Abstract
Immunoproliferative small intestinal disease (IPSID) is an extra-nodal B-cell lymphoma most commonly described in the Mediterranean, Africa, and Asia. It is associated with poverty and poor sanitation, and is rarely encountered in developed countries. A 26-year-old previously healthy, Marshallese male was transferred to our facility with a 6-month history of watery diarrhea, weakness, and cachexia refractory to multiple short courses of oral antibiotics. Stool cultures grew Campylobacter jejuni and Vibrio fluvialis. Endoscopic evaluation showed histologic evidence of Helicobacter pylori gastritis and gross evidence of whipworm infection found in the colon. Mesenteric lymph node biopsy cultures grew Escherichia coli. Histopathology and immunohistochemical stains of the small intestine were consistent with IPSID. He subsequently transformed to diffuse large B-cell lymphoma (DLBCL) with tonsillar involvement despite treatment with rituximab and an extended course of antibiotics. Systemic chemotherapy with six cycles of rituximab, cyclophosphamide, vincristine, doxorubicin, prednisone, and lenalidomide, resulted in remission of his diffuse B cell lymphoma. This case is illustrative of IPSID developing in a previously healthy individual due to overwhelming polymicrobial gastrointestinal infection by C. jejuni and other enteric pathogens with subsequent transformation to an aggressive DLBCL. IPSID should be considered in residents of developing countries presenting with refractory chronic diarrhea, weight loss, and mesenteric lymphadenopathy.
Introduction
Immunoproliferative small intestinal disease (IPSID), also known as Mediterranean lymphoma or α-heavy chain disease, is a rare extra-nodal marginal zone B-cell lymphoma occurring primarily in the proximal small intestine. It is a variant of mucosa-associated lymphoid-tissue lymphoma (MALT) described in young adults from the developing world, and is characterized by lymphoplasmocytic intestinal infiltrates with monotypic α-heavy chain expression.1 Its pathogenesis is attributed to antigenic stimulation by gastrointestinal infections; most notably Campylobacter jejuni, as identified by retrospective molecular analyses of tissue biopsies.2,3 We describe the first reported case of IPSID in a Pacific Islander who presented with C. jejuni, Vibrio fluvialis, Helicobacter pylori, and Trichuris trichiura gastrointestinal infections, as well as Escherichia coli mesenteric lymphadenitis with subsequent transformation to diffuse large B-cell lymphoma (DLBCL).
Case
A 26-year-old previously healthy sanitation worker from the Marshall Islands was seen at our tertiary care facility for a 6-month history of copious diarrhea (10–20 watery stools per day), persistent hypokalemia, and 40-lb weight loss. His diarrhea had failed to respond to multiple courses of empiric oral antibiotics. He denied any fevers, chills, night sweats, nausea, vomiting, abdominal pain, rashes, chest pain, or shortness of breath. On examination, he was cachectic and ill-appearing with bitemporal wasting, appearing older than his stated age. He had global weakness, but no focal neurologic abnormalities, organomegaly, or lymphadenopathy was noted.
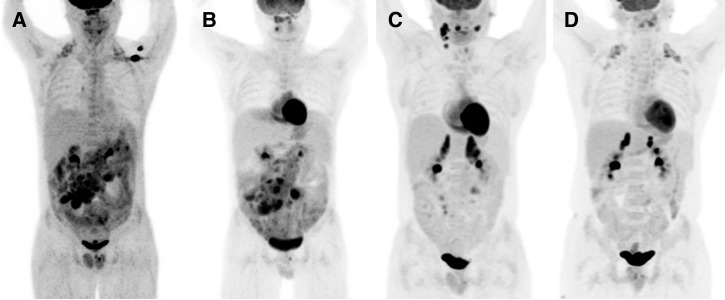
Initial laboratory investigations noted leukocytosis, mild anemia, and multiple biochemical abnormalities, including hypokalemia, hyponatremia, and a non-anion gap metabolic acidosis (Table 1). Additional laboratory tests, including an extensive serologic workup, are presented in Table 2. Stool cultures grew V. fluvialis and C. jejuni. Contrasted computerized tomography (CT) of the abdomen and pelvis demonstrated massive mesenteric and retroperitoneal lymphadenopathy with diffuse bowel-wall edema and mild hepatomegaly. Subsequent positron emission tomography-computerized tomography (PET-CT) revealed extensive small bowel and mesenteric hypermetabolic activity (Figure 1A ).
Table 1.
Initial hematologic and biochemical laboratory results
| Laboratory test | Result | Reference range |
|---|---|---|
| WBCs (109/L) | 19.1 | 3.9–10.6 |
| Neutrophil % | 74.1 | 42.2–75.2 |
| Hemoglobin (g/dL) | 13.4 | 13.3–17.7 |
| Platelets (109/L) | 438 | 150–440 |
| Na+ (mmol/L) | 129 | 136–145 |
| K+ (mmol/L) | 2.2 | 3.5–5.3 |
| HCO3- (mmol/L) | 12 | 23–29 |
| Albumin (g/dL) | 3.4 | 3.5–5.0 |
| Alkaline phosphatase (U/L) | 647 | 40–150 |
| Lactate dehydrogenase (U/L) | 205 | 121–247 |
| IgA (mg/dL) | 586 | 63–404 |
| IgG (mg/dL) | 944 | 540–1822 |
| IgM (mg/dL) | 23 | 22–293 |
IgA = immunoglobulin A; IgG = immunoglobulin G; IgM = immunoglobulin M; WBC = white blood cells.
Table 2.
Initial inflammatory and serologic test results
| Assay/test | Result |
|---|---|
| Erythrocyte sedimentation rate (mm/hour) | 34 |
| C-reactive protein (mg/L) | 8.3 |
| Procalcitonin (ng/mL) | 0.21 |
| Fecal leukocytes | Negative |
| Stool ova and parasite | Negative |
| Cryptosporidium DFA | Negative |
| Giardia DFA | Negative |
| Strongyloides Ab | Negative |
| Clostridium difficile toxin B | Negative |
| Treponema pallidum ELISA | Negative |
| HIV | Negative |
| Interferon gamma release assay | Negative |
| CMV IgG/IgM | Positive/negative |
| Hepatitis A IgG/IgM | Negative |
| Hepatitis B core Ab | Positive |
| Hepatitis B surface Ab | Negative |
| Hepatitis B surface Ag | Negative |
| Hepatitis B e Ag | Negative |
| Hepatitis C Ab | Negative |
| Antinuclear Ab | Negative |
| Anti-mitochondrial Ab | Negative |
| Anti-smooth muscle Ab | Positive |
| Tissue transglutaminase IgA | Negative |
Ab = Antibody; Ag = antigen; ELISA = enzyme-linked immunosorbent assay; HIV = human immunodeficiency virus; IgG = immunoglobulin G; IgM = immunoglobulin M; DFA = direct fluorescent antibody; CMV = cytomegalovirus.
Figure 1.
Three-dimensional coronal positron-emission tomography with 18-fluoro-2-deoxyglucose (FDG) composite images taken during the patient's evaluation and treatment. Panel A shows increased uptake in the small bowel and mesenteric lymph nodes on initial presentation. Panel B shows improvement in the small bowel FDG-avidity, but worsening mesenteric lymphadenopathy 3 weeks after completion of the initial 14-day antibiotic course. Panel C demonstrates improvement in the mesenteric lymph node uptake after 3 months of antibiotic therapy and four cycles of rituximab for immunoproliferative small intestinal disease, but also demonstrates new FDG uptake in the right cervical lymph node chain and right palatine tonsil. (D) Following completion of systemic chemotherapy for diffuse large B-cell lymphoma, the patient had complete resolution of the hypermetabolic lymphadenopathy.
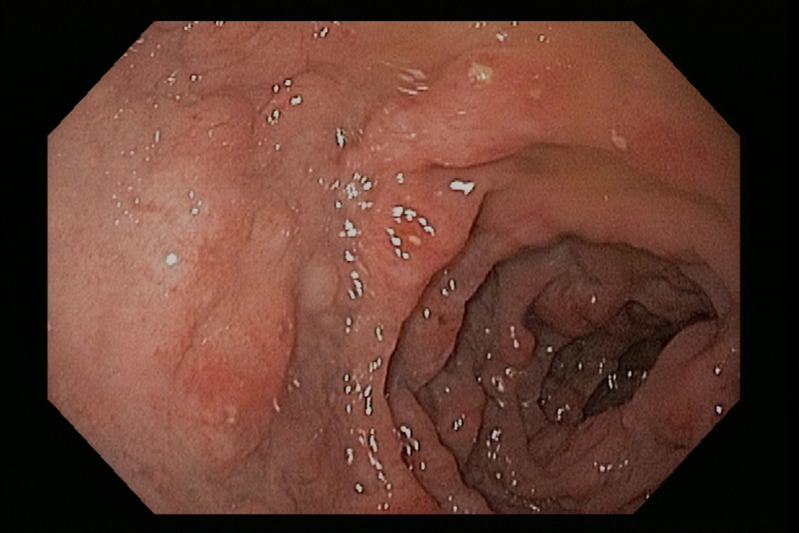
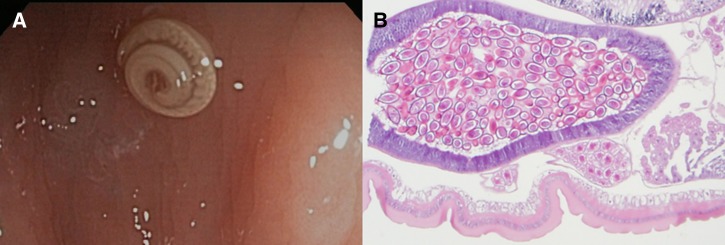
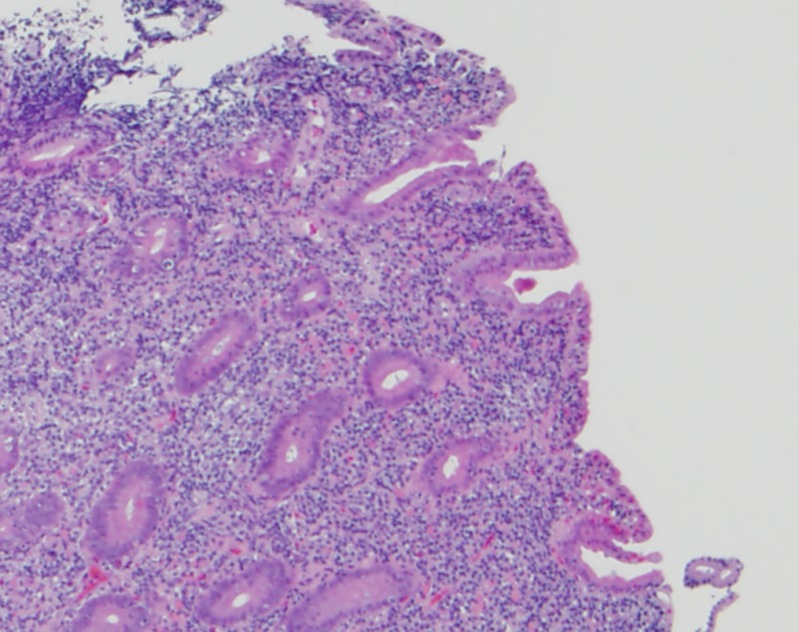
He was treated with piperacillin-tazobactam for 14 days for the C. jejuni and V. fluvialis infection, and for empiric coverage of enteric pathogens due to concerns of bacterial translocation from the diffuse colitis. He had resolution of his diarrhea with weight gain after completion of therapy. However, a PET-CT done 3 weeks later showed worsening of his mesenteric lymphadenopathy despite his clinical improvement (Figure 1B). A CT-guided mesenteric lymph node biopsy showed necrotic debris with admixed macrophages and clusters of gram-negative bacilli consistent with suppurative lymphadenitis. Aerobic and anaerobic tissue culture grew pan-sensitive E. coli. Esophagogastroduodenoscopy showed atrophic, nodular mucosa in the duodenum and colonoscopy showed nodular mucosa in the terminal ileum (Figure 2 ) with a T. trichiura worm attached to the cecum (Figure 3 ). Gastric mucosal biopsies showed high-density H. pylori infection with mild chronic gastritis, while the duodenal bulb and terminal ileum showed lymphoid expansion of the lamina propria with severe blunting of the villi and focal crypt destructive lesions with large immunoblastic lymphoid infiltrates (Figure 4 ). Immunohistochemical stains indicated increased monotypic α-heavy chain expression diagnostic of IPSID.
Figure 2.
Upper endoscopic image showing duodenal mucosal atrophy, nodularity, and ulcerations.
Figure 3.
(A) Trichuris trichiura attaching to the terminal ileum lumen on endoscopy. (B) Hematoxylin and eosin-stained longitudinal section of posterior end of worm with gravid uterus. Annulation in the cuticle as well as ova with bipolar plugs characteristic of T. trichiura are shown.
Figure 4.
Hematoxylin and eosin-stained duodenal biopsy demonstrating lymphoplasmacytic infiltration of the lamina propria, villous blunting, and focal crypt destruction.
The patient received a 28-day course of intravenous ceftriaxone for his E. coli lymphadenitis in conjunction with a 14-day course of metronidazole, azithromycin, and pantoprazole for his H. pylori infection. Repeat gastric biopsy and fecal antigen testing confirmed H. pylori eradication. His trichiuriasis was successfully treated with oral albendazole. In the middle of this antimicrobial therapy, he had a hepatitis B flare that was controlled with oral entecavir, which was continued throughout his chemotherapy treatment. He was transitioned to oral moxifloxacin for continued suppression of enteric pathogens in an effort to minimize antigenic stimulation. Despite 12 weeks of antibiotic therapy, repeat CT showed worsening of his mesenteric lymphadenopathy, and four cycles of rituximab was added for the IPSID in accordance with current clinical practice guidelines for localized extra-nodal marginal zone lymphomas.4 Six weeks later, PET-CT showed new hypermetabolic activity in the right cervical lymph nodes and right palatine tonsil (Figure 1C). Excisional biopsy of the tonsils showed sheets of atypical, large lymphoid cells with immunohistochemistry diagnostic (CD10 negative, BCL-6 trace positive, and MUM1 positive) of non-germinal center DLBCL with heavy IgA expression suggesting transformation from the underlying IPSID (Figure 5 ).5 He underwent systemic chemotherapy with six cycles of rituximab, lenalidomide, cyclophosphamide, doxorubicin, vincristine, and prednisone (R2-CHOP) for his DLBCL. This regimen was chosen based on reported improved progression-free and overall survival in patients with non-germinal center lymphomas at 24 months.6 Upon completion of chemotherapy, PET-CT demonstrated complete resolution of the previously hypermetabolic lymphadenopathy, resolution of colitis, and he had marked clinical improvement with cessation of diarrhea and 56-lb weight gain since his initial presentation (Figure 1D). He returned to the Marshall Islands to complete an additional 6 months of antibiotic therapy with moxifloxacin and entecavir. Telemedicine follow-up at 3 months after completion of chemotherapy was conducted with his primary care physician in the Marshall Islands and the patient is reported to be back at work in his prior sanitation job.
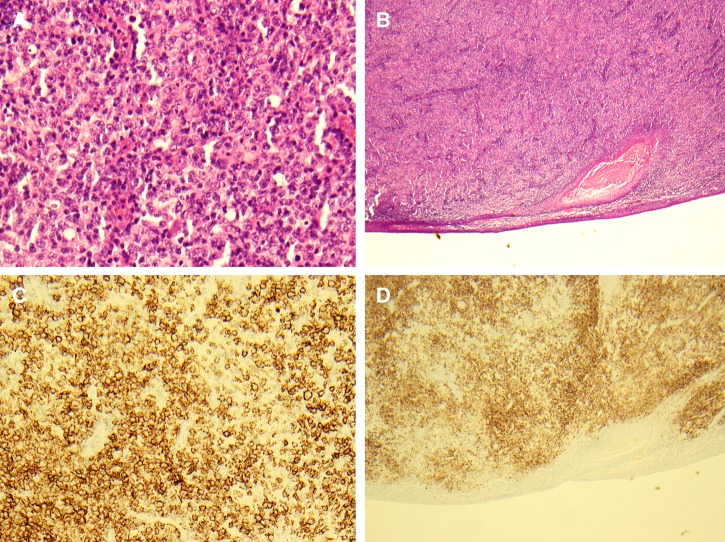
Figure 5.
Histopathology of the excised right palatine tonsil. (A and B) Hematoxylin and eosin–stained section of tonsillar biopsy (×40 and ×10, respectively) showing sheets of large, atypical lymphoid cells and obliteration of normal nodal architecture consistent with diffuse large B-cell lymphoma. (C and D) CD20 immunostaining positivity in both higher power (×40) and lower power (×10) magnification, respectively.
Discussion
We report the first case of IPSID in a Pacific Islander. The majority of IPSID cases are seen in patients from the Middle East, North Africa, and Asia, although cases from Central and South America are described.7 Adolescents and young adults (age range 10–35) are primarily affected with a male predominance of 2:1.1 The most common predisposing factors are poor hygiene, and lack of adequate sanitation and clean drinking water.8 Lankarani and others reported a significant decrease in IPSID incidence after major infrastructure improvements to deliver clean drinking water and improve sanitation systems in the 25-year period after the Islamic Revolution in Iran.9 Recurrent episodes of pediatric enterocolitis has also been reported as a predisposing factor.10 With the reported association between hygiene and IPSID, this patient's occupation as a sanitation worker likely placed him at increased risk for developing recurrent gastrointestinal infections, particularly if regular handwashing was not routinely practiced.
Typically, patients present with a malabsorption syndrome characterized by abdominal pain, weight loss, and biochemical abnormalities including hypoproteinemia and electrolyte derangements due to chronic diarrhea. In this case, the patient required supplemental potassium for 6 months. Endoscopy revealed edema, erythema, and villous atrophy of the small bowel with sparing of the gastric mucosa and large intestine. Mucosal ulcerations, cobblestone appearance, as well as lymphangiectasias have been reported.3,11 Pathology shows extensive lymphoplasmacytic infiltration with blunting or absence of the villi and destruction of the crypts, and in later stages the infiltrate can be marked by atypical lymphoid cells.1,7 In advanced disease, mesenteric, abdominal, and retroperitoneal lymph nodes can become involved, but involvement of other abdominal organs (liver, spleen) is uncommon.7
The pathogenesis of IPSID is not fully elucidated, although is believed to result from chronic antigenic stimulation due to recurrent or chronic gastrointestinal infections. To date, no causative agent has been identified. Small intestinal bacterial overgrowth, giardiasis, and other parasitic infections, including one case of trichiuriasis, have been reported in patients with IPSID.12 Campylobacter jejuni has been identified as a possible cause based on post hoc molecular detection of C. jejuni DNA in IPSID histopathology specimens.2,3 Puri and others reported a case of severe C. jejuni gastroenteritis in a patient undergoing chemotherapy for advanced IPSID, but this was seen as a complication of immunosuppression rather than an associated causal factor.13 In contrast, this is the first case where C. jejuni was cultured from stool of a patient with IPSID before diagnosis. Recently, Campylobacter coli was cultured from a patient with IPSID, suggesting a possible association with the disease.14 The exact mechanism of how Campylobacter would precipitate IPSID is unknown, but Al-Saleem and Al-Mondhiry hypothesized that exposure to a C. jejuni toxin, in conjunction with previous exposure to cholera toxin could lead to the monoclonal lymphoplasmacytic expansion seen on histopathology.1 In contrast to H. pylori, Campylobacter is rarely a colonizing pathogen, although long-term infections have been reported in immunocompromised patients, and asymptomatic C. jejuni infection has been reported in pediatric patients in developing countries.15,16 Interestingly, this patient was also infected with H. pylori, a well-known microbial cause of gastric MALT. The degree to which H. pylori contributes to the development of IPSID is unclear; two cases have reported regression of disease following successful eradication of H. pylori with antibiotic therapy.17,18 Vibrio fluvialis has not previously been reported in a case of IPSID, and it is unclear the extent to which it might augment malignant transformation. In conjunction with the whipworm infection, the isolation of V. fluvialis in the stool is suggestive of poor sanitation and lack of clean drinking water. The presence of multiple recurrent and concurrent enteric infections resulted in chronic intestinal inflammation that led to IPSID. Further studies are needed to evaluate the role of individual versus multiple pathogens and the degree of immune activation in the development of IPSID.
Treatment of IPSID is based on staging. In early disease, with minimal bowel wall involvement, extended courses of antibiotics (commonly tetracycline) have been shown to demonstrate clinical remission.1,19–21 Advanced disease is marked by involvement of the mesenteric lymph nodes, local and/or regional spread, or development of bowel obstruction from bulky tumor. Transformation to lymphoma, notably DLBCL, has been described although this is typically found on intestinal biopsy and has been associated with bowel obstruction and gastrointestinal hemorrhage.22,23 As in this patient, advanced cases with regional or distant metastases are treated with cyclophosphamide, vincristine, doxorubicin, and prednisone-based regimens with concurrent antibiotics, with reported 60–70% disease-free survival at 3 years.1 The role of rituximab has not yet been studied in IPSID directly, and was initially selected as treatment of a localized extra-nodal marginal zone B-cell lymphoma, consistent with the current guidelines. Monotherapy with rituximab was ineffective in this patient likely due to the rapid transformation to DLBCL. Further studies are needed to determine if rituximab has a role in less advanced cases with or without concomitant antibiotics.
We report the first case of IPSID in a Pacific islander which was associated with overwhelming gastrointestinal infection and subsequent transformation to DLBCL that responded to systemic chemotherapy and prolonged course of antibiotics. Although this disease is rarely seen in the developed world, it should be considered in patients presenting with chronic diarrhea, weight loss, and electrolyte abnormalities, particularly those emigrating from developing countries.
ACKNOWLEDGMENTS
We would like to thank Francis Gress and Harlan Rumjahn for their assistance with histology images and review of the case, as well as Integrated Oncology (Phoenix, AZ) for performing the immunohistochemistry studies. We would also like to thank Caleb Anderson, Kinsley Hubel, and Robert Pangilinan for providing excellent patient care during the patient's hospitalization.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Army, the Department of Defense, or the U.S. Government. Title 17 U.S.C. §105 provides that “copyright protection under this title is not available for any work of the United States Government”. Title 17 U.S.C. §101 defines a United States government work as “a work prepared by a military service member or employee of the United States government as part of that person's official duties”. This work was prepared as part of our official duties.
Footnotes
Authors' addresses: Evan C. Ewers, Department of Medicine, Tripler Army Medical Center, Honolulu, HI, E-mail: evan.c.ewers.mil@mail.mil. Robert L. Sheffler, Hematology/Oncology Service, Department of Medicine, Tripler Army Medical Center, Honolulu, HI, E-mail: robert.l.sheffler.mil@mail.mil. James Wang, Gastroenterology Service, Department of Medicine, Tripler Army Medical Center, Honolulu, HI, E-mail: james.y.wang.mil@mail.mil. Viseth Ngauy, Infectious Disease Service, Department of Medicine, Tripler Army Medical Center, Honolulu, HI, E-mail: viseth.ngauy.mil@mail.mil.
References
- 1.Al-Saleem T. Immunoproliferative small intestinal disease (IPSID): a model for mature B-cell neoplasms. Blood. 2005;105:2274–2280. doi: 10.1182/blood-2004-07-2755. [DOI] [PubMed] [Google Scholar]
- 2.Lecuit M, Abachin E, Martin A, Poyart C, Pochart P, Suarez F, Bengoufa D, Feuillard J, Lavergne A, Gordon JI, Berche P, Guillevin L, Lortholary O. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med. 2004;350:239–248. doi: 10.1056/NEJMoa031887. [DOI] [PubMed] [Google Scholar]
- 3.Mesnard B, De Vroey B, Maunoury V, Lecuit M. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. Dig Liver Dis. 2012;44:799–800. doi: 10.1016/j.dld.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Non-Hodgkin's Lymphoma (Version 5.2014) 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Available at. Accessed November 1, 2015. [Google Scholar]
- 5.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 6.Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, Thompson CA, Rivera CE, Inwards DJ, Micallef IN, Johnston PB, Porrata LF, Ansell SM, Gascoyne RD, Habermann TM, Witzig TE. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015;33:251–257. doi: 10.1200/JCO.2014.55.5714. [DOI] [PubMed] [Google Scholar]
- 7.Fine KD, Stone MJ. Alpha-heavy chain disease, Mediterranean lymphoma, and immunoproliferative small intestinal disease: a review of clinicopathological features, pathogenesis, and differential diagnosis. Am J Gastroenterol. 1999;94:1139–1152. doi: 10.1111/j.1572-0241.1999.01057.x. [DOI] [PubMed] [Google Scholar]
- 8.Rambaud JC. Small intestinal lymphomas and alpha-chain disease. Clin Gastroenterol. 1983;12:743–766. [PubMed] [Google Scholar]
- 9.Lankarani KB, Masoompour SM, Masoompour MB, Malekzadeh R, Tabei SZ, Haghshenas M. Changing epidemiology of IPSID in southern Iran. Gut. 2005;54:311–312. doi: 10.1136/gut.2004.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khojasteh A, Haghshenass M, Haghighi P. Current concepts immunoproliferative small intestinal disease. A “Third-World lesion”. N Engl J Med. 1983;308:1401–1405. doi: 10.1056/NEJM198306093082309. [DOI] [PubMed] [Google Scholar]
- 11.Ersoy O, Akin E, Demirezer A, Atalay R, Buyukasik S. Capsule-endoscopic findings in immunoproliferative small-intestinal disease. Endoscopy. 2012;44((Suppl 2)):E61–E62. doi: 10.1055/s-0031-1291564. [DOI] [PubMed] [Google Scholar]
- 12.Ghoshal UC, Chetri K, Banerjee PK, Choudhuri G, Pal BB, Dabadghao S, Dhar K, Naik S, Naik SR. Is immunoproliferative small intestinal disease uncommon in India? Trop Gastroenterol. 2001;22:14–17. [PubMed] [Google Scholar]
- 13.Puri AS, Aggarwal R, Khan EM, Naik S, Ayyagari A, Pandey R, Naik SR. Explosive Campylobacter jejuni diarrhea in immunoproliferative small intestinal disease. Indian J Gastroenterol. 1992;11:141–143. [PubMed] [Google Scholar]
- 14.Coeuret S, de La Blanchardière A, Saguet-Rysanek V, Chèze S, Tavernier M, Arsène D, Criscuolo A, Brisse S, Vergnaud M, Verdon R, Lecuit M. Campylobacter coli cultured from the stools of a patient with immunoproliferative small intestinal disease. Clin Microbiol Infect. 2014;20:908–911. doi: 10.1111/1469-0691.12545. [DOI] [PubMed] [Google Scholar]
- 15.Perlman DM, Ampel NM, Schifman RB, Cohn DL, Patton CM, Aguirre ML, Wang WL, Blaser MJ. Persistent Campylobacter jejuni infections in patients infected with the human immunodeficiency virus (HIV) Ann Intern Med. 1988;108:540–546. doi: 10.7326/0003-4819-108-4-540. [DOI] [PubMed] [Google Scholar]
- 16.Bodhidatta L, McDaniel P, Sornsakrin S, Srijan A, Serichantalergs O, Mason CJ. Case-control study of diarrheal disease etiology in a remote rural area in western Thailand. Am J Trop Med Hyg. 2010;83:1106–1109. doi: 10.4269/ajtmh.2010.10-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutta U, Udawat H, Noor MT, Sidhu GS, Kochhar R, Vaiphei K, Singh K. Regression of immunoproliferative small intestinal disease after eradication of Helicobacter pylori. J Gastrointest Cancer. 2010;41:212–215. doi: 10.1007/s12029-010-9138-z. [DOI] [PubMed] [Google Scholar]
- 18.Fischbach W, Tacke W, Greiner A, Konrad H, Müller-Hermelink, Regression of immunoproliferative small intestinal disease after eradication of Helicobacter pylori. Lancet. 1997;349:31–32. doi: 10.1016/s0140-6736(05)62165-4. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto S, Kinoshita Y, Fukuda H, Waki S, Asahara M, Matsushima Y, Kawanami C, Fujimori T, Chiba T. “Mediterranean lymphoma” treated with antibiotics. Intern Med. 1996;35:961–965. doi: 10.2169/internalmedicine.35.961. [DOI] [PubMed] [Google Scholar]
- 20.Saghir el NS. Combination chemotherapy with tetracycline and aggressive supportive care for immunoproliferative small-intestinal disease lymphoma. J Clin Oncol. 1995;13:794–795. doi: 10.1200/JCO.1995.13.3.794. [DOI] [PubMed] [Google Scholar]
- 21.Foster LH, Portell CA. The role of infectious agents, antibiotics, and antiviral therapy in the treatment of extranodal marginal zone lymphoma and other low-grade lymphomas. Curr Treat Options Oncol. 2015;16:28. doi: 10.1007/s11864-015-0344-6. [DOI] [PubMed] [Google Scholar]
- 22.Pervez S, Mumtaz K, Ullah SS, Akhtar N, Ali N, Aaqil H. Immunoproliferative small intestinal disease (IPSID) J Coll Physicians Surg Pak. 2011;21:57–58. [PubMed] [Google Scholar]
- 23.Stratigos P, Kouskos E, Kouroglou M, Chrisafis I, Fois L, Mavrogiorgis A, Axiotis E, Zamtrakis S. Emergency pancreatoduodenectomy (whipple procedure) for massive upper gastrointestinal bleeding caused by a diffuse B-cell lymphoma of the duodenum: report of a case. Surg Today. 2007;37:680–684. doi: 10.1007/s00595-007-3465-0. [DOI] [PubMed] [Google Scholar]