Abstract
Human taeniasis/cysticercosis caused by the pork tapeworm Taenia solium has been identified as a potentially eradicable disease by the International Task Force for Disease Eradication of the World Health Organization. In southeast Asia, T. solium taeniasis/cysticercosis is considered one of the major neglected tropical diseases afflicting the region. In the last few decades, a considerable effort has been invested toward establishing the epidemiology and burden of disease in several southeast Asian countries. Moreover, further evidence is emerging as to understanding the dynamics of disease transmission and cultural, political, and socioeconomic factors influencing the success of control and eradication efforts within the region. However, despite major collaborations by several champion groups, advances have been slow and little remains known about the complete epidemiology of taeniasis/cysticercosis and the barriers to programmatic success. This review article aims to address the above issues with a further focus on the challenges to control and eradicate taeniasis/cysticercosis within the southeast Asia region.
Introduction
Human taeniasis/cysticercosis is a zoonotic disease caused by the pork tapeworm Taenia solium. The World Health Organization (WHO) designated taeniasis/cysticercosis as one of the 17 neglected tropical diseases (NTDs) affecting the poorest people in the world.1,2 An estimated 2.5 million people are infected with T. solium, and there are 50,000 deaths annually due to neurocysticercosis.3 Neurocysticercosis is also the leading cause of epilepsy in many low- and medium-income countries; it is thought to contribute up to 30% of epilepsy cases in the endemic areas, with an estimated disease burden of 2–5 million loss in disability-adjusted life years (DALYs).2,4 Taeniasis/cysticercosis can thus be considered a poverty-perpetuating disease with adverse economic consequences to the affected populations.
In 1992, the International Task Force for Disease Eradication (IFTDE) of WHO declared T. solium as potentially eradicable.5 Since this declaration, several pilot programs on case detection and management of taeniasis/cysticercosis have emerged in many countries worldwide, but all with varying success rates.6 However, taking into account the definitions proposed by Molyneux and others7 where “eradication” is defined as the permanent reduction to zero of the worldwide incidence of infection, current available evidence suggests that eradication of T. solium remains far from possible in a foreseeable future.6 Nevertheless, in 2013, IFTDE reaffirmed their opinion regarding the eradicability of T. solium, putting forth further key recommendations to interrupt the parasite life cycle and disease transmission.8
In southeast Asia (SEA), taeniasis/cysticercosis is moderately endemic and has been identified as one of the major NTDs afflicting the region.9–11 SEA is a politically, socioeconomically, ethnoreligiously, and culturally diverse region consisting of 10 Association of Southeast Asian Nations (ASEAN) member states bound by the 2008 Charter in efforts to regional cooperation on many top level issues including that of population health.12 In the last three decades, while many ASEAN member nations have undergone rapid economic growth with significant improvements in health-care and living standards, certain countries such as Cambodia, Laos People's Democratic Republic (Lao PDR), and Myanmar remain on the United Nation's list of least developed countries.13 Further, socioeconomic and health status disparity still exists in many remote areas within some of the nations. Already marginalized and living under the strain of poverty, the ongoing burden of taeniasis/cysticercosis continue to pose a major threat to human and livestock health and economic productivity in many of these communities.14,15
To date, despite major collaborative efforts by several champion groups, advances in the control of taeniasis/cysticercosis have been slow and little is known about the complete epidemiology, burden of disease, transmission dynamics, and barriers to programmatic success across this region. This review article aims to address the above issues with a further focus on the diagnostic challenges in obtaining accurate epidemiological information and the emerging barriers to the control of taeniasis/cysticercosis within the SEA region. Clinical aspects and individual treatment options for taeniasis/cysticercosis are not considered in depth here.
Literature Search Methods
For review of literature on epidemiology and control measures, all published articles in English language from 2000 to June 2015 were searched using PubMed, Embase, and GoogleScholar using search terms “taeniasis,” “cysticercosis,” “neurocysticercosis,” “prevalence,” “epidemiology,” “control,” “elimination,” “eradication,” in combination with “Southeast Asia” or names of individual countries within the region (Brunei, Cambodia, East Timor, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand, and Vietnam). Articles were further hand searched using citations from referenced articles. Particular emphasis was given to publications within the last 5 years, since a comprehensive review by Willingham and others has previously summarized the epidemiological data till 2010.14 The search method identified 28 epidemiological studies and eight publications on control measures relevant to SEA region.
T. Solium Life Cycle
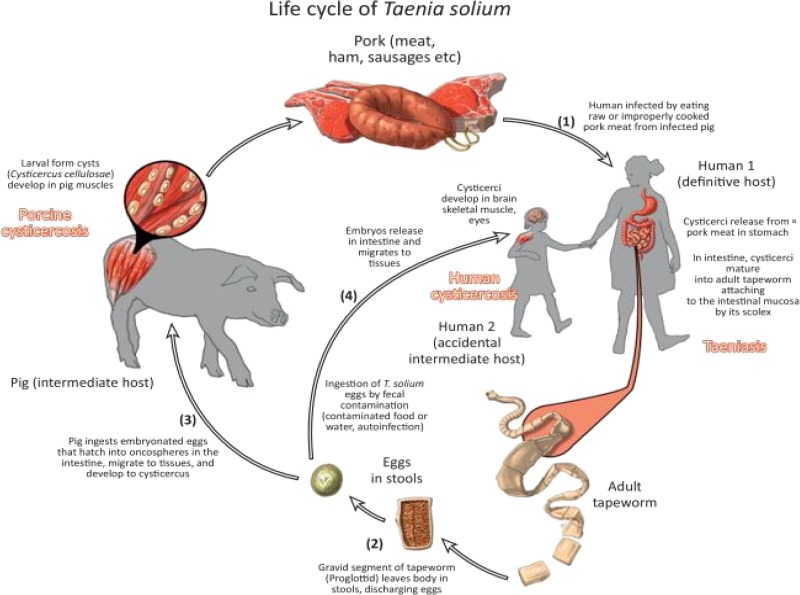
Figure 1 demonstrates the life cycle and transmission of T. solium. Adult tapeworms complete the sexual stages of life cycle in humans as definitive hosts. Pigs serve as an intermediate host where metacestodes (larva stage) survive in the muscles and other tissues as cysticerci. However, T. solium is unable to mature to adult stages in pigs to complete the reproductive cycle. While pigs acquire T. solium from ingesting Taenia eggs present in the human feces, humans acquire the infection directly from eating viable cysticerci present in uncooked pork or through ingestion of contaminated food or water containing eggs or proglottids.16 There is no reservoir in wildlife; however, dogs can become infected with T. solium cysts and may also play a role in transmission in areas where dog meat is consumed.16
Figure 1.
Life cycle of Taenia solium.
Clinical Disease
In humans, T. solium can manifest as asymptomatic or minimally symptomatic intestinal carriage (taeniasis) and/or as infection with cysticerci (cysticercosis), predominantly in the subcutaneous, muscle, eyes, and neural tissues (Figure 1). This occurs through hematogenous dissemination of cysts from the intestinal mucosa.
People with taeniasis may report daily passage of proglottids in stools. People with subcutaneous and muscle cysticerci are usually asymptomatic, but may serve as a clue to diagnosing the presence of disease in the communities. Involvement of neural tissues gives rise to neurocysticercosis. A majority of people with neurocysticercosis remain asymptomatic for life but where clinical symptoms are present, they may manifest mainly as epileptic seizures, headaches with or without accompanying intracranial hypertension, focal neurological symptoms, and cognitive disabilities.17–21
The main burden of human disease is conferred by death and disability from neurocysticercosis. In addition to neurological symptoms, neurocysticercosis can contribute to childhood learning disabilities, functional loss from resultant motor weakness, sensory deficits or chronic pain, early onset dementia, neuropsychiatric manifestations, and social stigmatization, thereby leading to reduced human productivity and economic losses.22–28 Several cases of accidental drowning and burns have also been linked to epileptic seizures.29,30 However, from the perspective of disease control and eradication, taeniasis cases are of importance as they play a key role in perpetuating the T. solium life cycle.
Risk Factors for Transmission
With the exception of Islamic countries such as Indonesia and Malaysia, pig production is central to many rural communities in SEA (pig density map available at: http://www.fao.org/ag/againfo/resources/en/glw/Modelled_maps/pigs_modelled-2005.jpg). Pork is the main source of protein as well as family income, with 78% of pigs in SEA produced under mixed crop–livestock smallholder farmers.31 Over the last decades, regional demands for pig production have also increased significantly.32 However, in many of these communities, intensification of industry has not yet been achieved and animal husbandry and farming practices still remain primitive and unregulated.32 SEA region also hosts one of the world's highest concentrations of free-ranging pigs, and they being coprophagic, eat human feces that may contain T. solium eggs.14 Several other risk factors have also been identified as potentially contributing to ongoing transmission of T. solium infection specific to this region. These include poor hygiene and sanitation practices such as open defecation, lack of health hardware such as functioning latrines, lack of access to clean water, lack of basic education and knowledge on disease transmission, feeding human waste to pigs, ancestral sacrifices and consumption of raw pork, and lack of meat inspection practices at slaughter houses.14,29,33,34 In addition, household contacts may acquire the infection through direct or indirect contact with carrier's feces, and clustering of neurocysticercosis cases around carriers has been further identified.14,35 There have also been reports suggesting a link between cysticercosis and the use of human feces and “wastewater” for fertilizing crops.36,37
Co-Endemicity and Polyparasitism
SEA is also endemic to other Taenia species, namely Taenia saginata (beef tapeworm) and Taenia asiatica, both of which can cause human taeniasis without cysticercosis.32 The burden of human taeniasis by these species is however negligible.14 Although controversies still exist as to whether T. asiatica can result in human cysticercosis, given that it shares a similar life cycle to that of T. solium in pigs as intermediate hosts and also causes liver cysticerci; the evidence has thus far been inconclusive.38–40 Transmission of T. saginata occurs through eating raw beef, whereas T. asiatica is acquired through consumption of uncooked pork viscera, but not meat.29 Dog tapeworm, Taenia hydatigena, on the other hand is not known to cause human disease but pigs can serve as intermediate hosts.41 Interestingly, all Taenia species could potentially influence the transmission dynamics of T. solium through competitive density-dependent crowding mechanisms in the gut in both humans and animals.32,41,42
In addition, polyparasitism is the norm in SEA with several other protozoan and helminthic infections such as Entamoeba, liver flukes (Opisthorchis and Clonorchis), hookworms, Ascaris, Trichuris, Schistosoma, and Trichinella causing high burden of human disease and sharing similar risk factors for transmission, such as poor hygiene, sanitation, and animal husbandry practices.4,43 Therefore, wider control efforts toward taeniasis/cysticercosis may have additional impact on these parasitic and zoonotic infections and thus reduce the burden of diseases collectively.34
Epidemiology
The prevalence data on taeniasis/cysticercosis in SEA has been extensively reviewed by Willingham and others in 201014 and most of these data are not replicated here. Newer findings are presented instead. Such epidemiologic data are important in analyzing critical information about the transmission of disease and other relevant risk factors, and therefore can be used to design effective control programs.11 However, caution should be exercised in interpreting these data because of several limitations, namely, small sample sizes, lack of strong study methodology, lack of national or regional representativeness, paucity of clinical data particularly in association with seizures, varied laboratory diagnostic methods, heavy reliance on serologic data, and intrinsic limitations of diagnostic tests.14
Diagnostic Methods and Impact on Epidemiology
Several challenges must first be recognized in diagnosing cases and therefore determining the accurate epidemiology of human taeniasis/cysticercosis in SEA. Table 1 provides a brief summary of diagnostic methods that can be used to determine the epidemiology of human taeniasis/cysticercosis. In general, clinical symptoms of taeniasis/cysticercosis are poorly defined and health-seeking behaviors of communities are largely unknown. Lack of education and social stigmatization may also lead to underreporting of symptoms.34,44 Moreover, appropriate imaging modalities such as computed tomography are not readily available in many rural areas of SEA. Clinical assessment is thus mainly based on the history of seizures, passage of proglottids in stool, and the presence of subcutaneous cysticerci nodules on examination. However, these clinical methods lack sensitivity and specificity.17 Nevertheless, the prevalence of epileptic seizures has previously been shown to correlate with the prevalence of neurocysticercosis in other endemic areas, with neurocysticercosis as an etiologic cause in a predictable proportion of epileptic patients. Therefore, the number of seizure cases could potentially serve as a useful estimate, provided the attributable proportion is known for a specific area.21,45,46
Table 1.
Diagnostic methods for epidemiologic studies of human taeniasis/cysticercosis
| Clinical |
| History of seizures |
| History of passage of proglottids in stool |
| Palpation for subcutaneous and muscle cysticerci |
| Neuroimaging (e.g., computed tomography) |
| Ultrasound-guided cyst aspiration |
| Stool examination (for example expulsion with antihelminthic treatment increases sensitivity) |
| Coprology: microscopy for Taenia eggs and proglottids* |
| Coproantigen ELISA |
| DNA hybridization techniques for eggs detection |
| Polymerase chain reaction techniques |
| Serological |
| EITB (antibody detection) |
| ELISA detecting metacestode antigens (Ag-ELISA) |
EITB = enzyme-linked immunoelectrotransfer blot; ELISA = enzyme-linked immunosorbent assay.
Egg morphology cannot be used to differentiate between Taenia species but proglottids morphology can be useful in differentiation.
Current immunological diagnostic tools used in cysticercosis diagnosis can be classified into 1) tests detecting antibodies directed against T. solium cysticerci and 2) tests detecting circulating antigens produced by living cysticerci.47 WHO recommends the use of lentil lectin-purified glycoproteins in enzyme-linked immunoelectrotransfer blot (EITB) format for antibody testing,48 while detection of circulating antigens is achieved via enzyme-linked immunosorbent assay (Ag-ELISA) detecting T. solium metacestode antigens (B158/B60 or HP10).11,49,50 The presence of antibodies indicates past or present infection, therefore usually overestimates the true prevalence, whereas the presence of circulating antigens indicates viable cysticerci in the human host and is usually reflective of active disease burden. Newer QuickELISA™ (Immunetics Inc., Boston, MA) assays also look promising due to the ease of use, but their performance has not been widely validated in various field conditions including SEA.51 In addition, diagnostic tools such as coprology, coproantigen ELISA, and molecular methods (such as polymerase chain reactions) vary widely in their performance in identification of taeniasis cases (with or without accompanying cysticercosis).52 Coprology methods struggle with sensitivity and species identification; this is especially problematic in SEA where sympatric infections with other Taenia species are common. Coproantigen method is usually only specific to the genus level, although species-specific assays for T. solium have been developed.53 However, molecular methods can achieve species identification but they are not widely available.
Moreover, the prevalence of T. solium disease in pigs also needs to be considered to construct effective control and eradication programs. The prevalence is determined by meat inspection methods and immunological investigations, each with their own limitations.14,54 However, the epidemiology in pigs are not further discussed in this review.
Prevalence of Taeniasis and Cysticercosis in Sea
Recent publication by Coral-Almeida and others11 analyzed epidemiologic studies that used only the WHO-recommended EITB or Ag-ELISA methods and found that the seroprevalence of T. solium antibodies in Asia was estimated at 15.7% (95% confidence interval [CI] = 10.3–23.2). However, only one study from Bali, Indonesia, represented the SEA region with estimated prevalence of 12.6% (95% CI = 10.3–15.2).55 Since this original study, the prevalence of T. solium infection in Bali appeared to be on the decline.56 On the other hand, analysis of studies using Ag-ELISA method estimated an overall prevalence of 3.9% (95% CI = 2.8–5.6) of T. solium cysticercosis in Asia.11 Only four studies from two countries within SEA region were represented in this analysis (Vietnam and Lao PDR).
Although being predominantly an Islamic country with little pork consumption, well-defined areas of human taeniasis are present in Indonesia, especially among the ethnic and religious minorities. Such information has previously been extensively reviewed through collaborative control efforts between Indonesian Ministry of Health and Asahikawa Medical College of Japan.14,29,56–58 In summary, T. solium has been reported mainly from Papua (former Irian Jaya) and Bali, T. saginata from Bali, and T. asiatica from Samosir Island in northern Sumatra.56,59 Despite control efforts, Papua is still considered to be a high-endemic area with the reported prevalence of T. solium taeniasis ranging from 0% to 13% and cysticercosis ranging from 1.1% to 45.8% (reviewed by Wandra and others).29,56,60,61 Through health education programs, the prevalence of T. solium in Bali has decreased to virtually 0% since 2002, except in Gianyar (low-level seroprevalence) and Karangasem districts.56,62
In Lao PDR, a recent national survey of > 55,000 people using coprology methods showed that Taenia eggs were present in 1.5% (845/55,038) of participants. Only 126 adult tapeworms were recovered, and of these, only three were identified as T. solium by subsequent molecular methods, with others being T. saginata.63 A number of studies in Lao PDR over the last 25 years, employing mainly coprology methods, also found that the prevalence of taeniasis ranged from 0% to 14%, with a degree of spatial and temporal variation (reviewed by Conlan and others64). However, species identification was again not achieved in these studies. Nevertheless, a high-endemic focus of T. solium infection appears to be present in the northern Lao PDR with reported seroprevalences for taeniasis and cysticercosis by EITB method in a village in this region being 46.7% and 66.7%, respectively.65 Further coproantigen ELISA testing of 92 human fecal samples confirmed taeniasis prevalence of 26.1% (95% CI = 18.2–35.9), and T. solium was confirmed by molecular methods in all five samples deemed suitable for further testing.65
A study of two villages in Kanchanaburi Province, Thailand, close to the border with Myanmar, found 0.6% (4/667) taeniasis cases by coprology alone or by a combination of clinical history, expulsion with antihelminthic treatment and coprology methods.66 Only 10 proglottids were recovered in this study, and of these only one was identified as T. solium by molecular methods (others being one T. asiatica and eight T. saginata). Serological testing of cysticercosis by ELISA method revealed 5.7% (9/159) seropositive, but only four were subsequently confirmed by immunoblotting.66 An earlier study of 24 taeniasis cases in the same province by the same author identified 11 cases (45.8%) with T. solium in stools by mitochondrial DNA analysis.67 Nevertheless, overall prevalence of taeniasis in Thailand is estimated to be probably < 2%, with the exception of northern provinces where the reported prevalence was as high as 5.9% (reviewed by Anantaphruti and others and Waikagul and others66,68).
In Vietnam, the prevalence of taeniasis ranges from 0.5% to 12% with probable spatial variation across the country (reviewed by van De and others69). Of the Taenia samples collected, T. solium was detected in 0–23.2%.70,71 The prevalence of human cysticercosis was found to be 1.0–7.2% in the northern and 4.3% in the southern regions of the country (reviewed by van De and others69). Similarly, an earlier study in Vietnam found that circulating T. solium antigens by Ag-ELISA method were detected in 5.3% (16/303) of people in three communities in the mountainous regions, where eating uncooked pork is common, but almost virtually absent in those from the costal and urban regions.72 A recent study on 513 Vietnamese patients with epilepsy also found that Ag-ELISA was positive in 9% (95% CI = 6–11).73
Being an Islamic country, the prevalence of taeniasis/cysticercosis in Malaysia is presumably low, but similar to Indonesia, pockets of endemicity may exist in certain areas where non-Muslim minority communities live.14,74 With urbanization, Singapore experiences only imported cases of neurocysticercosis, mostly in migrant workers from neighboring SEA countries.75 Only one study employing a nonstandard serological assay is available from a Schistosoma japonicum co-endemic region in the Philippines, which found seroprevalence of 24.6% (95% = CI 20.8–28.6) for cysticercosis.76 Recent data on the prevalence of taeniasis/cysticercosis is lacking from Brunei, Cambodia, and Myanmar.
All in all, given several limitations in individual studies, the prevalence data in SEA thus far contend to produce an accurate picture of T. solium taeniasis/cysticercosis in an individual community, geographical area, or country. For example, studies that used antibody detection methods may have overestimated the true prevalence of the disease while estimation of prevalence via observation of eggs and proglottids in stools tended to underestimate the disease burden. Many of these studies on taeniasis also failed to differentiate T. solium infection from other sympatric Taenia species, which confer negligible burden of disease in humans. In addition, extrapolating the results from a single community to a regional or even global level can be a hazardous exercise.11 Therefore, a cautious approach should be taken on the generalizability of an individual study. Nevertheless, taken together, a few useful general conclusions can still be drawn: 1) T. solium taeniasis/cysticercosis is probably common in many SEA countries; 2) the prevalence varies widely between neighboring countries and also within an individual country; 3) rural and remote communities are at the highest risk; 4) there are known highly endemic areas, especially in northern Laos PDR and Papua, Indonesia; 5) the prevalence also varies with religious and cultural practices within each country; 6) the true disease burden is still largely unknown; and 7) local control/eradication measures are likely achievable, as evident by the situation in Bali.
Control Strategies and Challenges
In theory, it is easy to interrupt the transmission of T. solium by 1) preventing/treating pig infection, 2) treating human carriers (i.e., taeniasis cases) who are definitive hosts, and 3) preventing human (re)infections. The simplest solution therefore is to stop T. solium transmission from human to pigs by providing latrines and clean water to prevent open defecation, ensuring hygienic animal husbandry practices and also to stop transmission from pigs back to humans through veterinary sanitary measures such as regulation of meat inspection practices and not eating raw pork.44 Other components of community control strategies are further highlighted in Table 2. However, in practice, it has been difficult to achieve these control measures.
Table 2.
Components for the control and eradication of human taeniasis/cysticercosis
| Prevention and treatment of pig infection |
| Proper pig management facilities: no free-ranging or scavenging pigs |
| No feeding human waste or fecally contaminated feeds to pigs |
| Pig vaccinations |
| Mass drug administration (e.g., praziquantel, niclosamide, or oxfendazole) |
| Provision of health hardware: functioning toilets and clean water access in villages, ongoing maintenance of these facilities |
| No open defecation by humans |
| Health education and social mobilization measures for change in hygiene and sanitation practices |
| Treatment of humans with taeniasis/cysticercosis* |
| Identification of infected/carriers through active/passive surveillance measures |
| Individual treatment |
| Mass drug administration (e.g., praziquantel, niclosamide, or albendazole) |
| Prevention of human infections |
| Hand and food hygiene measures |
| Strict meat inspection practices and regulations |
| Sufficient cooking of pork |
| Changes in cultural and religious practices on consumption of raw pork |
Cysticercosis alone does not contribute to perpetuation of Taenia solium life cycle. However, cysticercosis cases may also have concurrent taeniasis.
For control programs to be effective and sustainable, cost-effective combination of simple interventions simultaneously targeting both pigs (intermediate hosts) and humans (definitive hosts) need to be in place.14,44 Engagement of stakeholders from various relevant sectors and interested parties, including veterinary, environmental, and medical workers, should be promoted from the outset to help ensure success.3,14 In other words, an integrated One Health approach is necessary with political commitment and inter-sectoral collaboration at local, national, and international levels.29,44,77
However, to date, high-quality evidence on the impact of community-based control strategies is largely lacking. A recent study searched all published literature and analyzed the effectiveness of community-based control strategies, employing either a single or a combination of multiple interventions that used pre- and post-intervention designs.6 Unfortunately, it found paucity of high-quality data to definitively support any form of intervention. Available data from only two community-based randomized controlled trials suggested that education program alone or a combination of porcine and human mass treatment reduce porcine cysticercosis only in the short term.78,79 Of note, none of these studies were based in SEA.
Although pig vaccination with TSOL16-18 or S3PV seems to be effective at preventing pig infection, the evidence on its effectiveness is still lacking at the community level.6,80 In addition, there has been no pig vaccine trial in SEA to date and sustainability of vaccination in resource-limited settings is also questionable. A theoretical transmission dynamics modeling suggested that improved sanitation and pig management are more effective than vaccination, human or porcine mass treatment.81 However, this transmission model was based on limited empiric data from epidemiological and interventional studies originating in countries outside SEA region, and therefore the generalizability of findings to SEA countries, where transmission dynamics are largely unstudied, may be limited.
Several key challenges also exist in implementing control strategies in many parts of SEA. Although it is likely that taeniasis/cysticercosis is becoming less of a problem in many SEA countries that are economically progressing toward affluence, health care continues to struggle in poorer counterparts such as Cambodia, Laos PDR, and Myanmar.
At the national level, lack of political will, agenda setting and commitment, inadequate basic health-care and diagnostic facilities, lack of funding for surveillance and control programs, lack of public health champions, and poor national literacy rates may serve as key barriers.82,83 As the true epidemiology of T. solium taeniasis/cysticercosis is still largely unknown in many areas, it also poses difficulties in influencing government policies and local and regional health-care priorities. This further gives rise to difficulties in attracting international donors for funding of projects. Establishing shared national or regional surveillance programs may potentially overcome this issue.84 in addition, as suggested by a recent study, mass drug administration with broad-spectrum antihelminthic agents such as albendazole may confer additional benefits toward treating other parasitic infections plaguing this region, thereby collectively reducing their burden of disease.85 However, outcomes from further studies on control strategies targeting polyparasitism are needed before such approach can be recommended.
At the community level, in many rural parts of SEA, open defecation is still culturally and socially acceptable.34,86 Furthermore, consumption of raw pork remains a common tradition in many areas.29,34,72 In addition, poverty and religious beliefs prevail people's unwillingness to discard or cook cyst-infected meat, particularly during sacrificial ceremonies in certain communities.33 Moreover, instead of interrupting the disease transmission, meat inspection practices may counterintuitively produce a negative impact by contributing to further economic losses in pig farmers through stigmatization, thereby forcing them to avoid the regulated markets and sell at a cheaper price directly to the lowest income earners.14 All these factors perpetuate the vicious cycle of taeniasis/cysticercosis. Human behavioral changes required to interrupt the T. solium life cycle are therefore difficult to achieve due to long ingrained religious beliefs and traditions, although not impossible through education and social mobilization measures.16,87 Further studies are thus desperately needed from the social sciences perspectives.
All in all, it remains unclear as to what the best approach is in controlling taeniasis/cysticercosis in this region. As highlighted, political commitment, sustained funding, and public health infrastructure need to be securely in place before intervention measures can be carried out. With control strategies, no single method (mass drug administration, pig vaccination, changes in farming methods, etc.) may yield better results than the other. Nevertheless, it is likely that a combination of multiple interventions, implemented either concurrently or in tandem, will have a sustained impact on reducing transmission. However, high-level evidence on the effectiveness of this approach is yet to be seen.
Eradication
By biological and technical feasibility criteria for eradication of a disease,88 T. solium taeniasis/cysticercosis is far from achieving regional eradication in SEA or global eradication. Several of these criteria have been highlighted in this review, namely, lack of simple and accurate diagnostic tools, sensitive surveillance strategies, effective intervention measures, and effective mode of delivery for these measures. Nevertheless, within SEA, Indonesia has set an aspiring example by demonstrating that eradication may be achievable, as shown by a significant decrease in the prevalence of taeniasis/cysticercosis in Bali over the last two decades (cysticercosis prevalence decreased from 13% in 199155 to 0% by 2014 except for two districts (summary data by Wandra and others56) through sustained public health measures including political commitment, national and international collaboration, ongoing surveillance, health education, and changes in animal husbandry and meat inspection practices, all without the need to use expensive vaccination or mass drug administration strategies.56 Only a small area of Karangasem in Bali remains problematic. Although Bali is still struggling with T. saginata taeniasis, the current situation on T. solium infection looks promising, and only time will tell if the disease eradication is possible.
Conclusions
Despite IFTDE's declaration, T. solium taeniasis/cysticercosis has thus far proven to be a challenging disease for control and eradication. This is not only due to the difficulties in meeting biological and technical feasibility criteria for eradication of a disease, but also due to complex interactions between poverty, political and economic factors, human behaviors, cultural and religious beliefs, and the disease itself. Such complex dynamics are very much evident within the SEA region. Many factors still need to be addressed urgently for control strategies to be successfully implemented. These include establishment of better diagnostic and surveillance systems (and hence, a more accurate picture on the epidemiology), securing political commitment, funding allocation, fostering collaborative partnerships, identification of local champions to implement social mobilization measures, and above all, improving the basic health-care needs (such as providing health hardware) of many impoverished communities in SEA. Evolving evidence suggests that control and elimination of taeniasis/cysticercosis from SEA region remains a possibility in the foreseeable future.
ACKNOWLEDGMENTS
We would like to thank Associate Professor Jeffrey Hanna of James Cook University, Townsville, Australia, for invaluable comments on this manuscript and providing Figure 1.
Footnotes
Authors' addresses: Ar Kar Aung, Department of General Medicine and Infectious Diseases, The Alfred Hospital, Victoria, Australia, and School of Medicine, Monash University, Melbourne, Australia, E-mail: arkaraung@yahoo.com. Denis W. Spelman, Department of Infectious Diseases and Microbiology, The Alfred Hospital, Victoria, Australia, and Microbiology Unit, Monash University, Victoria, Australia, E-mail: d.spelman@alfred.org.au.
References
- 1.Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Sachs SE, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Taeniasis/Cysticercosis. 2015. http://www.who.int/mediacentre/factsheets/fs376/en/ Available at. Accessed July 27, 2015.
- 3.Pawlowski Z, Allan J, Sarti E. Control of Taenia solium taeniasis/cysticercosis: from research towards implementation. Int J Parasitol. 2005;35:1221–1232. doi: 10.1016/j.ijpara.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Torgerson PR, Macpherson CN. The socioeconomic burden of parasitic zoonoses: global trends. Vet Parasitol. 2011;182:79–95. doi: 10.1016/j.vetpar.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep. 1993;42:1–38. [PubMed] [Google Scholar]
- 6.Carabin H, Traoré AA. Taenia solium taeniasis and cysticercosis control and elimination through community-based interventions. Curr Trop Med Rep. 2014;1:181–193. doi: 10.1007/s40475-014-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molyneux DH, Hopkins DR, Zagaria N. Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol. 2004;20:347–351. doi: 10.1016/j.pt.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.The Carter Center Summary of the Twenty-First Meeting of the International Task Force for Disease Eradication (II) 2013. http://www.cartercenter.org/resources/pdfs/news/health_publications/itfde/ITFDE-summary-071013.pdf Available at.
- 9.Hotez PJ, Bottazzi ME, Strych U, Chang LY, Lim YA, Goodenow MM, AbuBakar S. Neglected tropical diseases among the Association of Southeast Asian Nations (ASEAN): overview and update. PLoS Negl Trop Dis. 2015;9:e0003575. doi: 10.1371/journal.pntd.0003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpio A. Neurocysticercosis: an update. Lancet Infect Dis. 2002;2:751–762. doi: 10.1016/s1473-3099(02)00454-1. [DOI] [PubMed] [Google Scholar]
- 11.Coral-Almeida M, Gabriël S, Abatih EN, Praet N, Benitez W, Dorny P. Taenia solium human cysticercosis: a systematic review of sero-epidemiological data from endemic zones around the world. PLoS Negl Trop Dis. 2015;9:e0003919. doi: 10.1371/journal.pntd.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association of Southeast Asian Nations ASEAN Overview. 2014. http://www.asean.org/asean/about-asean Available at. Accessed July 27, 2015.
- 13.United Nations Conference on Trade and Development UN List of Least Developed Countries. 2013. http://unctad.org/en/Pages/ALDC/Least%20Developed%20Countries/UN-list-of-Least-Developed-Countries.aspx Available at. Accessed July 27, 2015.
- 14.Willingham AL, Wu HW, Conlan J, Satrija F. Combating Taenia solium cysticercosis in southeast Asia: an opportunity for improving human health and livestock production. Adv Parasitol. 2010;72:235–266. doi: 10.1016/S0065-308X(10)72009-1. [DOI] [PubMed] [Google Scholar]
- 15.Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis. 2012;6:e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization Meeting of the International Task Force for Disease Eradication—July 2013. Wkly Epidemiol Rec. 2013;88:429–436. [Google Scholar]
- 17.Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13:1202–1215. doi: 10.1016/S1474-4422(14)70094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia HH, Del Brutto OH. Cysticercosis Working Group in Peru Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 2005;4:653–661. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 19.Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, Stoner JA, Rainwater E, Dickey M. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis. 2011;5:e1152. doi: 10.1371/journal.pntd.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattarai R, Budke CM, Carabin H, Proaño JV, Flores-Rivera J, Corona T, Cowan LD, Ivanek R, Snowden KF, Flisser A. Quality of life in patients with neurocysticercosis in Mexico. Am J Trop Med Hyg. 2011;84:782–786. doi: 10.4269/ajtmh.2011.10-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, Dickey M, Reynolds S, Stoner JA. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales NM, Agapejev S, Morales RR, Padula NA, Lima MM. Clinical aspects of neurocysticercosis in children. Pediatr Neurol. 2000;22:287–291. doi: 10.1016/s0887-8994(99)00158-7. [DOI] [PubMed] [Google Scholar]
- 23.Praet N, Speybroeck N, Manzanedo R, Berkvens D, Nsame Nforninwe D, Zoli A, Quet F, Preux PM, Carabin H, Geerts S. The disease burden of Taenia solium cysticercosis in Cameroon. PLoS Negl Trop Dis. 2009;3:e406. doi: 10.1371/journal.pntd.0000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattarai R, Budke CM, Carabin H, Proaño JV, Flores-Rivera J, Corona T, Ivanek R, Snowden KF, Flisser A. Estimating the non-monetary burden of neurocysticercosis in Mexico. PLoS Negl Trop Dis. 2012;6:e1521. doi: 10.1371/journal.pntd.0001521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantu C, Barinagarrementeria F. Cerebrovascular complications of neurocysticercosis: clinical and neuroimaging spectrum. Arch Neurol. 1996;53:233–239. doi: 10.1001/archneur.1996.00550030039021. [DOI] [PubMed] [Google Scholar]
- 26.Callacondo D, Garcia H, Gonzales I, Escalante D, Nash T, Gilman RH, Tsang VC, Gonzalez A, Lopez MT, Gavidia CM. High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology. 2012;78:1394–1400. doi: 10.1212/WNL.0b013e318253d657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Andrade DC, Rodrigues C, Abraham R, Castro L, Livramento J, Machado L, Leite C, Caramelli P. Cognitive impairment and dementia in neurocysticercosis: a cross-sectional controlled study. Neurology. 2010;74:1288–1295. doi: 10.1212/WNL.0b013e3181d9eda6. [DOI] [PubMed] [Google Scholar]
- 28.Osakwe C, Otte WM, Alo C. Epilepsy prevalence, potential causes and social beliefs in Ebonyi State and Benue State, Nigeria. Epilepsy Res. 2014;108:316–326. doi: 10.1016/j.eplepsyres.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Wandra T, Ito A, Swastika K, Dharmawan NS, Sako Y, Okamoto M. Taeniases and cysticercosis in Indonesia: past and present situations. Parasitology. 2013;140:1608–1616. doi: 10.1017/S0031182013000863. [DOI] [PubMed] [Google Scholar]
- 30.Margono SS, Wandra T, Swasono MF, Murni S, Craig PS, Ito A. Taeniasis/cysticercosis in Papua (Irian Jaya), Indonesia. Parasitol Int. 2006;55:S143–S148. doi: 10.1016/j.parint.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Groenewold JP. Classification and Characterization of World Livestock Production Systems: Update of the 1994 Livestock Production Systems with Recent Data. Rome, Italy: FAO AGAL; 2004. [Google Scholar]
- 32.Conlan JV, Sripa B, Attwood S, Newton PN. A review of parasitic zoonoses in a changing southeast Asia. Vet Parasitol. 2011;182:22–40. doi: 10.1016/j.vetpar.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Bardosh K, Inthavong P, Xayaheuang S, Okello AL. Controlling parasites, understanding practices: the biosocial complexity of a One Health intervention for neglected zoonotic helminths in northern Lao PDR. Soc Sci Med. 2014;120:215–223. doi: 10.1016/j.socscimed.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Burniston S, Okello AL, Khamlome B, Inthavong P, Gilbert J, Blacksell SD, Allen J, Welburn SC. Cultural drivers and health-seeking behaviours that impact on the transmission of pig-associated zoonoses in Lao People's Democratic Republic. Infect Dis Poverty. 2015;4:11. doi: 10.1186/2049-9957-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang V, Rodriguez S, Moulton LH, Villaran MV, Montano SM, Gonzalez AE. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLoS Negl Trop Dis. 2009;3:e371. doi: 10.1371/journal.pntd.0000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran DS, Odermatt P, Le Oanh T, Huc P, Phoumindr N, Ito A, Druet-Cabanac M, Preux PM, Strobel M. Risk factors for epilepsy in rural Lao PDR: a case-control study. Southeast Asian J Trop Med Public Health. 2007;38:537–542. [PubMed] [Google Scholar]
- 37.Willingham A, De NV, Doanh NQ, Cong le D, Dung TV, Dorny P, Cam PD, Dalsgaard A. Current status of cysticercosis in Vietnam. Southeast Asian J Trop Med Public Health. 2002;34:35–50. [PubMed] [Google Scholar]
- 38.Ito A, Nakao M, Wandra T. Human taeniasis and cysticercosis in Asia. Lancet. 2003;362:1918–1920. doi: 10.1016/S0140-6736(03)14965-3. [DOI] [PubMed] [Google Scholar]
- 39.Galán-Puchades MT, Fuentes MV. Taenia asiatica: the most neglected human Taenia and the possibility of cysticercosis. Korean J Parasitol. 2013;51:51–54. doi: 10.3347/kjp.2013.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ale A, Victor B, Praet N, Gabriël S, Speybroeck N, Dorny P, Devleesschauwer B. Epidemiology and genetic diversity of Taenia asiatica: a systematic review. Parasit Vectors. 2014;7:1–11. doi: 10.1186/1756-3305-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson R, Conlan J. Emerging issues and parasite zoonoses in the SE Asian and Australasian region. Vet Parasitol. 2011;181:69–73. doi: 10.1016/j.vetpar.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Conlan JV, Vongxay K, Khamlome B, Dorny P, Sripa B, Elliot A, Blacksell SD, Fenwick S, Thompson RC. A cross-sectional study of Taenia solium in a multiple taeniid-endemic region reveals competition may be protective. Am J Trop Med Hyg. 2012;87:281–291. doi: 10.4269/ajtmh.2012.11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Utzinger J, Bergquist R, Olveda R, Zhou XN. Important helminth infections in southeast Asia: diversity, potential for control and prospects for elimination. Adv Parasitol. 2010;72:1–30. doi: 10.1016/S0065-308X(10)72001-7. [DOI] [PubMed] [Google Scholar]
- 44.Welburn S, Beange I, Ducrotoy M, Okello A. The neglected zoonoses—the case for integrated control and advocacy. Clin Microbiol Infect. 2015;21:433–443. doi: 10.1016/j.cmi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Montano S, Villaran M, Ylquimiche L, Figueroa J, Rodriguez S, Bautista C, Gonzalez A, Tsang V, Gilman R, Garcia H. Neurocysticercosis: association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–233. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 46.Moyano LM, Saito M, Montano SM, Gonzalvez G, Olaya S, Ayvar V, González I, Larrauri L, Tsang V, Llanos F, Rodríguez S, Gonzalez AE, Gilman RH, Garcia HH. Cysticercosis Working Group in Peru Neurocysticercosis as a cause of epilepsy and seizures in two community-based studies in a cysticercosis-endemic region in Peru. PLoS Negl Trop Dis. 2014;8:e2692. doi: 10.1371/journal.pntd.0002692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Praet N, Rodriguez-Hidalgo R, Speybroeck N, Ahounou S, Benitez-Ortiz W, Berkvens D, Van Hul A, Barrionuevo-Samaniego M, Saegerman C, Dorny P. Infection with versus exposure to Taenia solium: what do serological test results tell us? Am J Trop Med Hyg. 2010;83:413–415. doi: 10.4269/ajtmh.2010.10-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 49.Garcia H, Harrison L, Parkhouse R, Montenegro T, Martinez S, Tsang V, Gilman R. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. Trans R Soc Trop Med Hyg. 1998;92:411–414. doi: 10.1016/s0035-9203(98)91069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabriël S, Blocher J, Dorny P, Abatih EN, Schmutzhard E, Ombay M, Mathias B, Winkler AS. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl Trop Dis. 2012;6:e1851. doi: 10.1371/journal.pntd.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YM, Handali S, Hancock K, Pattabhi S, Kovalenko VA, Levin A, Rodriguez S, Lin S, Scheel CM, Gonzalez AE. Serologic diagnosis of human Taenia solium cysticercosis by using recombinant and synthetic antigens in QuickELISA™. Am J Trop Med Hyg. 2011;84:587–593. doi: 10.4269/ajtmh.2011.10-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Praet N, Verweij JJ, Mwape KE, Phiri IK, Muma JB, Zulu G, Lieshout L, Rodriguez-Hidalgo R, Benitez-Ortiz W, Dorny P, Gabriël S. Bayesian modelling to estimate the test characteristics of coprology, coproantigen ELISA and a novel real-time PCR for the diagnosis of taeniasis. Trop Med Int Health. 2013;18:608–614. doi: 10.1111/tmi.12089. [DOI] [PubMed] [Google Scholar]
- 53.Guezala MC, Rodriguez S, Zamora H, Garcia HH, Gonzalez AE, Tembo A, Allan JC, Craig PS. Development of a species-specific coproantigen ELISA for human Taenia solium taeniasis. Am J Trop Med Hyg. 2009;81:433–437. [PubMed] [Google Scholar]
- 54.Gonzalez A. Control of Taenia solium with porcine chemotherapy. In: Singh G, Prabhakar S, editors. Taenia solium Cysticercosis: From Basic to Clinical Science. Wallingford, United Kingdom: CABI Publishing; 2002. pp. 431–435. [Google Scholar]
- 55.Theis J, Goldsmith R, Flisser A, Koss J, Chioino C, Plancarte A, Segura A, Widjana D, Sutisna P. Detection by immunoblot assay of antibodies to Taenia solium cysticerci in sera from residents of rural communities and from epileptic patients in Bali, Indonesia. Southeast Asian J Trop Med Public Health. 1994;25:464–468. [PubMed] [Google Scholar]
- 56.Wandra T, Swastika K, Dharmawan NS, Purba IE, Sudarmaja IM, Yoshida T, Sako Y, Okamoto M, Diarthini NLPE, Laksemi DAAS, Yanagida T, Nakao M, Ito A. The present situation and towards the prevention and control of neurocysticercosis on the tropical island, Bali, Indonesia. Parasit Vectors. 2015;8:1–11. doi: 10.1186/s13071-015-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito A, Nakao M, Wandra T, Suroso T, Okamoto M, Yamasaki H, Sako Y, Nakaya K. Taeniasis and cysticercosis in Asia and the Pacific: present state of knowledge and perspectives. Southeast Asian J Trop Med Public Health. 2005;36:123–130. [PubMed] [Google Scholar]
- 58.Suroso T, Margono SS, Wandra T, Ito A. Challenges for control of taeniasis/cysticercosis in Indonesia. Parasitol Int. 2006;55:S161–S165. doi: 10.1016/j.parint.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 59.Wandra T, Sutisna P, Dharmawan N, Margono S, Sudewi R, Suroso T, Craig P, Ito A. High prevalence of Taenia saginata taeniasis and status of Taenia solium cysticercosis in Bali, Indonesia, 2002–2004. Trans R Soc Trop Med Hyg. 2006;100:346–353. doi: 10.1016/j.trstmh.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 60.Wandra T, Ito A, Yamasaki H, Suroso T, Margono SS. Taenia solium cysticercosis, Irian Jaya, Indonesia. Emerg Infect Dis. 2003;9:884–885. doi: 10.3201/eid0907.020709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wandra T, Depary A, Sutisna P, Margono SS, Suroso T, Okamoto M, Craig PS, Ito A. Taeniasis and cysticercosis in Bali and north Sumatra, Indonesia. Parasitol Int. 2006;55:S155–S160. doi: 10.1016/j.parint.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 62.Wandra T, Sudewi A, Swastika IK, Sutisna P, Dharmawan NS, Yulfi H, Darlan DM, Kapti IN, Samaan G, Sato MO, Okamoto M, Sako Y, Ito A. Taeniasis/cysticercosis in Bali, Indonesia. Southeast Asian J Trop Med Public Health. 2011;42:793–802. [PubMed] [Google Scholar]
- 63.Jeon HK, Yong TS, Sohn WM, Chai JY, Min DY, Yun CH, Rim HJ, Pongvongsa T, Banouvong V, Insisiengmay B, Phommasack B, Eom KS. Current status of human taeniasis in Lao People's Democratic Republic. Korean J Parasitol. 2013;51:259–263. doi: 10.3347/kjp.2013.51.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conlan J, Khounsy S, Inthavong P, Fenwick S, Blacksell S, Thompson RC. A review of taeniasis and cysticercosis in the Lao People's Democratic Republic. Parasitol Int. 2008;57:252–255. doi: 10.1016/j.parint.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Okello A, Ash A, Keokhamphet C, Hobbs E, Khamlome B, Dorny P, Thomas L, Allen J. Investigating a hyper-endemic focus of Taenia solium in northern Lao PDR. Parasit Vectors. 2014;7:134. doi: 10.1186/1756-3305-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anantaphruti MT, Okamoto M, Yoonuan T, Saguankiat S, Kusolsuk T, Sato M, Sato MO, Sako Y, Waikagul J, Ito A. Molecular and serological survey on taeniasis and cysticercosis in Kanchanaburi Province, Thailand. Parasitol Int. 2010;59:326–330. doi: 10.1016/j.parint.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Anantaphruti MT, Yamasaki H, Nakao M, Waikagul J, Watthanakulpanich D, Nuamtanong S, Maipanich W, Pubampen S, Sanguankiat S, Muennoo C, Nakaya K, Sato MO, Sako Y, Okamoto M, Ito A. Sympatric occurrence of Taenia solium, T. saginata, and T. asiatica, Thailand. Emerg Infect Dis. 2007;13:1413–1416. doi: 10.3201/eid1309.061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waikagul J, Dekumyoy P, Anantaphruti MT. Taeniasis, cysticercosis and echinococcosis in Thailand. Parasitol Int. 2006;55:S175–S180. doi: 10.1016/j.parint.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 69.Van De N, Le TH, Lien PTH, Eom KS. Current status of taeniasis and cysticercosis in Vietnam. Korean J Parasitol. 2014;52:125–129. doi: 10.3347/kjp.2014.52.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Somers R, Dorny P, Geysen D, Nguyen LA, Thach DC, Vercruysse J, Nguyen VK. Human tapeworms in North Vietnam. Trans R Soc Trop Med Hyg. 2007;101:275–277. doi: 10.1016/j.trstmh.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Vien H, Dao L, Manh N, Tan H, Nguyen D, Nhung V. Identification of Taenia spp. and cysticercus species using multiplex PCR. J Malaria Parasit Dis. 2008;1:62–69. [Google Scholar]
- 72.Somers R, Dorny P, Nguyen V, Dang T, Goddeeris B, Craig P, Vercruysse J. Taenia solium taeniasis and cysticercosis in three communities in North Vietnam. Trop Med Int Health. 2006;11:65–72. doi: 10.1111/j.1365-3156.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- 73.Trung DD, Praet N, Cam TDT, Lam BVT, Manh HN, Gabriël S, Dorny P. Assessing the burden of human cysticercosis in Vietnam. Trop Med Int Health. 2013;18:352–356. doi: 10.1111/tmi.12043. [DOI] [PubMed] [Google Scholar]
- 74.Noor Azian M, Hakim SL, Sumiati A, Norhafizah M. Seroprevalence of cysticercosis in a rural village of Ranau, Sabah, Malaysia. Southeast Asian J Trop Med Public Health. 2006;37:58–61. [PubMed] [Google Scholar]
- 75.Foo S, Selvan V, Clarke M, Shen E. Unusual cause of seizures in Singapore: neurocysticercosis. Singapore Med J. 2008;49:e147–e150. [PubMed] [Google Scholar]
- 76.Xu JM, Acosta LP, Hou M, Manalo DL, Jiz M, Jarilla B, Pablo AO, Ovleda RM, Langdon G, McGarvey ST, Kurtis JD, Friedman JF, Wu HW. Seroprevalence of cysticercosis in children and young adults living in a helminth endemic community in Leyte, the Philippines. J Trop Med. 2010;2010:603174. doi: 10.1155/2010/603174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabinowitz PM, Kock R, Kachani M, Kunkel R, Thomas J, Gilbert J, Wallace R, Blackmore C, Wong D, Karesh W, Natterson B, Dugas R, Rubin C. Stone Mountain One Health Proof of Concept Working Group Toward proof of concept of a One Health approach to disease prediction and control. Emerg Infect Dis. 2013;19:e130265. doi: 10.3201/eid1912.130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ngowi H, Carabin H, Kassuku A, Mlozi M, Mlangwa J, Willingham A. A health-education intervention trial to reduce porcine cysticercosis in Mbulu District, Tanzania. Prev Vet Med. 2008;85:52–67. doi: 10.1016/j.prevetmed.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 79.Garcia HH, Gonzalez AE, Rodriguez S, Gonzalvez G, Llanos-Zavalaga F, Tsang VC, Gilman RH. Grupo de Trabajo en Cisticercosis en Perú Epidemiology and control of cysticercosis in Peru. Rev Peru Med Exp Salud Publica. 2010;27:592–597. doi: 10.1590/s1726-46342010000400016. [DOI] [PubMed] [Google Scholar]
- 80.Lightowlers MW. Control of Taenia solium taeniasis/cysticercosis: past practices and new possibilities. Parasitology. 2013;140:1566–1577. doi: 10.1017/S0031182013001005. [DOI] [PubMed] [Google Scholar]
- 81.Kyvsgaard NC, Johansen MV, Carabin H. Simulating transmission and control of Taenia solium infections using a Reed-Frost stochastic model. Int J Parasitol. 2007;37:547–558. doi: 10.1016/j.ijpara.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 82.Bardosh K. Global aspirations, local realities: the role of social science research in controlling neglected tropical diseases. Infect Dis Poverty. 2014;3:35. doi: 10.1186/2049-9957-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manderson L, Aagaard-Hansen J, Allotey P, Gyapong M, Sommerfeld J. Social research on neglected diseases of poverty: continuing and emerging themes. PLoS Negl Trop Dis. 2009;3:e332. doi: 10.1371/journal.pntd.0000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murrell K, Dorny P, Flisser A, Geerts S, Kyvsgaard NC, McManus D, Nash T, Pawlowski Z. WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/Cysticercosis. Paris, France: OIE (World Organisation for Animal Health), WHO (World Health Organization) and FAO (Food and Agriculture Organization); 2005. [Google Scholar]
- 85.Ash A, Okello A, Khamlome B, Inthavong P, Allen J, Thompson RA. Acta Trop. 2015. Controlling Taenia solium and soil transmitted helminths in a northern Lao PDR village: impact of a triple dose albendazole regime.http://dx.doi.org/10.1016/j.actatropica.2015.05.018 Available at. [DOI] [PubMed] [Google Scholar]
- 86.Phongluxa K, Xayaseng V, Vonghachack Y, Akkhavong K, van Eeuwijk P, Odermatt P. Helminth infection in southern Laos: high prevalence and low awareness. Parasit Vectors. 2013;6:328. doi: 10.1186/1756-3305-6-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kar K. Facilitating “Hands-on” Training Workshops for Community-led Total Sanitation. Geneva, Switzerland: Water Supply and Sanitation Collaborative Council; 2010. [Google Scholar]
- 88.Aylward B, Hennessey KA, Zagaria N, Olivé JM, Cochi S. When is a disease eradicable? 100 years of lessons learned. Am J Public Health. 2000;90:1515–1520. doi: 10.2105/ajph.90.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]