Abstract
The first oral cholera vaccine (OCV) campaign, since its prequalification by the World Health Organization, in response to an ongoing cholera epidemic (reactive vaccination) was successfully conducted in a poor urban slum of approximately 70,000 inhabitants in Port-au-Prince, Haiti, in 2012. Vaccine coverage was 75% of the target population. This report documents the impact of OCV in reducing the number of culture-confirmed cases of cholera admitted to the Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) cholera treatment center from that community in the 37 months postvaccination (April 2012–April 30, 2015). Of 1,788 patients with culture-confirmed cholera, 1,770 (99%) were either from outside the vaccine area (1,400 cases) or from the vaccinated community who had not received OCV (370 cases). Of the 388 people from the catchment area who developed culture-confirmed cholera, 370 occurred among the 17,643 people who had not been vaccinated (2.1%) and the remaining 18 occurred among the 52,357 people (0.034%) who had been vaccinated (P < 0.001), for an efficacy that approximates 97.5%. Despite not being designed as a randomized control trial, the very high efficacy is a strong evidence for the effectiveness of OCV as part of an integrated package for the control of cholera in outbreak settings.
Introduction
A cholera outbreak, caused by Vibrio cholerae O1 biotype El Tor, was recognized in the Caribbean nation of Haiti in October 2010,1 10 months after a devastating earthquake destroyed public infrastructure and crippled government institutions. This was the first time cholera had been documented in Haiti, so the population was entirely naive. The first cases occurred in small villages along the Artibonite River and rapidly spread downstream along its tributaries. Within 1 month, cholera cases were documented across all 10 departments and at the end of 2010 Haiti reported more cases to the World Health Organization (WHO) than the rest of the world combined (187,377 versus 137,940).2,3
The epidemic began at a time of chaos, severely limited government capacity and a public health crisis with 1.5 million internally displaced people living in tents since the earthquake. In spite of this, the Haitian Ministry of Health (MOH) led a rapid response in collaboration with local nongovernmental institutions and international partners. Cholera treatment centers (CTCs) were established; medical personnel were trained; potable water, sanitation, and hygiene (WASH) practices were promoted; and use of antibiotics for moderate and severe cases was instituted, all quickly leading to a reduction in cholera-associated mortality from 4% to 1%.4 However, by December 2011, the cholera epidemic continued with 537,384 cases and 7,018 deaths reported.5 Seasonal peaks followed the rainy season associated with flooding and surface water contamination.6,7
Lack of sanitation and access to potable water are responsible for continued cholera outbreaks around the world. In 2012, the WHO estimated that 1.4 billion were at risk for cholera and that every year cholera accounts for 28,000–142,000 deaths worldwide.8 The explosive nature of the Haiti outbreak was linked to the introduction of cholera in a naive population with severely limited access to safe drinking water and basic sanitation and the massive internal migration that followed the earthquake.9 Establishment of adequate sanitation and potable water systems is ultimately critical for the effective containment of cholera. However, in a country where 40% of the population lives without access to clean water and only 17% have access to improved sanitation, the cost and time associated with WASH are enormous and efforts over the past 5 years have had limited success. The emergence of cholera in Haiti demanded looking at new immediate cholera control strategies.10
The magnitude of the Haiti outbreak led to intense debate over the use of oral cholera vaccine (OCV) during an ongoing epidemic (reactive vaccination).11–14 From April to July 2012, Haiti conducted the first demonstration project using newly WHO-approved OCV in the face of an ongoing outbreak. Under the leadership of the Haitian MOH, vaccine was given in two sites: in urban slums of Port-au-Prince by the GHESKIO Centers and in the rural Artibonite valley by Partners in Health/Zamni Lasante (PIH).15,16 Overall 97,774 participants were vaccinated with the bivalent heat-inactivated OCV, Shanchol: 52,357 by GHESKIO and 45,417 by PIH, with 91% receiving the recommended two doses. This intervention demonstrated the feasibility of integrating OCV in a comprehensive control package.15,16 The operational success of the Haiti project was instrumental in OCV being endorsed by the WHO for use in endemic and epidemic settings, in creation of a 2 million dose vaccine stockpile, and in OCV being scaled up so that 1.4 million doses are being used annually.5,17,18
Although confirmation was obtained on the feasibility of reactive vaccination as a control strategy, important research questions remained, including the field effectiveness of OCV, duration of protection, efficacy of one versus two doses, and impact of herd immunity. This article reports the positive impact through 37 months of OCV on the rate of culture-confirmed cases of cholera and overall diarrheal illness admitted to the GHESKIO referral CTC serving the vaccinated urban population.
Methods
Study population.
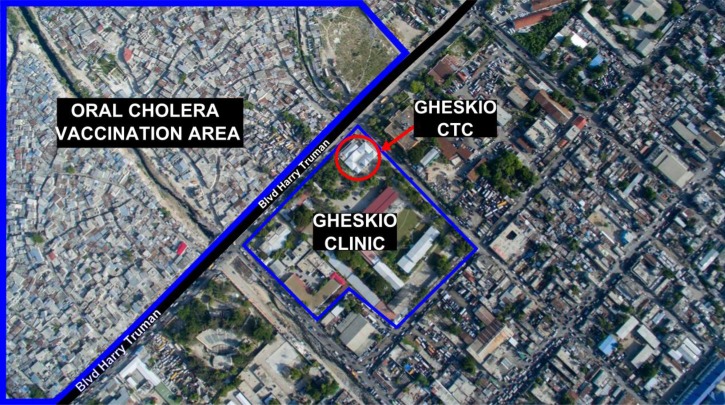
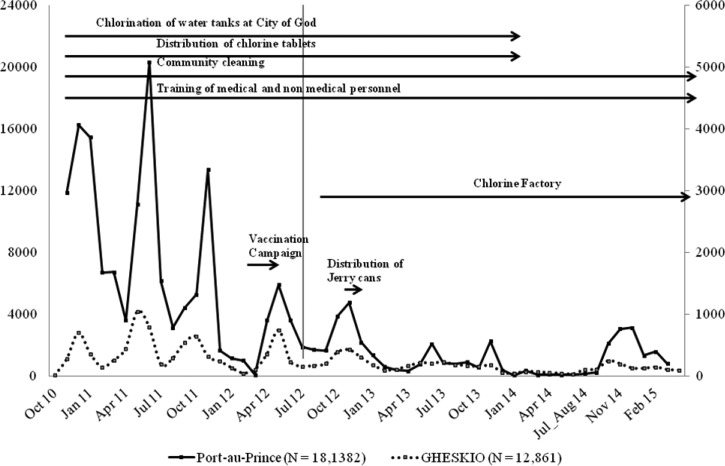
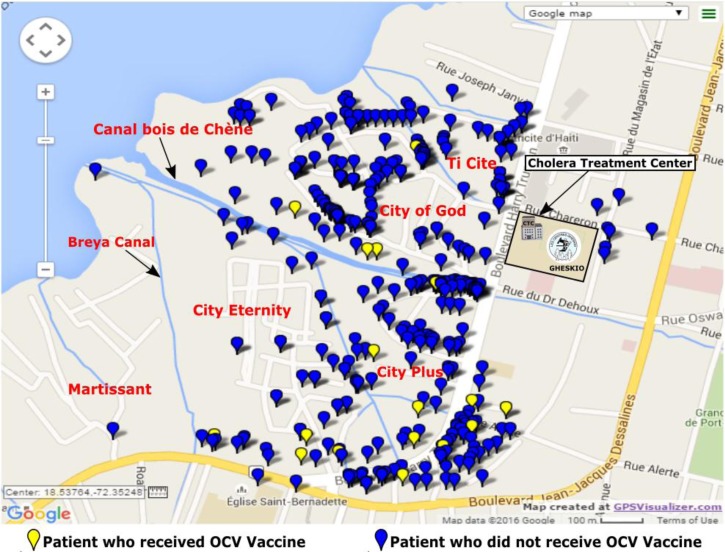
The slums adjacent to GHESKIO are densely populated areas of approximately 70,000 inhabitants with very poor access to basic hygiene, sanitation, and health-care services even before the earthquake (Figure 1 ). The slums are built on fill and refuse, and houses are below sea level so that pit latrines are impractical. Running water is not available inside the slums, so must be purchased from outside the community. High rates of illiteracy and unemployment are markers of the extreme poverty there. In response to the cholera outbreak, GHESKIO, which had assumed responsibility for many health and community services in these slums since the earthquake,19 developed a comprehensive model of prevention and care that included: 1) establishment of a CTC, 2) training medical and support personnel, 3) community sensitization and mobilization, 4) establishment of 15 oral rehydration points (ORPs) inside the slums, 5) promotion of hygiene, 6) water testing and chlorination of water vending points, 7) distribution of oral rehydration salts, 8) trash removal and street cleaning, 9) setting up a chlorine factory for increased chlorine distribution, and 10) distribution of Jerry cans to store water (Figure 2 ).
Figure 1.
Proximity of the Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes cholera treatment center (GHESKIO CTC) in relation to the target vaccination area.
Figure 2.
Distribution of patients with acute watery diarrhea admitted to the Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) cholera treatment center: October 2010–April 2015.
The MOH-GHESKIO urban OCV campaign was conducted over 6 months with an extensive prevaccination phase that included a door-to-door census of the community, establishment of an electronic database of the population, intense community sensitization and education, WASH promotion, and potable water distribution. Over 12 weeks, 52,357 individuals received one dose of Shanchol and 47,645 (91%) received the second dose. Postvaccination monitoring showed that OCV was well tolerated and accepted by the community, and vaccine coverage was estimated at 75% of the target population. GHESKIO continued to promote WASH practices, distribute chlorine, and encourage people to come for care at the GHESKIO CTC if they developed acute watery diarrhea. Patients with moderate to severe dehydration seen at ORPs within the community were referred to the CTC for continued care.
Study site.
The GHESKIO CTC was the first to open in Haiti's West department and has remained fully operational 24 hours/day since the outset of the epidemic. Throughout the study period, it has served as the main referral center receiving patients from nearby communities and referred from other CTCs that closed during low seasons. It was the only CTC available in such close proximity (half a mile) to the vaccinated community (Figure 1). All patients presenting with acute watery diarrhea were assessed and triaged by trained medical personnel who initiated prompt rehydration as per national guidelines. Patients with moderate or severe dehydration were immediately admitted to receive standard of care including oral and intravenous hydration, a single 300-mg dose of doxycycline for adults or erythromycin 12.5 mg/kg four times daily for 3 days for children and education on WASH practices at discharge to prevent further spread. Sociodemographic characteristics, previous use of antibiotics, duration of symptoms prior to presentation, degree of dehydration, and OCV status were gathered at admission for all patients. Paper records were later entered into an electronic database. OCV status was ascertained by presentation of the patient's cholera vaccination card and verified in the GHESKIO database. Stool specimens were collected on all patients with moderate or severe dehydration prior to administration of antibiotics. Systematic human immunodeficiency virus (HIV) counseling and testing was offered to all patients admitted. HIV-infected patients were referred for care at the HIV clinic on the same campus.
Laboratory procedures.
Stools specimens were kept at 4°C during transport to the GHESKIO laboratory for culture. Specimens were inoculated in alkaline peptone water and plated on thiosulfate citrate bile sucrose media. Suspicious colonies were selected for further identification of V. cholerae on MacConkey or blood agar. Stool specimens were aliquoted into sterile vials, archived, and frozen at −80°C. Serum samples were tested as per national guidelines for HIV-1 antibodies with the Determine HIV1/2, Alere (rapid test), or Murex HIV 1.2.0 (enzyme-linked immunosorbent assay) with a confirmatory test done using Shangai Kehua HIV 1-2, BIO-ENGINEERING SARL (Shanghai, China).
Data collection and analysis.
Data on all patients admitted during the study period were extracted from the electronic database. The primary outcome was the frequency of positive cholera culture in patients with moderate and severe diarrhea. We compared the prevalence of cholera between vaccinated and unvaccinated patients from the OCV intervention area and the unvaccinated areas. For comparison of means and medians, Student's t test and Wilcoxon rank sum test were used, respectively. All statistical tests were two sided with a P value of 0.05 for significance. Vaccine efficacy (VE) was estimated using the Orenstein formula.† 20
Results
Diarrheal illness.
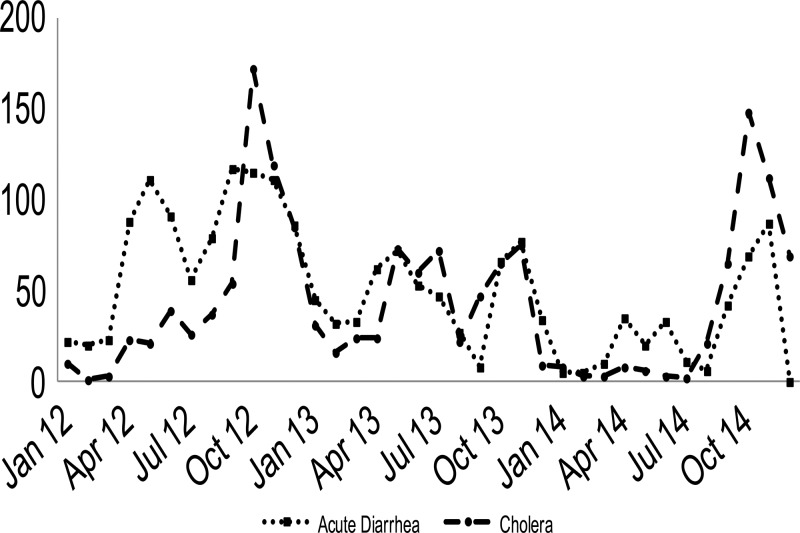
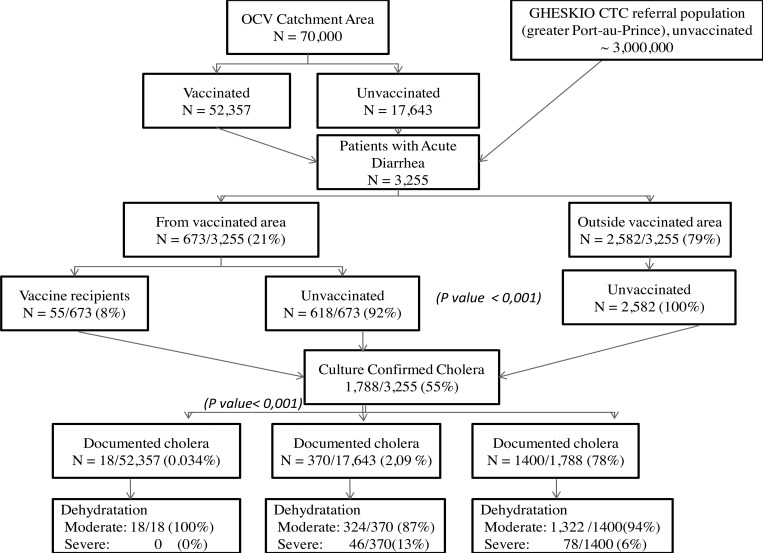
From April 13, 2012 to April 30, 2015, 3,255 patients with acute watery diarrhea were admitted to the GHESKIO CTC. Figure 2 depicts the distribution of admissions from October 2010 through April 2015. For the first 3 years, major peaks occurred twice a year with each rainy season. A significant decline in admissions is noted starting August 2012 with much smaller peaks occurring in 2013 and almost none in 2014. This parallels the curve of acute diarrhea cases for the Port-au-Prince area. Figure 3 shows the rate of culture-confirmed cholera among patients with acute diarrhea in Port-au-Prince. This data from the National Laboratory shows clearly that cholera is the driving force during the period of 2010–2014. In our CTC, 55% (1,788/3,255) of acute diarrhea was due to cholera (Figure 4 ). There were no major differences in patient characteristics between the vaccinated and unvaccinated groups in regards to age (94% older than 5 years) and gender (49% female) (Table 1).
Figure 3.
Rate of culture-confirmed cholera among patients with acute diarrhea in the Port-au-Prince area—Haiti National Laboratory (January 2012–November 2014).
Figure 4.
Impact of different interventions on acute watery diarrhea cases seen at the Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes cholera treatment center (GHESKIO CTC).
Table 1.
Characteristics of patients admitted at the GHESKIO CTC: April 2012–April 2015
| Acute diarrhea | Total (%) | Confirmed cholera cases | Total (%) | |||
|---|---|---|---|---|---|---|
| OCV area | Out of OCV area | OCV area | Out of OCV area | |||
| Female | 334 | 1,267 | 1,601/3,255 (49) | 197 | 674 | 871/1,788 (49) |
| Male | 339 | 1,315 | 1,654/3,255 (51) | 191 | 726 | 917/1,788 (51) |
| Age > 5 years | 592 | 2,319 | 2,911/3,255 (89) | 364 | 1,316 | 1,680/1,788 (94) |
| Age < 5 years | 81 | 263 | 344/3,255 (11) | 24 | 84 | 108/1,788 (6) |
| Total | 673 | 2,582 | 3,255 | 388 | 1,400 | 1,788 |
| HIV testing performed on N = 3,036 patients | ||||||
| HIV+ | 44 | 153 | 197 | 21 | 46 | 67 (34) |
GHESKIO CTC = Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes cholera treatment center; HIV = human immunodeficiency virus; OCV = oral cholera vaccine.
Overall, the OCV catchment area had significantly less cases of acute watery diarrhea and less cholera compared with admissions from outside the vaccination area. Indeed, of the 3,255 admissions to the CTC, 2,582 (79%) were from outside the vaccinated area and 673 (21%) from the OCV catchment area (P < 0.001) (Figure 4). Most admissions from the vaccinated area (92%) had not received the vaccine (618/673) and only 18 of the 52,357 vaccine recipients (0.034%) had culture-confirmed cholera compared with 370 of the 17,643 unvaccinated (2.09%), P value < 0.001. Patients from outside the vaccine area comprised 78% of culture-confirmed cholera cases (1,400/1,788). Finally, severe dehydration at admission was only seen in those who had not received OCV, either from the catchment area or from outside, suggesting a protective effect of OCV on severity of illness.
HIV and diarrhea.
Ninety-three percent of admissions (3,036) were screened for HIV and 197 (6.5%) confirmed positive (Table 1). HIV was found in 67 of 1,277 (5.2%) cholera culture positive cases compared with 130 of 1,759 (7.4%) culture negative cases. None of the HIV-infected individuals with cholera had received OCV compared with four vaccinated HIV-infected patients with noncholera diarrhea. Due to small numbers, estimation of OCV protection in HIV was not possible.
Outcomes postvaccination.
Two important things can be appreciated during the study period: first, all-cause watery diarrhea was significantly less in patients from the OCV catchment area compared with those from outside the vaccinated area, 673 versus 2,582, respectively (P value < 0.001). Second, in admissions from the OCV catchment area of 70,000 inhabitants, culture-confirmed cholera was significantly less frequent in vaccine recipients (18/52,357) compared with unvaccinated cases (370/17,643), P value < 0.001. Furthermore, cholera was more frequently confirmed in slums with lower OCV coverage (Figure 6 and Table 2), further supporting the impact of OCV vaccination in areas with poor sanitary conditions. The overall VE against culture-confirmed cholera using Orenstein‡ model20 was estimated at 97.5% when compared with the unvaccinated population from the same area. Finally, though numbers are small, there were only 18 cases of cholera in patients with at least one dose of vaccine and 10 cases of cholera in those with two doses of vaccine.
Figure 6.
Distribution of patients with culture-confirmed cholera within the Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes cholera treatment center (GHESKIO) oral cholera vaccine catchment area: April 2012–April 2015.
Table 2.
Rate of culture-confirmed cholera versus OCV vaccination coverage by slum in OCV catchment area
| Culture-confirmed cholera vs. vaccination coverage by slum | ||||||
|---|---|---|---|---|---|---|
| Slum | Population census | No. of OCV dose 1 | No. of OCV dose 2 | Vaccination coverage (%) | No. of culture-confirmed cholera cases | Cholera rate (%) |
| Village de Dieu | 11,747 | 7,004 | 6,113 | 60 | 186 | 1.6 |
| Ti Cité | 499 | 243 | 147 | 49 | 20 | 4.0 |
| Cité Plus | 11,990 | 9,764 | 8,700 | 81 | 76 | 0.6 |
| Cité de l'Eternel | 15,789 | 11,314 | 10,431 | 72 | 50 | 0.3 |
| Martissant | 21,326 | 17,448 | 16,430 | 82 | 56 | 0.3 |
| Mobile population around GHESKIO | 8,333 | 6,584 | 5,699 | 79 | 0 | 0.0 |
| Total | 69,684 | 52,357 | 47,520 | 75 | 388 | 0.56 |
GHESKIO = Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes; OCV = oral cholera vaccine.
Discussion
This article documents a sustained impact over 3 years of the first reactive vaccination campaign conducted in urban slums of Haiti. Although not designed as a case-controlled study since Shanchol's efficacy has already been proven,21–24 this article reports on the strong field evidence of OCV efficacy when used in conjunction with other preventative measures.
Since its introduction in Haiti, cholera continues to be an important cause of acute watery diarrhea (Figure 3). Data from Haiti's National Laboratory published in 2013 showed that 1,675 (62%) of 2,703 specimens collected within the 10 departments since 2010 were culture-confirmed cholera.25 Furthermore, their passive surveillance data (unpublished) from 25 public and private sites (April 2012–December 2014) reported cholera in 61% of the 2,938 stools specimens received (Figure 4).
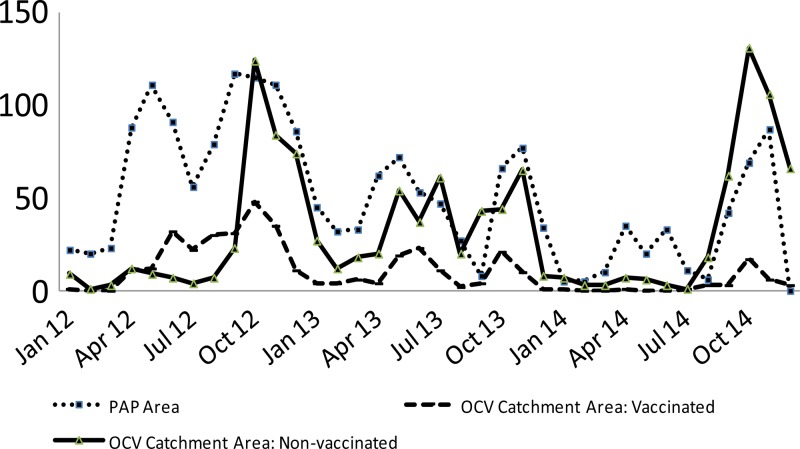
The major decrease in admissions seen at the GHESKIO CTC after July 2012 coincided with two major interventions—the introduction of OCV and the opening of the GHESKIO chlorine factory resulting in increased chlorine distribution in the slums. Although cholera continued to be documented in the vaccine catchment area, the vaccinated group had strikingly lower rates of cholera with an effect that continues up to 3 years after vaccination. No case of cholera has been documented in a vaccine recipient since September 2013 (Figure 5 ). The synergistic effect of OCV plus WASH led to a dramatic reduction of not only cholera but also all-cause watery diarrhea in the vaccinees compared with those who were not vaccinated in the same area. Vaccine recipients were sensitized and even more receptive to the WASH interventions occurring in the slum during the vaccination campaign compared with their unvaccinated neighbors or conversely the same factors that led to avoidance of vaccination contributed to an increased risk of subsequent diarrhea.
Figure 5.
Rate of culture-confirmed cholera in National Laboratory specimens compared with Groupe Haïtien d'Etude du Sarcome de Kaposi et des Infections Opportunistes (GHESKIO) oral cholera vaccine (OCV) catchment area vaccine and nonvaccine recipients.
Strengths.
There are a number of strengths to the study, which allow an estimation of VE. Stool specimens were systematically collected and cultured prior to antibiotics on every admission with moderate to severe diarrhea. A door-to-door census had been conducted in the neighborhood prior to vaccination, so dwelling place was well documented both prior to vaccination and in presentation at the CTC. The proximity of the GHESKIO facility to the slums and closing of other CTCs in the area meant that patients from the vaccinated areas were highly likely to come to the GHESKIO CTC with illness. During the study period, it was the only operational CTC in the metropolitan area within 5 miles. Although cholera was waning, GHESKIO continued as the primary care center for all cases of acute diarrhea during the study. A specific V. cholerae microbiologic diagnosis could be made in 55% of admissions.
The high field effectiveness reported here is not unique as seen in a recent case-controlled study in Guinea that reported field effectiveness of OCV as high as 86% after two doses.22 Our colleagues in the Artibonite valley have recently reported an estimated VE of 65% at their site using a case–control design.26 Our study reports that only 18 cases of cholera occurred in the patients who had received at least one dose. Although a small group, this argues for effectiveness of even a single dose of vaccine.
Finally, the higher HIV prevalence in this study (6.5% versus 2.2% in the general population) is similar to the at-risk adult population presenting to GHESKIO's voluntary counseling and testing center. This suggests that patients presenting with acute watery diarrhea should be targeted for routine HIV screening in HIV-endemic countries.
Limitations.
The efficacy of OCV having already been demonstrated before,22 this study was not designed as a case–control study and thus is susceptible to bias and overestimation of VE. Our study was aimed at evaluating the larger impact of the vaccine on a highly vulnerable population. Potential biases include the impact of natural immunity to cholera, heterogeneity of risk for cholera within the catchment area, impact of migration, and passive surveillance for acute diarrhea cases. OCV was given to a population in which cholera had been circulating for 16 months already, so increasing natural immunity may have contributed to decline in cases. However, people from outside the OCV catchment area were also from marginalized areas of town, where the same conditions favoring cholera transmission prevail. Furthermore, people in the OCV catchment area who had received the vaccine live in the same conditions like those who were not vaccinated (Figure 6). Although many in the OCV catchment area may have been immune from previous exposure to cholera, no differences in age or gender between the vaccinated and unvaccinated were identified and rates of cholera were actually higher in slums with lowest OCV coverage (Table 2). Although passive surveillance was used and people could have presented elsewhere for care, GHESKIO was the only operational CTC within a 5-mile radius and is well known and accepted by the vaccinated community. It is also probable that asymptomatic or mild cases did not present to the GHESKIO CTC and that we only captured the most severe cases that can lead to severe dehydration and death. Migration of population in and out of the slum may also impact the estimated herd immunity. In the most recent increase in cases seen in the September–October 2014 rainy season, all culture-confirmed cases of cholera from the vaccinated community were among unvaccinated individuals who had recently moved there. Finally, the impact of OCV may be hard to separate from the impact of other WASH interventions. However, despite the community sensitization, education, water distribution, and chlorination efforts by GHESKIO since the onset of the epidemic, cholera cases from the slums had continued with peaks roughly every 6 months. The integration of OCV in the arsenal of cholera control strategies was the final step.
Conclusion
The first ever use of OCV in urban and rural areas during an outbreak (reactive vaccination) since its prequalification by WHO proved to be feasible and now is shown to be highly effective. The combination of WASH and OCV is an effective model for the control of cholera even for the most at-risk populations. This study and the parallel study in the Artibonite valley supported and encouraged the Haitian MOH to integrate OCV in its national cholera control strategy, and 600,000 OCV doses have been administered since 2012 in two other campaigns in Haiti's North and Central departments. These findings support the integration of OCV in strategies for the global control of cholera.27,28
ACKNOWLEDGMENTS
We thank the medical staff and community health agents of Les Centres GHESKIO for their hard work and dedication and the patients and community leaders for their continued trust. We are also grateful to Dr. Roger Glass for his judicious comments.
Footnotes
Financial support: This study was supported by United Nations Children's Fund (grant Renforcement et Maintien des Activités dans le Cadre de la Réponse pour le Choléra), the Centers for Disease Control and Prevention (5U2GGH000545-02), and the National Institutes of Health (5D43TW009606).
Authors' addresses: Karine Sévère, Vanessa Rouzier, Stravinsky Benedict Anglade, Claudin Bertil, Patrice Joseph, Alexandra Deroncelay, and Marie Marcelle Mabou, Les Centres GHESKIO (Groupe Haitien d'Etude du Sarcome de Kaposi et des Infections Opportunistes), Port-au-Prince, Haiti, E-mails: karinesevere@gheskio.org, vrouzier@gheskio.org, vegatch@hotmail.com, claudinbert1902@gmail.com, pjoseph@gheskio.org, minister@mspp.gouv.ht, and mmabou@gheskio.org. Peter F. Wright, Department of Pediatrics, Dartmouth Medical School, Hanover, NH, E-mail: peter.f.wright@dartmouth.edu. Florence Duperval Guillaume, Ministry of Public Health and Population, Port-au-Prince, Haiti. Jean William Pape, Les Centres GHESKIO (Groupe Haitien d'Etude du Sarcome de Kaposi et des Infections Opportunistes), Port-au-Prince, Haiti, and Center for Global Health, Department of Medicine, Weill Cornell Medical College, New York, NY, E-mail: jwpape@gheskio.org.
Vaccine efficacy (VE) = (ARU − ARV)/ARU × 100
where ARU = attack rate in the unvaccinated population; ARV = attack rate in the vaccinated population.
Vaccine efficacy (VE) = (ARU − ARV)/ARU × 100
where ARU = attack rate in the unvaccinated population; ARV = attack rate in the vaccinated population.
References
- 1.Centers for Disease Control and Prevention Cholera outbreak—Haiti, October 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1411. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Update: outbreak of Cholera—Haiti. MMWR Morb Mortal Wkly. Rep. 2010;59:1586–1590. [PubMed] [Google Scholar]
- 3.World Health Organization Cholera Annual Report, 2010. Wkly Epidemiol Rec. 2011;31:325–340. [Google Scholar]
- 4.Date KA, Vicari A, Hyde TB, Mintz E, Danovaro-Holliday MC, Henry A, Tappero JW, Roels TH, Abrams J, Burkholder BT, Ruiz-Matus C, Andrus J, Dietz V. Considerations for oral cholera vaccine use during outbreak after earthquake in Haiti, 2010–2011. Emerg Infect Dis. 2011;17:2105–2112. doi: 10.3201/eid1711.110822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Cholera Annual Report, 2011. Wkly Epidemiol Rec. 2012;87:289–304. [Google Scholar]
- 6.Valcin CL, Severe K, Riche CT, Anglade BS, Moise CG, Woodworth M, Charles M, Li Z, Joseph P, Pape JW, Wright PF. Predictors of disease severity in patients admitted to a cholera treatment center in urban haiti. Am J Trop Med Hyg. 2013;89:625–632. doi: 10.4269/ajtmh.13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charles M, Delva GG, Boutin J, Severe K, Peck M, Mabou MM, Wright PF, Pape JW. Importace of cholera and other etiologies of acute diarrhea in post-earthquake Port-au-Prince, Haiti. Am J Trop Med Hyg. 2014;90:511–517. doi: 10.4269/ajtmh.13-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis. 2015;9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNICEF Statistics at a glance: Haiti. http://www.unicef.org/infobycountry/haiti_statistics.html Available at. Accessed September 30, 2014.
- 10.Ivers LC, Farmer P, Almazor CP, Léandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010;376:2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 11.Reyburn R, Deen JL, Grais RF, Bhattacharya SK, Sur D, Lopez AL, Jiddawi MS, Clemens JD, von Seidlein L. The case for reactive mass oral cholera vaccinations. PLoS Negl Trop Dis. 2011;5:e952. doi: 10.1371/journal.pntd.0000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Von Seidlein L, Deen JL. Considerations for oral cholera vaccine use during outbreak after earthquake in Haiti, 2010–2011. Emerg Infect Dis. 2012;18:1211–1214. doi: 10.3201/eid1807.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao DL, Halloran ME, Longini IM., Jr Vaccination strategies for epidemic cholera in Haiti with implications for the developing world. Proc Natl Acad Sci USA. 2011;108:7081–7085. doi: 10.1073/pnas.1102149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivers LC, Farmer P, Pape JW. Oral cholera vaccine and integrated cholera control in Haiti. Lancet. 2012;379:2026–2028. doi: 10.1016/S0140-6736(12)60832-0. [DOI] [PubMed] [Google Scholar]
- 15.Ivers LC, Teng JE, Lascher J, Raymond M, Weigel J, Victor N, Jerome JG, Hilaire IJ, Almazor CP, Ternier R, Cadet J, Francois J, Guillaume FD, Farmer PE. Use of oral cholera vaccine in Haiti: a rural demonstration project. Am J Trop Med Hyg. 2013;89:617–624. doi: 10.4269/ajtmh.13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouzier V, Severe K, Juste MA, Peck M, Perodin C, Severe P, Deschamps MM, Verdier RI, Prince S, Francois J, Cadet JR, Guillaume FD, Wright PF, Pape JW. Cholera vaccination in urban Haiti. Am J Trop Med Hyg. 2013;89:671–681. doi: 10.4269/ajtmh.13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin S, Costa A, Perea W. Stockpiling oral cholera vaccine. Bull World Health Organ. 2012;90:714. doi: 10.2471/BLT.12.112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin S, Lopez AL, Bellos A, Deen J, Ali M, Alberti K, Anh DD, Costa A, Grais RF, Legros D, Luquero FJ, Ghai MB, Perea W, Sack DA. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ. 2014;92:881–893. doi: 10.2471/BLT.14.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pape JW, Deschamps MM, Ford H, Joseph P, Johnson WD, Jr, Fitzgerald DW. The GHESKIO refugee camp after the earthquake in Haiti–dispatch 2 from Port-au-Prince. N Engl J Med. 2010;362:e27. doi: 10.1056/NEJMpv1001785. [DOI] [PubMed] [Google Scholar]
- 20.Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63:1055–1068. [PMC free article] [PubMed] [Google Scholar]
- 21.Schnirring L, Roos R. Cholera vaccine worked well during outbreak. Center for Infectious Disease Research and Policy. CIDRAP News. 2014. May 29, 2014.
- 22.Luquero FJ, Luquero FJ, Grout L, Ciglenecki I, Sakoba K, Traore B, Heile M, Diallo AA, Itama C, Page AL, Quilici ML, Mengel MA, Eiros JM, Serafini M, Legros D, Grais RF. Use of Vibrio cholerae vaccine in an outbreak in Guinea. N Engl J Med. 2014;370:2111–2120. doi: 10.1056/NEJMoa1312680. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, Sah B, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Dhingra MS, Donner A, Nair GB, Lopez AL, Wierzba TF, Clemens JD. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2013;13:1050–1056. doi: 10.1016/S1473-3099(13)70273-1. [DOI] [PubMed] [Google Scholar]
- 24.Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Han SH, Attridge S, Donner A, Ganguly NK, Bhattacharya SK, Nair GB, Clemens JD, Lopez AL. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis. 2011;5:e1289. doi: 10.1371/journal.pntd.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barzilay EJ, Schaad N, Magloire R, Mung KS, Boncy J, Dahourou GA, Mintz ED, Steenland MW, Vertefeuille JF, Tappero JW. Cholera surveillance during the Haiti epidemic—the first 2 years. N Engl J Med. 2013;368:599–609. doi: 10.1056/NEJMoa1204927. [DOI] [PubMed] [Google Scholar]
- 26.Ivers LC, Hilaire IJ, Teng JE, Almazor CP, Jerome JG, Ternier R, Boncy J, Buteau J, Murray MB, Harris JB, Franke MF. Effectiveness of reactive oral cholera vaccination in rural Haiti: a case-control study and bias-indicator analysis. Lancet Glob Health. 2015;3:e162–e168. doi: 10.1016/S2214-109X(14)70368-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinman AR, Farmer PE. The coalition for cholera prevention and control meeting. Vaccine. 2013;31:2323. doi: 10.1016/j.vaccine.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Pape JW, Rouzier V. Embracing oral cholera vaccine–shifting response to cholera. N Engl J Med. 2014;370:2067–2069. doi: 10.1056/NEJMp1402837. [DOI] [PubMed] [Google Scholar]