Abstract
Background
Heart rate variability (HRV) is a validated method to establish autonomic nervous system (ANS) activity. Rheumatoid arthritis (RA) is accompanied by ANS imbalance. We hypothesized that ANS dysfunction may precede the development of RA, which would suggest that it plays a role in its etiopathogenesis.
Methods
First, we assessed HRV parameters in supine (resting) and upright (active) position in healthy subjects (HS, n = 20), individuals at risk of developing arthritis (AR subjects, n = 50) and RA patients (RA, n = 20). Next, we measured resting heart rate (RHR), a parasympathetic HRV parameter, in an independent prospective cohort of AR subjects (n = 45). We also evaluated expression levels of the parasympathetic nicotinic acetylcholine receptor type 7 (α7nAChR) on circulating monocytes.
Findings
Both AR subjects (68 beats per minute (bpm), interquartile range (IQR) 68–73) and RA patients (68 bpm, IQR 62–76) had a significantly higher RHR compared to HS (60 bpm, IQR 56–63). RHR was significantly higher at baseline in individuals who subsequently developed arthritis. Expression levels of α7nAChR were lower in AR subjects with RHR ≥ 70 bpm compared to those with RHR < 70 bpm, consistent with reduced activity of the parasympathetic cholinergic anti-inflammatory pathway.
Interpretation
These data support the notion that autonomic dysfunction precedes the development of RA.
Keywords: Heart rate variability, RA, Autonomic nervous system, Norepinephrine, Heart rate, Preclinical
Highlights
-
•
Individuals at risk of developing RA show autonomic dysfunction similar to established RA patients.
-
•
Autonomic dysfunction is a predictor of development of arthritis in subjects at risk of RA, suggesting a role in its etiopathogenesis.
The autonomous nervous system is a neurological control system that acts largely unconsciously and regulates a variety of bodily functions. We found that dysfunction of this system may precede and predict the development of rheumatoid arthritis (RA), a chronic inflammatory disease with great unmet need. These findings provide important insights into the changes in the nervous system contributing to the development of this condition. They also open up the perspective of potential measures aimed at prevention of RA by restoring the balance in the nervous system before arthritis develops, which would have major implications for patients as well as society.
1. Introduction
Autonomic imbalance, defined by increased heart rate (HR) and decreased heart rate variability (HRV), is associated with increased morbidity and mortality in patients with various diseases (Inoue et al., 2013). Autonomic imbalance has been demonstrated in autoimmune diseases like rheumatoid arthritis (RA), systemic lupus erythematosus, ankylosing spondylitis and inflammatory bowel disease (Aydemir et al., 2010, Borman et al., 2008, Sharma et al., 2009), where it has traditionally been considered a result of chronic inflammation. In RA, a prototypic immune-mediated inflammatory disease, several studies demonstrated reduced activity of the parasympathetic nervous system (PNS), whereas others found an overactive sympathetic nervous system in RA patients (Adlan et al., 2014, Koopman et al., 2011). HRV measurements were also shown to predict response to biological treatment in RA patients (Holman and Ng, 2008). These observations are relevant because activation of the PNS via stimulation of the vagus nerve and the nicotinic acetylcholine receptor type 7 (α7nAChR) can reduce inflammation, which has been termed as the cholinergic anti-inflammatory pathway (Borovikova et al., 2000, Van Maanen et al., 2009). In addition to measurement of HRV, assessment of sympathetic nervous system (SNS)-related hormones, such as epinephrine and norepinephrine, is informative about autonomic dysfunction (Igari et al., 1977, Vlcek et al., 2008).
We postulated that autonomic dysfunction may precede the development of RA, which would suggest that it plays a role in the etiopathogenesis of this disease. Previous studies in healthy individuals have shown that decreased HRV parameters are associated with development of disease, such as hypertension and diabetes, as an independent risk factor (Thayer and Lane, 2007). Individuals at risk of developing autoantibody positive RA can be identified by detection of the autoantibodies IgM-rheumatoid factor (IgM-RF) and anti-citrullinated protein antibodies (ACPA); the risk of developing arthritis is further increased by smoking, overweight and genetic factors (Klareskog et al., 2009, Gerlag et al., 2015). Not all identified individuals will develop RA, but the presence of multiple serum autoantibodies increases the risk of developing RA in two years up to ~ 40% (Bos et al., 2010). Identification of additional risk factors is necessary to provide insight into pathogenic mechanisms contributing to the development of clinically manifest disease, and might also lead to development of preventive strategies.
To evaluate whether autonomic dysfunction might contribute to the development of RA rather than being the result of chronic inflammation, we measured HRV as well as plasma levels of (nor)epinephrine, and the expression of α7nAChR on peripheral blood monocytes in individuals at risk of developing RA, healthy subjects and RA patients. In a validation cohort, we examined the relationship between heart rate at baseline and development of RA after follow-up.
2. Materials & Methods
2.1. Study Subjects
RA patients (n = 20), healthy subjects (HS, n = 20) and individuals at risk of developing RA (AR subjects, n = 50) were included in a prospective observational study (study cohort). A sample size calculation was performed, whereby 18 subjects in the RA and HS groups would have 80% power to detect a difference in mean high frequency (HF) of 14.6 assuming a standard deviation (SD) of 15 (Evrengul et al., 2004) using a two group t-test with 0.05 two-sided significance level. We expected a smaller difference between HS and AR subjects, and RA and AR subjects, and have therefore included more AR subjects. AR subjects were defined as being positive for IgM-RF, ACPA or both, having either arthralgia, or a positive family history for RA (phase (c) + (d) or (c) + (a), but without any evidence of arthritis upon standardized physical examination according to European League Against Rheumatism (EULAR) recommendations (Gerlag et al., 2012, Scott et al., 1996). RA patients were classified according to the 2010 American College of Rheumatology and EULAR classification criteria (Aletaha et al., 2010) and had arthritis in at least one joint, despite a stable dose of methotrexate and in some patients prednisone (n = 4, median dose 6.25 mg per day, interquartile range (IQR) 5–9.4 mg per day) for at least one month. HS were negative for IgM-RF and ACPA. Individuals with a history of cardiovascular or neurological disease, diabetes, anti-hypertensive treatment, active infection or use of antibiotics in the 7 days preceding study assessments were excluded. The study was performed according to the principles of the Declaration of Helsinki, approved by the institutional review board of the Academic Medical Center (AMC), the Netherlands (reference NL34802.018.10), and all study subjects gave written informed consent.
A second, independent at risk (AR) cohort (validation cohort) was used to validate the results. This cohort was started at the Department of Clinical Immunology and Rheumatology AMC in 2005 and has been described before (de Hair et al., 2013, de Hair et al., 2014). Similar in- and exclusion criteria were used as described above.
2.2. Study Design
In the study cohort, during one single visit demographics, anthropometric data, smoking and alcohol status, medical history, RA medication history and current medication were obtained between 8:30 and 10 am, after patients had fasted for 8 to 10 h. Continuous heart rate (HR) and blood pressure (BP) measurements were obtained non-invasively using finger arterial pressure waveform recording of the left hand by Nexfin (Edwards Lifesciences, BMEYE B.V. Amsterdam, the Netherlands), a validated method to assess HRV parameters (Rang et al., 2004, Prevoo et al., 1995). The recording session contained two parts: ten-minute period in supine (resting) position, and a ten-minute period of orthostatic stress in upright (active) position. Individuals were allowed to equilibrate to the positional change before data were collected, followed by assessment of RA disease activity (Disease Activity Score of 28 joints (DAS28) with erythrocyte sedimentation rate (ESR) (Prevoo et al., 1995)) and a blood draw.
In the validation cohort we determined vital signs at yearly visits, including resting heart rate (RHR) in sitting position, using an automated monitor system. This measurement took place in non-fasting state either in the morning or the afternoon.
2.3. Analysis of Heart Rate Variability Parameters
HRV recordings were analyzed as described before (Rang et al., 2004) according to Task Force Guidelines (Anon., 1996). A period of ≥ 250 consecutive heart beats without interfering signals was selected for both positions. HRV parameter analysis is categorized into time and frequency domain (TD, FD) analysis. TD parameters are: HR, BP, respiratory rate (RR), standard deviation of all beat-to-beat intervals (SDNN) and root mean square of successive differences (RMSSD). RHR and SDNN are mainly influenced by the PNS, while in upright position the SNS becomes more active. RMSSD reflects PNS activity in both positions. FD parameters of the pulse interval were determined as described before (Zhang et al., 2013) by in-house developed digital Fourier transform (DFT, Matlab, courtesy W.J. Stok, MSc). The FD parameter for PNS is HF (0.15–0.40 Hz) and of both SNS and PNS are low frequency (LF: 0.04–0.15 Hz) and LF/HF ratio. LF expressed in normalized units (nu) instead of ms2 shows the influence of the SNS more distinctly, as it is controlled for total power and very low frequency (TP and VLF) (Anon., 1996). Furthermore, gain of the baroreceptor reflex sensitivity (BRS) was calculated from the transfer from systolic BP to heart period in the LF-band, representing ability of the cardiovascular system to counteract beat-to-beat changes in BP. Overall, lowered vagal efferent results in lower HRV parameters, except for HR, RR and LF/HF ratio which increase.
2.4. Measurement of Norepinephrine, Epinephrine and α7nAChR Expression in Peripheral Blood
Norepinephrine and epinephrine in peripheral blood could be measured in 16/20 (80%) HS, 43/50 (86%) AR subjects and 19/20 (95%) RA patients from the study cohort. Fourteen AR subjects gave informed consent for additional peripheral blood samples to measure α7nAChR expression on monocytes. Assays are described in the Methods Appendix.
2.5. Statistics
Data are presented as median with IQR. Demographic data from individuals enrolled in the study and validation cohorts were analyzed with non-parametric tests (Kruskal–Wallis test, Mann–Whitney U test) if not normally distributed or by parametric tests (analysis of variance (ANOVA), Student's t-test) if normally distributed. Categorical demographic variables were tested using Pearson's Chi-Square test. HRV parameters and SNS-related hormones were analyzed using multivariate linear regression analysis with following confounding factors: age, gender, body mass index (BMI), smoking pack years and plasma triglycerides. Confounders were chosen based on the literature (Valentini and Parati, 2009) and differences between the groups in the study cohort. The study cohort was designed to evaluate changes in AR subjects compared to RA patients and HS rather than to compare RA and HS. In the validation cohort Cox's proportional hazard regression analysis was performed to evaluate the association of RHR with arthritis development, taking follow-up time and the following potentially confounding factors into account: age, gender, BMI and smoking pack years. Plasma triglycerides were available in a subset of individuals from the validation cohort and these were not included as confounder. Medication was considered as confounding factor as well. There is no known influence of disease-modifying antirheumatic drugs (DMARDs), or prednisone on HRV parameters. Nonsteroidal anti-inflammatory drugs (NSAIDs) are known to influence the cardiovascular system (Bhala et al., 2013, Antman et al., 2007) and could therefore influence HRV. Adding NSAIDs as a confounder did not alter results of the study in either cohort, and was therefore not included in the multivariate regression analysis. SNS-related hormones are not influenced by DMARD or NSAID use, but prednisone use may lower (nor)epinephrine levels (Harle et al., 2006, Golczynska et al., 1995), and prednisone was included as an additional confounder in the analysis of these parameters. P-values < 0.05 were considered statistically significant.
2.6. Role of the Funding Source
The study was funded by the FP7 HEALTH program under grant agreement FP7-HEALTH-F2-2012-305549 and the Dutch Arthritis Foundation grant 09-1-307. None of the funding sources had any role in design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
3. Results
3.1. Demographics Study Cohort
Demographics of the study cohort are described in Table 1. As expected, AR subjects tended to be younger than RA patients. Significantly more ‘ever smoker's’ and ‘smoking pack years’ were identified in AR subjects and RA patients compared to HS. Plasma triglyceride levels were higher in AR subjects and RA patients than in HS, but there was no difference in other lipid levels. RA patients had active disease, as defined by DAS28 above 3.2 (Prevoo et al., 1995). The mean follow-up time of study cohort was 18.2 months (standard deviation (SD) 7.6 months) during 4/50 individuals (8%) developed arthritis.
Table 1.
Demographics study cohort.
| HS (n = 20) | AR subjects (n = 50) | RA (n = 20) | P-valuea | |
|---|---|---|---|---|
| Age (years) | 49 (39–56) | 47 (40–53) | 56 (45–64) | 0.078 |
| Female: n (%) | 12 (60) | 38 (76) | 13 (65) | 0.36 |
| BMI (kg/m2) | 23.8 (22.7–26.5) | 25.3 (22.1–27.3) | 25.9 (21.9–28.3) | 0.59 |
| Alcohol use: n (%) | 15 (75) | 27 (54) | 10 (50) | 0.20 |
| Current smoker: n (%) | 2 (10) | 14 (28) | 4 (20) | 0.25 |
| Ever smoker: n (%) | 3 (15) | 21 (42) | 10 (52.6) | 0.038 |
| Cigarettes/day | 0 (0–0) | 0 (0–3) | 0 (0–0) | 0.29 |
| Packyears | 0.0 (0.0–0.0) | 0.0 (0.0–11) | 2.5 (0.0–15.0) | 0.054 |
| Cholesterol total (mmol/L) | 5.3 (4.8–6.2) | 5.5 (4.7–6.3) | 5.20 (4.5–5.7) | 0.28 |
| HDL (mmol/L) | 1.8 (1.4–2.0) | 1.6 (1.2–1.9) | 1.5 (1.2–1.7) | 0.31 |
| LDL (mmol/L) | 3.3 (2.6–3.8) | 3.4 (2.7–4.0) | 3.2 (2.7–3.7) | 0.48 |
| Triglycerides (mmol/L) | 0.7 (0.6–1.0) | 0.9 (0.7–1.1) | 1.1 (0.9–1.4) | 0.032 |
| Total cholesterol/HDL ratio | 3.1 (2.7–4.3) | 3.5 (2.8–4.1) | 3.5 (2.9–3.9) | 0.71 |
| Glucose (mmol/L) | 5.0 (4.7–5.4) | 5.1 (4.8–5.4) | 5.1 (4.8–5.7) | 0.84 |
| RF positive: n (%) | 0 (0) | 31 (62) | 15 (75) | < 0.0001 |
| RF titer (kU/L) | 3 (1–12) | 92 (18–174) | 217 (53–379) | < 0.0001 |
| ACPA positive: n (%) | 0 (0) | 33 (66) | 16 (80) | < 0.0001 |
| ACPA titer (kAU/L) | 4 (1–5) | 96 (4–512) | 220 (57–1187) | < 0.0001 |
| Disease duration (months) | NA | NA | 20 (4–91) | |
| VAS GDA (mm) | NA | 26 (13–53) | 44 (25–70) | < 0.0001 |
| TJC28 (n) | 0 (0–0) | 1 (0–2) | 5 (3–8) | < 0.0001 |
| SJC28 (n) | 0 (0–0) | 0 (0–0) | 2 (2–4) | < 0.0001 |
| ESR (mm/h) | 5 (2–11) | 5 (2–10) | 15 (9–27) | < 0.0001 |
| CRP (mg/L) | 1.8 (1.0–2.7) | 1.5 (0.8–3.9) | 5.6 (1.9–10.5) | 0.001 |
| DAS28 | NA | NA | 4.32 (3.67–4.72) | |
| NSAIDs: n (%) | 0 (0) | 13 (27) | 10 (50) | 0.001 |
| Corticosteroids: n (%) | 0 (0) | 0 (0) | 4 (20) | < 0.0001 |
| Methotrexate: n (%) | 0 (0) | 0 (0) | 15 (75) | < 0.0001 |
| HCQ: n (%) | 0 (0) | 0 (0) | 2 (10) | 0.028 |
Abbreviations: AR: at risk, HS: healthy subjects; RA: rheumatoid arthritis; BMI: body mass index; HDL: high-density lipoprotein; LDL: low-density lipoprotein; RF: rheumatoid factor; ACPA: anti-citrullinated protein antibodies; VAS GDA: visual analogue scale (range 0–100 mm) global disease activity; TJC28: tender joint count of 28 joints; SJC28: swollen joint count of 28 joints; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; DAS28: disease activity score of 28 joints; NSAIDs: non-steroidal anti-inflammatory drugs; HCQ: hydroxychloroquine; NA: not applicable.
Analysis with non-parametric tests (Kruskal–Wallis test) if not normally distributed or parametric tests (analysis of variance (ANOVA)) if normally distributed. Categorical demographic variables tested using Pearson's Chi-Square test. Data presented as median (interquartile range) or number (percentage).
3.2. Lower Parasympathetic Activity in AR Subjects
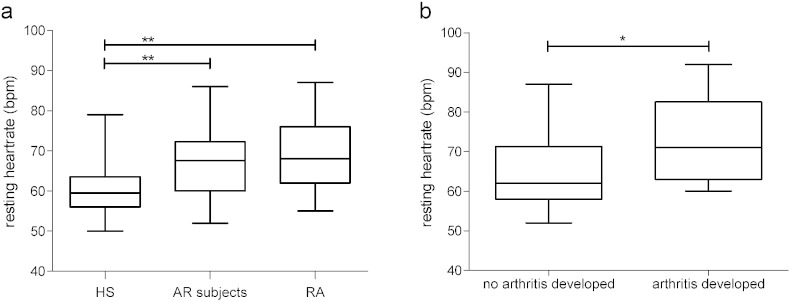
AR subjects (study cohort) had a significantly higher RHR (68 beats per minute (bpm)) compared to HS (60 bpm, p = 0.006), and similar RHR compared to RA patients (68 bpm, p = 0.38, Fig. 1A, Table 2). HR in upright position was also significantly higher in AR subjects than in HS (80 bpm versus 75 bpm, p = 0.045), and similar to RA patients (81 bpm). Elevated RHR is mainly a consequence of lower PNS (vagus tone) activity and elevated HR in upright position is a result of autonomic imbalance with lower PNS and higher SNS activity. Other HRV parameters in AR subjects were numerically lower compared to HS, but still higher compared to RA patients (Table 2), indicating that the autonomic changes in the AR subjects were not as pronounced on group level compared to the RA group. This would be consistent with the fact that not all AR subjects will develop disease. Next we used a cut-off rate for RHR of 70 bpm, as previous work has shown that RHR ≥ 70 bpm is an undesirable prognostic sign for morbidity and mortality in cardiovascular studies (Inoue et al., 2013). RHR was ≥ 70 bpm in 3/20 (15%) of the HS, 20/50 (40%) of AR subjects and 10/20 (50%) of RA patients. When dichotomizing AR subjects at RHR of 70, it became clear that those with a RHR ≥ 70 bpm displayed lower parasympathetic activity with significantly lower SDNN, RMSSD, LF (ms2), HF (ms2) and BRS in both positions. LF (nu), HF (nu), MAP, RR and LF/HF ratio were not altered (Table 3).
Fig. 1.
Resting heart rate in healthy subjects, AR subjects and RA patients (A) and resting heart rate in AR subjects that developed arthritis after follow-up (B).
Abbreviations: AR: at risk, HS: healthy subjects; RA: rheumatoid arthritis; bpm: beats per minute, *p < 0.05, **p < 0.01.
Table 2.
Heart rate variability parameters in supine (Sup, resting) and upright (Upr, active) position in the study cohort, as measured by continuous heart rate and blood pressure recordings comparing AR subjects with HS and RA.
| HS |
AR subjects |
RA |
P-valuea |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sup | Upr | Sup | Upr | Sup | Upr | Sup HS vs AR | Upr HS vs AR | Sup AR vs RA | Upr AR vs RA | |
| Time domain | ||||||||||
| Heart Rate (bpm) | 60 (56–63) | 75 (67–79) | 68 (61–73) | 80 (74–87) | 68 (62–76) | 81 (74–92) | 0.006 | 0.045 | 0.38 | 0.28 |
| MAP (mm Hg) | 85 (79–95) | 95 (89–100) | 88 (79–97) | 96 (89–110) | 88 (79–95) | 101 (87–105) | 0.66 | 0.38 | 0.19 | 0.75 |
| RR (breaths/min) | 14 (12–17) | 13 (12–16) | 14 (12–17) | 14 (13–16) | 15 (11–17) | 16 (14–18) | 0.64 | 0.27 | 0.83 | 0.23 |
| SDNN (ms) | 36 (30–46) | 38 (33–42) | 37 (26–52) | 31 (25–41) | 26 (20–39) | 26 (19–32) | 0.58 | 0.045 | 0.14 | 0.45 |
| RMSSD (ms) | 23 (20–45) | 17 (13–22) | 26 (18–38) | 15 (12–20) | 21 (16–35) | 11 (10–15) | 0.17 | 0.014 | 0.75 | 0.18 |
| Frequency domain | ||||||||||
| LF (ms2) | 355 (212–492) | 385 (161–827) | 267 (128–704) | 246 (124–465) | 154 (113–296) | 113 (71–186) | 0.59 | 0.074 | 0.67 | 0.24 |
| LF (nu) | 31 (22–45) | 44 (26–59) | 30 (19–45) | 40 (31–55) | 39 (27–45) | 37 (27–44) | 0.77 | 0.79 | 0.12 | 0.37 |
| HF (ms2) | 286 (177–987) | 181 (91–355) | 313 (165–628) | 155 (96–263) | 158 (124–301) | 126 (60–166) | 0.29 | 0.12 | 0.39 | 0.45 |
| HF (nu) | 41 (25–53) | 25 (16–36) | 42 (32–52) | 28 (22–37) | 46 (31–55) | 31 (21–36) | 0.26 | 0.46 | 0.43 | 0.85 |
| LF/HF ratio | 0.7 (0.5–1.6) | 1.6 (1.1–3.0) | 0.8 (0.5–1.2) | 1.5 (0.9–2.0) | 0.9 (0.4–1.5) | 1.1(0.7–1.7) | 0.45 | 0.75 | 0.55 | 0.63 |
| BRS (ms/mm Hg) | 8.4 (5.6–12.1) | 4.5 (3.5–7.4) | 6.9 (4.6–10.5) | 4.2 (2.6–5.5) | 5.9 (4.2–7.9) | 3.1(2.5–3.7) | 0.19 | 0.13 | 0.72 | 0.39 |
Abbreviations: AR: at risk subjects, HS: healthy subjects; RA: rheumatoid arthritis; Sup: supine (resting) position: Upr: upright (active) position; bpm: beats per minute; MAP: mean arterial pressure; mm Hg: millimeter of mercury; RR: respiratory rate; SDNN: standard deviation of the NN intervals; ms: milliseconds; RMSSD: the square root of the mean squared differences of successive NN intervals; LF: low frequency; nu: normalized units; HF: high frequency; BRS: baroreceptor reflex sensitivity; ms/mm Hg: milliseconds per millimeter of mercury.
Multivariate linear regression analysis comparing AR subjects with HS and RA patients taking the following confounding factors into account: age, gender, BMI, smoking pack years and plasma triglycerides. Data presented as median with interquartile range.
Table 3.
Resting heart rate (RHR) divided in < and ≥ 70 beats per minute (bpm) in AR subjects (study cohort).
| AR subjects RHR < 70 bpm |
AR subjects RHR ≥ 70 bpm |
P-valuea |
||||
|---|---|---|---|---|---|---|
| Sup | Upr | Sup | Upr | Sup | Upr | |
| Time domain | ||||||
| MAP (mm Hg) | 88 (78–95) | 95 (88–109) | 88 (83–99) | 96 (90–112) | 0.71 | 0.80 |
| RR (breaths/min) | 14.2 (12–16.8) | 14.7 (12.9–17.1) | 13.1 (11.7–18.2) | 13.4 (12.3–16.2) | 0.89 | 0.50 |
| SDNN (ms) | 42.1 (33.1–62.1) | 34.1 (27.6–44.9) | 27.2 (21.6–37.6) | 26.1 (19.3–35.5) | < 0.0001 | < 0.0001 |
| RMSSD (ms) | 29.4 (20.1–53.6) | 16.3 (13.5–23.4) | 19.4 (13.1–31.8) | 12.5 (9.7–18) | < 0.0001 | 0.002 |
| Frequency domain | ||||||
| LF (ms2) | 327 (197–986) | 362 (174–588) | 194 (64–465) | 175 (86–344) | 0.003 | 0.012 |
| LF (nu) | 25 (17–41) | 44 (27–59) | 35 (21–48) | 38 (31–48) | 0.19 | 0.62 |
| HF (ms2) | 491 (247–1210) | 162 (111–316) | 192 (98–407) | 127 (67–231) | < 0.0001 | 0.022 |
| HF (nu) | 41 (29–54) | 26 (22–34) | 43 (33–49) | 32 (24–38) | 0.32 | 0.63 |
| LF/HF ratio | 0.71 (0.47–1.16) | 1.74 (0.93–2.6) | 0.87 (0.47–1.38) | 1.24 (0.95–1.73) | 0.12 | 0.68 |
| BRS (ms/mm Hg) | 9.3 (6.7–15.5) | 5.1 (3.6–6.1) | 4.6 (3.5–6.7) | 2.7 (2.1–4.4) | < 0.0001 | < 0.0001 |
Abbreviations: AR: at risk, RHR: resting heart rate; bpm: Sup: supine (resting) position: Upr: upright (active) position; bpm: beats per minute; MAP: mean arterial pressure; mmHg: millimeter of mercury; RR: respiratory rate; SDNN: standard deviation of the NN intervals; ms: milliseconds; RMSSD: the square root of the mean squared differences of successive NN intervals; LF: low frequency; nu: normalized units; HF: high frequency; BRS: baroreceptor reflex sensitivity.
Multivariate linear regression analysis with age, gender, BMI, smoking pack years and plasma triglycerides as confounders. Data presented as median with interquartile range.
3.3. Higher Risk of Arthritis Development With Increased Heart Rate at Baseline
Having shown higher resting HR in AR subjects in the study cohort, we next evaluated RHR in a similar, independent validation cohort, and related RHR at baseline to development of arthritis. Demographics of the validation cohort are shown in Appendix Table 1; 14/45 (31.1%) individuals developed arthritis after follow-up (31.7 months, SD 25.0 months). RHR at baseline was significantly higher in individuals who developed arthritis after follow-up (n = 14, 73.8 vs 65.8 bpm, p = 0.018, Fig. 1B) compared to those who did not develop arthritis during follow-up (n = 31). In a Cox's regression analysis, taking follow-up time and confounders in consideration, RHR was associated with arthritis development over time (hazard ratio 1.098: 95% CI 1.012–1.191, p = 0.025). Thus, these data are consistent with the notion that altered autonomic function precedes the development of clinically manifest RA.
3.4. Lower α7nAChR Expression in AR Subjects with RHR ≥ 70 bpm
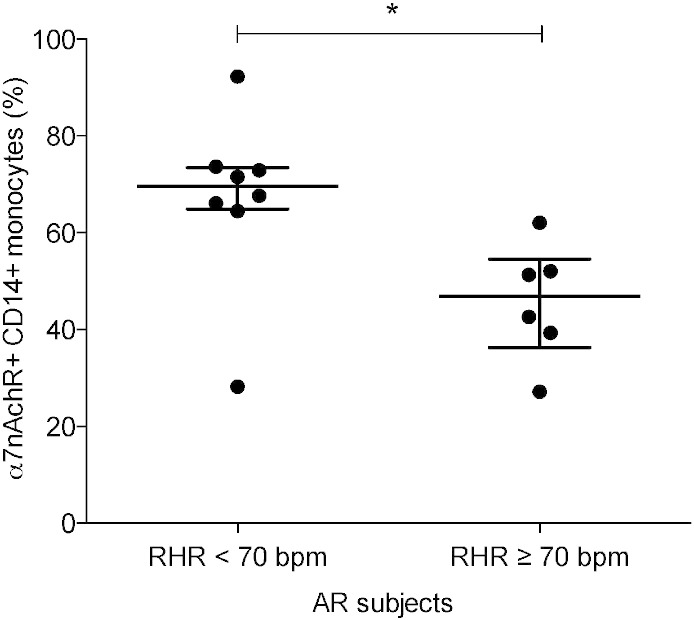
AR subjects with RHR ≥ 70 bpm (n = 6) had significantly lower α7nAChR expression levels on peripheral blood monocytes (p = 0.013) than AR subjects with RHR < 70 bpm (n = 8), consistent with decreased activity of the cholinergic anti-inflammatory reflex. Alpha-7nAChR was expressed on 47% (IQR 36.3–54.6) of monocytes in AR subjects with RHR ≥ 70 bpm, while in AR subjects with RHR < 70 bpm 69.6% (IQR 64.9–73.5) of monocytes were positive for α7nAChR (Fig. 2).
Fig. 2.
Expression levels of the parasympathetic receptor, nicotinic acetylcholine receptor type 7 (α7nAChR) on peripheral blood CD14 + monocytes AR subjects with resting heart rate < and ≥ 70 bpm.
Abbreviations: α7nAChR: nicotinic acetylcholine receptor type 7, AR: at risk, RHR: resting heart rate, bpm: beats per minute, *p < 0.05.
3.5. Hormonal Changes: AR Subjects Have Increased Norepinephrine Levels
Norepinephrine levels were higher in AR subjects compared to HS (p = 0.012), and there was a non-significant trend towards higher levels compared to RA patients (Table 4). There was no difference in epinephrine levels between the groups.
Table 4.
Levels of the sympathetic hormones norepinephrine and epinephrine, measured in venous blood samples of the study cohort.
| HS | AR subjects | RAb | P-valuea HS vs at risk | P-valuea at risk vs RA | |
|---|---|---|---|---|---|
| Norepinephrine (nmol/L) | 2.15 (1.55–2.60) | 3.02 (2.42–4.20) | 2.41 (1.43–2.89) | 0.012 | 0.056 |
| Epinephrine (nmol/L) | 0.14 (0.06–0.17) | 0.16 (0.05–0.24) | 0.17 (0.03–0.35) | 0.43 | 0.33 |
Abbreviations: AR: at risk, HS: healthy subjects; RA: rheumatoid arthritis; nmol/L: nanomoles/liter.
Multivariate linear regression analysis using age, gender, BMI, smoking pack years, plasma triglycerides and prednisone use as confounding factors. Data presented as median with interquartile range.
Levels of sympathetic hormones with and without prednisone use in RA patients are as follows (median with interquartile range): norepinephrine 1.57 (1.18–1.94) vs 2.45 (1.53–4.38) nmol/L; epinephrine 0.03 (0.03–0.10) vs 0.26 (0.10–0.41) nmol/L.
3.6. Lower Parasympathetic Activity in Established RA Patients
RA patients had significantly higher HR in both positions (supine: p = 0.002, upright: p = 0.010) compared to HS, indicating less PNS activity. SDNN, RMSSD, HF and BRS were all lower in RA compared to HS (Table 2 and Appendix Table 2) in upright position. LF (ms2) was significantly lower in RA patients compared to HS and AR subjects in upright position, but evaluating LF (nu) did not show significant changes.
4. Discussion
Results presented here show that lower parasympathetic activity, as revealed by lower HRV and elevated RHR in AR subjects and RA patients compared to healthy subjects. Decreased vagus nerve tone was similar in AR subjects and established RA patients. In a similar, independent validation cohort RHR was significantly higher in individuals who subsequently developed arthritis. AR subjects with RHR ≥ 70 bpm demonstrate lower α7nAChR levels on peripheral monocytes than AR subjects with RHR < 70 bpm. Furthermore, the sympathetic hormone norepinephrine is elevated in AR subjects. Taken together, these prospective observational studies indicate an altered autonomic function preceding the development of clinically manifest disease, which could therefore be part of the pathogenesis of RA development.
In the resting state, HR is mostly under inhibitory control by the PNS, while in active, upright position the SNS becomes more active to the vasculature and their balance regulates appropriate HR increase (Valentini and Parati, 2009). Elevated RHR in AR subjects and RA patients clearly indicates lower parasympathetic activity compared to HS. HRV parameters are dependent on HR; e.g. increased HR results in decreased HRV parameters (Monfredi et al., 2014). Elevated RHR (≥ 70 bpm) is therefore accompanied by significantly lower HRV parasympathetic parameters in both positions. Increased RHR at baseline was associated with subsequent arthritis development, as we could show in an independent cohort of individuals at risk of RA.
Expression levels of the parasympathetic receptor α7nAChR on peripheral blood monocytes could be evaluated in a subset of AR subjects. Expression levels were significantly decreased in individuals with RHR ≥ 70 bpm compared to those with RHR < 70 bpm. Alpha-7nAChR is expressed on many different immune cells (Koopman et al., 2011); it is currently not known how expression levels are regulated. Binding of acetylcholine, produced by the vagus nerve (PNS) and immune cells, to α7nAChR has an immunosuppressive effect (Koopman et al., 2014). Decreased levels of α7nAChR are in line with reduced activity of the cholinergic anti-inflammatory reflex preceding the development of clinically manifest RA.
Levels of the sympathomimetic hormone norepinephrine were significantly increased in AR subjects compared to HS, and there was a trend towards higher levels compared to RA patients. Norepinephrine levels in peripheral blood depend on release, turn over and clearance, and may with care be interpreted as an estimate of overall sympathetic activity (Adlan et al., 2014). Norepinephrine levels in established RA have been reported to be increased (Igari et al., 1977, Vlcek et al., 2008) or similar compared to HS (Palm et al., 1992, Vlcek et al., 2012). The role of increased norepinephrine levels in AR subjects is not clear. Catecholamine depletion before onset of arthritis in animal models of RA has an anti-inflammatory effect, suggesting that catecholamines may promote the immune response before onset of disease (Koopman et al., 2011). In our study norepinephrine levels are relatively low, which could result in activation of the alpha adrenergic receptor types (α-AR), mainly expressed on innate immune cells (Koopman et al., 2011). Activation of α-AR could result in a more pro-inflammatory status, but data are limited (Koopman et al., 2011). Epinephrine levels tend to be lower (Igari et al., 1977, Vlcek et al., 2008) in RA compared to healthy controls or show similar levels (Vlcek et al., 2012, Hirano et al., 2001). In our study we did not find differences in epinephrine levels between the groups.
Several studies have evaluated the autonomic balance in established RA patients and demonstrated autonomic changes using various HRV parameters (Aydemir et al., 2010, Evrengul et al., 2004, Goldstein et al., 2007, Lazzerini et al., 2013). Most of these studies did not adjust for confounding factors of HRV parameters with multivariable analysis, despite known influence of confounders, such as age and sex identified in large cohorts (Valentini and Parati, 2009). In our study PNS activity was attenuated in RA patients compared to HS, as HR was significantly higher in both positions. As a result, other HRV parameters were lowered, but only statistically significant in active position. LF (nu) as a more distinct parameter of the SNS was higher in RA patients at rest, but the difference did not reach statistical significance. Overall, these findings are consistent with decreased parasympathetic activity in RA patients with little indication of an overactive sympathetic system, which is in line with the literature (Adlan et al., 2014).
A limitation of this study is that continuous BP and HR measurements were only available in the study cohort. However, automated BP and HR measurements are also used in other studies and we were able to show excellent correlation between continuous and automated BP and HR measurements in the study cohort (Pearson's test r = 0.78, p < 0.001), indicating that single BP and HR measurements provide reliable data. Finally, we did not investigate the level of physical activity or chronic stress in the study subjects, which could have provided interesting information with regard to the cause of the decreased vagus nerve activity.
Data presented here are consistent with a pathogenic role of the autonomic nervous system in the development of RA. Could these findings help to develop preventive strategies? It is tempting to speculate that decreasing RHR by for instance exercise or meditation (Janse van Rensburg et al., 2012, Nesvold et al., 2012, Routledge et al., 2010) could reduce the risk of transition of preclinical RA to clinically manifest RA. Alternatively, a bioelectronics approach aimed at stimulation of the vagus nerve might be explored as a preventive intervention in subjects at high risk of developing RA (Koopman et al., 2014). Clearly, the possible value of such interventions needs to be demonstrated in future studies.
In conclusion, we have found that autonomic imbalance precedes the development of established RA, which could also be relevant for other immune-mediated inflammatory diseases. This work supports the rationale for future studies on measures aimed at prevention of RA by restoring the balance in the nervous system before arthritis develops.
Conflict of interest statements
PPT and DMG are currently also employees of GlaxoSmithKline (GSK). GSK was not involved in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Authors' contributions
FK contributed to study design, collection and interpretation of data and writing the article. MWT, JV and IYC collected data and reviewed the article. MJH and MJV contributed to the interpretation of data and reviewed the article. JMK contributed to the study design, interpretation of data, writing and reviewing of the article. DMG contributed to the study design and reviewed the article. PPT contributed to the study design, data interpretation, writing and reviewing of the article.
Ethics committee approval
The study was approved by the institutional review board of the Academic Medical Center (AMC), the Netherlands with reference NL34802.018.10.
Acknowledgments
We would like to thank J. Visscher (Amsterdam Rheumatology and Immunology Center | Department of Clinical Immunology & Rheumatology, Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands) for clinical assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.02.029.
Appendix A. Supplementary data
Supplementary material.
References
- Adlan A.M., Lip G.Y., Paton J.F., Kitas G.D., Fisher J.P. Autonomic function and rheumatoid arthritis—a systematic review. Semin. Arthritis Rheum. 2014 doi: 10.1016/j.semarthrit.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Aletaha D., Neogi T., Silman A.J., et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- Anon. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- Antman E.M., Bennett J.S., Daugherty A., Furberg C., Roberts H., Taubert K.A. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- Aydemir M., Yazisiz V., Basarici I., et al. Cardiac autonomic profile in rheumatoid arthritis and systemic lupus erythematosus. Lupus. 2010;19(3):255–261. doi: 10.1177/0961203309351540. [DOI] [PubMed] [Google Scholar]
- Bhala N., Emberson J., Merhi A., et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman P., Gokoglu F., Kocaoglu S., Yorgancioglu Z.R. The autonomic dysfunction in patients with ankylosing spondylitis: a clinical and electrophysiological study. Clin. Rheumatol. 2008;27(10):1267–1273. doi: 10.1007/s10067-008-0906-0. [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Zhang M., et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bos W.H., Wolbink G.J., Boers M., et al. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann. Rheum. Dis. 2010;69(3):490–494. doi: 10.1136/ard.2008.105759. [DOI] [PubMed] [Google Scholar]
- de Hair M.J., Landewe R.B., van de Sande M.G., et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann. Rheum. Dis. 2013;72(10):1654–1658. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hair M.J., van de Sande M.G., Ramwadhdoebe T.H., et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: implications for understanding preclinical rheumatoid arthritis. Arthritis Rheum. 2014;66(3):513–522. doi: 10.1002/art.38273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrengul H., Dursunoglu D., Cobankara V., et al. Heart rate variability in patients with rheumatoid arthritis. Rheumatol. Int. 2004;24(4):198–202. doi: 10.1007/s00296-003-0357-5. [DOI] [PubMed] [Google Scholar]
- Gerlag D.M., Raza K., van Baarsen L.G., et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann. Rheum. Dis. 2012;71(5):638–641. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlag D.M., Norris J.M., Tak P.P. RA: from risk factors and pathogenesis to prevention: towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology. 2015 doi: 10.1093/rheumatology/kev347. Sep 15, pii: kev347 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golczynska A., Lenders J.W., Goldstein D.S. Glucocorticoid-induced sympathoinhibition in humans. Clin. Pharmacol. Ther. 1995;58(1):90–98. doi: 10.1016/0009-9236(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Goldstein R.S., Bruchfeld A., Yang L., et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 2007;13(3–4):210–215. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle P., Straub R.H., Wiest R., et al. Increase of sympathetic outflow measured by neuropeptide Y and decrease of the hypothalamic–pituitary–adrenal axis tone in patients with systemic lupus erythematosus and rheumatoid arthritis: another example of uncoupling of response systems. Ann. Rheum. Dis. 2006;65(1):51–56. doi: 10.1136/ard.2005.038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano D., Nagashima M., Ogawa R., Yoshino S. Serum levels of interleukin 6 and stress related substances indicate mental stress condition in patients with rheumatoid arthritis. J. Rheumatol. 2001;28(3):490–495. [PubMed] [Google Scholar]
- Holman A.J., Ng E. Heart rate variability predicts anti-tumor necrosis factor therapy response for inflammatory arthritis. Auton. Neurosci. 2008;143(1–2):58–67. doi: 10.1016/j.autneu.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Igari T., Takeda M., Obara K., Ono S. Catecholamine metabolism in the patients with rheumatoid arthritis. Tohoku J. Exp. Med. 1977;122(1):9–20. doi: 10.1620/tjem.122.9. [DOI] [PubMed] [Google Scholar]
- Inoue T., Iseki K., Ohya Y. Heart rate as a possible therapeutic guide for the prevention of cardiovascular disease. Hypertens. Res. 2013;36(10):838–844. doi: 10.1038/hr.2013.98. [DOI] [PubMed] [Google Scholar]
- Janse van Rensburg D.C., Ker J.A., Grant C.C., Fletcher L. Effect of exercise on cardiac autonomic function in females with rheumatoid arthritis. Clin. Rheumatol. 2012;31(8):1155–1162. doi: 10.1007/s10067-012-1985-5. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Catrina A.I., Paget S. Rheumatoid arthritis. Lancet. 2009;373(9664):659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- Koopman F.A., Stoof S.P., Straub R.H., Van Maanen M.A., Vervoordeldonk M.J., Tak P.P. Restoring the balance of the autonomic nervous system as an innovative approach to the treatment of rheumatoid arthritis. Mol. Med. 2011;17(9–10):937–948. doi: 10.2119/molmed.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman F.A., Schuurman P.R., Vervoordeldonk M.J., Tak P.P. Vagus nerve stimulation: a new bioelectronics approach to treat rheumatoid arthritis? Best Pract. Res. Clin. Rheumatol. 2014;28(4):625–635. doi: 10.1016/j.berh.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Lazzerini P.E., Acampa M., Capecchi P.L., et al. Association between high sensitivity C-reactive protein, heart rate variability and corrected QT interval in patients with chronic inflammatory arthritis. Eur. J. Intern. Med. 2013;24(4):368–374. doi: 10.1016/j.ejim.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Monfredi O., Lyashkov A.E., Johnsen A.B., et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64(6):1334–1343. doi: 10.1161/HYPERTENSIONAHA.114.03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvold A., Fagerland M.W., Davanger S., et al. Increased heart rate variability during nondirective meditation. Eur. J. Prev. Cardiol. 2012;19(4):773–780. doi: 10.1177/1741826711414625. [DOI] [PubMed] [Google Scholar]
- Palm S., Hinrichsen H., Barth J., et al. Modulation of lymphocyte subsets due to psychological stress in patients with rheumatoid arthritis. Eur. J. Clin. Investig. 1992;22(Suppl. 1):26–29. [PubMed] [Google Scholar]
- Prevoo M.L., van 't Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- Rang S., Wolf H., van Montfrans G.A., Karemaker J.M. Serial assessment of cardiovascular control shows early signs of developing pre-eclampsia. J. Hypertens. 2004;22(2):369–376. doi: 10.1097/00004872-200402000-00022. [DOI] [PubMed] [Google Scholar]
- Routledge F.S., Campbell T.S., McFetridge-Durdle J.A., Bacon S.L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010;26(6):303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D.L., Choy E.H., Greeves A., et al. Standardising joint assessment in rheumatoid arthritis. Clin. Rheumatol. 1996;15(6):579–582. doi: 10.1007/BF02238547. [DOI] [PubMed] [Google Scholar]
- Sharma P., Makharia G.K., Ahuja V., Dwivedi S.N., Deepak K.K. Autonomic dysfunctions in patients with inflammatory bowel disease in clinical remission. Dig. Dis. Sci. 2009;54(4):853–861. doi: 10.1007/s10620-008-0424-6. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007;74(2):224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Valentini M., Parati G. Variables influencing heart rate. Prog. Cardiovasc. Dis. 2009;52(1):11–19. doi: 10.1016/j.pcad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Van Maanen M.A., Vervoordeldonk M.J., Tak P.P. The cholinergic anti-inflammatory pathway: towards innovative treatment of rheumatoid arthritis. Nat. Rev. Rheumatol. 2009;5(4):229–232. doi: 10.1038/nrrheum.2009.31. [DOI] [PubMed] [Google Scholar]
- Vlcek M., Rovensky J., Blazicek P., et al. Sympathetic nervous system response to orthostatic stress in female patients with rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2008;1148:556–561. doi: 10.1196/annals.1410.026. [DOI] [PubMed] [Google Scholar]
- Vlcek M., Rovensky J., Eisenhofer G., et al. Autonomic nervous system function in rheumatoid arthritis. Cell. Mol. Neurobiol. 2012;32(5):897–901. doi: 10.1007/s10571-012-9805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., de Peuter O.R., Kamphuisen P.W., Karemaker J.M. Search for HRV-parameters that detect a sympathetic shift in heart failure patients on beta-blocker treatment. Front. Physiol. 2013;4:81. doi: 10.3389/fphys.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.