Abstract
Podoconiosis is a neglected tropical disease caused by long-term barefoot exposure to volcanic clay soil. Our previous qualitative research identified various domains of beliefs about the causes of podoconiosis held by members of the community. This cross-sectional survey, conducted in southern Ethiopia, aimed to quantitatively evaluate the prevalence of these beliefs and to assess their association with observed shoe-wearing behavior. A total of 1,800 adult respondents (600 from affected families and 1,200 from unaffected families of an index child aged between 3 and 6 years) took part in the survey. Two standardized versions of an enumerator-administered survey were created, with “all day, everyday” shoe-wearing status of the index child assessed in parallel for the affected and unaffected household respondents. Associations between measures were assessed using logistic regression. Accuracy of understanding about podoconiosis was significantly lower among respondents from unaffected than affected households (P < 0.001). Among affected respondents, beliefs about heredity were negatively associated with reported shoe wearing of the index child (odds ratio = 0.67, 95% confidence interval = 0.55–0.83). In both groups, associations of causal beliefs with shoe wearing were moderated by risk perceptions. Interventions aimed at preventing podoconiosis and improving shoe wearing should consider family-oriented education on hereditary susceptibility that targets affected and unaffected families in resource-limited settings.
Introduction
Numerous studies conducted in low- and middle-income countries (LMICs) have shown the importance of considering target groups' beliefs about the causes of disease when designing efficacious public health interventions.1,2 For example, studies of malaria prevention, maternal and child health, and neglected tropical diseases all show that uptake of preventive practices are challenged by low levels of understanding and misconceptions about the causes of these conditions.3–5 Research in LMICs has shown that even when interventions and therapies are accessible, some populations do not effectively implement these strategies, in part due to their misunderstanding of disease causes.6
In these populations, research has documented limited knowledge and misconceptions about how diseases are transmitted. Beliefs that diseases run “in the blood” or are inherited have been reported in diverse cultural settings for an array of disease conditions.7–9 While in some cases, these conditions are indeed influenced by genetic susceptibility, low literacy and poor understanding of gene–environment interactions compounded with cultural beliefs can result in fatalistic perceptions that these conditions are unavoidable. Such beliefs may lead to stigma and diminish motivation to adopt risk-reducing behaviors.
Some studies suggest a positive association between risk perceptions and adoption of preventive behaviors. Research on onchocerciasis in Ethiopia and Uganda has shown a positive association between compliance with treatment and perceived risk of the condition.10–14 However, other studies have not found risk perceptions to be associated with motivation or adoption of preventive behaviors.15,16
The research described in this report was conducted in rural southern Ethiopia, in a location in which podoconiosis is endemic. Podoconiosis is a noninfectious form of lymphedema (leg swelling) that arises due to the joint contributions of genetic and environmental factors among susceptible people who have long-term barefoot exposure to irritant tropical soils.17–20 It is a chronic, progressive, and disabling disease clinically distinguished from lymphatic filariasis through exclusively affecting the lower limbs and usually causing bilateral and asymmetrical lymphedema.21 Evidence suggests that mineral particles in red clay soils are absorbed through the skin of the foot and engulfed by macrophages in the lower limb lymphatic system, inducing an inflammatory response in the lymphatic vessels, which results in fibrosis and obstruction of the vessel lumen.21,22 This situation leads to a progressive bilateral swelling of the lower legs. Affected individuals can develop disfiguring symptoms, such as nodular skin changes, a mossy appearance around the base of the foot, or foul-smelling wounds.17,20
Recent evidence suggests that heightened susceptibility to soil exposure clusters in families, with heritability estimated at 63%.19 Siblings of patients have a five times higher risk of developing podoconiosis than people in general.19 A recent study, using a genome-wide approach, found an association of podoconiosis with genetic variants in the human leukocyte antigen class II locus suggesting susceptibility to podoconiosis.23 However, the disease is considered to be entirely preventable if genetically high-risk individuals consistently protect their feet from exposure to irritant particles by wearing shoes starting at a young age.17,19 Recent evidence indicates that shoe-wearing prevalence is low and inconsistent.24
In our previous qualitative research, we identified three domains of beliefs about the causes of podoconiosis wrongly held by members of the community.25 Some participants observed that podoconiosis tended to affect multiple family members and concluded that the disease was entirely inherited, making it inevitable. Others noted that not all family members contracted the condition—some developed the disease while others did not—leading them to conclude that the condition was contagious and not caused by heredity. A third group thought these observations meant that multiple causes including heredity and environmental influences caused the condition. In turn, these beliefs were associated with whether community members thought shoe wearing was or was not important as a strategy to prevent podoconiosis. Participants who believed the condition to be inevitable were less inclined to consider shoe wearing to be important. Finally, those who believed the condition to be contagious were concerned about shoes as a means of transmitting the condition.
The purpose of this report is to quantitatively evaluate the prevalence of each of these beliefs and to assess their association with observed shoe-wearing behavior in a large cross-sectional survey. Specifically, we address three questions:
-
1)
How common are the previously identified beliefs and misconceptions about causes of podoconiosis in a representative sample of people from communities in which the disease is endemic?
-
2)
Are beliefs about the causes of podoconiosis (inherited versus contagious) associated with decreased perceptions that it can be prevented, decreased perceptions that shoe wearing is important, or decreased shoe-wearing behavior?
-
3)
Are the associations of beliefs with shoe-wearing behavior moderated by perceptions of risk for podoconiosis?
Materials and Methods
Study setting and participants.
The survey was conducted in 2013 in six rural communities in Wolaita zone, southern Ethiopia, and formed the baseline assessment for a subsequent intervention trial. Ethical clearance was obtained for all study procedures from the Institutional Review Board of the College of Health Sciences, Addis Ababa University, and the National Human Genome Research Institute.
The communities were selected from 15 clinic sites in which Mossy Foot International (MFI) was operating. MFI is a nongovernment organization that has engaged in podoconiosis treatment and prevention in Wolaita zone since 1996. The 15 clinic sites cover all the districts in Wolaita zone. Six communities were selected based on the number of affected families registered at the clinic and their distance from the zonal capital, Wolaita Sodo. As the communities in the zone are relatively homogeneous in sociocultural, religious, and geographic settings, distance from the capital was taken as the criterion for the selection of these communities. Three sites were relatively close (≤ 25 km), whereas three were more distant (≥ 50 km).
A total of 1,800 participants took part in the surveys, 300 at each site, of which 100 were from affected families and 200 from unaffected families. Affected households were identified from MFI's shoe distribution ledgers. The ledgers served as a sampling frame of affected households from which a random sample of children aged 3–6 years were identified. Where more than one age-eligible child was present, the child with the “next birthday” was selected as the “index child” for the caregiver to consider when answering survey questions. This age group was chosen because the study intended to measure parents' understanding of the importance of consistent shoe wearing from the age at which most children start activities leading to significant soil exposure.
Two neighboring unaffected households were identified for each affected household. The neighboring households were selected on the basis that: 1) no one in the neighboring household was a blood relative of anyone living in the corresponding affected household; 2) neither caregiver in the neighboring household was a first degree relative of an individual with podoconiosis; 3) the neighboring household included at least one child in the age group 3–6 years; 4) the neighboring household was within 500-m radius of the affected household selected; and 5) an adult in the neighboring household agreed to participate in the study.
Data collection.
Three data collectors with a minimum of high school education and a good command of the local language were recruited from each of the six selected sites. They underwent training for 3 days and learned to conduct household enumeration and survey assessment. Data collectors visited identified households and obtained oral and written consent from caregivers. From each household, one caregiver who spent the “most time with the index child and knew the child's daily habits the best” was asked to complete the baseline survey with the index child in mind. Data collectors administered the survey to the participants. The baseline data collection was carried out over 15 days in each of the six sites in February 2013.
Survey instruments.
Two versions of the survey were created, with measures assessed in parallel for the affected and unaffected household participants. The survey included questions assessing the participant's age, education and gender, their beliefs about the preventability of podoconiosis, their confidence to ensure that the index child wore shoes consistently, their knowledge and attitudes about the importance of the index child wearing shoes, and their reports of the child's frequency of shoe wearing.
Measures.
Three categories of beliefs were assessed: endorsement that podoconiosis is caused by “heredity,” by “contagion,” or “holding both beliefs equally.” Three statements were posed about heredity (e.g., “If there's podoconiosis in the family, there is nothing that can be done to prevent the disease”; agreement with each statement was summed and ranged from 0 to 3). Two statements assessed beliefs about contagion as a cause of podoconiosis (e.g., “Podoconiosis is contagious,” summed agreement ranged from 0 to 2). In addition, we coded each of the five statements as “true” (1) or “false” (0) to compute accuracy of understanding which ranged from 0 to 5. Perceived risk for the index child was assessed based on the level of agreement with the statement “It is likely that index child will eventually develop podoconiosis in his/her lifetime” (yes versus no). “All day every day” shoe wearing was based on responses to two items assessing the number of days and daily duration of shoe wearing by the index child in the past 7 days. This variable was dichotomized with 0 indicating no use of shoes or response less than shoe wearing for the whole duration of every day and 1 indicating shoe wearing every day for the duration of the whole day.
Data analysis.
Data entry and cleaning was done by a qualified consultant using EPI Info software, and then reviewed and cross-checked by data analysts. Associations between measures were assessed using logistic regression models. To test for differences between affected and unaffected households on accuracy of understanding of the causes of podoconiosis and perception that the index child was at risk, a t test and a χ2 test were used, respectively. Grouping affected and unaffected households separately. Logistic regression models were used to test associations of beliefs about the causes of podoconiosis with shoe wearing after adjusting for gender of respondent, gender of child, and an indicator of whether the respondent had attended school. To test whether risk perceptions moderated relationships between causal beliefs and shoe wearing, interaction terms were added to logistic regression models.
Results
Sample characteristics.
Of the 1,800 participants (600 from affected households and 1,200 from unaffected) approached, 1,784 (596 affected [99.3%] and 1,188 unaffected [99%]) completed the baseline survey (giving an overall response rate of 99.1%). The demographic and other descriptive characteristics of the study participants are shown in Table 1. The majority of respondents were mothers of the index child. Males accounted for 15% and 13% of affected household and unaffected household respondents, respectively. However, the gender distribution of index children was nearly equal (50.3% male children and 49.7% female children in the affected households; 51.8% males and 48.2% females in the unaffected households, P = 0.57). The mean age of caregivers in affected households was 33.9 years (with a range 18–70 and standard deviation [SD] = 8.73 years) while that of unaffected household respondents was significantly lower at 31.3 years (ranging from 18–80, SD = 7.42, P < 0.001). Less than half (43.3%) of the affected household respondents and just over half (54.1%) of the unaffected household respondents had ever attended formal schooling. In both groups, the household size ranged from 2 to 12, with the mean size significantly higher in the affected households (6.4, SD = 1.93) than in unaffected households (5.8, SD = 1.73, P < 0.001). The mean proportion reporting “all day, every day” shoe wearing of the index child was significantly higher in affected households (31%) than in unaffected households (20%, P < 0.001).
Table 1.
Descriptive characteristics of the respondents
| Characteristics | Affected (N = 596) | Unaffected (N = 1,188) | P value |
|---|---|---|---|
| % Male | 15 | 13 | 0.149 |
| % Ever gone to school | 43 | 54 | 0.001 |
| Mean age (SD) | 33.91 (8.73) | 31.32 (7.42) | 0.001 |
| Average household size (SD) | 6.40 (1.93) | 5.80 (1.73) | 0.001 |
| % Shoe-wearing status of index child, “all day every day” | 31 | 20 | 0.001 |
| % Respondents' perceived risk that index child will develop podoconiosis (scale: 0 [low] to 1 [high]) | 37 | 48 | 0.001 |
| Mean strength of beliefs that podoconiosis is hereditary (scale: 0 [low] to 3 [high]) | 1.31 (0.9) | 1.85 (0.92) | 0.001 |
| Mean strength of beliefs that podoconiosis is contagious (scale: 0 [low] to 2 [high]) | 1.27 (0.75) | 1.67 (0.54) | 0.001 |
| Mean strength of belief that podoconiosis is both hereditary and contagious (scale: 0 [low] to 1 [high]) | 0.32 (0.47) | 0.50 (0.50) | 0.001 |
| Mean accuracy of knowledge about podoconiosis (scale: 0 [low] to 5 [high]) | 2.43 (1.4) | 1.47 (1.18) | 0.001 |
SD = standard deviation.
Causal beliefs and risk perceptions related to podoconiosis.
All causal beliefs were less strongly endorsed by affected household respondents (mean scores for heredity: 1.31, SD = 0.9 and contagion: 1.27, SD = 0.75) than by unaffected household respondents (heredity: 1.85, SD = 0.92, and contagion: 1.67, SD = 0.54, P for both comparisons < 0.001). Thirty-two percent of the affected household respondents and 50% of the unaffected household respondents reported beliefs that both heredity and contagion could cause podoconiosis. The overall mean score for accuracy of these beliefs was relatively low for respondents from both affected and unaffected households (2.43, SD = 1.4 and 1.47, SD = 1.2; range = 0–5), but was significantly lower among the unaffected than the affected (P < 0.001). However, affected family respondents were less likely to perceive the index child to be at risk for podoconiosis than unaffected family respondents (37% versus 48%, respectively, P < 0.001).
Association of beliefs (heredity versus contagion) with perceptions that podoconiosis is preventable, shoe-wearing attitudes and behavior.
The logistic regression analysis in Table 2 shows that among the affected household respondents, beliefs about heredity were negatively associated with reported shoe wearing of the index child (odds ratio [OR] = 0.67, 95% confidence interval [CI] = 0.55–0.83). Belief that podoconiosis was contagious or that both heredity and contagion were causes were not significantly associated with the index child's shoe wearing in the affected group (OR = 0.87, 95% CI = 0.68–1.11 and OR = 0.77, 95% CI = 0.52–1.14).
Table 2.
Association of causal beliefs about podoconiosis with “all day everyday” shoe-wearing status of the index child among affected and unaffected households*
| Causal beliefs | Likelihood of shoe wearing | |||
|---|---|---|---|---|
| Affected | Unaffected | |||
| OR | 95% CI | OR | 95% CI | |
| Belief that podoconiosis is contagious | 0.89 | 0.68–1.11 | 0.85 | 0.66–1.088 |
| Belief that podoconiosis is hereditary | 0.67 | 0.55–0.83 | 0.95 | 0.82–1.11 |
| Belief that podoconiosis is both hereditary and contagious | 0.77 | 0.52–1.14 | 0.74 | 0.56–0.98 |
CI = confidence interval; OR = odds ratio.
Analysis adjusted for respondent's gender, level of education, and child's gender.
Among unaffected household respondents, a negative association was observed between beliefs in both heredity and contagion as a cause for podoconiosis and reported shoe wearing of the index child (OR = 0.74, 95% CI = 0.56–0.98). Beliefs in contagion or heredity alone were not associated significantly with the index child's shoe wearing in these households (OR = 0.85, 95% CI = 0.66–1.09 and OR = 0.95, 95% CI = 0.82–1.11).
Respondents' level of education was significantly associated with reported shoe wearing of the index child in both affected and unaffected groups. The higher the level of education of the respondent, the more likely the index child was to wear shoes “all day every day.” Gender of respondents in both groups was associated with reported shoe wearing by the index child, with male caregivers being more likely to report children wearing shoes regularly than female caregivers (P < 0.001). In unaffected households, there was a significant difference in reported regular shoe wearing by gender of index child, with male index children more likely to be wearing shoes than female index children (P < 0.05).
Associations of beliefs with shoe-wearing behavior and risk perceptions.
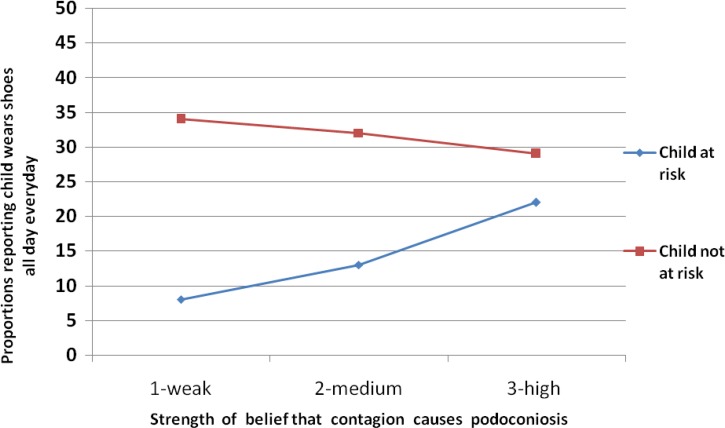
Interactions were tested to assess whether risk perceptions moderated the association of respondents' causal beliefs and index child's shoe wearing. Two significant interactions were observed for affected participants, as illustrated in Figures 1 and 2 . As affected household respondents' beliefs in contagion increased, the likelihood of shoe wearing by the index child also increased but only among respondents who perceived the index child to be at risk for podoconiosis (Figure 1).
Figure 1.
Interaction of beliefs about contagion as a cause with perceived risk of podoconiosis for index child to predict consistent shoe wearing (P < 0.05).
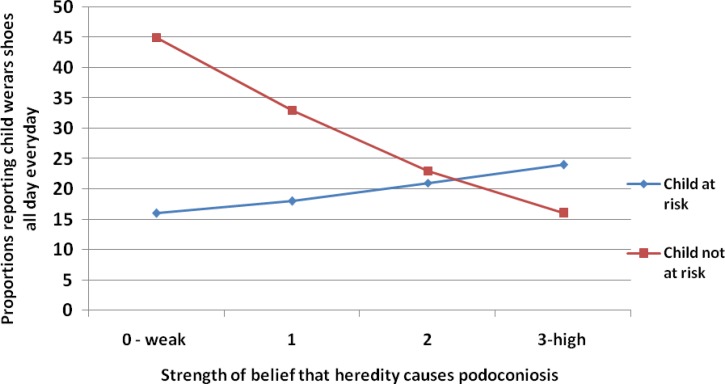
Figure 2.
Interaction of beliefs about heredity as a cause with perceived risk of podoconiosis for index child to predict consistent shoe wearing (P < 0.05).
Among affected household respondents, the association of beliefs about heredity with shoe wearing occurred only among those who perceived the child not to be at risk for podoconiosis (Figure 2). This association was negative such that stronger beliefs in heredity resulted in less shoe wearing among those who perceived the index child to be at lower risk.
Discussion
This study aimed to examine the prevalence of beliefs about podoconiosis and their association with reported shoe wearing in a large representative sample of community members. In addition, these results provide insight into how beliefs about the cause of podoconiosis are influenced by parents' perceived level of risk to their children in both affected and unaffected households. Consistent with our qualitative study, affected respondents were less likely to recognize heredity as a cause of podoconiosis than those who were not affected by the condition.25 Beliefs about heredity were negatively associated with reported shoe wearing of the index child in affected households. When these beliefs were coded for accuracy, affected individuals were more accurate in their knowledge than unaffected respondents. Index children in affected households were significantly more likely to wear shoes “all day, every day” compared with children in unaffected households. Greater knowledge and more regular shoe wearing in affected households may reflect the decade-long community-based interventions run by MFI, which solely targets affected families with treatment and prevention. On the other hand, the low level of accuracy of knowledge and shoe-wearing practice in unaffected households suggests the importance of considering intervention approaches targeting both types of family for more sustainable outcomes in preventing podoconiosis.
Our findings also showed that beliefs in contagion were not associated with reported shoe wearing of the index child in either affected or unaffected families. Although contagion was considered to be a cause of podoconiosis by many respondents, little evidence was obtained that this influenced shoe wearing. This finding is in contrast to our previous qualitative study in which we found that those who believed podoconiosis to be contagious were concerned that shoes might transmit the condition.25
We found that the caregiver's gender was significantly associated with reported shoe wearing by the index child. Female caregivers reported less regular shoe wearing among index children than male caregivers. In unaffected families only, there was a significant association between the gender of the index child and reported shoe wearing, with male children being more likely to wear shoes than female children. This finding is consistent with previous studies in podoconiosis-endemic communities of Ethiopia22,26 in which more women were found to be barefoot than men, and women were wearing lower quality shoes than men. These gender differences may be attributed to deep-rooted social norms regarding women's dependence on men in making decisions in relation to resources. Both may exacerbate women's exposure to the irritant soil and hence their likelihood of developing the condition. Recent studies have suggested higher prevalence of podoconiosis among women than men,27 which may reflect greater discrepancies in access to shoes by gender. The finding is also consistent with the results of the nationwide mapping in Ethiopia which showed higher proportions of men wearing shoes than women at the time of the interview.24
Level of education was also associated with shoe wearing in both affected and unaffected groups. Although it is difficult to disentangle cause and effect, our earlier qualitative work suggested that shoes have social value and facilitate school attendance.25 Alternatively, level of education might influence norms about shoe wearing and foot protection.
Affected respondents reported lower levels of perceived risk of podoconiosis for the index child than unaffected respondents. Because the study was cross-sectional, we cannot determine whether lower risk perceptions are a result of wearing shoes more frequently. However, among respondents in affected families, shoe wearing was not associated with accuracy of understanding of the role of hereditary susceptibility to soil. This may be because affected families are reluctant to acknowledge heredity and family history as possible causes of podoconiosis. In our previous qualitative study, we found that affected families downplayed heredity as a cause for podoconiosis due to fear of stigma.25 This situation calls for sensitivity in designing programs that raise awareness of hereditary susceptibility in high-risk population. Some recent studies support the usefulness of family-oriented education with messages tailored to familial risks in resource-limited communities.28
Our findings also suggest that for affected households, the association of respondents' causal beliefs with child shoe-wearing behavior depended on whether the index child was perceived to be at risk for podoconiosis. Although beliefs about contagion were positively associated with shoe wearing when the child was perceived to be at risk, beliefs about heredity were negatively associated with shoe wearing when the child was perceived to be at risk. This finding suggests that respondents have misconceptions about the cause of podoconiosis. Risk perceptions have long been considered as critical in promoting health behaviors.29 As noted earlier, MFI has worked for over a decade in affected communities to raise awareness that podoconiosis is preventable as a way of increasing preventive actions. However, in contexts typified by low literacy, the notion of preventability is likely to be strongly and negatively associated with perceiving heredity as a cause. Our prior study showed that genetic causal beliefs were relatively common in these communities. Our results suggest that the MFI messages in this context may be understood in a contradictory manner. The challenge is to help communities understand that heritability and preventability are not mutually exclusive concepts. Improving understanding of gene-by-environment contributions to disease risk must consider the low literacy of many LMIC contexts.
There are several limitations to this study. Firstly, our approach to interviewing the child's main caregiver meant that very few male respondents took part in the study. Secondly, this study was conducted in homogenous communities in southern Ethiopia and the results may not be generalizable to other settings. However, despite these limitations, the results provide empirical support for the associations of causal beliefs and risk perceptions with shoe wearing among a large sample of community members living in a low-income setting.
In conclusion, these findings suggest a number of directions for future interventions, including communication approaches to raise awareness of the role of heritability in disease development. Better understanding of causal beliefs may be important in improving prevention of neglected tropical diseases in communities with limited literacy. This study demonstrates the challenges of conceptualizing heritability and shows the importance of developing contextually sensitive educational programs with clear, simple messages on the joint role of gene and environment in podoconiosis. It also suggests the need for further inquiry into causal beliefs in diverse cultural settings to develop locally effective strategies to prevent podoconiosis.
ACKNOWLEDGMENTS
We thank Mossy Foot International in southern Ethiopia and all study participants, Ejigayehu General Trading and Development Consultancy P.L.C. for data encoding, and Cristofer Price of Abt Associates Inc. for analyzing the data.
Footnotes
Financial support: This project was supported by the Intramural Research Program of the Social and Behavioral Research Branch (SBRB). The SBRB is supported by the National Human Genome Research Institute, National Institutes of Health.
Authors' addresses: Desta Ayode, College of Social Sciences, Addis Ababa University, Addis Ababa, Ethiopia, E-mail: destaayode@yahoo.com. Abebayehu Tora, Department of Sociology, Wolaita Sodo University, Sodo, Ethiopia, E-mail: abezed@yahoo.com. David Farrell, People Designs Inc., Durham, NC, E-mail: dfarrell@peopledesigns.com. Getnet Tadele, College of Social Sciences, Addis Ababa University, Addis Ababa, Ethiopia, E-mail: getnett2001@yahoo.com. Gail Davey, Brighton and Sussex Medical School, Falmer, Brighton, East Sussex, United Kingdom, E-mail: G.Davey@bsms.ac.uk. Colleen M. McBride, Department of Behavioral Sciences and Health Education, Rollins School of Public Health, Emory University, Atlanta, GA, E-mail: cmmcbri@emory.edu.
References
- 1.Bauman AE, Sallis JF, Dzewaltowski DA, Owen N. Toward a better understanding of the influences on physical activity: the role of determinants, correlates, causal variables, mediators, moderators, and confounders. Am J Prev Med. 2002;23:5–14. doi: 10.1016/s0749-3797(02)00469-5. [DOI] [PubMed] [Google Scholar]
- 2.Ryan RM, Patrick H, Deci EL, Williams GC. Facilitating health behaviour change and its maintenance: interventions based on self-determination theory. European Health Psychologist. 2008;10:2–5. [Google Scholar]
- 3.King R, Mann V, Boone PD. Knowledge and reported practices of men and women on maternal and child health in rural Guinea Bissau: a cross sectional survey. BMC Public Health. 2010;10:319. doi: 10.1186/1471-2458-10-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dye TD, Apondi R, Lugada ES, Kahn JG, Smith J, Othoro C. “Before we used to get sick all the time”: perceptions of malaria and use of long-lasting insecticide-treated bed nets (LLINs) in a rural Kenyan community. Malar J. 2010;9:345. doi: 10.1186/1475-2875-9-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musuva RM, Awiti A, Omedo M, Ogutu M, Secor WE, Montgomery SP, Alaii J, Mwinzi PN. Community knowledge, attitudes and practices on schistosomiasis in western Kenya: the SCORE project. Am J Trop Med Hyg. 2014;90:646–652. doi: 10.4269/ajtmh.13-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng'ang'a PN, Jayasinghe G, Kimani V, Shililu J, Kabutha C, Kabuage L, Githure J, Mutero C. Bed net use and associated factors in a rice farming community in central Kenya. Malar J. 2009;8:64. doi: 10.1186/1475-2875-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos S, Bizzo N. From “new genetics” to everyday knowledge: ideas about how genetic diseases are transmitted in two large Brazilian families. Sci Educ. 2005;89:564–576. [Google Scholar]
- 8.Lock M, Freeman J, Sharples R, Lloyd S. When it runs in the family: putting susceptibility genes in perspective. Public Underst Sci. 2006;15:277–300. [Google Scholar]
- 9.Walter FM, Emery J, Braithwaite D, Marteau TM. Lay understanding of familial risk of common chronic diseases: a systematic review and synthesis of qualitative research. Ann Fam Med. 2004;2:583–594. doi: 10.1370/afm.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waters EA, Hay JL, Orom H, Kiviniemi MT, Drake BF. “Don't know” responses to risk perception measures: implications for underserved populations. Med Decis Making. 2013;33:271–281. doi: 10.1177/0272989X12464435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan W, Yin L, Qian H-Z, Li D, Shao Y, Vermund SH, Ruan Y, Zhang Z. HIV risk perception among HIV negative or status-unknown men who have sex with men in China. BioMed Res Int. 2014;2014:232451. doi: 10.1155/2014/232451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuwaha F, Okware J, Ndyomugyenyi R. Predictors of compliance with community-directed ivermectin treatment in Uganda: quantitative results. Trop Med Int Health. 2005;10:659–667. doi: 10.1111/j.1365-3156.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- 13.Lavielle P, Wacher N. The predictors of glucose screening: the contribution of risk perception. BMC Fam Pract. 2014;15:108. doi: 10.1186/1471-2296-15-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yirga D, Deribe K, Woldemichael K, Wondafrash M, Kassahun W. Factors associated with compliance with community directed treatment with ivermectin for onchocerciasis control in southwestern Ethiopia. Parasit Vectors. 2010;3:48. doi: 10.1186/1756-3305-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce E, Bauai L, Sapuri M, Kaldor JM, Fairley CK, Keogh LA. HIV knowledge, risk perception, and safer sex practices among female sex workers in Port Moresby, Papua New Guinea. Int J Womens Health. 2011;3:53–61. doi: 10.2147/IJWH.S14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos EM, Lourenco MTC, Rossi BM. Risk perception among Brazilian individuals with high risk for colorectal cancer and colonoscopy. Hered Cancer Clin Pract. 2011;9:4. doi: 10.1186/1897-4287-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price EW. Non-filarial elephantiasis: confirmed as a geochemical disease, and renamed podoconiosis. Ethiop Med J. 1988;26:151–153. [PubMed] [Google Scholar]
- 18.Davey G, Newport M. Podoconiosis: the most neglected tropical disease? Lancet. 2007;369:888–889. doi: 10.1016/S0140-6736(07)60425-5. [DOI] [PubMed] [Google Scholar]
- 19.Davey G, Gebrehanna E, Adeyemo A, Rotimi C, Newport M, Desta K. Podoconiosis: a tropical model for gene-environment interactions? Trans R Soc Trop Med Hyg. 2007;101:91–96. doi: 10.1016/j.trstmh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Korevaar D, Visser B. Podoconiosis, a neglected tropical disease. Neth J Med. 2012;70:210–214. [PubMed] [Google Scholar]
- 21.Davey G. Podoconiosis, non-filarial elephantiasis and lymphology. Lymphology. 2010;43:168–177. [PubMed] [Google Scholar]
- 22.Alemu G, Tekola Ayele F, Daniel T, Ahrens C, Davey G. Burden of podoconiosis in poor rural communities in Gulliso woreda, west Ethiopia. PLoS Negl Trop Dis. 2011;5:e1184. doi: 10.1371/journal.pntd.0001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tekola Ayele F, Adeyemo A, Finan C, Hailu E, Sinnott P, Burlinson ND, Aseffa A, Rotimi CN, Newport MJ, Davey G. HLA class II locus and susceptibility to podoconiosis. N Engl J Med. 2012;366:1200–1208. doi: 10.1056/NEJMoa1108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deribe K, Brooker SJ, Pullan RL, Sime H, Gebretsadik A, Assefa A, Kebede A, Hailu A, Rebollo MP, Shafi O, Bockarie MJ, Aseffa A, Reithinger R, Cano J, Enquselassie F, Newport MJ, Davey G. Epidemiology and individual, household and geographical risk factors of podoconiosis in Ethiopia: results from the first nationwide mapping. Am J Trop Med Hyg. 2015;92:148–158. doi: 10.4269/ajtmh.14-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayode D, McBride CM, de Heer H, Watanabe E, Gebreyesus T, Tadele G, Tora A, Davey G. The association of beliefs about heredity with preventive and interpersonal behaviors in communities affected by podoconiosis in rural Ethiopia. Am J Trop Med Hyg. 2012;87:623–630. doi: 10.4269/ajtmh.2012.12-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molla YB, Le Blond JS, Wardrop N, Baxter P, Atkinson PM, Newport MJ, Davey G. Individual correlates of podoconiosis in areas of varying endemicity: a case-control study. PLoS Negl Trop Dis. 2013;7:e2554. doi: 10.1371/journal.pntd.0002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tekola Ayele F, Alemu G, Davey G, Ahrens C. Community-based survey of podoconiosis in Bedele Zuria woreda, west Ethiopia. Int Health. 2013;5:119–125. doi: 10.1093/inthealth/iht003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tekola Ayele F, Adeyemo A, Rotimi C. Using a “genomics tool” to develop disease prevention strategy in a low-income setting: lessons from the podoconiosis research project. J Community Genet. 2012;3:303–309. doi: 10.1007/s12687-012-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein ND. What does it mean to understand a risk? Evaluating risk comprehension. J Natl Cancer Inst Monogr. 1999;25:15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024192. [DOI] [PubMed] [Google Scholar]