, virtually all mammals are susceptible to infection with Leptospira spp.10,11 Burkholderia pseudomallei, a saprophytic, gram-negative bacillus causes melioidosis, was recently shown to be in PR.12,13 Excluding WNV, where mammals are dead-end hosts, it is unknown if free-ranging primates in PR are capable of being infected with any of these endemic pathogens or whether they might contribute to their transmission cycles in PR.
Several government agencies have programs established to remove these free-ranging nonhuman primates. These programs provided the opportunity to obtain specimens to test for pathogens affecting humans. Serum specimens obtained during the removal program were tested for evidence of DENV, WNV, Leptospira spp., and B. pseudomallei infection.
Materials and Methods
In 2010 and 2012, blood from 24 nonhuman primates from southwestern PR was obtained from participating agencies as part of the wildlife damage control program by cardiac puncture, performed soon after death. Blood was transferred to a sterile collection tube, allowed to clot for 15 minutes, centrifuged at 1,500 rpm for 10 minutes, and serum was stored at −20°C until diagnostic testing was performed.
Serum specimens were heat-treated for 30 minutes at 56°C to inactivate any other possible adventitious virus in the specimen.14 Specimens were tested for the presence of anti-DENV antibodies by microneutralization test (MNT), which is an adaptation of the plaque-reduction neutralization test (PRNT).15 Primary DENV infection was defined by a single reciprocal titer ≥ 40; samples with a reciprocal titer ≥ 40 to more than one DENV type were characterized as having evidence of second or greater DENV infection or having DENV cross-reactive antibodies. Specimens were also tested for evidence of WNV neutralizing antibodies by PRNT90. WNV neutralizing PRNT90 reciprocal titers of ≥ 20 indicated previous exposure to WNV. Evidence of Leptospira spp. exposure was determined by microscopic agglutination test (MAT). Individual animals with a reciprocal MAT titer ≥ 100 were considered positive for Leptospira antibody.16 Indirect hemagglutination assay was used to detect anti-B. pseudomallei antibodies, and seropositivity was defined by a reciprocal titer ≥ 40.17
Results
Out of 24 nonhuman primates from four clans that were captured in southwestern PR, 22 were patas monkeys and two were rhesus macaques (Table 1). Sex and approximate age was determined for 11 patas monkeys, of which nine (82%) were female and eight (73%) were adults. Of the two rhesus macaques, one was female and both were adults.
Table 1.
Characteristics and DENV, WNV, Leptospira, and Burkholderia pseudomallei serologic diagnostic test results of nonhuman primates from Puerto Rico
| Species | Location sample collected | Date sample collected | Sex | Age | DENV type microneutralization titers | WNV plaque reduction neutralization | Leptospira serogroup (highest titer) | Leptospira highest titer | B. pseudomallei titer | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | |||||||||
| Erythrocebus patas | A | March 2010 | Female | Adult | < 40 | < 40 | 80 | 40 | 40 | Neg. | < 100 | < 40 |
| A | March 2010 | ND | ND | < 40 | < 40 | 40 | 40 | < 20 | Neg. | < 100 | < 40 | |
| A | March 2010 | ND | ND | < 40 | < 40 | < 40 | 320 | 160 | Neg. | < 100 | < 40 | |
| A | March 2010 | Female | Adult | < 40 | < 40 | 80 | 80 | 20 | Neg. | < 100 | < 40 | |
| B | June 2010 | Female | Adult | < 40 | < 40 | < 40 | 160 | NT | Ballum | 100 | < 40 | |
| B | June 2010 | ND | ND | < 40 | < 40 | 80 | 40 | 20 | Neg. | < 100 | < 40 | |
| C | June 2010 | Male | Adult | < 40 | < 40 | < 40 | 320 | NT | Bataviae | 800 | < 40 | |
| C | June 2010 | ND | ND | < 40 | < 40 | < 40 | 40 | NT | Neg. | < 100 | 80 | |
| C | June 2010 | ND | ND | < 40 | < 40 | < 40 | 160 | < 20 | Neg. | < 100 | < 40 | |
| D | June 2010 | Female | Juvenile | < 40 | < 40 | < 40 | 80 | NT | Neg. | < 100 | < 40 | |
| D | June 2010 | Male | Adult | < 40 | < 40 | 40 | 80 | < 20 | Neg. | < 100 | < 40 | |
| D | June 2010 | ND | ND | < 40 | < 40 | 40 | 80 | 80 | Bataviae | 800 | < 40 | |
| D | June 2010 | ND | ND | < 40 | < 40 | 160 | 320 | 640 | Neg. | < 100 | < 40 | |
| E | June 2010 | Female | Adult | < 40 | < 40 | 160 | 160 | < 20 | Bataviae | 800 | < 40 | |
| E | June 2010 | Female | Juvenile | 80 | < 40 | < 40 | 40 | < 20 | Neg. | < 100 | < 40 | |
| E | June 2010 | ND | ND | < 40 | < 40 | 160 | 80 | < 20 | Ballum | 100 | < 40 | |
| E | June 2010 | ND | ND | < 40 | < 40 | 320 | 320 | NT | Neg. | < 100 | < 40 | |
| E | June 2010 | Female | Adult | < 40 | < 40 | 320 | 80 | 40 | Icterohaemorrhagiae | 100 | < 40 | |
| E | June 2010 | Female | Juvenile | 80 | < 40 | < 40 | 40 | NT | Neg. | < 100 | < 40 | |
| E | June 2010 | ND | ND | < 40 | < 40 | 40 | 80 | < 20 | Ballum | 100 | < 40 | |
| E | June 2010 | ND | ND | < 40 | < 40 | 40 | 320 | < 20 | Ballum | 800 | < 40 | |
| F | April 2012 | Female | Adult | NT | NT | NT | NT | 20 | Icterohaemorrhagiae/Autumnalis/Australis | 400 | < 40 | |
| Macaca mulatta | G | April 2012 | Male | Adult | < 40 | < 40 | 40 | 80 | NT | Neg. | < 100 | < 40 |
| H | April 2012 | Female | Adult | < 40 | < 40 | < 40 | 40 | 320 | Bataviae | 400 | < 40 | |
DENV = dengue virus; WNV = West Nile virus. ND indicates that variable characteristic was not determined (sex, age); NT indicates that the specimen was not tested (DENV, WNV); and Neg. indicates that the specimen sample was negative (Leptospira serogroup).
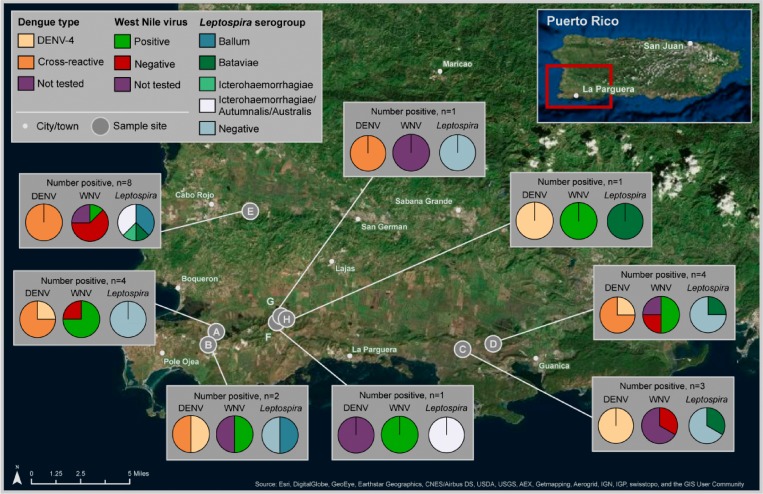
All 23 of the monkeys tested had evidence of prior DENV infection by MNT (one specimen could not be tested). Seven (30%) monkeys had evidence of a primary infection with DENV-4, which included six patas monkeys and one rhesus macaque (Table 1). In the remaining animals, two (9%) had elevated anti-DENV MNT titers against both DENV-1 and DENV-4 (both patas), and 14 (61%) had elevated MNT titers against DENV-3 and DENV-4 (Table 1). Infection with DENV-4 was not associated with any particular clan or geographic area; however, the two monkeys positive for both anti-DENV-1 and anti-DENV-4 neutralizing antibodies came from Cabo Rojo region in the southwest of the island (Figure 1 ). None of the animals had anti-DENV-2 neutralizing antibodies.
Figure 1.
Geographic location and serologic test results for dengue virus and Leptospira spp. in nonhuman primates from Puerto Rico.
Nine of the 17 (53%) monkeys tested had neutralizing antibodies against WNV. Of these nine monkeys, two had high PRNT90 reciprocal titers of 320 and 640, respectively.
Ten (42%) of 24 monkeys had antibodies to Leptospira spp. MAT titers were highest for serogroups Bataviae (4), Ballum (4), Icterohaemorrhagiae (1), and one specimen in which antibody was equally reactive to serogroups Icterohaemorrhagiae, Autumnalis, and Australis. Dominant serogroups were not associated with geographic regions from which the monkeys were captured.
One patas monkey of unknown age and sex had evidence of previous exposure to B. pseudomallei.
Discussion
In this study, antibodies against DENVs were detected in 23 free-ranging nonhuman primates in southwestern PR. As there is no evidence of sylvatic DENV transmission in the Americas, the most likely explanation for these findings is spillback of human DENV into this small, nonhuman primate population, as has been observed in French Guiana and the Philippines.18,19 Our findings suggest that DENV spillback may be common in PR. Anti-DENV antibodies were detected in four geographically disparate clans of monkeys that have limited interaction with one another, suggesting that transmission from humans to nonhuman primates has occurred on more than one occasion. During 1992 to 2012, all four DENV types have circulated in humans in southwestern PR, where the dominant types were DENV-1, DENV-2, DENV-4, followed by DENV-3. It is interesting that all of the monkeys that were tested had either a primary DENV-4 infection or had cross-reactive antibodies to DENV-4. Surprisingly, no monkeys in PR had evidence of past infection with DENV-2.5,20
We were not able to conclusively determine if the DENV serologic results found in this study were a result of regular virus spillback from humans to monkeys or if a stable enzootic DENV transmission cycle exists in the absence of humans. Transmission of DENV from humans to nonhuman primates should occur in a similar ecological setting as sylvatic DENV emergence, a “zone of emergence” as described in Vasilakis and others,8 where environmental conditions enable overlap between vectors, humans, and nonhuman primates. Conditions around southwestern PR seem favorable for an enzootic cycle of human DENV transmission due to: 1) patas monkeys, a known host of sylvatic DENV in West Africa and 2) the presence of competent mosquito vectors of DENV in PR.21–24
In addition to the presence of neutralizing antibodies to DENV, the monkey serum also had neutralizing antibodies against WNV. In 2007, WNV was first isolated in PR following the seroconversion of sentinel chickens in municipality of Ceiba.25 This chicken surveillance was dismantled at the end of 2007 leaving PR with no method of identifying active WNV transmission. Since 2007, there has been sporadic evidence of WNV transmission identified in blood donors; however, no locally acquired human cases have been found in PR.25 Previous research in 2007 did not find evidence of WNV-infected rhesus macaques on the island of Cayo Santiago, PR.26 Differences in the local environment and availability of competent vectors on the island may explain this finding.
Nearly half of the nonhuman primates had been previously exposed to Leptospira spp. bacteria. Patas monkeys are terrestrial, and rhesus macaques are both terrestrial and arboreal, making it possible for both to come into contact with contaminated soil or water while foraging for food on the ground. Moreover, because patas monkeys have been observed to venture into areas occupied by humans and consume crops grown in agricultural fields, humans (e.g., agricultural workers, consumers) could potentially come into contact with soil or agricultural crops that have been contaminated with monkey urine. Nonetheless, the public health importance of infection with Leptospira spp. bacteria in nonhuman primates is unknown since it is not known whether monkeys shed the bacteria once infected and serve as reservoirs.
Though also transmitted through contact with contaminated soil and water, just one monkey had evidence of B. pseudomallei exposure. This finding may be due to either infrequent presence of the bacteria in soil, decreased susceptibility of nonhuman primates to infection, and/or false-positive serologic test results.17,27
Because patas monkeys and rhesus macaques are invasive species in PR, their importance in the local disease ecology has not been previously studied and not well understood. Feral monkeys often come in close proximity to humans; with a home range that often exceeds 20 km2 and the ability to travel more than 2 km a day, infected nonhuman primates could be both reservoirs and mechanisms for disease dispersal.1 Further study is required to better understand the mosquito species and conditions leading to DENV exposure of monkeys in PR, and how frequently and close nonhuman primates and humans come into contact with each other in natural settings. If a stable enzootic cycle exists between mosquitoes and nonhuman primates, the possibility for DENV elimination in PR may be difficult, lacking the complete elimination of nonhuman primates.6 Additional efforts should be made to isolate DENV from arboreal mosquitoes and nonhuman primates, as well as elucidating why DENV-4 was the predominant type of DENV infecting nonhuman primates. Furthermore, the potential of nonhuman primates as sentinels for WNV in PR and the role of nonhuman primates as a reservoir for Leptospira spp. and B. pseudomallei in PR merits further investigation.
ACKNOWLEDGMENTS
We thank Frank Boyd and Gaston Wesson from the U.S. Department of Agriculture/Wildlife Services for coordinating sample retrieval. We also thank Elvira McIntyre and Olivia Leach from the Geospatial Research, Analysis and Services Program, a division of the Agency for Toxic Substances and Disease Registry at the Centers for Disease Control and Prevention, for assistance with figure preparation. We also thank Jessica Carrion and Manuela Beltran for providing technical support for dengue virus and West Nile virus testing. Finally, we thank Harold S. Margolis for critical review of the manuscript.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Centers for Disease Control and Prevention or the U.S. Government.
Footnotes
Financial support: This study was supported by the Centers for Disease Control and Prevention.
Authors' addresses: Ryan R. Hemme, Entomology and Ecology Activity, Dengue Branch, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mail: rhemme@cdc.gov. Ricardo Lopez-Ortiz, Fish and Wildlife Bureau, Puerto Rico Department of Natural and Environmental Resources, San Juan, Puerto Rico, E-mail: rlopez@drna.gobierno.pr. Brenda Rivera Garcia, Epidemiology and Research Office, Puerto Rico Department of Health, San Juan, Puerto Rico, E-mail: brendarivera@salud.pr.gov. Tyler M. Sharp, Epidemiology Activity, Dengue Branch, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mail: tsharp@cdc.gov. Renee L. Galloway and Mindy G. Elrod, Zoonoses and Select Agent Laboratory, Bacterial Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: zul0@cdc.gov and wzg0@cdc.gov. Elizabeth A. Hunsperger, Immunodiagnostic, Development, and Research Laboratory, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mail: enh4@cdc.gov.
References
- 1.Gonzalez-Martinez J. The introduced free-ranging rhesus and patas monkey populations of southwestern Puerto Rico. P R Health Sci J. 2004;23:39–46. [PubMed] [Google Scholar]
- 2.USDA, PRDA, USFWS, U.S. Department of Agriculture/Animal and Plant Health Inspection Service/Wildlife Services, Puerto Rico Department of Natural and Environmental Resources, Puerto Rico Department of Agriculture, U.S. Fish and Wildlife Service . Environmental Assessment: Management of Feral and Free-ranging Patas and Rhesus Monkey Populations to Reduce Threats to Human Health and Safety, Agriculture, Nuisances, and Impacts to Native Wildlife Species in the Commonwealth of Puerto Rico. Washington, DC: USDA/Animal and Plant Health Inspection Service; 2008. p. 87. [Google Scholar]
- 3.CDC Notes from the field: investigation of leptospirosis underreporting - Puerto Rico, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:421. [PubMed] [Google Scholar]
- 4.Sanders EJ, Rigau-Perez JG, Smits HL, Deseda CC, Vorndam VA, Aye T, Spiegel RA, Weyant RS, Bragg SL. Increase of leptospirosis in dengue-negative patients after a hurricane in Puerto Rico in 1996 [correction of 1966] Am J Trop Med Hyg. 1999;61:399–404. doi: 10.4269/ajtmh.1999.61.399. [DOI] [PubMed] [Google Scholar]
- 5.Sharp TM, Hunsperger E, Santiago GA, Munoz-Jordan JL, Santiago LM, Rivera A, Rodriguez-Acosta RL, Gonzalez Feliciano L, Margolis HS, Tomashek KM. Virus-specific differences in rates of disease during the 2010 dengue epidemic in Puerto Rico. PLoS Negl Trop Dis. 2013;7:e2159. doi: 10.1371/journal.pntd.0002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292–311. doi: 10.1016/j.meegid.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 8.Vasilakis N, Cardosa J, Hanley KA, Holmes EC, Weaver SC. Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nat Rev Microbiol. 2011;9:532–541. doi: 10.1038/nrmicro2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chancey C, Grinev A, Volkova E, Rios M. The global ecology and epidemiology of West Nile virus. BioMed Res Int. 2015;2015:376230. doi: 10.1155/2015/376230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 11.Greene CE, Miller MA, Brown CA. Leptospirosis. In: Greene CE, editor. Infectious Diseases of Small Animals. Philadelphia, PA: W. B. Saunders Co.; 1998. pp. 273–281. [Google Scholar]
- 12.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doker TJ, Sharp TM, Rivera-Garcia B, Perez-Padilla J, Benoit TJ, Ellis EM, Elrod MG, Gee JE, Shieh WJ, Beesley CA, Ryff KR, Traxler RM, Galloway RL, Haberling DL, Waller LA, Shadomy SV, Bower WA, Hoffmaster AR, Walke HT, Blaney DD. Contact investigation of melioidosis cases reveals regional endemicity in Puerto Rico. Clin Infect Dis. 2014;60:243–50. doi: 10.1093/cid/ciu764. [DOI] [PubMed] [Google Scholar]
- 14.WHO . Guidelines for Plaque Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Geneva, Switzerland: World Health Organization; 2007. [DOI] [PubMed] [Google Scholar]
- 15.Vorndam V, Beltran M. Enzyme-linked immunosorbent assay-format microneutralization test for dengue viruses. Am J Trop Med Hyg. 2002;66:208–212. doi: 10.4269/ajtmh.2002.66.208. [DOI] [PubMed] [Google Scholar]
- 16.Dikken H, Kmety E. Serological typing methods of leptospires. In: Bergan T, Norris J, editors. Methods in Microbiology. London, United Kingdom: Academic Press; 1978. p. 259.p. 307. [Google Scholar]
- 17.Alexander AD, Huxsoll DL, Warner AR, Jr, Shepler V, Dorsey A. Serological diagnosis of human melioidosis with indirect hemagglutination and complement fixation tests. Appl Microbiol. 1970;20:825–833. doi: 10.1128/am.20.5.825-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Thoisy B, Lacoste V, Germain A, Munoz-Jordan J, Colon C, Mauffrey JF, Delaval M, Catzeflis F, Kazanji M, Matheus S, Dussart P, Morvan J, Setien AA, Deparis X, Lavergne A. Dengue infection in neotropical forest mammals. Vector Borne Zoonotic Dis. 2009;9:157–170. doi: 10.1089/vbz.2007.0280. [DOI] [PubMed] [Google Scholar]
- 19.Kato F, Ishida Y, Kawagishi T, Kobayashi T, Hishiki T, Miura T, Igarashi T. Natural infection of cynomolgus monkeys with dengue virus occurs in epidemic cycles in the Philippines. J Gen Virol. 2013;94:2202–2207. doi: 10.1099/vir.0.055343-0. [DOI] [PubMed] [Google Scholar]
- 20.Tomashek KM, Rivera A, Munoz-Jordan JL, Hunsperger E, Santiago L, Padro O, Garcia E, Sun W. Description of a large island-wide outbreak of dengue in Puerto Rico, 2007. Am J Trop Med Hyg. 2009;81:467–474. [PubMed] [Google Scholar]
- 21.Barrera R, Bingham AM, Hassan HK, Amador M, Mackay AJ, Unnasch TR. Vertebrate hosts of Aedes aegypti and Aedes mediovittatus (Diptera: Culicidae) in rural Puerto Rico. J Med Entomol. 2012;49:917–921. doi: 10.1603/me12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox J, Grillet ME, Ramos OM, Amador M, Barrera R. Habitat segregation of dengue vectors along an urban environmental gradient. Am J Trop Med Hyg. 2007;76:820–826. [PubMed] [Google Scholar]
- 23.Gubler DJ, Novak RJ, Vergne E, Colon NA, Velez M, Fowler J. Aedes (Gymnometopa) mediovittatus (Diptera: Culicidae), a potential maintenance vector of dengue viruses in Puerto Rico. J Med Entomol. 1985;22:469–475. doi: 10.1093/jmedent/22.5.469. [DOI] [PubMed] [Google Scholar]
- 24.Poole-Smith BK, Hemme RR, Delorey M, Felix G, Gonzalez AL, Amador M, Hunsperger EA, Barrera R. Comparison of vector competence of Aedes mediovittatus and Aedes aegypti for dengue virus: implications for dengue control in the Caribbean. PLoS Negl Trop Dis. 2015;9:e0003462. doi: 10.1371/journal.pntd.0003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunsperger EA, McElroy KL, Bessoff K, Colon C, Barrera R, Munoz-Jordan JL. West Nile virus from blood donors, vertebrates, and mosquitoes, Puerto Rico, 2007. Emerg Infect Dis. 2009;15:1298–1300. doi: 10.3201/eid1508.090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phoutrides E, Jusino-Mendez T, Perez-Medina T, Seda-Lozada R, Garcia-Negron M, Davila-Toro F, Hunsperger E. The utility of animal surveillance in the detection of West Nile virus activity in Puerto Rico, 2007. Vector Borne Zoonotic Dis. 2011;11:447–450. doi: 10.1089/vbz.2010.0011. [DOI] [PubMed] [Google Scholar]
- 27.Miller WR, Pannell L, Cravitz L, Tanner WA, Rosebury T. Studies on certain biological characteristics of Malleomyces mallei and Malleomyces pseudomallei. II. Virulence and infectivity for animals. J Bacteriol. 1948;55:127–135. [PMC free article] [PubMed] [Google Scholar]