Abstract
Background
Angiosarcomas are rare malignant tumors of vascular origin that represent a genuine therapeutic challenge. Recently, the combination of metronomic chemotherapy and drug repositioning has been proposed as an attractive alternative for cancer patients living in developing countries.
Methods
In vitro experiments with transformed endothelial cells were used to identify synergistic interactions between anti-hypertensive drug propranolol and chemotherapeutics. This led to the design of a pilot treatment protocol combining oral propranolol and metronomic chemotherapy. Seven consecutive patients with advanced/metastatic/recurrent angiosarcoma were treated with this combination for up to 12 months, followed by propranolol-containing maintenance therapy.
Findings
Gene expression analysis showed expression of ADRB1 and ADRB2 adrenergic receptor genes in transformed endothelial cells and in angiosarcoma tumors. Propranolol strongly synergized with the microtubule-targeting agent vinblastine in vitro, but only displayed additivity or slight antagonism with paclitaxel and doxorubicin. A combination treatment using bi-daily propranolol (40 mg) and weekly metronomic vinblastine (6 mg/m2) and methotrexate (35 mg/m2) was designed and used in 7 patients with advanced angiosarcoma. Treatment was well tolerated and resulted in 100% response rate, including 1 complete response and 3 very good partial responses, based on RECIST criteria. Median progression-free and overall survival was 11 months (range 5–24) and 16 months (range 10–30), respectively.
Interpretation
Our results provide a strong rationale for the combination of β-blockers and vinblastine-based metronomic chemotherapy for the treatment of advanced angiosarcoma. Furthermore, our study highlights the potential of drug repositioning in combination with metronomic chemotherapy in low- and middle-income country setting.
Funding
This study was funded by institutional and philanthropic grants.
Keywords: Angiosarcoma, Vascular tumor, Metronomic chemotherapy, Propranolol, Adrenergic receptor
Highlights
-
•
A strong synergism was identified between propranolol and vinblastine in an in vitro model of angiosarcoma.
-
•
Adrenergic receptor expression was detected in angiosarcoma tumors providing a molecular target for propranolol.
-
•
Propranolol and vinblastine-based metronomic chemotherapy led to 100% response in 7 patients with inoperable angiosarcoma.
-
•
This treatment resulted in prolonged survival of angiosarcoma patients and warrants further investigation in larger trials.
Angiosarcomas are rare, but very aggressive tumors of vascular origin. Prognosis for advanced angiosarcoma patients is dismal. Here, we used an in vitro model of angiosarcoma and identified a very potent combination of chemotherapy agent vinblastine and anti-hypertensive drug propranolol. This led to the design of an innovative and inexpensive treatment protocol, which was evaluated in 7 consecutive patients with advanced angiosarcoma. This treatment resulted in 100% response and prolonged survival, thus warranting further validation in larger clinical trials and highlighting the potential of this type of therapeutic approach for both developing and high-income countries.
1. Introduction
Drug repositioning or repurposing, which consists in using already approved drugs for new medical applications, provides a unique opportunity to effectively develop and rapidly implement new treatment modalities for cancer patients (Yap et al., 2010, Blatt and Corey, 2013, André et al., 2013, Bertolini et al., 2015). By relying on drugs with well-known pharmacokinetic properties and toxicity profiles, drug repositioning can significantly lower the risks of failure and decrease the time needed to translate pre-clinical results into the clinic, thus considerably reducing costs. These advantages are perfectly illustrated by the recent repositioning of β-blockers for the treatment of severe hemangiomas. Indeed, the serendipitous discovery of the efficacy of the non-selective β-blocker, propranolol, in treating infantile hemangioma (Léauté-Labrèze et al., 2008) in 2008 has completely revolutionized the management of this common pathology (Léauté-Labrèze et al., 2015). Although hemangiomas are benign vascular tumors, this breakthrough led us to hypothesize that β-blockers may be able to increase the efficacy of chemotherapy against malignant tumors when used in combination. Thus, we recently demonstrated that β-blockers could potentiate the anti-proliferative and anti-angiogenic properties of certain chemotherapy agents in vitro, especially microtubule-targeting drugs, and increase their anti-tumor efficacy in animal models of triple-negative breast cancer (Pasquier et al., 2011) and neuroblastoma (Pasquier et al., 2013). Interestingly, our findings were consistent with an increasing body of retrospective analyses showing that the use of β-blockers for the treatment of hypertension may be associated with significant benefits in cancer patients in terms of prolonged survival and/or decreased risk of metastasis or relapse (Powe et al., 2010, Choi et al., 2014, Grytli et al., 2014). Collectively, these studies strongly suggest that β-blockers may prove useful in the treatment of drug-refractory cancers.
Angiosarcomas are a rare form of malignant endothelial cell tumors of vascular or lymphatic origin, accounting for 2–3% of all soft-tissue sarcomas (Young et al., 2010). Radical surgery with complete resection is the standard of care for local disease. For patients with unresectable and/or metastatic disease, treatment may involve radiation and chemotherapy with either doxorubicin or paclitaxel but despite aggressive therapy, prognosis remains dismal (Young et al., 2010, Fury et al., 2005). There is no randomized trial available and very few prospective studies. As a result, no evidence-based recommendation can be made for specific angiosarcoma subtypes and given clinical settings. Several small studies have evaluated the efficacy of anti-angiogenic agents, such as sorafenib and bevacizumab, but with very limited success (Maki et al., 2009, Von Mehren et al., 2012, Agulnik et al., 2013). Response rate was low (5–11%) and median progression-free and overall survival was poor (3–5 months and 13–15 months, respectively). Therefore, alternative therapeutic options are urgently needed, particularly in patients who present with metastatic disease.
Accumulating pre-clinical and clinical evidence suggest that β-adrenergic receptor blockade may prove useful in the treatment of angiosarcoma. Chisholm et al. first reported detectable expression of adrenergic receptors in vascular tumors (Chisholm et al., 2012). This finding was then confirmed by Stiles et al., who also demonstrated the therapeutic potential of propranolol in an animal model of angiosarcoma (Stiles et al., 2013). More recently, clinical data further corroborated the therapeutic potential of β-adrenergic receptor blockade. First, we reported sustained complete response to propranolol in combination with metronomic chemotherapy in a patient with a relapsing metastatic angiosarcoma (Banavali et al., 2015). Shortly after, Bryan and colleagues reported extensive tumor regression induced by propranolol in combination with paclitaxel poliglumex and radiotherapy in a patient with multi-focal angiosarcoma of the scalp and face (Chow et al., 2015).
Here we took our initial clinical experience to the bench and investigated potential synergistic interactions between propranolol and various chemotherapy agents. This led us to design a pilot treatment protocol combining propranolol and vinblastine-based metronomic chemotherapy, which was then used in 7 consecutive patients with advanced and/or metastatic angiosarcoma.
2. Material and Methods
2.1. Cell Culture
BMST (full name BMSVThTERT-4) and BMST-Ras (full name BMSVThTERT-4Nras) cell lines are bone marrow-derived endothelial cells that were successively transformed with SV40T and immortalized by ectopic expression of human telomerase reverse transcriptase (hTERT) (MacKenzie et al., 2002). BMST-Ras were also transfected with the vector LN-ras2 to express the N-ras oncogene. Both cell lines were previously characterized for expression of angiogenic markers, including CD31, VEGFR-2, CD34 and VE-Cadherin (MacKenzie et al., 2002). They were grown in Iscove's Modified Dulbecco's Medium (Invitrogen, Mount Waverley, Australia) containing 20% Fetal Calf Serum (FCS) and 2 mM L-glutamine and were routinely maintained in culture on 0.1% gelatin-coated flasks at 37 °C and 5% CO2. Both cell lines were regularly screened and are free from mycoplasma contamination.
2.2. Quantitative RT-PCR
The expression of adrenergic receptor genes ADRB1 and ADRB2 was examined in endothelial cell lines using real-time quantitative RT-PCR. Total RNA was extracted and DNAse treated using the Qiagen Mini RNeasy kit (Qiagen, Doncaster, Australia) and the RNA concentration was determined from the absorbance at 260 nm. cDNA synthesis was performed using High capacity cDNA reverse transcription kit with RNAse inhibitor (Applied Biosystem, Melbourne, Australia). Real time PCR was run on 7900HT Fast Real-Time PCR system using Power SYBR® green (Applied Biosystems) for ADRB1 and ADRB2 using DNA primer sequences previously described (Cao et al., 2010) and endogenous control gene GAPDH. Gene expression levels were determined using the ΔΔCt method, normalized to the GAPDH control gene (QT01192646) and expressed relative to a calibrator (Winer et al., 1999).
2.3. Growth Inhibition Assay
Growth inhibition assays were performed as previously described (Pasquier et al., 2011). Briefly, cells were seeded at 1500 cells/well in 96-well plates. After 24 h, cells were treated with a range of concentrations of chemotherapeutic drugs alone or in combination with propranolol and after 72 h drug incubation, metabolic activity was detected by addition of Alamar blue and spectrophotometric analysis. Cell proliferation was determined and expressed as a percentage of untreated control cells. The determination of IC50 values was performed by point-to-point fit spline analysis using GraphPad Prism 4 software (GraphPad Software Inc., La Jolla, CA). Combination index (CI) values were calculated for all tested drug concentrations according to the Chou and Talalay method (Chou, 2010) using the following equation:
where (D)1 and (D)2 represent the dose of agent 1 and 2 used in combination to induce X% growth inhibition, and (DX)1 and (DX)2 represent the dose of agent 1 and 2 required to reach X% growth inhibition when used alone. The CI theorem then provides quantitative definition for additive effects (0.9 ≤ CI ≤ 1.1), synergism (CI < 0.9) and antagonism (CI > 1.1) in drug combinations.
2.4. Tumor Spheroid Assay and PARP Cleavage
For the tumor spheroid assay, 5000 BMST-Ras cells were seeded in ultra-low attachment 96-well plates (Corning Inc., Corning, NY) and allowed to form even tumor spheroids of 600 μm in diameter for 48 h. Drug treatment was then initiated and photographs were taken every 24 h using the 5 × objective of an Axiovert 200 M fluorescent microscope coupled to an AxioCamMR3 camera driven by the AxioVision 4.7 software (Carl Zeiss, North Ryde, Australia). The volume of at least 10 tumor spheroids was determined using the AxioVision 4.7 software and the following formula: V = 4/3 × π × r3.
To assess apoptosis induction, tumor spheroids were lysed in RIPA buffer containing a cocktail of protease inhibitors (Sigma-Aldrich, Castle Hill, Australia) after 120 h of drug treatment. Total cellular proteins (50 μg) were resolved on 10% SDS-PAGE before electrotransfer onto nitrocellulose membrane. Immunoblotting was done using antibodies directed against glyceraldehyde-3-phosphate dehydrogenase (GADPH; Abcam, Cambridge, UK) and the large fragment of poly (ADP-ribose) polymerase (PARP) produced by caspase cleavage (cleaved PARP; Cell Signaling Technology, Beverly, MA, USA). The membranes were then incubated with horseradish peroxidase-conjugated IgG secondary antibodies, and protein was detected with ECL Plus (GE Healthcare Life Sciences, Uppsala, Sweden).
2.5. Patient Population
All patients had presented at the Tata Memorial Hospital, Mumbai and registered under the Bone and Soft Tissue Disease Management Group. Patients were evaluated with a biopsy for histopathological diagnosis and most patients got a PET–CT done to evaluate the extent of disease. These patients with advanced/metastatic/recurrent disease were then referred for metronomic therapy because of extremely poor prognosis with standard therapies. Therapy was started after explaining the experimental nature of treatment, discussing all the treatment options and obtaining written consent.
2.6. Gene Expression Analysis From FFPE Patient Samples
RNA was extracted from HeLa and SK-N-SH cell lines (positive controls for ADRB1 and ADRB2, respectively) by conventional Trizol method. Formalin-fixed, Paraffin Embedded (FFPE) sections of 14 μm were deparaffinized and processed for RNA extraction using the RNeasy FFPE kit (Qiagen) and following the manufacturer's instructions. RNA concentration was determined from the absorbance at 260 nm and 5 μg of RNA was used to synthesize cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). cDNA was then processed for PCR analysis using specific primers for ADRB1 (Forward: CCTCGTCCGTAGTCTCCTTC; Reverse: GCAGCTGTCGATCTTCTTCA) and ADRB2 (Forward: AGAGCCTGCTGACCAAGAAT; TAGCAGTTGATGGCTTCCTG). PCR products were then loaded onto a 2% agarose gel to verify the product size. Bands corresponding to the correct amplicon size (103 bp and 138 bp for ADRB1 and ADRB2, respectively) were collected and DNA was purified using the QIAquick Gel Purification Kit (Qiagen). PCR products were then used as template and cycle sequenced using both strands (forward & reverse) using the Big dye terminator kit on an Verity Thermal cycler (Applied Biosystems). Samples were finally loaded onto ABIPrism 3500 Genetic Analyzer (Applied Biosystems) for sequencing.
2.7. Treatment Protocol
Treatment consisted in the combination of bi-daily oral propranolol (40 mg) and weekly i.v. injections of vinblastine (6 mg/m2; maximum 6 mg) and methotrexate (35 mg/m2; maximum 50 mg) for up to 12 months. This was followed by oral maintenance therapy consisting of bi-daily oral propranolol (40 mg) in combination with daily oral etoposide (50 mg) and cyclophosphamide (50 mg) for 20 consecutive days in cycles of 30 days.
3. Results
3.1. NRAS-induced Transformation of Endothelial Cells Does not Alter β-adrenergic Receptor Gene Expression and Sensitivity to Propranolol
Immortalized and transformed endothelial cells were developed to better understand vascular tumors, such as angiosarcoma, and to evaluate the therapeutic potential of conventional and novel drugs against these malignancies (Wen and MacKenzie, 2013). Although immortalized murine endothelial cells have been shown to form benign hemangiomas in vivo, the introduction of oncogenic HRAS produced rapidly growing and poorly differentiated angiosarcomas (Cao et al., 2010). Here, we used bone-marrow derived endothelial cells that were immortalized and transformed by successive transfections with SV40 T antigen, the catalytic subunit of human telomerase (hTERT) and the N-ras oncogene, as an in vitro model of angiosarcoma. First, we assessed the expression of β-adrenergic receptor genes ADRB1 and ADRB2 by qRT-PCR in 3 immortalized (HMEC-1, BMhTERT-1 and BMST) and 1 Nras-transformed (BMST-Ras) endothelial cell lines (Fig. 1A). All four endothelial cell lines showed high ADRB2 mRNA expression, similar to positive control cell line HeLa. In contrast, they displayed varying levels of ADRB1 mRNA expression, with HMEC-1 cells showing high expression, BMST and BMST-Ras cells intermediate expression and BMhTERT-1 cells low expression. We then investigated the anti-proliferative effects of propranolol against BMST and BMST-Ras cells and found that propranolol inhibited the proliferation of immortalized and NRAS-transformed endothelial cells in a dose-dependent manner (Fig. 1B). The IC50 values were 157 ± 7 μM and 161 ± 7 μM for BMST and BMST-Ras cells, respectively.
Fig. 1.
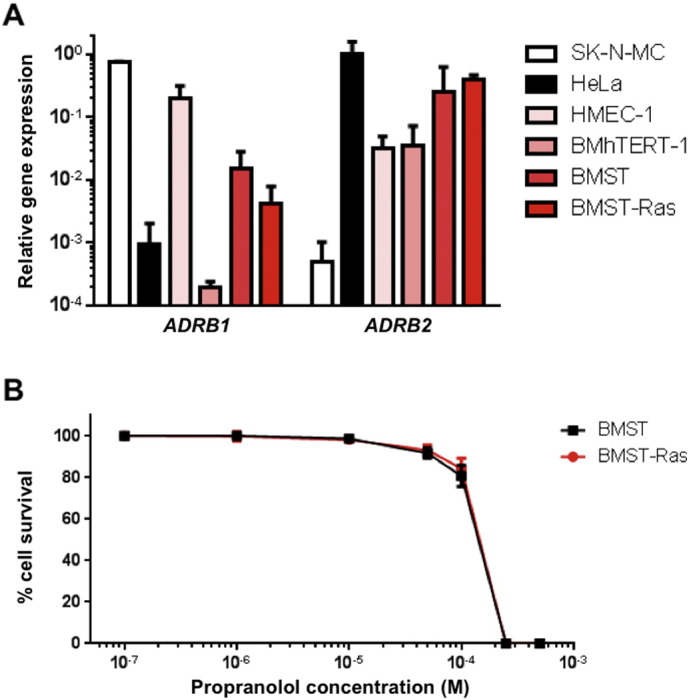
Expression of adrenergic receptor genes in immortalized and Ras-transformed vascular endothelial cells and in vitro sensitivity to propranolol. (A) Relative mRNA expression of ADRB1 and ADRB2 adrenergic receptor genes in immortalized (HMEC-1, BMhTERT-1 and BMST) and Ras-transformed (BMST-Ras) endothelial cell lines as determined by qRT-PCR using GAPDH as control gene. SK-N-MC neuroepithelioma cell line and HeLa cervical cancer cell line were included as positive controls for ADRB1 and ADRB2 expression, respectively. (B) Growth inhibition assay performed on BMST (black) and BMST-Ras (red) cell lines using Alamar Blue after 72 h incubation with propranolol. Points, % of cell proliferation as compared to untreated control cells, means of eight individual experiments; bars, 95% confidence interval; log scale for x axis.
3.2. Propranolol Synergizes With Vinblastine but not With Chemotherapeutic Drugs Commonly Used for the Treatment of Angiosarcoma
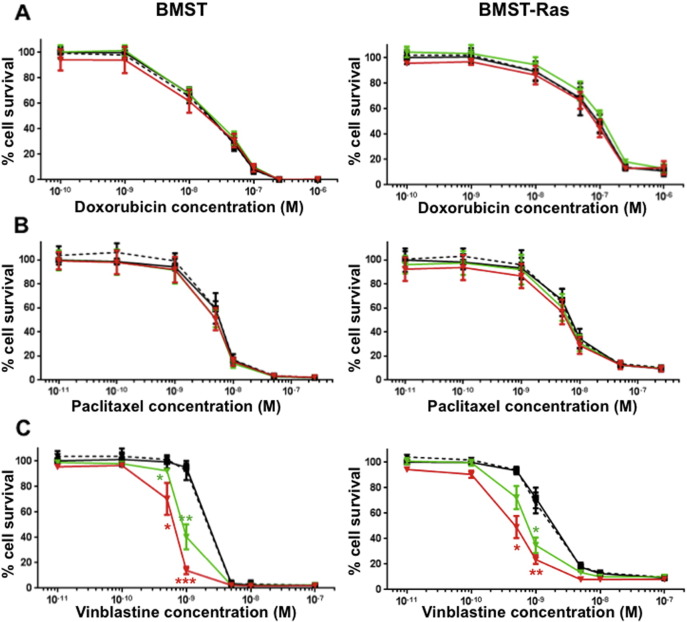
Drug combination studies using growth inhibition assay were performed to determine whether propranolol could potentiate the anti-proliferative effects of chemotherapeutic drugs commonly used in the treatment of angiosarcoma. BMST and BMST-Ras cells were treated with a range of concentrations of doxorubicin and paclitaxel alone or in combination with propranolol at 1 μM, 10 μM or 50 μM. As shown in Fig. 2, propranolol only marginally impacted the sensitivity of BMST and BMST-Ras cells to doxorubicin and paclitaxel. No significant change in the IC50 of both drugs was observed as a result of propranolol addition, except a 24% decrease in sensitivity to doxorubicin in BMST-Ras cells in presence of 10 μM propranolol and a 14% increase in sensitivity to paclitaxel in BMST cells the in presence of 10 or 50 μM propranolol (Fig. 3A–B; p < 0.05). In sharp contrast, propranolol increased the sensitivity of immortalized and Nras-transformed endothelial cells to vinblastine by 2.7 to 4.5 folds (Figs. 2C and 3C; p < 0.05). The interaction of propranolol with chemotherapy agents was quantified using the Chou and Talalay method (Chou, 2010). Combination indexes showed that the association of propranolol with doxorubicin and paclitaxel was slightly antagonistic and additive, respectively, while the interaction between propranolol and vinblastine was highly synergistic (Fig. 4). The synergism of the combination of propranolol and vinblastine was further evaluated using an in vitro 3D tumor spheroid model (Fig. 5). BMST-Ras cells were allowed to form spheroids of ~ 600 μm in diameter before treatment was initiated. When used alone, 10 μM propranolol and 1 nM vinblastine significantly slowed down the growth of tumor spheroids, resulting in a 19–20% decrease in spheroid volume after 5 days of treatment as compared to untreated spheroids (p < 0.001). Furthermore, the combination of propranolol and vinblastine completely suppressed the growth of tumor spheroids, leading to a 59% decrease in volume after 5 days as compared to control spheroids (p < 0.001). Interestingly, the growth inhibitory effect of the combination was due to apoptosis induction, as evidenced by the cleavage of the Poly ADP ribose polymerase (PARP) (Fig. 5C).
Fig. 2.
In vitro drug combination studies. Growth inhibition assays performed on BMST (left panel) and BMST-Ras (right panel) cell lines using Alamar Blue after 72 h incubation with doxorubicin (A), paclitaxel (B) and vinblastine (C) alone (black — solid line) or in combination with propranolol at 1 μM (black — broken line), 10 μM (green — solid line) and 50 μM (red — solid line). Points, % of cell proliferation as compared to untreated control cells, means of four individual experiments; bars, 95% confidence interval; log scale for x axis. Statistical analysis was performed by comparing the cytotoxic effect of chemotherapy alone and in combination with propranolol using Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Fig. 3.
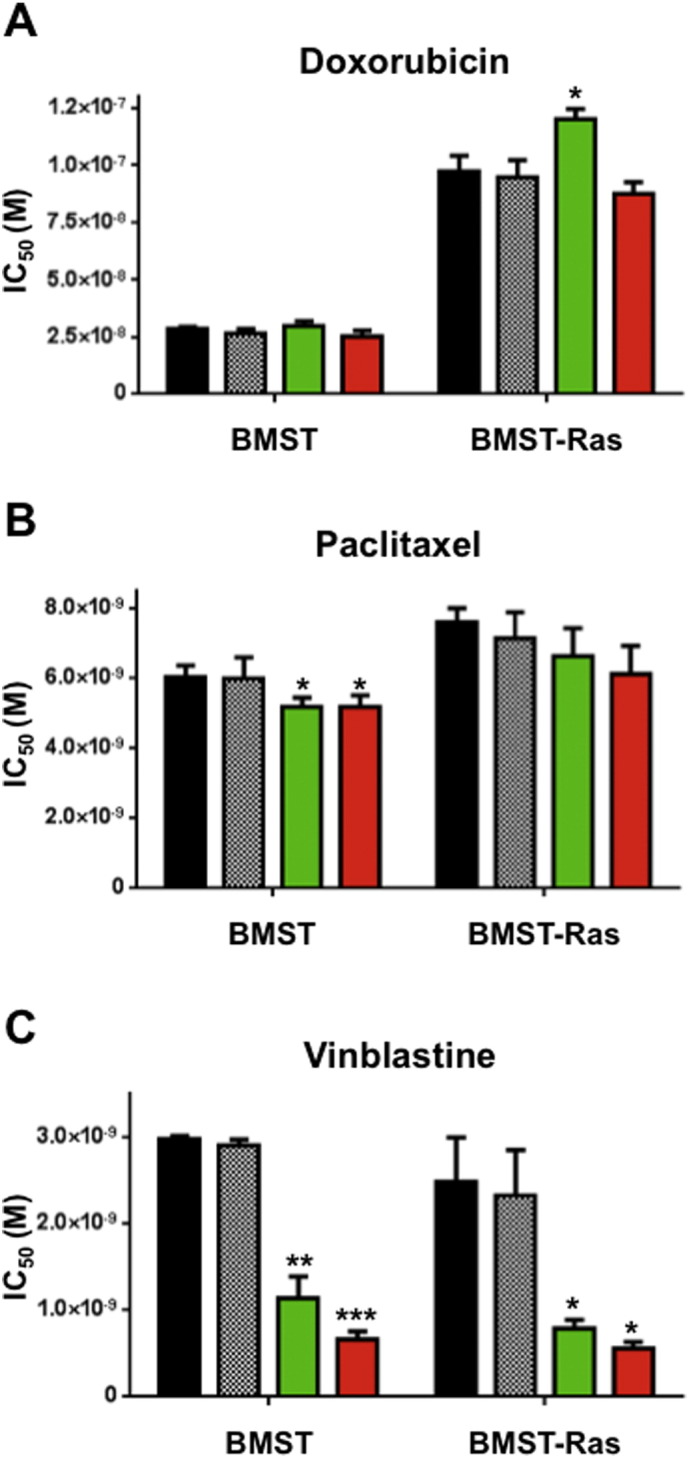
Changes in sensitivity to chemotherapy. Histogram representation of the molar concentration of doxorubicin (A), paclitaxel (B) and vinblastine (C) required to inhibit 50% (IC50) of cell proliferation after 72 h drug incubation in absence (black) or presence of propranolol at 1 μM (hashed), 10 μM (green) and 50 μM (red). Columns, means of four individual experiments; bars, SEM. Statistical analysis was performed by comparing the IC50 values of chemotherapy alone and in combination with propranolol using Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Fig. 4.
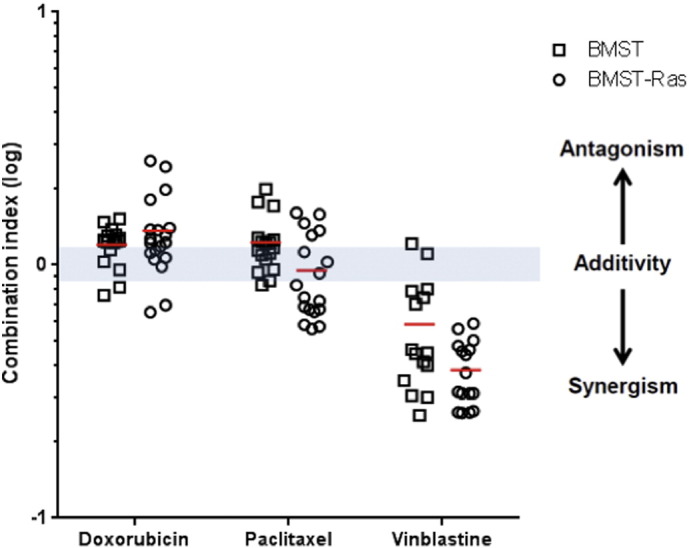
Combination indexes of propranolol with chemotherapy agents. Dot plot representation of the combination index of propranolol in association with doxorubicin, paclitaxel and vinblastine on BMST (□) and BMST-Ras (○) cell lines. Growth inhibition assays were performed using Alamar Blue after 72 h incubation with a range of chemotherapeutic drug concentrations in the presence or absence of propranolol at 50 μM. CI values were determined based on the Chou and Talalay method for all tested concentrations of chemotherapeutic drug. Bars, mean of at least three individual experiments; y axis, log scale.
Fig. 5.
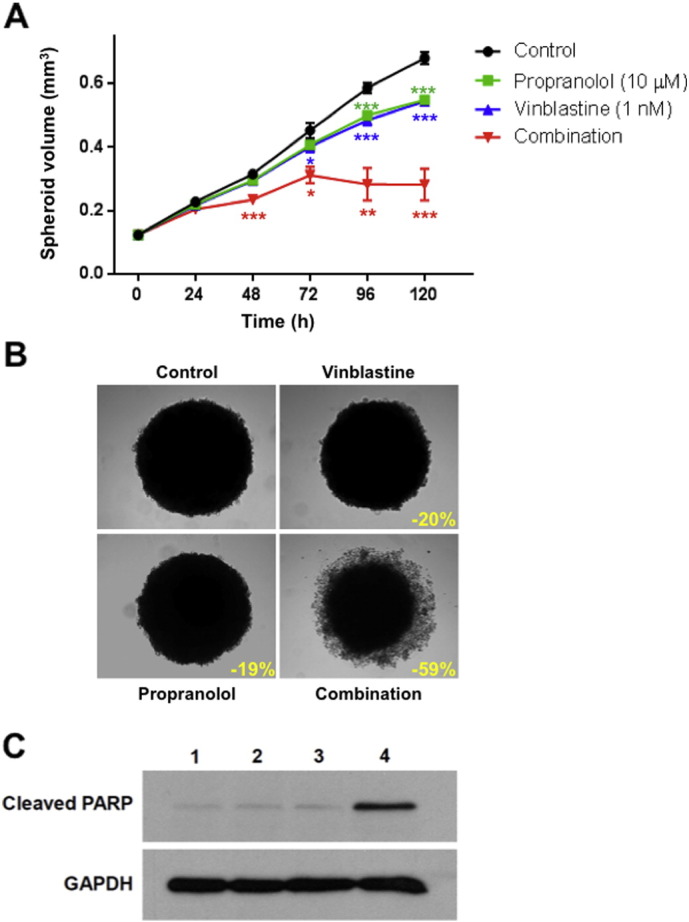
Combination of propranolol and vinblastine in tumor spheroids. (A) Quantitative analysis of the growth of tumor spheroids formed by BMST-Ras cells on ultra-low attachment plates by daily volume measurements. Tumor spheroids were either untreated (black) or treated with 10 μM propranolol (green), 1 nM vinblastine (blue) or the combination (red). Points, means of at least three individual experiments; bars, SEM. Statistical analysis was performed by comparing the volume of tumor spheroids in absence and presence of treatment using Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). (B) Representative photographs of BMST-Ras tumor spheroids after 120 h incubation with vinblastine and propranolol alone or in combination. Images were obtained using the 5 × objective of a Zeiss Axiovert 200 M. Inset, % of growth inhibition as compared to untreated tumor spheroids; scale bar, 200 μm. (C) Representative immunoblots of BMST-Ras tumor spheroid lysates after 120 h incubation with no drug (1), 10 μM propranolol (2), 1 nM vinblastine (3) or the combination (4). Membranes were probed with antibodies directed against cleaved PARP and GAPDH (loading control).
3.3. Propranolol in Combination With Metronomic Chemotherapy Results in Long-term Response in Advanced Angiosarcoma Patients
Our in vitro data showed that propranolol interacted synergistically with vinblastine. Therefore a combination of propranolol and vinblastine-based metronomic chemotherapy was designed and used in seven consecutive angiosarcoma patients. The characteristics of the patients are summarized in Table 1. Five were males, 2 females; the median age was 53 years (range 20 to 72 years); 4 were newly diagnosed, 3 previously treated. All patients presented with advanced/metastatic/recurrent tumors and had multiple adverse prognostic factors as noted in Table 1. Three tumors (patients #1, #3 and #4) were examined for β-adrenergic receptor gene expression by conventional PCR (Fig. 6). All 3 tested tumors showed detectable levels of ADRB1 expression while ADRB2 expression was variable, with patient #4 showing detectable expression while patients #1 and #3 showed very weak expression. Experimental treatment protocol consisted of bi-daily oral propranolol at 40 mg in combination with weekly vinblastine at 6 mg/m2 (maximum 10 mg) and weekly methotrexate at 35 mg/m2 (maximum 50 mg) administered intravenously. After 12 months, or upon patient request, this was followed by oral maintenance therapy, consisting of continuous bi-daily propranolol at 40 mg in combination with etoposide and cyclophosphamide both at 50 mg/day for 20 days in cycles of 30 days. Treatment duration and responses are summarized in Table 2.
Table 1.
Patient characteristics.
| Patient no. | Sex/ age |
New/ treated |
Sites involved | Adverse prognostic factors | Previous treatment | Date Rx A started⁎ |
|---|---|---|---|---|---|---|
| 1 | F/53 | Treated | (L) Breast primary. Orbit; bilateral breasts; cavernous sinus; ant. Chest wall; vertebrae | Recurrent/metastatic; size > 5 cm; no surgery | Surgery; weekly paclitaxel; thalidomide | 31/08/2011 |
| 2 | M/72 | New | Scalp primary. Multiple scalp lesions; large lesion on face | Size > 5 cm; distant lesions; no surgery | Nil | 01/03/2012 |
| 3 | M/54 | New | Scalp primary. Multiple scalp lesions; multiple vertebrae; mandible | Metastatic; size > 5 cm; tumor necrosis; no surgery | Nil | 05/09/2013 |
| 4 | M/62 | New | Para-vertebral masses primary. Multiple bones; bone-marrow; liver; spleen | Metastatic; hepatic primary; poor performance status; no surgery | Surgery (laminectomy) | 15/10/2013 |
| 5 | M/35 | Treated | (R) Hand primary; metastases to axilla; lung; pleural effusion | Recurrent/metastatic; no surgery | Surgery; doxorubicin & cisplatin chemotherapy; forequarter amputation (R) UE + metastatectomy of lung nodules |
20/12/2013 |
| 6 | F/49 | Treated | Supra-orbital Primary; temple; above eyelids; post-auricular mass | Recurrent/metastatic; no surgery | Radiotherapy; cisplatin | 10/07/2014 |
| 7 | M/20 | New | Lung primary. Para tracheal nodes; liver; multiple bones; bone marrow | Recurrent/metastatic; visceral; no surgery | Nil | 03/11/2014 |
Patients with skeletal involvement also received zoledronic acid.
Rx A: Propranolol /Vinblastine/Methotrexate.
Fig. 6.
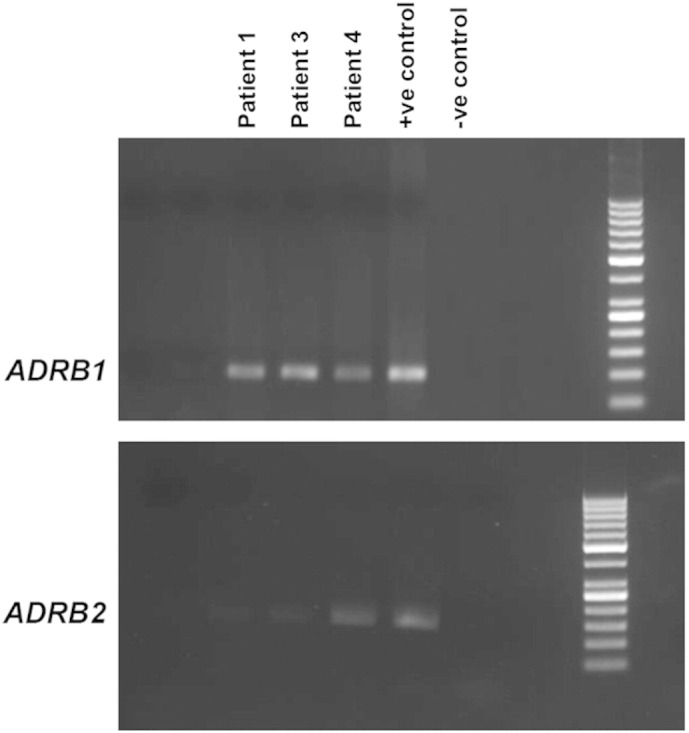
Expression of β-adrenergic receptor gene in angiosarcoma tumors. ADRB1 (top panel — 103 base pairs) and ADRB2 (bottom panel — 138 base pairs) RT-PCR products analyzed by 2% agarose gel electrophoresis followed by ethidium bromide staining using 50 bp DNA marker. mRNA was isolated from tumor material obtained from patients #1, #3 and #4. Positive control RNA was extracted from HeLa (ADRB1) and SK-N-SH (ADRB2) cell lines.
Table 2.
Treatment duration and responses.
| Patient no. | Duration of Rx A⁎ | Best response to Rx A⁎ | Duration oral maintenance⁎⁎ | PFS | Additional treatment | Status as of Feb. 2016 | OS |
|---|---|---|---|---|---|---|---|
| 1 | 3 months (stopped on request) | Very good partial response (VGPR) | 5 months | 7 months | EBRT to eye; doxorubicin; gemcitabine + cisplatin | Died of PD Oct. 2012 | 14 months |
| 2 | 12 months | Complete clinical response. MRI still showed some diffuse scalp thickening: VGPR. | 12 months | 24 months | EBRT to scalp | Died of PD Oct. 2014 | 30 months |
| 3 | 12 months | Complete clinical & metabolic response | 2 months | 14 months | EBRT to scalp, bones; paclitaxel; thalidomide | Died of PD Mar. 2015 | 19 months |
| 4 | 4 months (could not continue due to low platelet counts) | Bone marrow complete morphological response | Could not take much chemo due to autoimmune thrombo-cytopenia | 5 months | EBRT to bones; thalidomide | Died of PD Aug. 2014 | 10 months |
| 5 | 11 months | Complete response of residual lung nodules | 3 months | 11 months | Palliative care | Died of progresive disease Apr. 2015 | 16 months |
| 6 | 12 months | Very good partial response (VGPR) | 3 months | 19 + months | N/A | Alive on Rx with VGPR |
19 + months |
| 7 | 5 months (stopped due to logistics) | Partial response | Not started | 14 + months | Presently on thalidomide/oral etoposide | Alive on Rx with stable disease | 14 + months |
EBRT: external beam radio therapy; PD: progressive disease.
Rx A: Propranolol /Vinblastine/Methotrexate.
Maintenance Rx: oral etoposide/cyclophosphamide/propranolol.
Overall, treatment was well tolerated by most patients. Nearly all patients developed grade II fatigue by 6 months of injectable chemotherapy and the doses of injections were decreased by approximately 50% to a fixed dose of 5 mg vinblastine and 25 mg methotrexate. None of the patients developed febrile neutropenia nor required blood or platelet support, except for patient #4, who had bone marrow involvement at presentation. This patient had presented with pancytopenia (low hemoglobin, ANC & platelet count) and thus was started at a 50% reduced dose of chemotherapy. However, he continued to have low counts and received only 4 months of intermittent chemotherapy. Patient #1 developed grade II abdominal pain/diarrhea on week 2 of injectable chemotherapy and dose was therefore reduced by 25%. Patient #2 developed grade II neutropenia (ANC: 1.28 × 109/L) after the first injection of chemotherapy and dose was also decreased by 25%. In both cases, dose reduction was effective in abrogating toxicity.
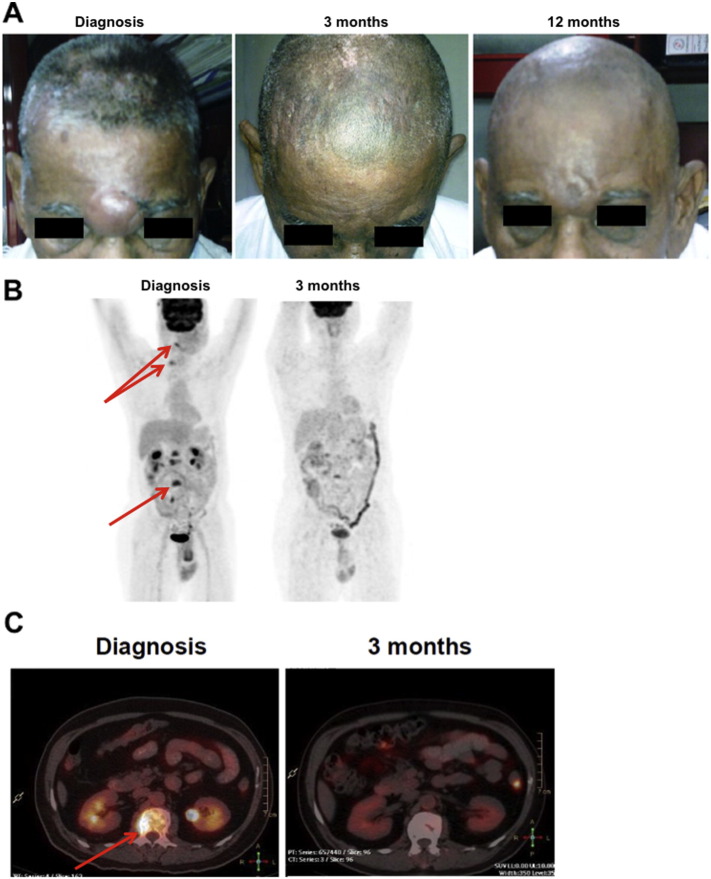
All 7 patients responded to treatment with propranolol in combination with metronomic chemotherapy, with best responses based on RECIST criteria classified as very good partial response in 3 and complete response in 1. Spectacular tumor regressions were observed in patients #2 and #3 (Fig. 7). Importantly, patients #1 and #5 experienced 7 and 11 months PFS as third line treatment, respectively. Two patients are still alive and on various stages of treatment. The median PFS is 11 months (range 5 to 24 months) and OS is 16 months (range 10 to 30 months). Collectively, these results show that the combination of propranolol with metronomic chemotherapy is a very promising strategy to manage recurrent and/or metastatic angiosarcoma.
Fig. 7.
Clinical response of angiosarcoma patients. (A) Photographs of patient #2 who presented with a large angiosarcoma in the periorbital region and multiple lesions on the scalp. Sustained complete clinical response was observed in this patient. (B–C) PET and PET–CT scan images of patient #3 who presented with a primary angiosarcoma of the scalp and multiple metastases located in the vertebrae (arrows). Sustained complete clinical and metabolic response was observed in this patient.
4. Discussion
Although quite rare, angiosarcoma remains a genuine challenge in medical oncology. When surgery is not possible because of the extent and/or localization of the disease, prognosis is very dismal. With standard treatment, usually based on taxanes and/or doxorubicin chemotherapy, median OS is around 9 months (Young et al., 2010). Given the vascular origin of angiosarcoma and the importance of angiogenesis in the biology of the disease (Young et al., 2010, Behjati et al., 2014), anti-angiogenic agents were investigated in clinical studies with great expectations. However, the results were disappointing, with low response rates and median PFS of 4–5 months (Young et al., 2010, Maki et al., 2009, Von Mehren et al., 2012). Overall, there is currently no curative option for advanced angiosarcoma and treatment is mostly palliative. In the present study, we report 100% response rate based on RECIST criteria, 11 months median PFS and 16 months median OS in 7 consecutive patients with advanced and/or metastatic/recurrent angiosarcoma using an inexpensive combination treatment designed from pre-clinical data.
Our in vitro experiments first confirmed the dose-dependent anti-proliferative effects of propranolol against transformed endothelial cells, as previously shown by Stiles et al.(Stiles et al. (2013)) It is however important to note that in the current study and previous ones, high micromolar concentrations of propranolol were required to inhibit angiosarcoma cell proliferation when used alone, which is not achievable in vivo with standard dosage. This suggests that the anti-tumor efficacy of propranolol alone is not directly mediated by its anti-proliferative activity against cancer cells, but rather through alternative mechanisms including angiogenesis inhibition and immunostimulatory effects (Cole and Sood, 2012). Importantly, we did not observe any significant difference in the sensitivity to propranolol between BMST and BMST-Ras cells, demonstrating that the introduction of oncogenic NRAS did not induce resistance to propranolol. This finding is particularly important given that Ras oncogenes (NRAS, KRAS and HRAS) are frequently mutated in angiosarcoma tumors (Murali et al., 2015).
We then sought potential synergism between propranolol and chemotherapy agents. In vitro combination studies showed that propranolol did not increase the efficacy of doxorubicin or paclitaxel but interacted synergistically with vinblastine. This synergism was further validated using 3D tumor spheroids and found to be associated with apoptosis induction. This finding is consistent with results we previously reported in neuroblastoma (Pasquier et al., 2013). Indeed, out of 7 chemotherapy agents tested, we found that β-blockers specifically synergized with vincristine against neuroblastoma cells. Collectively, these results suggest that β-adrenergic receptor blockade should be used in combination with Vinca alkaloids to maximize therapeutic efficacy.
We and others have recently reported the therapeutic benefits of propranolol in 2 patients with relapsing metastatic and multi-focal angiosarcoma, respectively (Banavali et al., 2015, Chow et al., 2015). Here, we extended these initial findings to 7 consecutive angiosarcoma patients with dismal prognosis. Treatment protocol design was based on the synergistic interaction of propranolol and vinblastine observed in vitro. In addition, methotrexate was included based on its anti-inflammatory properties and previous report of efficacy of low-dose methotrexate in combination with vinblastine against other forms of aggressive soft-tissue tumors, like inoperable fibromatosis (Azzarelli et al., 2001). The 100% response rate based on RECIST criteria and extended PFS and OS reported here are impressive given the advanced disease stage in all 7 patients and the common drug refractoriness of angiosarcomas (Young et al., 2010).
We tested 3 patient tumors for β-adrenergic receptor gene expression by RT-PCR and found that all 3 tumors expressed ADRB1 at similar levels while ADRB2 expression was more variable and barely detectable in 2 out of 3 patients. This is somewhat different from the results of tissue microarray immunostaining experiments that reported high ADRB2 expression in various vascular tumors, including angiosarcoma (Chisholm et al., 2012, Stiles et al., 2013). It is however important to note that antibodies directed towards G-protein coupled receptors notoriously lack specificity (Michel et al., 2009) and gene expression analysis may provide more reliable results than immunostaining of tumor sections. Elsewhere, stress-induced tumor growth, angiogenesis, metastasis and resistance to treatment has been directly linked to ADRB2 signaling (Cole and Sood, 2012). Future studies will need to address the contribution of the different adrenergic receptors in the synergism between propranolol and chemotherapy agents in angiosarcoma and other refractory tumors, such as triple-negative breast cancer and neuroblastoma (Pasquier et al., 2011, Pasquier et al., 2013).
Our study has a number of caveats that should not be ignored. First, this is an unpowered clinical study with a mixture of first line and relapse treatments. Secondly, treatment was slightly heterogeneous. For instance, Patients #3, #4, and #7 who had multiple bone metastases and were in pain at presentation, also received celecoxib 200 mg PO bid and weekly zoledronic acid 1 mg IV for the first 3 months of therapy. In addition, given the palliative nature of treatment, the dose of chemotherapy agents was decreased in the presence of grade II toxicities, in order to prevent the occurrence of grade III and IV toxicities. This heterogeneity in terms of clinical setting and treatment protocol does not allow rigorous comparison with historical controls. Finally, our study did not include the use of propranolol as monotherapy to demonstrate its potential anti-tumor activity, although recent in vivo data (Stiles et al., 2013) and a clinical case report (Chow et al., 2015) suggest it may also prove useful as a single agent. It is however important to note that previous studies reported positive results with the use of metronomic chemotherapy to treat malignant vascular tumors (Vogt et al., 2003, Mir et al., 2011), thus providing further rationale for our combination treatment.
5. Conclusions
Drug repositioning provides a unique opportunity to develop new treatment modalities that can be rapidly translated into the clinic. This approach is particularly attractive for low- and middle-income countries (LMIC), where the latest drugs and therapies developed in high-income countries (HIC) are unaffordable for the wide majority of patients (André et al., 2013). Here, despite the limitations of our study we produce strong evidence for the repositioning of β-adrenergic receptor antagonist, propranolol, in combination with vinblastine-based metronomic chemotherapy for the treatment of advanced angiosarcoma. The safety and efficacy of this treatment will now need to be further validated in a larger phase I/II clinical trial. Importantly, this type of treatment comes at a fraction of the cost of experimental treatments developed for angiosarcoma patients in HIC and it can be administered on an out-patient basis with manageable toxicities. It thus represents a very promising and economically viable strategy for patients living in LMIC, thus paving the way for the development of a fair, global oncology.
Contributions of Authors
EP, NA, MK and SB conceived the study; EP, JS and MM performed the in vitro work; EP, NA and SB designed the treatment protocol and analyzed the data; AC performed the molecular work on patient samples; BR was in charge of the anatomopathological analysis of patient samples; JG, DSJP and SB treated and monitored the patients; KLM provided the cell lines; EP, NA and SB wrote the manuscript; KLM and MK proofread it and provided feedback.
Acknowledgements
This project was funded in part by the Children's Cancer Institute, which is affiliated with the University of New South Wales (UNSW Australia) and Sydney Children's Hospital Network and by grants from the Balnaves Foundation (EP), Cancer Council New South Wales (MK), and NHMRC Senior Research Fellowships (MK; APP1058299). The authors would also like to thank LNlavie and Les copains de Charles for their support. EP is funded by a Marie Curie-Sklodowska Fellowship from the European Research Council (grant agreement number 626794) and MK is funded by the Australian Research Council Centre of Excellence in Convergent Bio-Nano Science and Technology (project number CE140100036). None of the funding bodies played any role in the study design, data collection, data analysis, interpretation or writing of the manuscript.
Contributor Information
Eddy Pasquier, Email: epasquier@ccia.unsw.edu.au.
Shripad D. Banavali, Email: banavali_2000@yahoo.com.
References
- Agulnik M., Yarber J.L., Okuno S.H., et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann. Oncol. 2013;24:257–263. doi: 10.1093/annonc/mds237. [DOI] [PubMed] [Google Scholar]
- André N., Banavali S., Snihur Y., Pasquier E. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013;14:e239–e248. doi: 10.1016/S1470-2045(13)70056-1. [DOI] [PubMed] [Google Scholar]
- Azzarelli A., Gronchi A., Bertulli R., et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer. 2001;92:1259–1264. doi: 10.1002/1097-0142(20010901)92:5<1259::aid-cncr1446>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Banavali S., Pasquier E., Andre N. Targeted therapy with propranolol and metronomic chemotherapy combination: sustained complete response of a relapsing metastatic angiosarcoma. Ecancermedicalscience. 2015;9:499. doi: 10.3332/ecancer.2015.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behjati S., Tarpey P.S., Sheldon H., et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat. Genet. 2014;46:376–379. doi: 10.1038/ng.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini F., Sukhatme V.P., Bouche G. Drug repurposing in oncology-patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015;12:732–742. doi: 10.1038/nrclinonc.2015.169. [DOI] [PubMed] [Google Scholar]
- Blatt J., Corey S.J. Drug repurposing in pediatrics and pediatric hematology oncology. Drug Discov. Today. 2013;18:4–10. doi: 10.1016/j.drudis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Cao D., et al. Role of beta1-adrenoceptor in increased lipolysis in cancer cachexia. Cancer Sci. 2010;101:1639–1645. doi: 10.1111/j.1349-7006.2010.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm K.M., Chang K.W., Truong M.T., Kwok S., West R.B., Heerema-McKenney A.E. β-Adrenergic receptor expression in vascular tumors. Mod. Pathol. 2012;25:1446–1451. doi: 10.1038/modpathol.2012.108. [DOI] [PubMed] [Google Scholar]
- Choi C.H., Song T., Kim T.H., et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J. Cancer Res. Clin. Oncol. 2014;140:1179–1188. doi: 10.1007/s00432-014-1658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.-C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Chow W., Amaya C.N., Rains S., Chow M., Dickerson E.B., Bryan B.A. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;16:1–4. doi: 10.1001/jamadermatol.2015.2554. [DOI] [PubMed] [Google Scholar]
- Cole S.W., Sood A.K. Molecular pathways: beta-adrenergic signaling in cancer. Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fury M.G., Antonescu C.R., Van Zee K.J., Brennan M.F., Maki R.G. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11:241–247. doi: 10.1097/00130404-200505000-00011. [DOI] [PubMed] [Google Scholar]
- Grytli H.H., Fagerland M.W., Fosså S.D., Taskén K.A. Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 2014;65:635–641. doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Léauté-Labrèze C., Dumas de la Roque E., Hubiche T., Boralevi F., Thambo J.B., Taïeb A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- Léauté-Labrèze C., Hoeger P., Mazereeuw-Hautier J., et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N. Engl. J. Med. 2015;372:735–746. doi: 10.1056/NEJMoa1404710. [DOI] [PubMed] [Google Scholar]
- MacKenzie K.L., Franco S., Naiyer S.L., et al. Multiple stages of malignant transformation of human endothelial cells modelled by co-expression of telomerase reverse transcriptase, SV40 T antigen and oncogenic N-ras. Oncogene. 2002;21:4200–4211. doi: 10.1038/sj.onc.1205425. [DOI] [PubMed] [Google Scholar]
- Maki R.G., D'Adamo D.R., Keohan M.L., et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J. Clin. Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M.C., Wieland T., Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedeberg's Arch. Pharmacol. 2009;379:385–388. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
- Mir O., Domont J., Cioffi A., et al. Feasibility of metronomic oral cyclophosphamide plus prednisolone in elderly patients with inoperable or metastatic soft tissue sarcoma. Eur. J. Cancer. 2011;47:515–519. doi: 10.1016/j.ejca.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Murali R., Chandramohan R., Möller I., et al. Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget. 2015;6:36041–36052. doi: 10.18632/oncotarget.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier E., Ciccolini J., Carré M., et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797–809. doi: 10.18632/oncotarget.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier E., Street J., Pouchy C., et al. β-Blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br. J. Cancer. 2013;108:2485–2494. doi: 10.1038/bjc.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe D.G., Voss M.J., Zanker K.S., et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J.M., Amaya C., Rains S., et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS ONE. 2013;8:e60021. doi: 10.1371/journal.pone.0060021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T., Hafner C., Bross K., et al. Antiangiogenetic therapy with pioglitazone, rofecoxib, and metronomic trofosfamide in patients with advanced malignant vascular tumors. Cancer. 2003;98:2251–2256. doi: 10.1002/cncr.11775. [DOI] [PubMed] [Google Scholar]
- Von Mehren M., Rankin C., Goldblum J.R., et al. Phase 2 Southwest Oncology Group-directed intergroup trial (S0505) of sorafenib in advanced soft tissue sarcomas. Cancer. 2012;118:770–776. doi: 10.1002/cncr.26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen V.W., MacKenzie K.L. Modeling human endothelial cell transformation in vascular neoplasias. Dis. Model. Mech. 2013;6:1066–1079. doi: 10.1242/dmm.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer J., Jung C.K., Shackel I., Williams P.M. Development and validation of real-time quantitative reverse transcriptase–polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal. Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Yap T.A., Sandhu S.K., Workman P., de Bono J.S. Envisioning the future of early anticancer drug development. Nat. Rev. Cancer. 2010;10:514–523. doi: 10.1038/nrc2870. [DOI] [PubMed] [Google Scholar]
- Young R.J., Brown N.J., Reed M.W., Hughes D., Woll P.J. Angiosarcoma. Lancet Oncol. 2010;11:983–991. doi: 10.1016/S1470-2045(10)70023-1. [DOI] [PubMed] [Google Scholar]