Abstract
Twenty-four quinoxaline derivatives were evaluated for their antimycobacterial activity using BacTiter-Glo microbial cell viability assay. Five compounds showed MIC values < 3.1 μM and IC50 values < 1.5 μM in primary screening and therefore, they were moved on for further evaluation. Compounds 21 and 18 stand out, showing MIC values of 1.6 μM and IC50 values of 0.5 and 1.0 μM respectively. Both compounds were the most potent against three evaluated drug-resistant strains. Moreover, they exhibited intracellular activity in infected macrophages, considering log-reduction and cellular viability. In addition, compounds 16 and 21 were potent against non-replicating M. Tb. and compound 21 was bactericidal. Therefore, quinoxaline derivatives could be considered for making further advances in the future development of antimycobacterial agents.
Keywords: tuberculosis, antimycobacterial, quinoxaline, intracellular activity
Graphical Abstract
Tuberculosis (TB) is a chronic infection caused by Mycobacterium tuberculosis (M. Tb.), which is the second cause of death from a single infectious agent. Due to its high infectivity it is estimated that a third of the world population is a reservoir of Mycobacterium.
In 2000, within the Millennium Development Goals (MDG6), the United Nations proposed to halt the spread and reverse the incidence of TB by 2015. Some of these objectives have already been achieved. The TB mortality rate has decreased 45% since 1990 and more people have access to effective treatments and to early and sensitive diagnostic methods. However, epidemiological data are still alarming1. According to World Health Organization (WHO) data, 9 million people were infected by tuberculosis bacillus in 2013, including 1.1 million cases among people living with HIV. In 2013, 1.5 million people died due to tuberculosis infection, with this being the main cause of death among people affected by HIV. In addition, the number of infected people with multidrug-resistant TB (MDR-TB) is increasing annually. The ability of M. Tb. to develop resistance to drugs and the difficulties faced in the treatment of the TB-disease has facilitated the development of resistant strains. MDR-TB has been spreading rapidly in the last decades, especially in recent years; the number of individuals infected with MDR-TB tripled between 2009 and 2013, and reached 136,000 worldwide2.
These data indicate the urgent need to continue working in the development of new compounds against new targets and with new mechanisms of action for treating MDR-TB. It is also important to shorten the long periods of treatment because they are a common reason for treatment discontinuation on the part of the patient, and stopping the treatment too early is related with the appearance of resistant strains3. With this aim, our group has been working for several years on the synthesis and biological evaluation of new structures derived from quinoxalines. Quinoxaline derivatives show very interesting biological properties. Several studies have been published which justify the interest in these derivatives as antibacterial agents. As a result of our anti-tuberculosis research project, several papers have been published in which both synthesis and biological activity assessments have been described for a large number of quinoxaline and quinoxaline 1,4-di-N-oxide derivatives with a variety of substituents. Some of them have shown growth inhibition values of 99% and 100%4-9. This indicates that these types of structures are very interesting for developing new anti-TB molecules. Furthermore, it has been reported that oxidation of the nitrogen in the quinoxaline ring provides a significant increase in the antibacterial biological activity.10 It has also been reported that the quinoxaline 1,4-di-N-oxide derivatives suffer a bioreduction process under hypoxic conditions11. This behavior could be interesting because in the caseous core of the tuberculous granulomas there is a low concentration of oxygen where non-replicating persistence (NRP) forms of M. Tb. bacilli can survive12. These forms are thought to be the reason behind the need for long treatments and the development of tolerance to the treatment13.
Chalcones are precursors of flavonoids and isoflavonoids, which consist in two aromatic rings linked by a 3 carbons chain presenting an α,β-unsaturated ketone system. Chalcones exhibit a wide range of biological activities such as anticancer, anti-leishmaniasis, anti-inflammatory, anti-oxidant and anti-tuberculosis. With regard to the anti-mycobacterial activity of chalcones, there are numerous publications that justify their use in the search for new anti-TB compounds14, 15.
Fluoroquinolones are an important group of broad spectrum antibiotics and they are present in many molecules with high antituberculosis activity such as gatifloxacin and moxifloxacin, which are under development in clinical trials16.
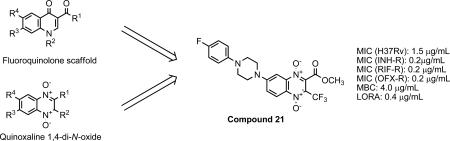
With the aim of developing new antitubercular drug candidates, we have synthesized and evaluated 24 quinoxaline derivatives. The design was based on the molecular hybridization of the quinoxaline 1,4-di-N-oxide with chalcone and fluoroquinolones scaffolds17.
We report the design and synthesis of the quinoxaline 1,4-di-N-oxide derivatives 1-24. The biological evaluation of the compounds included a M. Tb. H37Rv dose-response assay (primary screening) in which the IC50, IC90 and MIC against M. Tb. were determined. The most active compounds were moved on to a more advanced testing stage for antimycobacterial activity. These assays included the evaluation against single drug-resistant (SDR) strains of M. Tb., Minimum bactericidal concentration (MBC), Low Oxygen Recovery Assay (LORA) and intracellular drug activity. All results are reported and structure-activity relationships (SARs) are discussed.
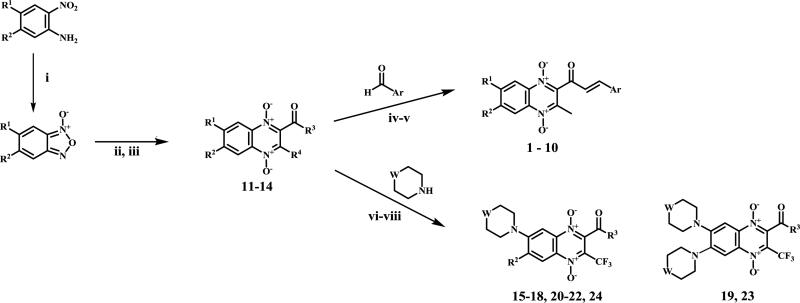
The quinoxaline 1,4-di-N-oxide derivatives were synthesized through the synthetic process illustrated in Scheme 1.
Scheme 1.
General synthesis of quinoxaline 1,4-di-N-oxide derivatives.
Reagents and conditions: (i) N,N-DMF, NaOCl, low temperature; (ii) ethanolamine, CaCl2; (iii) microwave assisted synthesis; (iv) NaOH, MeOH, ice bath; (v) NaOH, MeOH, freezing bath; (vi) N,N-DMF, reflux; (vii) CH3CN, Et3N, rt.; (viii) CH3CN, DBU, 50°C.
The starting benzofuroxans, were commercially available or were obtained by previously described methods18. The synthesis of quinoxaline 1,4-di-N-oxide intermediates were carried out by a variation of the Beirut reaction following the procedure described in the literature19-21.
The 10 novel chalcone analogs (1-10) were prepared by Claisen-Schmidt condensation22. Benzaldehyde, 5-nitro-2-furaldehyde and 5-nitro-2-thiophenecarboxylaldehyde were used as the starting aldehyde. The corresponding quinoxaline 1,4-di-N-oxide previously synthesized was used as the ketone. The new derivatives 1-10 were unsubstituted or substituted in R1 or R2 positions by methyl moiety as electron-releasing groups and by chlorine moiety as electron-withdrawing groups.
The methodologies for the synthesis of compounds 11-15, 17-20 and 22-24 were previously described by our group19. Compound 16 and 21 were obtained by nucleophilic aromatic substitution of chlorine or fluorine linked to R1/R2 substituent on the quinoxaline ring. Morpholine and 1-(4-fluorophenyl)piperazine were used as the nucleophilic amine. Compound 16 was obtained by reflux using N,N-DMF and compound 21 was obtained using DBU as base.
The identification of novel interesting compounds begins with a primary screening. This initial dose response assay evaluates the ability of compounds to inhibit the M. Tb. replication. The IC90, IC50 and MIC of the 24 quinoxaline 1,4-di-N-oxide derivatives were determined against M. Tb. H37Rv (ATCC 27294) using BacTiter-Glo (BTG) microbial cell viability assay.
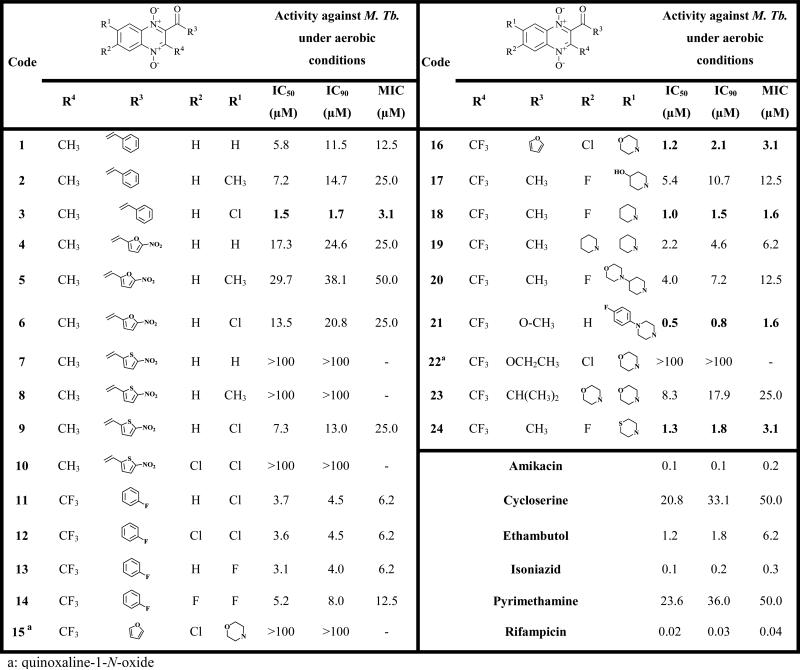
The structure and anti-tuberculosis activity data of the 24 compounds are shown in Table 1. Nine out of twenty-four evaluated compounds exhibited MIC values ≤ 6.2 μM and IC50 values < 3.7 μM. Compounds 3, 16, 18, 21 and 24 were the most active, showing MIC values among 1.6-3.1 μM and IC50 values among 0.5-1.5 μM. These MIC and IC50 values were lower than those of the second-line reference drugs cycloserine and pyrimethamine, and also by the first-line drug ethambutol. It is worth noting that 4 out of the 5 most active compounds (16, 18, 21 and 24) are fluoroquinolone analogs while compound 3 belongs to chalcone analogs.
Table 1.
Biological results against M. Tb. H37Rv dose-response assay
In comparison with fluoroquinolones, the presence of an electron-withdrawing substituent (fluorine or chlorine) in only one of the R1 and R2 positions in the quinoxaline ring is important for biological activity. This behavior is observed comparing the biological results obtained for compound 3 vs 1 and 2, 6 vs 5, 9 vs 7, 8 and 10, 13 vs 14, 18 vs 19 and 24 vs 23.
With respect to R4 substitutions, 8 out of 9 most active compounds present a trifluoromethyl group. The substitution of hydrogen by its bioisostere fluorine is a widely used strategy in the search for new compounds. The presence of fluorine atoms can change and radically modulate the physicochemical properties of organic compounds modifying its biological behavior 23. However, it is not possible to carry out a SAR study due to the limited structural variability in this position.
With regard to R3 position, it seems to be less important for the anti-mycobacterial activity.
With regard to N-oxide groups of the quinoxaline ring, these groups seem to be essential for the activity as we can see comparing compounds 15 vs 16. This important loss of activity confirmed again that the presence of the both N-oxide groups is a structural requirement for biological activity 24, 25.
According to obtained results, compounds 3, 16, 18, 21 and 24 (MIC < 6.25 μM) were submitted for advanced anti-mycobacterial susceptibility profiling. This advanced screening includes the determination of MIC against SDR strains of M. Tb., MBC, LORA as well as intracellular drug screening study in infected macrophages and a MTT cell proliferation study in uninfected macrophages at different concentrations. Table 2 shows the MIC values of the selected compounds against rifampin (RIF), isoniazid (INH) and ofloxacin (OFX) resistant strains and a retested MIC value against a susceptible strain.
Table 2.
Minimum inhibitory concentration against single-drug resistant strains of M. Tb.
| Code | Structure | H37Rv | INH-R M. Tb. | RIF-R M. Tb. | OFX-R M. Tb. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRI 1345 strain | SRI 1369 strain | SRI 1367 strain | SRI 4000 strain | |||||||||
| MIC μg/mL | % Inh(a) | MIC μg/mL | % Inh. | R(b) | MIC μg/mL | % Inh. | R(b) | MIC μg/mL | % Inh. | R(b) | ||
| 3 |
|
2 | 80 | 1 | 64 | 2 | 0.5 | 51 | 4 | 0.5 | 100 | 4 |
| 16 |
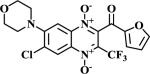
|
2 | 61 | 0.5 | 78 | 4 | 0.5 | 66 | 4 | 0.25 | 76 | 8 |
| 18 |
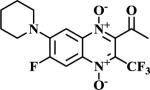
|
1 | 78 | 0.25 | 57 | 8 | 0.25 | 68 | 8 | 0.25 | 84 | 8 |
| 21 |
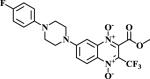
|
1.5 | 78 | 0.19 | 51 | 8 | 0.19 | 73 | 8 | 0.19 | 81 | 8 |
| 24 |
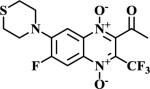
|
0.75 | 51 | 0.75 | 89 | 1 | 0.38 | 75 | 2 | 0.19 | 62 | 4 |
| RIF | 0.05 | 55 | 0.05 | 72 | 0.195 | 53 | ||||||
| INH | 0.02 | 55 | ||||||||||
Percentage inhibition at the MIC concentration.
R: Ratio of MIC H37Rv/MIC Resistant-strain
INR-R: isoniazid-resistant
RIF-R: rifampin-resistant
OFX-R: ofioxacin-resistant
In general, the OFX-resistant M. Tb. strain was the most sensitive to the 5 tested compounds. The susceptibilities of INH, RIF and OFX-resistant strains can be considered greater than those of H37Rv, as indicated by the ratios of MICs against non-resistant and resistant strains which are between 2 and 8. Only compound 24 showed low resistance with INH-resistant strain with a ratio = 1.
These results suggest that all compounds were active against RIF, INH and OFX-resistant M. Tb. strains. Therefore, there is no cross-resistance with the antitubercular drugs used in the assay, supporting the idea that our compounds act through a different mechanism of action. These results are promising for the development of new effective compounds against the growing number of drug-resistant strains.
A compound is considered as bactericidal if the ratio MBC/MIC is ≤ 426. This is important because bactericidal drugs can potentially shorten the standard treatment regimen, which is a minimum of 6 months for tuberculosis. Therefore and according to the results showed in Table 3, compound 21 could be considered as bactericidal due to the low ratio obtained. If a compound lacks bactericidal activity, many times the colony-forming units (CFUs) are too numerous to count (TNTC) as observed with compound 16 27.
Table 3.
Minimum bactericidal concentration and Low Oxygen Recovery Assay results.
| Code | MIC H37RV | MBC | LORA | |||
|---|---|---|---|---|---|---|
| μg/mL | % Inha | μg/mL | MBC/MICH37RV | μg/mL | MIC H37RV/LORA | |
| 3 | 2 | 80 | NA | - | UR | - |
| 16 | 2 | 61 | TNTC | - | 0.25 | 8 |
| 18 | 1 | 78 | NA | - | >8 | Nd |
| 21 | 1.5 | 78 | 4 | 2.6 | 0.375 | 4 |
| 24 | 0.75 | 51 | NA | - | >12 | Nd |
| RIF | 0.05 | 55 | 0.78 | 15.6 | 0.39 | - |
Percentage inhibition at the MIC concentration.
UR: Unreportable: Compound precipitated out of solution. NA: Not applicable: colony counts above the established rejection value ≥ 40. TNTC: Too numerous to count. Nd: Not determinated.
It is well known that quinoxaline 1,4-di-N-oxide possess a selective toxicity against hypoxic tumoral cells through a bioreductive activation process28. In the case of tuberculosis, the granulomatous lesions formed in the infected lungs present a hypoxic environment where TB bacilli are in a latent state of low metabolic activity. These nonreplicating persistence (NRP) M. Tb. bacilli is thought to be a main factor responsible for the long treatment periods and the emergence of antimicrobial tolerance29. Therefore, special attention should be paid to the NRP state of M. Tb. from the early stages of the drug discovery process. Therefore, the LORA assay, conducted under low oxygen conditions, has been included in the biological evaluation with the aim of identifying compounds able to acting on the non-replicative bacilli. As shown in Table 3, compounds 16 and 21 were more potent against NPR M. Tb. being 8-fold and 4-fold more active under anaerobic conditions than aerobic conditions respectively. These results offer an important approach for studying this family of compounds in these conditions due to the important role that NRP M. Tb. bacilli play in influencing the duration of treatment.
M. Tb. can evade the immune response of macrophages and has the ability to reside inside them in a latent state. This evasion mechanism makes the effectiveness of treatment difficult and increases the microbial resistance30, 31.
With the aim of assessing the intracellular effectiveness of the 5 selected compounds in this infection phase, the activity in J774 infected macrophages was evaluated. Compounds 3, 16, 18, 21 and 24 were tested at three 10-fold concentrations based on their MIC values against M. Tb. H37Rv.
All of the evaluated compounds showed high log-reduction in the burden of the intracellular bacilli (Table 4). However, it was observed that none of the evaluated compounds showed dose response log-reduction in contrast to RIF.
Table 4.
Intracellular activity in M. tb infected J774 macrophages.
| Code | Log reductiona | % of cellular viability | ||||
|---|---|---|---|---|---|---|
| Low concb | Mid concb | High concb | Low concb | Mid concb | High concb | |
| 3 | 2.11 | 1.68 | 1.76 | 70 | 81 | 26 |
| 16 | 1.68 | 1.57 | 2.67 | 88 | 37 | 37 |
| 18 | 1.74 | 1.5 | 2.66 | 83 | 53 | 65 |
| 21 | 1.43 | 1.41 | 3.86 | 60 | 64 | 47 |
| 24 | 2.17 | 1.66 | 1.76 | 70 | 81 | 26 |
| RIF | 0.48 | 1.73 | 2.68 | 93 | 87 | 80 |
Intracellular drug activity is reported as log reduction values calculated as reduction in Mtb concentration from zero hour to 7 days post-infection.
The three concentrations chosen were based on the MIC data generated in the HTS primary screen. The mid concentration bracketed the reported MIC with the lower concentration ten-fold below the mid and the higher concentration ten-fold above the mid.
At the lowest concentration, compounds 3 and 24 showed values > 2-log-reduction (> 99% inhibition) while compounds 16, 18 and 21 showed values > 1-log-reduction (> 90% inhibition). Therefore, the 5 evaluated compounds exhibited log-reduction among 3 to 4.5-fold more than RIF.
Compounds 16, 18 and 21 showed the highest log-reduction at the highest concentration and presented values of 26%, 65% and 47% of cellular viability respectively at this concentration. Therefore, compounds 18 and 21 stand out as being the most potent derivatives against intracellular bacilli, considering log-reduction and cell viability values.
In conclusion, we describe the synthesis of 12 new compounds and the antimycobacterial evaluation of 24 quinoxaline di-N-oxide derivatives. Five of the tested compounds passed the first cut-off established in the primary screening against H37Rv, thereby justifying the great interest in this family of compounds. The results obtained in the advanced screening (MIC against H37Rv and SDR-strains, MBC, LORA, intracellular activity and MTT cell proliferation) confirm the promising pharmacological profile of these derivatives.
Compounds 3, 16, 18, 21 and 24 were active against INH, RIF and OXF-resistant strains suggesting that they could act through a different mechanism of action that the reference drugs. Moreover, the MIC and LORA assay show that compound 21 is potent not only against replicating M. Tb but also against NRP M. Tb. In addition, compound 21 could be classified as bactericidal according to the MBC and has also stand out in the intracellular drug activity assay. The mechanism of action of these compounds has not yet been studied.
Supplementary Material
Acknowledgements
“This project has been funded in whole or in part with Federal funds from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201100009I”.
M.S. is indebted to Government of Navarra for grant “International Talent” and with University of Navarra for a grant. E.T. is indebted to the University of Navarra for a grant.
Abbreviations
- TB
tuberculosis
- M. Tb.
Mycobacterium tuberculosis
- BTG
BacTiter-Glo microbial cell viability assay
- MDG
Millennium Development Goals
- BFX
benzofuroxan
- NRP
non-replicating
- MBC
Minimal bactericidal concentration
- LORA
Low Oxygen Recovery Assay
- SDR
single drug-resistant
- FBS
bovine serum
- NA
Not available
- RIF
Rifampicin
- INH
Isoniazid
- OFX
Ofloxacin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare no conflict of interest.
Supplementary data:
References
- 1.The Millennium Development Goals Report. United Nations: 2015. p. 72. http://www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20(July%201).pdf, 2015. [Google Scholar]
- 2.WHO. World Health Organization. 2015 Jul 12; http://www.who.int/mediacentre/factsheets/fs104/en/
- 3.Mitra PP. The Indian journal of tuberculosis. 2012;59:194. [PubMed] [Google Scholar]
- 4.Vicente E, Villar R, Burguete A, Solano B, Perez-Silanes S, Aldana I, Maddry JA, Lenaerts AJ, Franzblau SG, Cho SH, Monge A, Goldman RC. Antimicrob Agents Chemother. 2008;52:3321. doi: 10.1128/AAC.00379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villar R, Vicente E, Solano B, Perez-Silanes S, Aldana I, Maddry JA, Lenaerts AJ, Franzblau SG, Cho SH, Monge A, Goldman RC. J antimicrob chemoter. 2008;62:547. doi: 10.1093/jac/dkn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicente E, Perez-Silanes S, Lima LM, Ancizu S, Burguete A, Solano B, Villar R, Aldana I, Monge A. Bioorganic & medicinal chemistry. 2009;17:385. doi: 10.1016/j.bmc.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 7.Ancizu S, Moreno E, Solano B, Villar R, Burguete A, Torres E, Perez- Silanes S, Aldana I, Monge A. Bioorganic & medicinal chemistry. 2010;18:2713. doi: 10.1016/j.bmc.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Moreno E, Ancizu S, Perez-Silanes S, Torres E, Aldana I, Monge A. Eur J Med Chem. 2010;45:4418. doi: 10.1016/j.ejmech.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Torres E, Moreno E, Ancizu S, Barea C, Galiano S, Aldana I, Monge A, Perez-Silanes S. Bioorganic & medicinal chemistry letters. 2011;21:3699. doi: 10.1016/j.bmcl.2011.04.072. [DOI] [PubMed] [Google Scholar]
- 10.Crawford PW, Scamehorn RG, Hollstein U, Ryan MD, Kovacic P. Chemico-biological interactions. 1986;60:67. doi: 10.1016/0009-2797(86)90018-9. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury G, Kotandeniya D, Daniels JS, Barnes CL, Gates KS. Chem. Res. Toxicol. 2004;17:1399. doi: 10.1021/tx049836w. [DOI] [PubMed] [Google Scholar]
- 12.Moreno E, Pérez-Silanes S, Gouravaram S, Macharam A, Ancizu S, Torres E, Aldana I, Monge A, Crawford PW. Electrochimica Acta. 2011;56:3270. [Google Scholar]
- 13.Thompson AM, Blaser A, Anderson RF, Shinde SS, Franzblau SG, Ma Z, Denny WA, Palmer BD. J. Med. Chem. 2009;52:637. doi: 10.1021/jm801087e. [DOI] [PubMed] [Google Scholar]
- 14.Chiaradia LD, Mascarello A, Purificacao M, Vernal J, Sechini Cordeiro MN, Zenteno ME, Villarino A, Nunes RJ, Yunes RA, Terenzi H. Bioorg. Med. Chem. Lett. 2008;18:6227. doi: 10.1016/j.bmcl.2008.09.105. [DOI] [PubMed] [Google Scholar]
- 15.Bia Ventura TL, Calixto SD, Abrahim-Vieira B. d. A., Teles de Souza AM, Palmeira Mello MV, Rodrigues CR, de Mariz e Miranda LS, Mendonca Alves de Souza RO, Ramos Leal IC, Lasunskaia EB, Muzitano MF. Molecules. 2015;20:8072. doi: 10.3390/molecules20058072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stop TB Partnership-Working Group on new TB drugs-Drug Pipeline. 2015 Aug 3; http://www.newtbdrugs.org/pipeline.php.
- 17.Viegas-Junior C, Danuello A, Bolzani V. d. S., Barreir EJ, Manssour Fraga CA. Curr. Med. Chem. 2007;14:1829. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]
- 18.Gaughran RJ, Picard JP, Kaufman JVR. J Am Chem Soc. 1954;76:2233. [Google Scholar]
- 19.Pérez-Silanes S, Torres E, Arbillaga L, Varela J, Cerecetto H, González M, Azqueta A, Moreno-Viguri E. Bioorg. Med. Chem. Lett. 2016;26:903. doi: 10.1016/j.bmcl.2015.12.070. [DOI] [PubMed] [Google Scholar]
- 20.Burguete A, Pontiki E, Hadjipavlou-Litina D, Ancizu S, Villar R, Solano B, Moreno E, Torres E, Perez S, Aldana I, Monge A. Chem Biol Drug Des. 2011;77:255. doi: 10.1111/j.1747-0285.2011.01076.x. [DOI] [PubMed] [Google Scholar]
- 21.Jaso A, Zarranz B, Aldana I, Monge A. Eur J Med Chem. 2003;38:791. doi: 10.1016/s0223-5234(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 22.Burguete A, Pontiki E, Hadjipavlou-Litina D, Villar R, Vicente E, Solano B, Ancizu S, Perez-Silanes S, Aldana I, Monge A. Bioorganic & medicinal chemistry letters. 2007;17:6439. doi: 10.1016/j.bmcl.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Hagmann WK. J. Med. Chem. 2008;51:4359. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 24.Sainz Y, Montoya ME, Martinez-Crespo FJ, Ortega MA, Lopez de Cerain A, Monge A. Arzneimittel-Forschung. 1999;49:55. doi: 10.1055/s-0031-1300359. [DOI] [PubMed] [Google Scholar]
- 25.Ortega MA, Morancho MJ, Martinez-Crespo FJ, Sainz Y, Montoya ME, Lopez de Cerain A, Monge A. Eur J Med Chem. 2000;35:21. doi: 10.1016/s0223-5234(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 26.White EL, Suling WJ, Ross LJ, Seitz LE, Reynolds RCJ. Antimicrob. Chemother. 2002;50:111. doi: 10.1093/jac/dkf075. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulou MV, Bloomer WD, Rosenzweig HS, Arena A, Arrieta F, Rebolledo JCJ, Smith DK. Antimicrob Agents Chemother. 2014;58:6828. doi: 10.1128/AAC.03644-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monge A, Palop JA, Decerain AL, Senador V, Martinezcrespo FJ, Sainz Y, Narro S, Garcia E, Demiguel C, Gonzalez M, Hamilton E, Barker AJ, Clarke ED, Greenhow DT. J. Med. Chem. 1995;38:1786. doi: 10.1021/jm00010a023. [DOI] [PubMed] [Google Scholar]
- 29.Cho SH, Warit S, Wan B, Hwang CH, Pauli GF, Franzblau SG. Antimicrob Agents Chemother. 2007;51:1380. doi: 10.1128/AAC.00055-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang WS, Kim S, Podder B, Jyoti MA, Nam K-W, Lee B-E, Song H-Y. J. Microbiol. Biotechn. 2015;25:946. doi: 10.4014/jmb.1412.12023. [DOI] [PubMed] [Google Scholar]
- 31.Jayachandran R, Scherr N, Pieters J. Expert Review of Anti-Infective Therapy. 2012;10:1007. doi: 10.1586/eri.12.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.