Abstract
The paradigm that all blood cells are derived from hematopoietic stem cells (HSCs) has been challenged by two findings. First, there are tissue-resident hematopoietic cells, including subsets of macrophages that are not replenished by adult HSCs, but instead are maintained by self-renewal of fetal-derived cells. Second, during embryogenesis, there is a conserved program of HSC-independent hematopoiesis that precedes HSC function and is required for embryonic survival. The presence of waves of HSC-independent hematopoiesis as well as fetal HSCs raises questions about the origin of fetal-derived adult tissue-resident macrophages. In the murine embryo, historical examination of embryonic macrophage and monocyte populations combined with recent reports utilizing genetic lineage-tracing approaches has led to a model of macrophage ontogeny that can be integrated with existing models of hematopoietic ontogeny. The first wave of hematopoiesis contains primitive erythroid, megakaryocyte and macrophage progenitors that arise in the yolk sac, and these macrophage progenitors are the source of early macrophages throughout the embryo, including the liver. A second wave of multipotential erythro-myeloid progenitors (EMPs) also arises in the yolk sac . EMPs colonize the fetal liver, initiating myelopoiesis and forming macrophages. Lineage tracing indicates that this second wave of macrophages are distributed in most fetal tissues, although not appreciably in the brain. Thus, fetal-derived adult tissue-resident macrophages, other than microglia, appear to predominately derive from EMPs. While HSCs emerge at midgestation and colonize the fetal liver, the relative contribution of fetal HSCs to tissue macrophages at later stages of development is unclear. The inclusion of macrophage potential in multiple waves of hematopoiesis is consistent with reports of their functional roles throughout development in innate immunity, phagocytosis, and tissue morphogenesis and remodeling. Understanding the influences of developmental origin, as well as local tissue-specific signals, will be necessary to fully decode the diverse functions and responses of tissue-resident macrophages.
Keywords: Macrophage, yolk sac, microglia, erythro-myeloid progenitor, EMP, fetal
Graphical Abstract
1. Introduction
In the adult, all of the red blood cells, platelets, granulocytes, monocytes, and lymphoid cells that circulate in the bloodstream are continuously replenished from hematopoietic stem cells (HSCs). HSCs are functionally defined by their capacity to regenerate all of these circulating blood cells upon transplantation into a myeloablated recipient. Differentiating HSCs undergo restrictions in lineage fate to generate multipotential, and ultimately, unipotential progenitor cells that, in turn, generate mature blood cells. The capacity of hematopoietic progenitors to give rise to colonies of blood cells in semisolid media has revealed hierarchical lineage relationships within the hematopoietic system. Macrophage formation in the adult is characterized by progressive differentiation of bipotential granulocyte and macrophage (GM-CFC) progenitors to monocyte progenitors, and mature monocytes that differentiate into macrophages upon stimulation [1].
Studies of hematopoiesis in the adult organism led to the concept that all blood cells, including macrophages, are ultimately derived from HSCs. This widely held concept was also applied to the embryo, leading to the concept that HSCs arise in the yolk sac, the place where blood cells first emerge during development. However, functional transplantation studies in the mouse embryo two decades ago revealed the emergence of HSC beginning at embryonic day 10.5 (E10.5) in the AGM region of the embryo proper [2, 3]. In contrast, the onset of hematopoiesis is characterized by the development of maturing primitive erythroid cells in the yolk sac at beginning at E7.5, as well as macrophage cells and megakaryocytes by E9.0. Analysis of functional hematopoietic progenitors, capable of colony-forming activity, has revealed a complex pattern of emergence in embryonic time and space, which is characterized by two distinct, but partially overlapping waves of erythroid progenitors initiating in the yolk sac prior to the onset of a functional vasculature and adult-repopulating HSC [4, 5]. Macrophage colony-forming potential is associated temporally and spatially with each of these waves of primitive and definitive erythroid progenitors [4]. Recent cell marking studies have revealed that subsets of tissue-resident blood cells, including some tissue-resident macrophages, do not appear to be replenished by HSCs postnatally, and are instead regenerated by self-renewal of fetal-derived cells [6-11]. In this review, we will consider the developmental origin of fetal-derived macrophages in the context of the various waves of hematopoietic potential that emerge during early embryogenesis.
2. Emergence of hematopoiesis in the fetus
2.1 Emergence of hematopoietic stem cells
The first adult-repopulating HSCs in mouse embryos are found associated with the appearance of cell clusters in the aorta, umbilical artery, and vitelline artery between E10.5 and E11.5 [2, 12]. In both mouse and zebrafish, rounded hematopoietic cells form directly from the endothelium through an endothelial-to-hematopoietic transition [13-16]. Murine HSCs then colonize the liver, the site of fetal hematopoiesis, and begin to expand in numbers from E12.5 to E16.5 [3, 17]. Similarly, at 5 weeks post-conception in the human embryo, aortic cell clusters arise along with HSC-like cells that can repopulate adult immune-deficient mice [18, 19]. Studies in the mouse indicate a large discrepancy between the number of cell clusters and the number of functional HSC [2, 3, 20, 21]. While the reasons for this discrepancy remain unclear, it is evident that bona fide adult-repopulating HSC activity is extremely rare in the embryo before E12.5 [3, 17] and likely does not significantly contribute to fetal hematopoiesis until well after this time.
2.2 HSC-independent hematopoiesis
The first cells to circulate in the embryonic bloodstream consist of primitive erythroid cells that emerge in blood islands in the yolk sac of mammalian and avian embryos soon after the onset of gastrulation, well before HSC formation [22-24]. The several million primitive erythroid cells that mature in the murine embryo are subsequently replaced by hundreds of millions of smaller definitive erythrocytes produced in the fetal liver [25]. Primitive erythroid cells are distinguished from their later definitive counterparts not only by their large size, but also their expression of embryonic globin genes, for review see [26].
Foundational insights into the complexity of HSC-independent hematopoiesis have been chiefly derived from the analysis of erythropoiesis [4, 27]. The first few primitive erythroid progenitors (EryP-CFCs) emerge in the nascent yolk sac of the late streak mouse embryo at E7.25, peak in number by E8.5, and subsequently differentiate into a wave of maturing erythroblasts in the bloodstream. At E8.25, just before the first cardiac contractions and the onset of circulation, the first few definitive erythroid progenitors (BFU-Es) begin to emerge, also in the yolk sac [4, 27] and then increase in numbers by E9.5. Unlike the transient wave of EryP-CFCs, BFU-Es are also detected in the fetal bloodstream and in the early fetal liver by E10.5, which is prior to the liver colonization by adult-repopulating HSCs [4, 17]. These studies of erythroid colony-forming progenitors initially raised the hypothesis that two distinct (primitive and definitive) waves of hematopoietic progenitors emerge in the yolk sac prior to HSC emergence.
2.3 The first wave of HSC-independent hematopoiesis: Primitive
Coincident with primitive EryP-CFC, the first Meg-CFC and Mac-CFC are also found emerging at E7.25 in the yolk sac and increase in numbers by E8.5 [4, 28, 29]. Subsequently, significant numbers of maturing megakaryocyte and macrophage precursors are found in the yolk sac contemporary with primitive erythroblasts, at a time when only the first few BFU-E have begun to emerge [4, 5, 30, 31]. These include small acetylcholinesterase-positive and GP1β-positive megakaryocytes identified in the yolk sac of E8.5 and E9.5 mouse embryos, respectively [28, 29] that have formed platelets by E10.5 [29, 32]. As will be discussed in more detail below, the first immature embryonic macrophages have also been identified in blood islands of the early murine yolk sac by E9.0 [33-36].
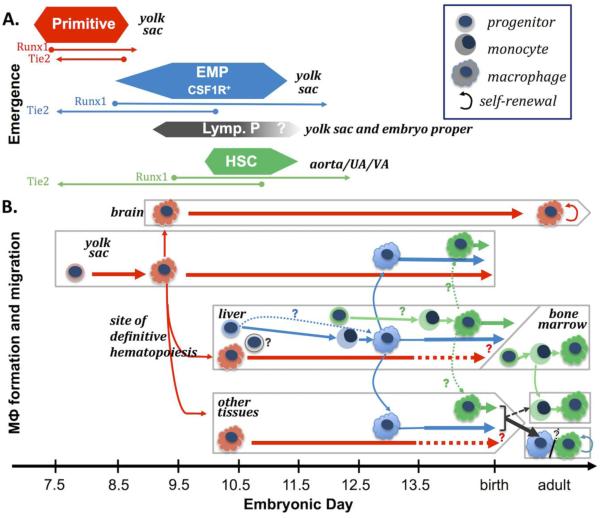
In the adult bone marrow, the definitive erythroid and megakaryocyte lineages share a common bipotential progenitor (MEP). Interestingly, MEP that give rise to primitive erythroid cells and megakaryocytes are also present in the early murine yolk sac [29], and in human iPS cell cultures [37]. Unlike the bipotential primitive MEP, early macrophage potential is associated with monopotent progenitors [4, 30], suggesting that if there is a common primitive hematopoietic trilineage progenitor, it must be rare and/or very transient in the embryo. Macrophage potential in the murine embryo is not a component of bi- and multilineage progenitors until E8.25 and these progenitors also contain definitive erythroid and/or granulocyte potential and can be immunophenotypically distinguished from the more abundant unipotential macrophage progenitors and primitive erythroid progenitors at this time [4, 5, 30]. Interestingly, in the zebrafish embryo, primitive macrophages first arise in the head, spatially distinct both from primitive erythroid potential and from the subsequent wave of definitive erythro-myeloid progenitors (see below)[38]. While it is not clear if these rapidly produced primitive hematopoietic progenitors possess the same hierarchal relationship as later waves of hematopoiesis, they are distinct from later definitive hematopoietic progenitors, and their timing of emergence and their rapid maturation link the primitive erythroid and coincident megakaryocyte and macrophage lineages as a "primitive" wave of hematopoiesis (Fig. 1A).
Figure 1.
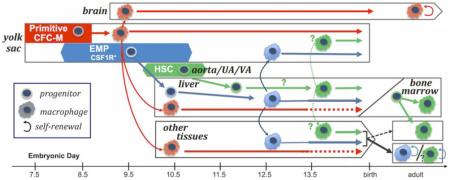
Temporal model of murine embryonic hematopoiesis and macrophage generation. A. Emergence of waves of hematopoietic progenitors. Emergence of primitive hematopoietic progenitors (red) is closely followed by the emergence of definitive EMPs (blue) in the yolk sac and primitive hematopoietic progenitors expand in numbers by E8.5 and quickly mature past progenitors stage, while EMP expand through E9.5 and continue to emerge through E11.5.. HSC (green) emergence begins slightly later in the aorta, umbilical artery (UA) and vitelline artery (VA). Although they possess distinct hematopoietic potential, production of all these waves significantly overlap and involves a transition between Tie2-expressing cells to Runx1 expressing cells (lines under emergence). Additionally, EMPs despite having both erythroid and granulocyte potential, uniquely express CSF1R, which is normally restricted to the macrophage lineage. A discrete wave of a small numbers of lymphoid (T and B-cell) progenitors (grey) also emerges at E9.0 in both the yolk sac and embryo proper although it is not known specifically how long they continue to be generated. For review see [50, 86] B. Paradigm of macrophage (MФ) maturation and migration. Primitive hematopoietic (red) progenitors rapidly develop into macrophages in the yolk sac, before monocytes are observed [5, 34]. By E9.5, macrophages are found in the brain, and by E10.5, they are broadly distributed in all tissues, including the fetal liver [47, 51, 54]. The timing relative to progenitor numbers and lineage mapping correlations indicate these early macrophages (pink) are largely derived from the primitive progenitors. EMPs (blue) colonize the fetal liver beginning at E10, and produce the first fetal monocytes there, although it is not known if EMPs can also directly differentiate into macrophages [4, 5, 47, 51]. HSC-independent lymphoid progenitors (grey) are also found in the early fetal liver, although evidence indicates they do not contribute to tissue macrophages [10]. HSCs (green) begin to accumulate in the liver by E12.5, where they subsequently expand in numbers, generate myeloid progenitors, and differentiate into macrophages [17]. Given the overlap of markers and timing of emergence of HSCs with EMPs, it remains unclear when HSC-derived monocytes and macrophages first appear. Lineage tracing studies suggest that these first primitive macrophages persist as adult microglia in the brain (Fig. 2A). In other tissues, the prevalence of early produced primitive macrophages decreases after mid-gestation, but whether they are lost, or simply diluted by the subsequent waves of definitive macrophages is not known [51]. Lineage tracing studies indicate that later definitive macrophages are found in non-brain tissues by E13.5 and suggest that these are EMP-derived, although an HSC contribution is likely to occur later in development (Fig. 2A). While shown here as colonizing macrophages, the EMP and HSC-derived monocytes may also colonize tissues and may generate macrophages there [34, 51]. In this current model, the adult brain has predominately primitive-derived macrophages while in other tissues EMP/HSC fetal-derived macrophages are replaced by adult HSC-derived macrophages in some tissues, while other tissues are maintained by fetal-derived macrophage self-renewal [66] for review [70].
2.4 Definitive HSC-independent hematopoiesis: Erythro-myeloid progenitors (EMP)
Analysis of hematopoietic progenitors in E8.25-E8.5 mouse embryos revealed not only the onset of definitive erythroid (BFU-E), but also the first occurrence of bipotential GM-CFC, mast cell progenitors (Mast-CFC), and multilineage high proliferative potential colony-forming cells (HPP-CFC), which give rise in vitro to macrophages, granulocytes, and mast cells [4, 39]. This diverse myeloid potential appears to arise from a cohort of “erythro-myeloid progenitors” (EMPs) that express significant Kit and CD41 on their cell surface [40, 41], distinguishing them from the KitloCD41lo primitive erythroid and unipotential macrophage colony-forming activity at E8.5 [40]. By E9.5, EMPs can be prospectively identified as a Kit+CD41+CD16/32+ cell population that can be distinguished from maturing populations of Kitneg CD16/32+CD45hi primitive macrophages and Kitneg CD41hi Gp1β+ primitive megakaryocytes [5, 31]. Consist with the emergence of HPP-CFC, clonal analyses have confirmed that single E9.5 EMPs have the capacity to generate multiple myeloid lineages in vitro, as well as definitive erythroid potential [5]. Unlike HSCs, EMP do not contain B-lymphoid potential [5]. Like HSCs, EMPs arise via an endothelial-to-hematopoietic transition in the yolk sac, and do so in a Runx1-dependent manner [31, 42-44]. Importantly, EMP emergence continues through E11.5, overlapping temporally with HSC production in the major arteries (Fig. 1A) [31, 45].
EMPs are found in the bloodstream and in the fetal liver of the mouse embryo by E10.5 [4, 5]. EMPs that seed the fetal liver produce the first enucleated definitive erythrocytes and also granulocytes by E11.5, although many EMPs are also present in the embryonic circulation through E12.5 [5, 46, 47]. In human embryos, BFU-E and GM-CFC also first arise in the yolk sac by 4 to 5 weeks and are then found in the liver before HSC colonization [18, 19, 22]. In mouse embryos lacking Cbfβ, a critical binding partner of Runx1, rescue of Runx1 function transgene during emergence of EMPs, but not HSCs, rescued normal numbers of macrophage progenitors in mid-gestation fetal livers [43, 44], supporting the concept that EMPs play a significant role in myeloid production in the mid-gestation murine fetal liver. Importantly, the rescue of EMPs also extended survival of Cbfβ-null embryos until birth, despite the absence of functional HSCs [44].
EMPs have also been identified in zebrafish embryos temporally distinct from HSCs and primitive hematopoiesis [30, 38]. However, granulocyte potential in the zebrafish may be associated not only with EMPs but also with the primitive wave of hematopoiesis [38, 48].
2.5 Definitive HSC-independent hematopoiesis: Lymphoid
Small numbers of lymphoid progenitors have also been detected prior to HSC emergence. Progenitors with B-cell and T-cell potential emerge around E9.0 both in the yolk sac and the embryo proper [9-11, 49]. These lymphoid progenitors migrate to and differentiate in the early fetal liver, and are thought to provide unique subsets of lymphoid cells that persist in the adult, for review see [50]. It is not clear how these progenitors contribute to the lymphoid/myeloid progenitor hierarchy found in the fetal period [51]. However, lymphoid progenitors arise in a spatial pattern that differs from that of EMP and EMPs lack B-cell potential before E10.5 [5, 49]. In addition, these early lymphoid progenitors do not generate significant macrophages when transplanted, and Rag2 lineage tracing fails to label tissue macrophages [9, 10, 49]. While lymphoid progenitors contribute to the complexity of HSC-independent hematopoiesis, current evidence suggests that they do not contribute to adult tissue macrophage production.
3. Source of tissue-resident macrophage populations in the fetus
Our current understanding of macrophage ontogeny is largely based on two experimental approaches. The first approach is the spatial and temporal correlation of macrophage progenitors derived from primitive, EMP and HSC hematopoiesis with the production of macrophages and monocytes using immunophenotypic analysis, which have also been validated through microscopy and functional assays [7, 34, 47, 51-55]. The second approach is in vivo lineage tracing driven by genes involved in myeloid differentiation, or more generally, hematopoietic emergence. Cre-recombinase expressed under the control of these various promoters activates fluorescent protein expression, effectively labeling all daughter cells. A temporal restriction in lineage mapping can also be introduced through the use of cre-recombinase fused to, and dependent on, the activity of an estrogen receptor (ER-Cre), which is only activated in the presence of tamoxifen [56].
3.1 Macrophage and monocyte production during development
The first macrophages observed in the murine embryo are a small, transient wave of mature CD45+F4/80+Kitneg maternal macrophages detected at E7.5-8.0 [30, 55]. These maternally-derived macrophages lack colony-forming ability and are markedly diminished in numbers by E8.5-9.0. The first embryonic-derived immature macrophages are found in yolk sac blood islands at E9.0 [33-35]. More mature macrophages are subsequently found in the yolk sac and brain by E9.5, and throughout the rest of the embryo by E10.5 [6, 30, 36, 47, 54]. The maturation of these macrophages by E9.5 in the mouse embryo is suggestive of an HSC-independent origin. In circulation-deficient embryos, these macrophages fail to colonize the embryo proper and brain, but remain in the yolk sac, consistent with their exclusive origin in the yolk sac and their requirement for blood flow to colonize embryonic tissues [6, 57]. While the detection of these first embryonic macrophages is after the initiation of both primitive and EMP hematopoiesis, their maturation by E9.0 makes it most likely that they are derived from the earlier primitive macrophage progenitors, which emerge beginning at E7.25, and have expanded to hundreds of colony forming units by E8.5, rather than EMPs, which only produce a few progenitors by E8.5 (Fig. 1A and B) [4, 5].
Intriguingly, monocytes have not been observed in the murine conceptus until after these early macrophages are already present in fetal tissues. Specifically, monocytes are found at E12.5 in the bloodstream, liver and many other tissues, but not the brain [7, 34, 47, 51]. Consistent with these observations, lineage mapping utilizing a promoter active in adult monocytes (S100a4) labels macrophages in the fetus after E12.5, and by E16.5, these labeled macrophages become the most abundant macrophages in fetal tissues outside of the brain [51]. While it has been assumed that all monocyte-independent macrophages are derived from primitive hematopoiesis, it remains possible that EMPs as well as fetal HSCs, also have the capacity to directly generate macrophages. By E9.5, the transient wave of primitive hematopoiesis has matured past the progenitor stage and the macrophage progenitor activity in the yolk sac is now found in the EMP population [4, 5]. There are no known markers that distinguish macrophages based on hematopoietic source, thus once fetal liver myelopoiesis has begun further delineation of macrophage contributions from the different hematopoietic waves has relied on lineage-tracing approaches.
3.2 Lineage tracing of macrophage sources
Lineage-tracing is a very powerful tool because it allows progenitor cell fate to be assessed in vivo. However, there are important considerations in the interpretation of these studies, particularly given the overlapping timing and promoter usage in emerging hematopoietic waves and their progeny. First, ER-Cre activity occurs over a range of developmental time that depends both on tamoxifen dosage and metabolism, and has inherent variability due to the inter-litter and intra-litter differences that occur in rapid murine embryonic development. Second, there are caveats associated with some of the promoter constructs. While Runx1 and Kit are associated with emergence of hematopoiesis, they are also active in more mature hematopoietic progenitors, and it isn't clear why these other hematopoietic populations are not labeled with later tamoxifen treatment [58-60]. Additionally, Runx1 promoter constructs inactivate one Runx1 allele, which can cause ectopic HSC formation, as well as abnormalities in lineage decisions [59, 61]. While Flt3-cre can lineage-trace all HSC progeny in adults, it does not efficiently label early fetal HSC [51, 62]. Third, many promoters are active in more than one wave of hematopoiesis. Important examples of this are the CX3CR1 and the CSF1R (M-CSF receptor) promoters. CX3CR1 is expressed on subclasses of macrophages in the adult, including many tissue-resident macrophages, as well as early embryonic macrophages and, in addition, the CX3CR1 promoter has been used to identify emerging macrophages [6, 30, 63]. CSF1R becomes active in maturing primitive macrophages after E8.5 as well as in EMP and HSC derived macrophages [47, 64]. While CSF1R is not active when primitive hematopoietic progenitors arise and does not lineage-trace primitive erythroid cells, it is expressed along with another myeloid genes, such as CD16/32, in EMPs by E9.5 and consequently lineage-traces diverse EMP progeny, including definitive erythroid and granulocyte potential in the fetal liver though E14.5 [5, 31, 47, 51]. Because primitive macrophage maturation and EMP emergence occur at the same time, CSF1R lineage-traced cells collectively represent the macrophage progeny of both waves of hematopoiesis.
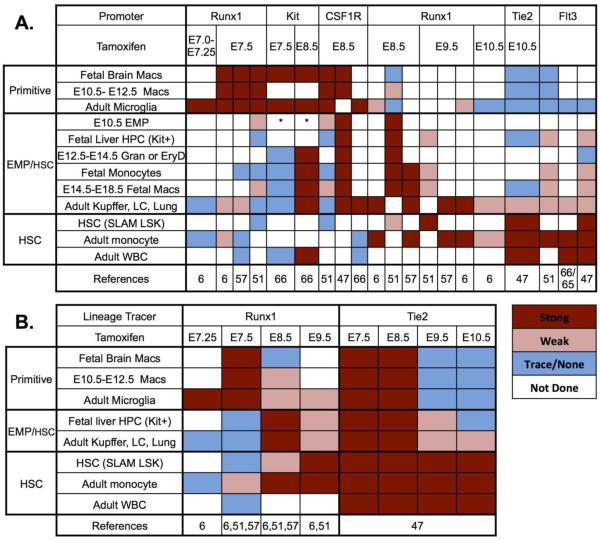
Despite these limitations, a pattern emerges when all of the recent lineage tracing results are compared to one another (Fig. 2A). Temporal studies utilizing ER-Cre activation highlight the existence of overlapping patterns of macrophage labeling, as well as the co-labeling of specific subsets of fetal and adult macrophages. There is a strong correlation between labeling of adult microglia and conditions that also highly label (early) fetal macrophages between E10.5 and E12.5. In contrast, conditions that strongly label (later) fetal macrophages between E14.5 and birth, consistently label non-microglial, fetal-derived adult tissue-resident macrophages. Importantly, correlation of these staining patterns with lineage labeling of other hematopoietic cells, as well as immunophenotypically defined EMP or HSC, have helped to shed light on the parent progenitor populations that give rise to these macrophage populations.
Figure 2. Lineage-tracing of macrophages and other hematopoietic cells.
Promoters driving ER-Cre are listed above the time of tamoxifen administration except for the Flt3 promoter which was driving Cre without a temporal limit. Colors in the boxes indicate levels of labeling. References indicated by the numbers at the bottom of the tables. Note that calls of strong or weak are based on maximum labeling observed within that experiment. A. Progressive labeling of cohorts of tissue macrophages and other hematopoietic cells. Cells whose labeling is best correlated overall are grouped together and the proposed hematopoietic sources of these cells listed on the left. Fetal macrophages are divided into three groups. Microglia, fetal brain and broad spread early (E10.5 to E12.5) macrophages are included in the upper group. The middle group contains fetal-derived adult tissue macrophages (Liver Kupffer cells, skin Langerhans cells and alveolar macrophages), E16.5 to E18.5 non-brain fetal macrophages, immunophenotypic EMP and other components of fetal hematopoiesis. The lower group has adult HSC-derived hematopoietic cells as well as immunophenotype defined fetal or adult HSC. EMP boxes labeled with * indicate that these investigators reported EMP labeling with an immunophenotype (Sca1hi) that conflicts with other reports of EMP and early fetal progenitor labeling [5, 92]. B. Comparison of Runx1 and Tie2 ER-cre labeling experiments. Runx1 is first expressed in hematopoietic waves as they emerge and then may label less well depending on the expression of Runx1 in more mature hematopoietic progeny. In contrast, Tie2 is expressed on all the endothelial-like precursors of hematopoiesis by E7.5 and then is turned off in hematopoietic cells after they emerge and thus labeling is sequentially lost in primitive hematopoietic, EMP and finally HSC-derived hematopoiesis, for review [92]. Abbreviations: Mac=macrophage, Gran=granulocyte, EryD=definitive erythroblasts, HPC= hematopoietic progenitor cells, Kit+ not HSC. SLAM HSC=lin-Kit+scaI+CD150+, LC=Langerhans Cells, Adult WBC=circulating granulocytes or lymphoid cells.
3.3 Microglia and early primitive hematopoietic-derived macrophages
Microglia, the tissue macrophages of the brain, have a very low rate of replacement in steady state in the adult as evidenced by transplantation, parabiosis and adult lineage-tracing studies [6-8, 65, 66]. Additionally, among adult tissue-resident macrophages known to be derived from the fetus, only microglia are not labeled by lineage mapping with the S100a4 adult monocyte promoter [51, 57, 67]. Tamoxifen administration between E7.0 to E7.5 to activate Runx1-driven ER-Cre labels microglia as well as the first macrophages in other tissues of the E10.5 to E12.5 murine embryo (Fig. 2A). [6, 51, 57]. Furthermore, administration of tamoxifen between E7.0 and E7.75 revealed a gradual decrease in labeling efficiency both of adult microglia and of E10.5 yolk sac macrophages with a little to no labeling of fetal or adult microglia with treatment at E8.5 [6, 51]. These data, together with the timing of primitive progenitor production and early macrophage formation discussed above, suggest a model where primitive hematopoiesis generates macrophages found in multiple tissues in the E10.5 murine embryo that persist in the brain as microglia (Fig. 1B).
3.4 EMP- and HSC-derived fetal definitive hematopoiesis
Administration of tamoxifen from E8.5 to E9.5 to activate Runx1 or CSF1R promoter-driven labeling results in increased labeling of non-brain fetal macrophages from E13.5 to birth, adult fetal-derived macrophages except microglia, as well as fetal liver monocytes, granulocytes, and E10.5 EMPs (Fig. 2A) [47, 51, 57]. One exception to this pattern is a recent genetic tracing study utilizing a Kit promoter that reported the labeling of EMPs following tamoxifen administration at E7.5, but only weak labeling of fetal liver hematopoietic derivatives and non-microglia adult tissue-resident macrophages [66]. However, it is unlikely that a significant number of EMPs would be efficiently labeled by this treatment, as Kit expression is only on a minority of EMPs before E9.5, and there is continued production of Kit+ EMPs through E11.5 [4, 5, 31]. Additionally, EMP-derived granulocytes in the early fetal liver were not effectively labeled with E7.5 tamoxifen treatment in this study [66].
Particularly illuminating is comparison of lineage-tracing by the Runx1 promoter, which labels hematopoietic cells as they are specified, and labeling with the Tie2 promoter, which ceases to be expressed on hematopoietic progenitors after they have emerged from endothelial-like precursors (Fig. 2B) [6, 47, 51, 57]. As expected from macrophages primarily derived from primitive hematopoietic progenitors, microglia and fetal macrophages were efficiently labeled from E10.5-E12.5 with E7.5 Runx1-driven lineage-tracing, but ceased to be labeled with activation of the Tie2 promoter after E9.5 consistent with the exhaustion of primitive progenitor activity described above (Fig. 2B). In contrast, fetal liver hematopoietic progenitors and non-microglia adult tissue-resident macrophages become increasingly labeled by activation of the Runx1 promoter at E8.5, but are decreasingly labeled by the Tie2 promoter when tamoxifen is administered at E9.5, and are almost undetectable with tamoxifen exposure at E10.5. Interestingly, this diminution of fetal macrophage labeling after E9.5 occurs despite efficient labeling of HSCs and their derivatives. Additionally, E8.5 activation of CSF1R promoter-driven lineage tracing does not label HSCs and their derivatives, but instead labels a significant proportion of fetal macrophages [47]. Taken together, these data suggest that with the exception of the brain, fetal-derived tissue-resident macrophages originate in large part from EMPs. Fetal HSCs may also contribute to these tissue-resident macrophages, however the extent and timing of this contribution has yet to be determined (Fig. 1B). Ultimately, further progenitor- and HSC-labeling studies throughout embryogenesis are needed to confirm and refine this model.
3.5 Adult tissue macrophages
Collectively, data from lineage tracing studies suggest that many tissues can be sequentially colonized by emerging waves of macrophages, and that the time window of colonization depends on the tissue. In the mouse, the brain normally limits colonization of macrophages to early development and only accepts new macrophages in the setting of injury, which in turn largely limits the source of adult microglia to primitive hematopoiesis. This process of sequential colonization of tissues by macrophages has also been observed during zebrafish development. However, in zebrafish, the brain continues to be colonized by new macrophages, resulting in the replacement of embryonic-derived microglia with adult-derived microglia [68]. In all other tissues examined in the murine embryo, macrophages derived from primitive hematopoiesis are replaced, or diluted, by macrophages derived from EMPs, or eventually, HSCs. Lineage mapping in the late fetus provides evidence that there is variation in the time and degree that each tissue becomes resistant to further macrophage colonization [57, 66]. Tissues no longer "open" to significant colonization by adult circulating monocytes retain self - renewing fetal-derived tissue-resident macrophage populations, including Kupffer cells, alveolar macrophages and Langerhans cells. Other adult tissues, including regions of the skin and intestine, continue to be colonized by monocytes, so that these tissue-resident macrophages are ultimately HSC-derived (Fig. 1B) [66, 69] for review see [70].
4. Roles for macrophages during embryogenesis
Development of complex organ systems and tissues in metazoans requires coordination and execution of cell differentiation, self-renewal, and apoptosis. As professional scavengers of apoptotic cells and debris, as well as potent sources of secreted signaling molecules, macrophages play critical roles in various developmental processes for review see [71]. One important role of fetal macrophages is to support the extensive erythropoiesis in the fetal liver that sustains the rapid growth of the fetus, for review see [26]. In both the fetal liver and adult bone marrow, maturing erythroid precursors are in direct contact with macrophages in “erythroblast islands” for review see [72]. These specialized macrophages are thought to promote erythroblast proliferation and maturation [73], and phagocytose the nucleated pyrenocyte, the remainder of the final cell division that creates the enucleated reticulocyte. Both circulating primitive erythroblasts and maturing definitive erythroblasts enucleate in the developing fetal liver [25, 74, 75]. The significance of these macrophage-erythroblast interactions is highlighted by the fetal lethality due to anemia in mice with mutations that affect the ability of macrophages to assemble in erythroblast islands or to digest erythroblast nuclei [76-79]. Many of the other roles of macrophages in development involve removing apoptotic cells generated during limb development, where macrophages enter the remodeling digits of the limb bud and digest apoptotic cells as early as E12.5 [80]. However, macrophages may also be instructive, as demonstrated by the role for microglia in neuronal development and migration, and macrophage involvement in angiogenesis during testis formation [81, 82].
Macrophages also contribute to the innate immune system in the embryo, which may be particularly important before the adaptive immune system becomes functional. As early as E10.5, macrophages are responsive to toll-like-receptor agonists, can ingest bacteria, and secrete a variety of proinflammatory cytokines and chemokines, including those recently implicated in triggering the formation of adult-repopulating hematopoietic stem cells in mouse and zebrafish embryos [53, 83-85]. Studies of macrophage response to injury has shown that prior to E14.5, macrophages do not appear to efficiently enter wound sites, although they can participate in phagocytosis at the injury site after healing has commenced [80]. After E14.5, macrophages more rapidly enter sites of injury [80], but it is unknown if these temporal differences in macrophage response are the result of altered recruitment cues or to recruitment of ontogenically distinct macrophage populations. The diverse roles of macrophages during development may explain why they are among the first blood cells that emerge in the embryo and why macrophage potential is a component of primitive, EMP and HSC waves of hematopoiesis.
5. Nomenclature/paradigms
It is perhaps inevitable that when two fields of study are bridged challenges with the integration of nomenclature occur. Macrophages have often been categorized into two populations: primitive or definitive, Myb-dependent or -independent, yolk sac- or fetal liver-derived. However, these delineations are inherently inadequate when extrapolated to the multiple developmental origins of macrophages.
The term EMP was originally proposed in a study of macrophage ontogeny to distinguish the yolk sac definitive erythroid/myeloid progenitor wave from the earlier primitive hematopoietic wave as well as from the later HSC-derived definitive wave of hematopoiesis, [30, for review 86]. Recently, the primitive hematopoietic wave has also been referred to as "EMP" [51, 87]. While primitive hematopoiesis contains both erythroid and macrophage lineages, we favor restricting the term "EMP", as originally defined by Bertrand et al., [30, 38] to the definitive hematopoietic wave in the yolk sac for two reasons. First, referring to primitive hematopoietic progenitors as "EMP" reduces the classification of three waves of hematopoietic potential (primitive, EMP, and HSC) again to two terms. Second, it has not been determined that a common primitive erythroid-macrophage progenitor exists in vivo, and in the zebrafish embryo, these lineages arise in different regions, suggesting they may not come from a common progenitor [4, 30, 38]. There has been some confusion caused by assuming primitive macrophage potential is contained within an "EMP". This terminology was used when cultures of E8.0 yolk sac Kit+/lo cells produce both macrophage and erythroid cells [55], but clonal analysis was not performed so these data are consistent with the known presence of individual primitive erythroid and macrophage progenitors at this time [4, 55]. Additional confusion has come from the simultaneous labeling of both primitive macrophages (and thus microglia), and EMPs (and thus later fetal macrophages), by the CSF1R promoter [7, 47]. Thus, "CSF1R+, myb-independent primitive EMPs" [51, 87] are likely the combination of two distinct progenitors, the first being primitive macrophage progenitors, without erythroid potential, that generate microglia, and the second being CSF1R+ myb+ definitive erythroid producing EMPs that are also a source of later fetal macrophages (Fig. 1). While the data and models of macrophage formation formed from these seminal, technically challenging experiments are critical for understanding macrophage ontogeny, they can not be extrapolated to other hematopoietic lineages when the clonal progenitor potential, and most notably, the nature of the associated erythropoiesis, has not been determined.
The terms “Myb-dependent” and “Myb-independent” have also been used to categorize embryonic macrophages. It is known that a functional Myb gene is required for the development of definitive, but not primitive, erythrocytes [88]. Furthermore, conditional deficiency of Myb in adults demonstrates a requirement for maintenance of adult HSCs [7]. The detection of macrophages in the Myb-null embryo has helped to establish the HSC-independent developmental origin, and maintenance by self-renewal, of a subset of tissue-resident macrophages [7]. The deficiency of Kit+ hematopoietic progenitors in the fetal liver of Myb-null mouse embryos suggests that definitive hematopoiesis is widely defective in the absence of Myb [7, 88]. It is not known if EMP or EMP-derived macrophages emerge in the absence of Myb. The detection of of some Kit+ cells in the yolk sac of Myb-null embryos raises the possibility that some Myb-independent EMPs might emerge and persist [7]. Furthermore, Myb-null embryos die when primitive macrophages are normally still present in tissues, so it remains unclear if the normal replacement of early macrophages with later fetal macrophages would occur if the embryo survived. Thus, it is not yet known if all definitive macrophage potential is Myb-dependent.
Finally, yolk sac versus fetal liver locations have been used to delineate the origin of different macrophages. However, these tissues are not themselves restricted to a particular wave of hematopoiesis. The site of maturation of primitive macrophages is in the early yolk sac, while EMPs and HSCs both mature in the fetal liver. Monocytes are also detected in the yolk sac later in development, raising the possibility that the yolk sac is ultimately colonized by myeloid cells that have matured in the fetal liver [34, 35]. Additionally, early lineage tracing studies indicate that the fetal liver contains both primitive yolk sac-matured macrophages, as well as a significant myelopoiesis derived from EMPs that also originated from the yolk sac. Hematopoietic progenitor lineage potential, genetic requirements, and sites of synthesis and maturation are all important aspects of fetal macrophage biology that need to be better understood.
Studies to date have highlighted the complexity of macrophage origins in the fetus. The roles of hematopoietic source, timing of emergence, sites of colonization, and sites of maturation need further investigation to better understand the diversity of tissue-resident macrophages. Recent data from ectopically transplanted macrophages have demonstrated the importance of microenvironmental signals in shaping the tissue-specific enhancer repertoire of adult tissue-resident macrophages [89]. However, these data also indicate that a significant portion of enhancers are not reprogrammed after transplantation. In agreement with the concept of distinct roles for fetal versus adult macrophages, recent data have demonstrated that fetal-derived tissue macrophages in the heart are replaced after birth by adult-derived tissue macrophages, and this is associated with decreased ability to repair myocardial damage [90], for review see [91]. Thus, both inherent ontogenic differences, and local environmental signals are likely to influence the function of tissue macrophages. Understanding the influences of both will be necessary to fully decode the functional capabilities of tissue-resident macrophages.
Highlights.
Multiple sources of tissue-resident macrophage emerge during embryogenesis
Adult microglia originate from unipotential yolk sac primitive macrophage progenitors
Later yolk sac-derived EMP initiate definitive myelopoiesis in the fetal liver
Most fetal-derived adult tissue-resident macrophages originate from EMP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, et al. Origin of monocytes and macrophages in a committed progenitor. Nat Immunol. 2013;14:821–830. doi: 10.1038/ni.2638. [DOI] [PubMed] [Google Scholar]
- [2].Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- [3].Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- [4].Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- [5].McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, et al. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell Rep. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- [8].Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boiers C, Carrelha J, Lutteropp M, Luc S, Green JC, Azzoni E, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- [11].Kobayashi M, Shelley WC, Seo W, Vemula S, Lin Y, Liu Y, et al. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfbeta for their development. Proc Natl Acad Sci U S A. 2014;111:12151–12156. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].de Bruijn MFTR, Speck NA, Peeters MCE, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO Journal. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- [14].Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lam EY, Hall CJ, Crosier PS, Crosier KE, Flores MV. Live imaging of Runx1 expression in the dorsal aorta tracks the emergence of blood progenitors from endothelial cells. Blood. 2010;116:909–914. doi: 10.1182/blood-2010-01-264382. [DOI] [PubMed] [Google Scholar]
- [16].Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- [17].Ema H, Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95:2284–2288. [PubMed] [Google Scholar]
- [18].Tavian M, Coulombel L, Luton D, Clemente HS, Dieterlen-Lievre F, Peault B. Aorta-associated CD34+ hematopoietic cells in the early human embryo. Blood. 1996;87:67–72. [PubMed] [Google Scholar]
- [19].Ivanovs A, Rybtsov S, Welch L, Anderson RA, Turner ML, Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med. 2011;208:2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yokomizo T, Dzierzak E. Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development. 2010;137:3651–3661. doi: 10.1242/dev.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boisset JC, Clapes T, Klaus A, Papazian N, Onderwater J, Mommaas-Kienhuis M, et al. Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta. Blood. 2015;125:465–469. doi: 10.1182/blood-2014-07-588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Migliaccio G, Migliaccio AR, Petti S, Mavilio F, Russo G, Lazzaro D, et al. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac to liver transition. J Clin Invest. 1986;78:51–60. doi: 10.1172/JCI112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maximow AA. Untersuchungen uber blut und bindegewebe 1. Die fruhesten entwicklungsstadien der blut- und binde-gewebszellan bein saugetierembryo, bis zum anfang der blutbilding unden leber. Arch Mikroskop Anat. 1909;73:444–561. [Google Scholar]
- [24].Sabin FR. Studies on the origin of blood vessels and red blood corpuscles as seen in the living blastoderm of chicks during the second day of incubation. Contributions to Embryology. 1920;9:213–262. [Google Scholar]
- [25].Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- [26].Palis J. Primitive and definitive erythropoiesis in mammals. Front Physiol. 2014;5:3. doi: 10.3389/fphys.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wong PMC, Chung S-H, Reicheld SM, Chui DHK. Hemoglobin switching during murine embryonic development: evidence for two populations of embryonic erythropoietic progenitor cells. Blood. 1986;67:716–721. [PubMed] [Google Scholar]
- [28].Xu MJ, Matsuoka S, Yang FC, Ebihara Y, Manabe A, Tanaka R, et al. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood. 2001;97:2016–2022. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- [29].Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KK, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- [31].Frame JM, Fegan KH, Conway SJ, McGrath KE, Palis J. Definitive Hematopoiesis In The Yolk Sac Emerges from Wnt-responsive Hemogenic Endothelium Independently Of Circulation and Arterial Identity. Stem Cells. 2015 doi: 10.1002/stem.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Potts KS, Sargeant TJ, Markham JF, Shi W, Biben C, Josefsson EC, et al. A lineage of diploid platelet-forming cells precedes polyploid megakaryocyte formation in the mouse embryo. Blood. 2014;124:2725–2729. doi: 10.1182/blood-2014-02-559468. [DOI] [PubMed] [Google Scholar]
- [33].Cline MJ, Moore MA. Embryonic origin of the mouse macrophage. Blood. 1972;39:842–849. [PubMed] [Google Scholar]
- [34].Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. Journal of leukocyte biology. 1989;45:87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- [35].Naito M, Umeda S, Yamamoto T, Moriyama H, Umezu H, Hasegawa G, et al. Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. Journal of leukocyte biology. 1996;59:133–138. doi: 10.1002/jlb.59.2.133. [DOI] [PubMed] [Google Scholar]
- [36].Morris L, Graham CF, Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991;112:517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- [37].Klimchenko O, Mori M, Distefano A, Langlois T, Larbret F, Lecluse Y, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114:1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- [38].Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Palis J, Chan RJ, Koniski AD, Patel R, Starr M, Yoder MC. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc Natl Acad Sci U S A. 2001;98:4528–4533. doi: 10.1073/pnas.071002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- [41].Mikkola HKA, Fujiwara Y, Schlaeger TM, Traver D, Orkin SH. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- [42].North T, Gu TL, Stacy T, Wang Q, Howard L, Binder M, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- [43].Miller J, Horner A, Stacy T, Lowrey C, Lian JB, Stein G, et al. The core-binding factor beta subunit is required for bone formation and hematopoietic maturation. Nat Genet. 2002;32:645–649. doi: 10.1038/ng1049. [DOI] [PubMed] [Google Scholar]
- [44].Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tober J, Yzaguirre AD, Piwarzyk E, Speck NA. Distinct temporal requirements for Runx1 in hematopoietic progenitors and stem cells. Development. 2013;140:3765–3776. doi: 10.1242/dev.094961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, et al. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood. 2011;117:4600–4608. doi: 10.1182/blood-2010-12-325357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Glenn NO, Schumacher JA, Kim HJ, Zhao EJ, Skerniskyte J, Sumanas S. Distinct regulation of the anterior and posterior myeloperoxidase expression by Etv2 and Gata1 during primitive Granulopoiesis in zebrafish. Dev Biol. 2014;393:149–159. doi: 10.1016/j.ydbio.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lin Y, Yoder MC, Yoshimoto M. Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem Cells Dev. 2014;23:1168–1177. doi: 10.1089/scd.2013.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hoeffel G, Chen J, Lavin Y, Low D, Almeida FF, See P, et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Takahashi K, Naito M, Takeya M. Development and heterogeneity of macrophages and their related cells through their differentiation pathways. Pathol Int. 1996;46:473–485. doi: 10.1111/j.1440-1827.1996.tb03641.x. [DOI] [PubMed] [Google Scholar]
- [53].Balounova J, Vavrochova T, Benesova M, Ballek O, Kolar M, Filipp D. Toll-like receptors expressed on embryonic macrophages couple inflammatory signals to iron metabolism during early ontogenesis. Eur J Immunol. 2014;44:1491–1502. doi: 10.1002/eji.201344040. [DOI] [PubMed] [Google Scholar]
- [54].Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- [55].Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- [56].Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- [57].Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].North TE, de Bruijn MF, Stacy T, Talebian L, Lind E, Robin C, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- [59].North TE, Stacy T, Matheny CJ, Speck NA, de Bruijn MF. Runx1 is expressed in adult mouse hematopoietic stem cells and differentiating myeloid and lymphoid cells, but not in maturing erythroid cells. Stem Cells. 2004;22:158–168. doi: 10.1634/stemcells.22-2-158. [DOI] [PubMed] [Google Scholar]
- [60].Challen GA, Boles N, Lin KK, Goodell MA. Mouse hematopoietic stem cell identification and analysis. Cytometry A. 2009;75:14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing RJ, et al. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity. 2000;13:423–431. doi: 10.1016/s1074-7613(00)00042-x. [DOI] [PubMed] [Google Scholar]
- [62].Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Evrard M, Chong SZ, Devi S, Chew WK, Lee B, Poidinger M, et al. Visualization of bone marrow monocyte mobilization using Cx3cr1gfp/+Flt3L−/− reporter mouse by multiphoton intravital microscopy. Journal of leukocyte biology. 2015;97:611–619. doi: 10.1189/jlb.1TA0514-274R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lichanska AM, Browne CM, Henkel GW, Murphy KM, Ostrowski MC, McKercher SR, et al. Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood. 1999;94:127–138. [PubMed] [Google Scholar]
- [65].Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of "resident" myeloid cells: the case of the microglia. Glia. 2013;61:112–120. doi: 10.1002/glia.22393. [DOI] [PubMed] [Google Scholar]
- [66].Sheng J, Ruedl C, Karjalainen K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43:382–393. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- [67].Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xu J, Zhu L, He S, Wu Y, Jin W, Yu T, et al. Temporal-Spatial Resolution Fate Mapping Reveals Distinct Origins for Embryonic and Adult Microglia in Zebrafish. Dev Cell. 2015;34:632–641. doi: 10.1016/j.devcel.2015.08.018. [DOI] [PubMed] [Google Scholar]
- [69].Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lavin Y, Merad M. Macrophages: gatekeepers of tissue integrity. Cancer Immunol Res. 2013;1:201–209. doi: 10.1158/2326-6066.CIR-13-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Harvey NL, Gordon EJ. Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis. Vasc Cell. 2012;4:15. doi: 10.1186/2045-824X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jacobsen RN, Perkins AC, Levesque JP. Macrophages and regulation of erythropoiesis. Curr Opin Hematol. 2015;22:212–219. doi: 10.1097/MOH.0000000000000131. [DOI] [PubMed] [Google Scholar]
- [73].Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].McGrath KE, Kingsley PD, Koniski AD, Porter RL, Bushnell TP, Palis J. Enucleation of primitive erythroid cells generates a transient population of "pyrenocytes" in the mammalian fetus. Blood. 2008;111:2409–2417. doi: 10.1182/blood-2007-08-107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Isern J, Fraser ST, He Z, Baron MH. The fetal liver is a niche for maturation of primitive erythroid cells. Proc Natl Acad Sci U S A. 2008;105:6662–6667. doi: 10.1073/pnas.0802032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292:1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- [77].Liu XS, Li XH, Wang Y, Shu RZ, Wang L, Lu SY, et al. Disruption of palladin leads to defects in definitive erythropoiesis by interfering with erythroblastic island formation in mouse fetal liver. Blood. 2007;110:870–876. doi: 10.1182/blood-2007-01-068528. [DOI] [PubMed] [Google Scholar]
- [78].Kusakabe M, Hasegawa K, Hamada M, Nakamura M, Ohsumi T, Suzuki H, et al. c-Maf plays a crucial role for the definitive erythropoiesis that accompanies erythroblastic island formation in the fetal liver. Blood. 2011;118:1374–1385. doi: 10.1182/blood-2010-08-300400. [DOI] [PubMed] [Google Scholar]
- [79].Soni S, Bala S, Gwynn B, Sahr KE, Peters LL, Hanspal MS. Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. Journal of Biological Chemistry. 2006;281:20181–20189. doi: 10.1074/jbc.M603226200. [DOI] [PubMed] [Google Scholar]
- [80].Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107:1159–1167. doi: 10.1242/jcs.107.5.1159. Pt 5. [DOI] [PubMed] [Google Scholar]
- [81].Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, et al. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- [82].DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111:E2384–2393. doi: 10.1073/pnas.1400057111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 2014;28:2597–2612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Espin-Palazon R, Stachura DL, Campbell CA, Garcia-Moreno D, Del Cid N, Kim AD, et al. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159:1070–1085. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sawamiphak S, Kontarakis Z, Stainier DY. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev Cell. 2014;31:640–653. doi: 10.1016/j.devcel.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Frame JM, McGrath KE, Palis J. Erythro-myeloid progenitors: "definitive" hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood Cells Mol Dis. 2013;51:220–225. doi: 10.1016/j.bcmd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hoeffel G, Ginhoux F. Ontogeny of Tissue-Resident Macrophages. Front Immunol. 2015;6:486. doi: 10.3389/fimmu.2015.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- [89].Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Weinberger T, Schulz C. Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front Physiol. 2015;6:107. doi: 10.3389/fphys.2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]