Abstract
Background
Type 2 diabetes (T2D) is a risk factor for dysregulation of glomerular filtration rate (GFR) and albuminuria. However, whether the association is causal remains unestablished.
Research Design and Methods
We performed a Mendelian Randomization (MR) analysis in 11,502 participants aged 40 and above, from a well-defined community in Shanghai during 2011–2013, to explore the causal association between T2D and decreased estimated GFR (eGFR) and increased urinary albumin-to-creatinine ratio (uACR). We genotyped 34 established T2D common variants in East Asians, and created a T2D-genetic risk score (GRS). We defined decreased eGFR as eGFR < 90 ml/min/1.73 m2 and increased uACR as uACR ≥ 30 mg/g. We used the T2D_GRS as the instrumental variable (IV) to quantify the causal effect of T2D on decreased eGFR and increased uACR.
Results
Each 1-standard deviation (SD, 3.90 points) increment in T2D_GRS was associated with decreased eGFR: odds ratio (OR) = 1.18 (95% confidence interval [CI]: 1.01, 1.30). In the MR analysis, we demonstrated a causal relationship between genetically determined T2D and decreased eGFR (OR = 1.47, 95% CI: 1.15, 1.88, P = 0.0003). When grouping the genetic loci according to their relations with either insulin secretion (IS) or insulin resistance (IR), we found both IS_GRS and IR_GRS were significantly related to decreased eGFR (both P < 0.02). In addition, T2D_GRS and IS_GRS were significantly associated with Log-uACR (both P = 0.04).
Conclusion
Our results provide novel evidence for a causal association between T2D and decreased eGFR by using MR approach in a Chinese population.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; 2 h PG, 2-hour post-loading plasma glucose; GRS, genetic risk score; HDL, high-density lipoprotein; HWE, Hardy–Weinberg equilibrium; HOMA, homeostasis model assessment; IR, insulin resistance; IS, insulin secretion; IV, instrumental variable; LDL, low-density lipoprotein; MR, Mendelian Randomization; OGTT, oral glucose tolerance test; OR, odds ratio; RCT, randomized controlled trial; SBP, systolic blood pressure; SNP, single nucleotide polymorphism; TC, total cholesterol; T2D, type 2 diabetes; TG, triglyceride; uACR, urinary albumin-to-creatinine ratio
Keywords: Type 2 diabetes, Genetic epidemiology, Albuminuria, Renal function, Causal modeling
Highlights
-
•
A MR study was conducted to explore the causal association between T2D and decreased renal function.
-
•
Weighted T2D genetic risk score (GRS) was used as the instrumental variable (IV).
-
•
A higher T2D_GRS was associated with higher risk of decreased glomerular filtration rate (GFR).
-
•
T2D estimated by the IV may causally associate with risk of decreased renal function in the MR analysis.
Epidemiological and clinical studies show that type 2 diabetes (T2D) is a risk factor for dysregulation of kidney function and albuminuria, intensive diabetes treatment was associated with better renal outcomes, suggesting a possible causal link between T2D and renal outcomes. Recently, the Mendelian Randomization (MR) approach using genetic variants as the instrumental variable has been widely used for assessing causality in population studies, because the genetic alleles are allocated randomly during gamete formation and are inherited independent of potential confounding factors and represented as a life-long exposure, which could oppose the bias such as confounding or reverse causation, or short-term intervention. We found that in 11,502 community dwelling Chinese adults, a genetic risk score representing the susceptibility to T2D but not other metabolic traits was significantly associated with decreased renal function, in dependent of obesity, lipids and high blood pressure. These findings for the first time provided novel evidence for a causal relationship between genetically determined T2D and decreased kidney function by using MR.
1. Introduction
With a rapid rise in number of type 2 diabetes (T2D) patients, diabetic nephropathy has become the leading cause of chronic kidney disease (CKD). In recent decades, CKD has been an important public health problem in both developed and developing countries (Levey and Coresh, 2012, Zhang et al., 2012). The evaluation of diabetic nephropathy depends on assessment of two markers, albumin excretion rate and glomerular filtration rate (GFR) (Jerums et al., 2009). It is generally believed that microalbuminuria is an early clinical manifestation of diabetic nephropathy, and that decreased GFR occurs secondarily, mainly in individuals with longstanding diabetes (Reutens, 2013). Randomized controlled trials (RCTs) have also shown that intensive diabetes treatment was associated with better renal outcomes, including lowering risk of albuminuria and increasing estimated GFR (eGFR) (de Boer et al., 2011, DCCT/EDIC research group, 2014, de Boer, 2014), suggesting a possible causal link between diabetes and renal outcomes. However, because conventional epidemiological studies are subject to a variety of bias such as confounding or reverse causation, and the RCTs are largely limited by short-term intervention, other novel approaches are needed to investigate the causal relation between T2D and renal dysfunction.
Recently, the Mendelian Randomization (MR) approach has been widely used for assessing causality in population studies (Jansen et al., 2014, Ding et al., 2009). Because the genetic alleles are allocated randomly during gamete formation and are inherited independent of potential confounding factors and represented as a life-long exposure, using the genetic variants as the instrumental variable (IV) have become a widely-used approach for causal inference (Lawlor et al., 2008).
In the present study, we performed a MR analysis to explore the causal relation between T2D and decreased eGFR and increased uACR, in a large community-based sample of Chinese participants. In order to reduce the statistical errors with multi-testing, we created a T2D genetic risk score (GRS) by using 34 T2D associated common variants that were identified in East Asians and represented the comprehensive genetic susceptibility of T2D, to be used as the IV (Palmer et al., 2012, Liu and Song, 2010). In addition, we also explored the genetic variations according to their roles in insulin secretion and insulin resistance.
2. Material and Methods
2.1. Study Participants
The present analysis was one part of an ongoing study of the Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study, which is a large, nationwide, prospective study involving 259,657 community-dwelling adults, aged 40 years or older. Details of the study rationale and profile have been published elsewhere (Ning, 2012, Bi et al., 2014). The study participants were recruited from two nearby communities at Baoshan district in Shanghai during 2011 and 2013. A standard questionnaire was used to collect information about lifestyle factors, disease and medical history. Anthropometric measurements, 75-g oral glucose tolerance tests (OGTT) were performed to determine the glucose metabolism status; blood and urine samples were collected for the measurements of interests.
11,935 participants (average age 63.5 years and 35.6% men) were recruited, in which genotype information was available in 11,837 ones (99.2%). Individuals with missing information on eGFR or urinary albumin-to-creatinine ratio (uACR) (n = 95) were excluded. We further excluded the participants who were missing more than two variants (n = 240). Thus, a total of 11,502 participants were included in the final analysis. The Institutional Review Board of Rui-Jin Hospital affiliated to Shanghai Jiao Tong University School of Medicine approved the study protocol. Written informed consent was obtained from each participant.
2.2. Anthropometric Information and Biochemical Measurements
A standard questionnaire was used to collect the social demographic information, the history of chronic diseases and medications, and lifestyle factors such as smoking and drinking status and physical activity. The current smoking or drinking was defined as 'yes' if the subject smoked at least one cigarette or consumed alcohol at least once a week in the past 6 month. Physical activity at leisure time was estimated by using the short form of the International Physical Activity Questionnaire (IPAQ) (Hagstromer et al., 2006). Trained investigators measured body height and body weight. Body mass index (BMI) was calculated as weight in kilograms divided by height squared in meters (kg/m2). Systolic and diastolic blood pressures (SBP and DBP) were measured in triplicate on the same day after at least ten-min rest by using an automated electronic device (OMRON Model HEM-752 FUZZY, Omron Company, Dalian, China), and the average value of the three measurements was used for analysis. The SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg, or those who were taking anti-hypertension medications were diagnosed as hypertension.
Fasting and 2-hour post-loading plasma glucose (FPG and 2 h PG) were measured by using hexokinase method on a clinical chemistry diagnostic system (C16000, Abbott Laboratories, Otawara-shi, Japan). Serum fasting insulin was measured by using the immunoassay diagnostic system (I2000, Abbott Laboratories, Dallas, USA). The homeostasis model assessment of insulin resistance index (HOMA-IR) was calculated as fasting insulin (μIU/ml) × fasting glucose (mmol/l) / 22.5 (Matthews et al., 1985). The homeostasis model assessment of β cell function or insulin secretion (HOMA-β) was calculated by using the formula: HOMA-β = [20 ∗ fasting insulin (uIU/ml)] / [fasting glucose (mmol/l) − 3.5] (Cersosimo et al., 2014). According to the 1999 World Health Organization diagnostic criteria (Alberti and Zimmet, 1998), type 2 diabetes was defined as FPG ≥ 7.0 mmol/l and/or 2 h-OGTT PG ≥ 11.1 mmol/l and/or treatment with anti-diabetic medication and/or previously diagnosed diabetes by physicians. Fasting serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-cholesterol) and low-density lipoprotein cholesterol (LDL-cholesterol) were measured by using the clinical chemistry diagnostic system (C16000, Abbott Laboratories, Otawara-shi, Japan).
2.3. Assessment of Decreased eGFR and Increased uACR
The abbreviated Modification of Diet in Renal Disease (MDRD) formula recalibrated for Chinese was used to estimate GFR (Ma et al., 2006). The formula was: eGFR = 186 × [serum creatinine × 0.011]− 1.154 × [age]− 0.203 × [0.742 if female] × 1.233, where 1.233 was the adjusting coefficient for Chinese and eGFR was expressed in ml/min/1.73 m2. Decreased eGFR was defined as eGFR < 90 ml/min/1.73 m2 (Inker et al., 2014), with mildly decreased eGFR defined as eGFR of 60–89 ml/min/1.73 m2, and moderately to severely decreased eGFR defined as eGFR < 60 ml/min/1.73 m2.
Urinary albumin and creatinine concentrations were determined in the first-void sterile urine samples in the early morning by rate nephelometry (Beckman Coulter, Fullerton, CA) and alkaline nitroxanthic acid method (Beckman LX/20, Brea, CA), respectively. The increased uACR was defined as a uACR ≥ 30 mg/g, with micro-albuminuria defined as a uACR of 30–299 mg/g, and macro-albuminuria as a uACR ≥ 300 mg/g (Reutens, 2013).
2.4. Selection of Genetic Loci, Genotyping, and Genetic Risk Score Construction
On considering the population specificity of genetic background, the selected variants or single nucleotide polymorphisms (SNPs) were either discovered in Europeans and replicated in East Asians (Cho et al., 2012a, Kato, 2013) or those identified and validated in meta-analysis including GWASs from East Asians (Cho et al., 2012b), including: PROX1 rs213985913, BCL11 rs60357484, GCKR rs27518370, IRS1 rs226229029, IGF2BP2 rs185793899, PPARG rs12351626, PSMD6 rs64062621, UBE2E2 rs23294959, MAEA rs1316113, CDKAL1 rs20685255, ZFAND3 rs38139068, DGKB rs15024684, GCC1/PAX4 rs127524904, JAZF1 rs28140937, SLC30A8 rs117172544, TP53INP1 rs94948283, CDKN2A/B rs22134095, GLIS3 rs4287466, PTPRD rs8879118, CDC123/CAMK1D rs12272998, CDC123/CAMK1D rs12286011, HHEX/IDE rs92703125, TCF7L2 rs112998590, CENTD2 rs72722053, KCNQ1 rs2670241, KCNQ1 rs2818521, KCNJ11 rs17387083, SPRY2 rs80143021, C2CD4A/C2CD4B rs62104190, FTO rs53779455, TCF2 (HNF1B) rs37738049, SRR rs2312964, PEPD rs33402102 and FITM2/R3HT2DL/HNF4A rs44318326 (Supplementary Table 1). They all reached a genome-wide significance level (P < 5 × 10− 8) and no linkage disequilibrium relationship existed among the above loci (r2 = 0.000, except it was 0.055 between rs10906115 and rs12779790 in CDC123/CAMK1D) according to the data of East Asian ancestries in the International HapMap release 21. For the GRS construction, we assumed the additive genetic model (Palmer et al., 2012) for each variant, applying a linear weighing of 0, 1, and 2 to genotypes containing 0, 1, or 2 risk alleles, respectively. The weighted GRS was calculated by weighting each risk allele with the effect size (the natural log of the odds ratios [ORs]) for risk of T2D summarized in the literature (Cho et al., 2012a). We excluded the participants who were missing more than two variants (n = 240). Thus, a total of 11,502 participants were included in the final analysis. With those who were missing one or two variants, we assigned them the average GRS. Using these 34 variants, we created a GRS ranging from 21.02–49.39 (weighted) and 22.00–48.42 (un-weighted). All the results in the present study were by using the weighted GRS, and the un-weighted GRS was used in the sensitivity analysis.
Additionally, we grouped the genetic variations according to their associations with insulin secretion or insulin resistance (Supplementary Table 2). Among the 34 variants, 16 were found significantly associated with Log-HOMA-β and 6 were associated with Log-HOMA-IR. Using the 16 insulin secretion related variants, we created a weighted IS-GRS ranging from 6.48 to 25.60 based on weighting each allele with the effect size (β) on association with Log-HOMA-β; and using the 6 insulin resistance related variants to create a weighted IR-GRS ranging from 3.20 to 12.00 based on weighting each allele with the effect size (β) on association with Log-HOMA-IR.
Blood white cells were collected for DNA extractions by using commercial blood genomic DNA extraction kit (OSR-M102-T1, TIANGEN BIOTECH CO, LTD, Beijing, China) on an automated nucleic acid extraction instrument (OSE-M48, TIANGEN BIOTECH CO, LTD, Beijing, China) according to the manufacturer's standard protocol. Specific assays were designed using the MassARRAY Assay Design software package (v3.1) (https://www.agenacx.com/Home). Mass determination was carried out with the MALDI-TOF mass spectrometer and data acquisition was used MassARRAY Type 4.0 software (SEQUENOM, CapitalBio Corporation, Beijing, China). The minimum call rate was 98.7%. The concordance rate is more than 99% based on 100 duplicates genotyping. Most of the SNPs were in Hardy–Weinberg equilibrium (HWE).
2.5. Statistical Analysis
SAS version 9.3 (SAS Institute, Cary, NC) was used for database management and statistical analysis. Serum TG, HOMA-IR and HOMA-β were normalized by logarithmic transformation because of skewed distributions. Continuous variables were given as age-, sex- and BMI-adjusted means ± standard error [SE]. Categorical variables were shown in proportions. Linear regression analysis and Cochran–Armitage trend chi-square test were used to test for trends across the T2D_GRS quartiles for continuous variables and categorical variables. Multivariable-adjusted linear regression models were fitted to evaluate the association of T2D-, IR- and IS-GRS with Log-eGFR and Log-uACR, respectively. Multivariable logistic regression models were used to assess the associations between the GRSs and decreased eGFR and increased uACR. The adjustment included age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l). Multinomial logistic regression models were fitted to assess the associations of T2D-GRS with mildly and moderately to severely decreased eGFR, as well as micro- and macro-albuminuria. Statistical significance was set to a two-sided P value of less than 0.05.
In the MR analysis, we used the IV estimators to measure the strength of the causal relationship between T2D and decreased eGFR and increased uACR. The IV estimate of causal OR was derived by using the Wald-type estimator (Fall et al., 2013) and then exponentiating to express as an OR. The computational formula was ORIV = exp (Ln (OR GRS − outcome) / Ln (OR GRS − T2D)), in which Ln (OR GRS − outcome) was βGRS - outcome and Ln (OR GRS − T2D) was βGRS - T2D. The SE for the IV estimators was estimated using the delta method.
Based on these estimates, we appeal to standard-normal asymptotics, with the resulting Wald test statistic and 95% confidence intervals (CIs) given as
The P-value for the H0: βIV = 0 was derived from the standard normal distribution. For the ORs associated with dichotomous traits (decreased eGFR or increased uACR), the 95% CI estimates were back-transformed through the exponentiation.
The IV estimates were adjusted for age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l).
3. Results
3.1. Characteristics of Study Participants
The characteristics of individual variants included in the T2D_GRS and their associations with T2D were shown in Supplementary Table 1. Mean value of the weighted T2D_GRS was 34.53 with a standard deviation (SD) of 3.90 (Supplementary Figure 1). Sociodemographic and clinical characteristics of participants according to quartiles of weighted T2D_GRS were displayed in Table 1. Among the 11,502 participants, 4101 (35.7%) were men and 2869 (24.9%) were T2D patients. The average age was 63.3 years (SD, 13.5). The levels of serum creatinine and uACR were increasing across T2D_GRS quartiles, while the eGFR levels were decreasing. The prevalence of decreased eGFR was increasing across the T2D_GRS quartiles (P for trend = 0.02).
Table 1.
Characteristics of the participants according to quartiles of weighted T2D_GRS.
| n | T2D_GRS |
P for trend |
|||
|---|---|---|---|---|---|
| Quartile 1 |
Quartile 2 |
Quartile 3 |
Quartile 4 |
||
| 2875 | 2875 | 2877 | 2875 | / | |
| Age, years | 63.2 ± 0.3 | 63.4 ± 0.3 | 63.3 ± 0.3 | 63.2 ± 0.3 | 0.91 |
| Male, n, % | 990 (34.3) | 981 (34.1) | 1056 (36.7) | 1074 (37.4) | 0.005 |
| Current smoker, n (%) | 395 (14.6) | 391 (14.5) | 411 (15.1) | 438 (16.1) | 0.10 |
| Current drinker, n (%) | 240 (8.9) | 245 (9.1) | 264 (9.8) | 280 (10.3) | 0.04 |
| BMI, kg/m2 | 25.44 ± 0.07 | 24.36 ± 0.07 | 25.20 ± 0.07 | 25.11 ± 0.07 | 0.0004 |
| FPG, mmol/l | 5.78 ± 0.03 | 5.97 ± 0.03 | 6.09 ± 0.03 | 6.20 ± 0.03 | < 0.0001 |
| 2 h PG, mmol/l | 8.32 ± 0.07 | 8.82 ± 0.07 | 9.06 ± 0.07 | 9.40 ± 0.07 | < 0.0001 |
| Log-HOMA-IR | 0.47 ± 0.01 | 0.49 ± 0.01 | 0.50 ± 0.01 | 0.50 ± 0.01 | 0.02 |
| Log-HOMA-β, % | 4.13 ± 0.01 | 4.06 ± 0.01 | 4.02 ± 0.01 | 3.97 ± 0.01 | < 0.0001 |
| Log-TG, mmol/l | 0.29 ± 0.009 | 0.28 ± 0.009 | 0.28 ± 0.009 | 0.29 ± 0.009 | 0.98 |
| Total cholesterol, mmol/l | 4.93 ± 0.02 | 4.94 ± 0.02 | 4.95 ± 0.02 | 4.96 ± 0.02 | 0.44 |
| SBP, mm Hg | 136.3 ± 0.4 | 136.9 ± 0.4 | 137.2 ± 0.4 | 137.2 ± 0.4 | 0.06 |
| DBP, mm Hg | 77.5 ± 0.2 | 77.2 ± 0.2 | 77.4 ± 0.2 | 76.9 ± 0.2 | 0.28 |
| Hypertension, n (%) | 1680 (58.5) | 1644 (57.2) | 1655 (57.5) | 1648 (57.4) | 0.47 |
| Diabetes, n (%) | 527 (18.3) | 689 (24.0) | 756 (26.3) | 897 (31.2) | < 0.0001 |
| Log-Creatinine, mg/dl | 4.17 ± 0.003 | 4.18 ± 0.003 | 4.18 ± 0.003 | 4.19 ± 0.003 | 0.04 |
| Log-eGFR, ml/min/1.73 m2 | 4.77 ± 0.004 | 4.76 ± 0.004 | 4.75 ± 0.004 | 4.75 ± 0.004 | 0.04 |
| Log-uACR, mg/g | 2.16 ± 0.02 | 2.19 ± 0.02 | 2.21 ± 0.02 | 2.23 ± 0.02 | 0.02 |
| Decreased eGFR, n (%) | 231 (8.0) | 257 (8.9) | 264 (9.2) | 283 (9.8) | 0.02 |
| Increased uACR, n (%) | 306 (10.6) | 318 (11.1) | 331 (11.5) | 340 (11.8) | 0.13 |
Data are age-, sex- and BMI-adjusted means ± standard errors (SE) for continuous variables or proportions for categorical variables. P for trend was calculated from the age-, sex- and BMI-adjusted linear regressions and Cochran–Armitage trend chi-square tests, respectively. T2D-GRS: type 2 diabetes genetic risk score; BMI: body mass index; FPG: fasting plasma glucose; 2 h PG: 2 h post-loading plasma glucose; HOMA-IR: homeostasis model assessment of insulin resistance; HOMA-β: homeostasis model assessment of β cell function; TG: triglyceride; uACR: urinary albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate. Decreased eGFR was defined as eGFR < 90 ml/min/1.73 m2; increased uACR was defined as uACR ≥ 30 mg/g.
3.2. Associations of T2D-GRS with Decreased eGFR and Increased uACR
As shown in Table 2, per SD (3.90 points) increase in T2D_GRS was associated with 12% increased risk of decreased eGFR after adjustment for age, sex and BMI (95% CI: 1.04, 1.20, P = 0.001). Further adjustment for current smoking and current drinking, physical activity, hypertension and serum lipids did not appreciably change the results (OR = 1.12, 95% CI: 1.04, 1.20, P = 0.002). The categorical analysis showed similar results. Compared with the lowest quartile of T2D_GRS, the second, third and highest quartiles were associated with 15%, 19% and 34% increased risk of decreased eGFR, respectively, after adjustment for age, sex, BMI and other covariates (P for trend = 0.005). We did not find positive results between T2D_GRS and increased uACR after adjusted for age, sex, BMI and other covariates. The associations of each SNP with decreased eGFR and increased uACR were shown in Supplementary Table 3.
Table 2.
The associations of weighted T2D_GRS with decreased eGFR and increased uACR.
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Decreased eGFR, eGFR < 90 ml/min/1.73 m2 | ||||
| Continuous variable of T2D_GRS, per SD, 3.90 | ||||
| 1.12 (1.04, 1.20) | 0.001 | 1.12 (1.04, 1.20) | 0.002 | |
| Categorical variable of T2D_GRS | ||||
| Q1 | 1.00 | 0.004 | 1.00 | 0.005 |
| Q2 | 1.16 (0.95, 1.41) | 1.15 (0.94, 1.41) | ||
| Q3 | 1.19 (0.98, 1.44) | 1.19 (0.97, 1.45) | ||
| Q4 | 1.33 (1.10, 1.61) | 1.34 (1.10, 1.12) | ||
| Increased uACR, uACR ≥ 30 mg/g | ||||
| Continuous variable of T2D_GRS, per SD, 3.90 | ||||
| 1.05 (0.99, 1.12) | 0.09 | 1.05 (0.98, 1.11) | 0.16 | |
| Categorical variable of T2D_GRS | ||||
| Q1 | 1.00 | 0.04 | 1.00 | 0.09 |
| Q2 | 1.06 (0.89, 1.25) | 0.99 (0.83, 1.19) | ||
| Q3 | 1.12 (0.95, 1.33) | 1.10 (0.92, 1.31) | ||
| Q4 | 1.17 (0.99, 1.38) | 1.13 (0.95, 1.35) | ||
Data are odds ratios (ORs) and 95% confidence intervals (CIs). P values were calculated from the multivariable-adjusted logistic regression models. Model 1, is adjusted for age, sex and BMI; Model 2, further adjusted for current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l). T2D_GRS: type 2 diabetes genetic risks score; eGFR: estimated glomerular filtration rate; uACR: urinary albumin-to-creatinine ratio; SD: standard deviation; Q1: quartile 1; Q2: quartile 2; Q3: quartile 3; Q4: quartile 4.
3.3. Associations of T2D with Decreased eGFR and Increased uACR: The MR Analysis
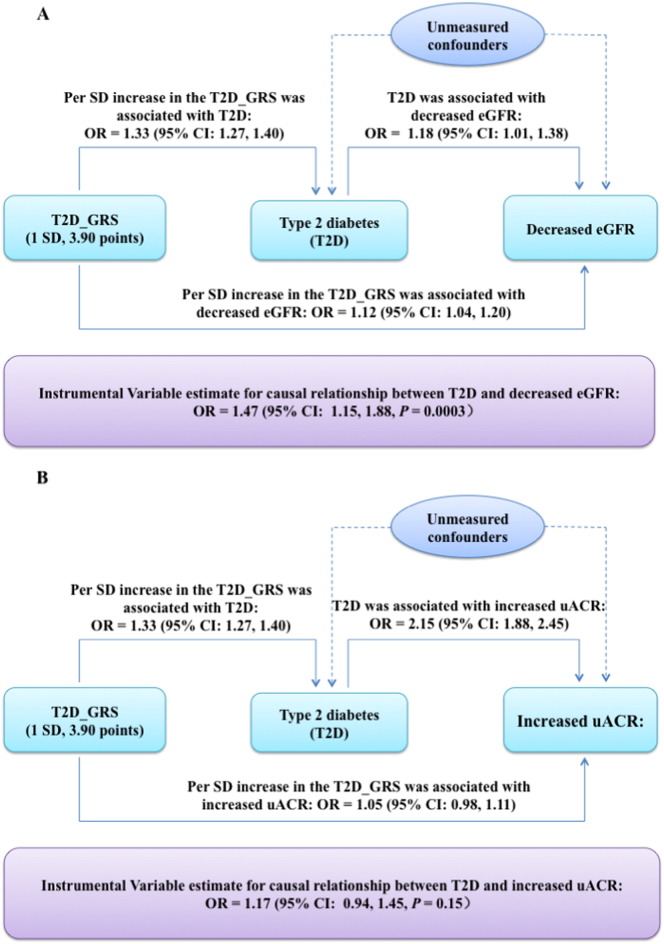
Fig. 1 Panel A shows the comparison of the observed and the IV causal estimated association of T2D with decreased eGFR and increased uACR. T2D was significantly associated with decreased eGFR OR = 1.18 (95% CI: 1.01, 1.38), after adjustment for age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l). In the MR analysis, we found a significant causal relationship between genetically determined T2D and decreased eGFR, with the OR of 1.47 (95% CI, 1.15, 1.88, P = 0.0003).
Fig. 1.
Observed versus the IV estimated association of T2D with decreased eGFR and increased uACR. In the MR framework, T2D_GRS-T2D association is assumed to be independent of confounders. All the analyses were adjusted for age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l).
Panel B shows the comparison of the observed and the IV causal estimated association of T2D with increased uACR. The observational analysis showed that T2D was significantly associated with increased uACR, OR = 2.15 (95% CI: 1.88, 2.45) after same adjustments as Panel A. While the MR analysis did not show a significant causal association, with the causal OR of genetically determined T2D for increased uACR, OR = 1.17 (95% CI: 0.94, 1.45).
We also showed the IV estimated results for associations of each individual SNP with decreased eGFR and increased uACR in Supplementary Table 4. The results were similar with the association study in Supplementary Table 3.
3.4. Association of Function-specific GRS with Decreased eGFR and Increased uACR
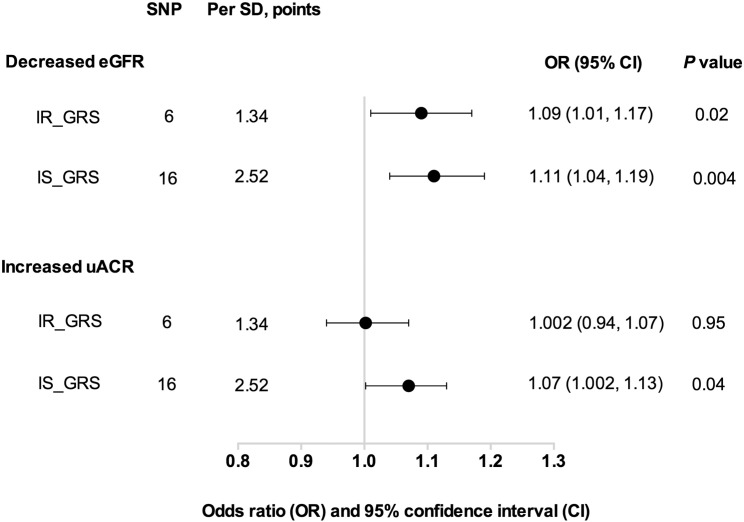
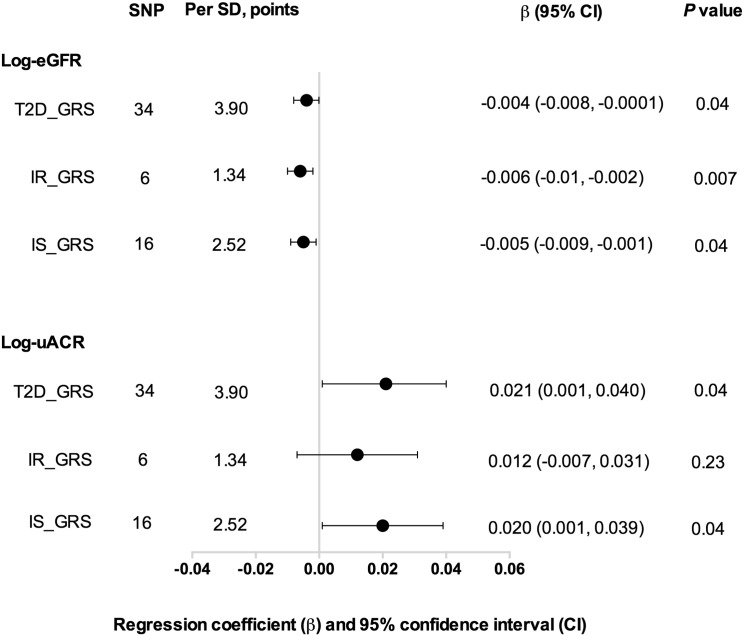
Fig. 2 shows that per SD (2.52 points) increase in IS_GRS was significantly associated with decreased eGFR, OR = 1.11 (95% CI: 1.04, 1.19, P = 0.004) and increased uACR, OR = 1.07 (95% CI: 1.002, 1.13, P = 0.04), after adjustment of age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l). Similarly, we found a significant association between IR_GRS with decreased eGFR (OR = 1.09, 95% CI: 1.01, 1.17, P = 0.02); while no significant association was found with increased uACR (P = 0.95). The IS_GRS was associated with Log-eGFR and Log-uACR (both P = 0.04). However, the IR_GRS was only associated with Log-eGFR (P = 0.007), but not Log-uACR (P = 0.23, Fig. 3).
Fig. 2.
Associations of IS_GRS and IR_GRS with decreased eGFR and increased uACR IS_GRS: insulin secretion associated genetic risk score; IR_GRS: insulin resistance associated genetic risk score. Data were presented as odds ratios (ORs) and 95% confidence intervals (CIs). P values were calculated from a multivariable logistic regression models after adjustment for age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l).
Fig. 3.
The associations of different GRSs with log-eGFR and log-uACR. Data are presented as regression coefficient (β) and 95% confidence interval (CI). P values were calculated from multivariable linear regression models, after adjustment for age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l). T2D_GRS: type 2 diabetes genetic risk score; IR_GRS: insulin resistance associated genetic risk score; IS_GRS: insulin secretion associated genetic risks score; eGFR: estimated glomerular filtration rate; uACR: urinary albumin-to-creatinine ratio.
When classified decreased eGFR as mildly decreased and moderately to severely decreased eGFR, we found that the T2D_GRS, IS_GRS and IR_GRS were significantly related to mildly decreased eGFR (all P ≤ 0.03), but not moderately to severely decreased eGFR (Supplementary Table 5). When classified increased uACR into micro- and macro-albuminuria, we found that both T2D_GRS and IS_GRS was significantly associated with macro-albuminuria (P ≤ 0.02), but not micro-albuminuria. We did not find any positive relationship of IR_GRS with micro-albuminuria or with macro-albuminuria (Supplementary Table 5).
3.5. Sensitivity Analysis
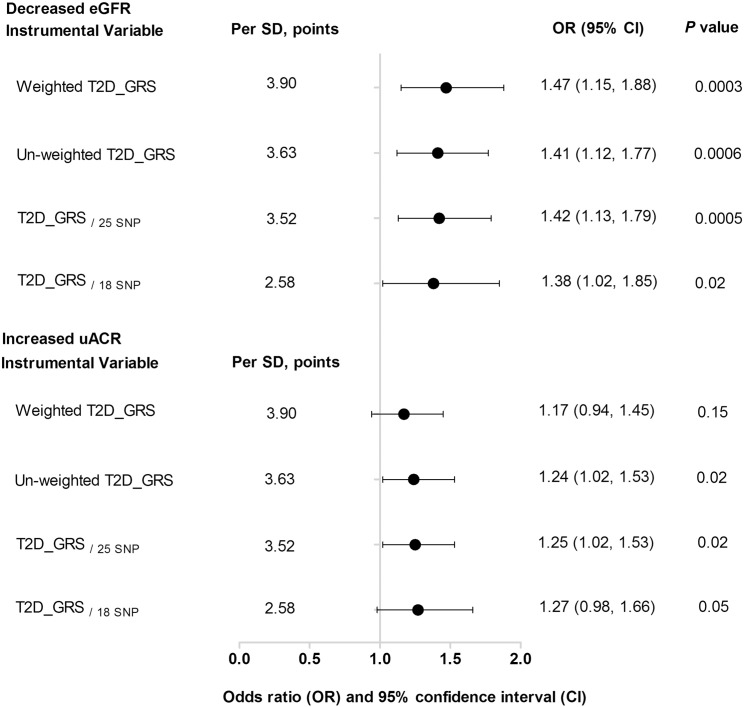
In agreement with the conjecture, we observed a consistent and significant association between genetically determined T2D and decreased eGFR with an OR of 1.41 (95% CI: 1.12, 1.77, P = 0.0006) using the un-weighted T2D-GRS as the IV, as shown in Fig. 4. When using the un-weighted T2D_GRS as the IV, we found that genetically determined T2D was causally associated with increased uACR (OR = 1.24, 95% CI: 1.02, 1.53, P = 0.02), though we did not find a causal association using the weighted T2D_GRS as the IV. We excluded the 9 SNPs that are not in HWE (Supplementary Table 1, rs1801282, rs831571, rs6467136, rs864745, rs13266634, rs89654, rs17584499, rs111187 and rs1552224) and created a new T2D_GRS/25 SNP with a mean of 26.10 (SD, 3.52). In the MR analysis, after adjustment of age, sex, BMI, current smoking, current drinking, physical activity, hypertension, total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides, the IV estimate for causal relationship between T2D and decreased eGFR was, OR = 1.42 (95% CI: 1.13–1.79, P = 0.0005) (Fig. 4 and Supplementary 2 Panel A).
Fig. 4.
Sensitivity analysis of the associations of genetically determined T2D with decreased eGFR and increased uACR. Data were presented as odds ratios (ORs) and 95% confidence intervals (CIs). P values were adjusted for age (years), sex, BMI (kg/m2), current smoking (yes or no), current drinking (yes or no), physical activity (mild, moderate or vigorous), hypertension (yes or no), total cholesterol (mmol/l), HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l) and triglycerides (mmol/l). T2D_GRS: type 2 diabetes genetic risk score; SD: standard deviation. T2D_GRS/25 SNP was calculated from the SNPs after excluding those were out of Hardy–Weinberg equilibrium (rs1801282, rs831571, rs6467136, rs864745, rs13266634, rs89654, rs17584499, rs111187 and rs1552224). T2D_GRS/18 SNP was calculated using the SNPs after excluding those may have potential pleiotropic effects (rs5215, rs4402960, rs1111875, rs243021, rs231362, rs10906115, rs3786897, rs9356744, rs17817449, rs6017317, rs17584499, rs2191349, rs1552224, rs7612463, rs13266634 and rs780094).
The T2D_GRS was found to relate to BMI and SBP, in addition to FPG, OGTT-2 h PG, HOMA-IR and HOMA-β (Supplemental Table 6). To lessen the potential influence of pleiotropic effects of the IV, we performed sensitivity analysis by excluding the 16 SNPs linking to BMI, SBP, DBP, TC and TG (Supplemental Table 7, rs5215, rs4402960, rs1111875, rs243021, rs231362, rs10906115, rs3786897, rs9356744, rs17817449, rs6017317, rs17584499, rs2191349, rs1552224, rs7612463, rs13266634 and rs780094), the T2D_GRS composing the SNPs only associated with T2D was associated with decreased eGFR (Supplementary Figure 3, Panel A). The instrumental variable estimate for causal relationship of T2D and decreased eGFR was: OR = 1.38 (95% CI: 1.02, 1.85, P = 0.02) (Fig. 4 and Supplementary 3).
4. Conclusions
In the present investigation including 11,502 community dwelling Chinese adults, we found that T2D_GRS composing of 34 T2D common variants discovered in East Asians was significantly associated with decreased eGFR. To the best of our knowledge, the results for the first time provided novel evidence for a causal relationship between genetically determined T2D and decreased kidney function by using MR.
The MR analysis is a recent improvement in genetic epidemiology (Jansen et al., 2014, Ding et al., 2009, Didelez and Sheehan, 2007). The independent distribution of alleles/genotypes means that the association of a disease with a genetic variation would not be affected by confounders (Qi, 2009). Moreover, because the random assignment of alleles/genotype transferred from parent to offspring occurs at the time of gamete formation, the association between a genetic variation and a disease is free of reverse causation. Besides, MR study is linked to a natural RCT according to the methodology and has particular superiority in assessing the effects of long-term or lifetime exposures (Qi, 2009). Previous MR studies have provided significant evidence to support causal links between biomarkers, traits and cardiovascular diseases (Davey Smith and Hemani, 2014, Jensen et al., 2013, Rasmussen-Torvik et al., 2011). Our results were consistent with the latest two MR studies which have provided a causal relationship between diabetes and coronary artery disease using diabetes GRS as the IV (Ross et al., 2015, Ahmad et al., 2015). Our study, for the first time, provided potential causal evidence between genetically determined T2D and mild renal sufficiency assessed by decreased eGFR using the MR approach.
Earlier stages of renal insufficiency can be detected through serum creatinine and eGFR (K/DOQI, 2002). Individuals with T2D and reduced eGFR have been shown to be at high risk of cardiovascular events, independent of albuminuria and metabolic control (So et al., 2006). Very recently, one study reported that serum urate was causally associated with improved renal function using GRS of uric acid transporters as the IV, implying the mechanism of the potential renal function protection mediated by xanthine oxidase inhibitors (Hughes et al., 2014). In the present investigation, we found that T2D_GRS was significantly associated with decreased eGFR, especially mildly decreased eGFR. Intriguingly, consistent results were obtained when restricting the MR analysis to genetic variations related to decreased insulin secretion and insulin resistance, suggesting that dysfunction in both insulin secretion and insulin sensitivity may causally affect renal insufficiency. Though further replications in other populations are needed, our findings suggest that that long-term treatment with T2D may be beneficial for prevention of diabetic renal impairment.
Measurement of urinary albumin excretion can identify patients with kidney damage (K/DOQI, 2002). Microalbuminuria is now considered as a marker for early diabetic nephropathy (Viberti et al., 1982) and an independent predictor for cardiovascular disease and mortality (Dinneen and Gerstein, 1997). Macroalbuminuria is the common manifestation in progressive renal disease, and is viewed as a measurement of the severity and determinant for diabetic renal disease progression (K/DOQI, 2002). In our present study, we only found a significant association between T2D_GRS and macroalbuminuria, but not microalbuminuria. Despite this, we also found that T2D_GRS was linearly related to Log-uACR. When using the un-weighted T2D_GRS, IS_GRS and GRS excluding those SNPs not in HWE (T2D_GRS/25 SNP) as the IVs, we found a causal association between genetically determined T2D and increased uACR in the sensitivity analysis. Thus, our data also suggest potential causal relation between genetically determined T2D and albuminuria. Additional larger sample size MR studies or systemically functional investigations are needed to confirm this conjecture.
Several assumptions should be well met in performing a MR analysis (Qi, 2009). Firstly, the IV must not be correlated with confounders. In our study, the T2D_GRS was found to be associated with diabetes and diabetes-associated traits, but not with smoking, or drinking habits, physical activity, lipids or blood pressure, which are major risk factors for the renal disease. Nevertheless, the T2D_GRS was found to be associated with BMI and sex. To lessen the potential influence of pleiotropic effects of the IV, we examined the association of each SNP with other traditional factors related to decreased eGFR and increased uACR, such as BMI, SBP, DBP, TG and TG and performed sensitivity analysis by excluding those SNPs that associated with these metabolic traits. The IV estimated causal relationship remained significant. Secondly, the IV was strongly associated with exposure of interest. All variants used in this study have previously been shown to be strong associated with T2D in large meta-analysis of GWAS and could mostly be replicated in our present study. Thirdly, the IV related to outcome only through the exposure of interest. There should be no direct effect of genotype on disease or any other mediated effectors other than through the exposure of interest. Though this assumption is considered largely untestable, further adjustments of other potential confounders/mediators did change the results slightly in our analysis. We hypothesized that the association between genetically determined T2D with risk of decreased GFR is due to a direct consequence of being diabetes. We could not exclude the possibility that the associations of the genetic variants with T2D and with decreased eGFR are through completely different mechanisms. Pathway analysis in large meta-GWAS study or animal studies is warranted for verifying this assumption.
The strength of our study included the relative large sample size, well-defined community setting and the highly homogeneous population, the MR study design and a created T2D_GRS representing the combined effect of the established common genetic variants of T2D used as the IV. Several limitations should be acknowledged. Firstly, we used serum creatinine estimated GFR as marker of renal function, which thought to be usually accurate when GFR is below 60 ml/min and less accurate at normal or high GFR (Jerums et al., 2009). The serum creatinine estimated GFR is widely used in the epidemiological studies due to good availability. Nevertheless, new methods such as the use of cystatin-C-based equations for estimating GFR would be considered in the future analysis. Secondly, the T2D_GRS only consisted of the validated common variants, which was considered to represent limited diabetes heritability so far. We were unable to assess the potential contribution of rare variants. Thirdly, though this study provided insights into the potentially causal effect of lifetime exposure of hyperglycemia, further investigations are needed to illustrate the precise mechanisms. Fourthly, because of the cross-sectional design, our findings may suggest the association is causal, further studies in prospective cohorts are needed to verify our findings. Finally, we created the T2D_GRS by using the common variants robustly associated with T2D in East Asians; it needs to be cautious to generalize the findings to other ethnicities or ethnic groups.
In conclusion, we found that a higher T2D_GRS was associated with higher risk of decreased eGFR. This analysis provides evidence for the biologically plausible causal relationship between genetically determined T2D and renal insufficient. Additional studies are needed to validate our findings and elucidate the mechanisms behind these findings.
Funding
This work was supported by grants from the China National Clinical Research Center for Metabolic Diseases (2013BAI09B13), the National High Technology Research and Development Program of China (863 Program) (2012AA020101), the 973 Program of China (2015CB553600), the National Natural Science Foundation of China (81561128019, 81471059, 81471062, 81321001, 81390352 and 81270877), the Joint Research Program for Important Diseases of the Shanghai Municipal Commission of Health and Family Planning (2013ZYJB1002), the Shu Guang Project of Shanghai Municipal Education Commission and Shanghai Education Development Foundation (12SG21) and the Gaofeng Clinical Medicine Grant Support from the Shanghai Municipal Education Commission (20152508). The funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Author Contributions
Min Xu designed the study, performed analysis, wrote the manuscript and contributed to the discussion. Yufang Bi contributed to the discussion and reviewed/edited the manuscript. Ya Huang researched data, performed analysis and wrote the manuscript. Lan Xie researched data and reviewed the manuscript. Mingli Hao researched data. Zhiyun Zhao researched data and reviewed/edited the manuscript. Yu Xu reviewed/edited the manuscript. Jieli Lu reviewed/edited the manuscript. Yuhong Chen reviewed/edited the manuscript. Yimin Sun researched data and reviewed the manuscript. Lu Qi contributed to the discussion, reviewed/edited the manuscript. Weiqing Wang reviewed/edited the manuscript. Guang Ning designed the study, contributed to the discussion, reviewed/edited the manuscript, and takes full responsibility for the work as a whole.
Conflict of Interest Statement
None declared.
Acknowledgments
We thank all the study participants for their participation and contribution.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.02.032.
Contributor Information
Yimin Sun, Email: ymsun@capitalbio.com.
Guang Ning, Email: gning@sibs.ac.cn.
Appendix A. Supplementary Data
Supplementary material.
References
- Ahmad O.S., Morris J.A., Mujammami M., et al. A Mendelian randomization study of the effect of type-2 diabetes on coronary heart disease. Nat. Commun. 2015;6:7060. doi: 10.1038/ncomms8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti K.G., Zimmet P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Bi Y., Lu J., Wang W., et al. Cohort profile: risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J. Diabetes. 2014;6:147–157. doi: 10.1111/1753-0407.12108. [DOI] [PubMed] [Google Scholar]
- de Boer I.H. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:24–30. doi: 10.2337/dc13-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer I.H., Sun W., Cleary P.A., et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N. Engl. J. Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo E., Solis-Herrera C., Trautmann M.E., et al. Assessment of pancreatic beta-cell function: review of methods and clinical applications. Curr. Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.S., Chen C.H., Hu C., et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat. Genet. 2012;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.S., Lee J.Y., Park K.S., et al. Genetics of type 2 diabetes in East Asian populations. Curr. Diab. Rep. 2012;12:686–696. doi: 10.1007/s11892-012-0326-z. [DOI] [PubMed] [Google Scholar]
- Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DCCT/EDIC research group Effect of intensive diabetes treatment on albuminuria in type 1 diabetes: long-term follow-up of the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications study. Lancet Diabetes Endocrinol. 2014;2:793–800. doi: 10.1016/S2213-8587(14)70155-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelez V., Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 2007;16:309–330. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- Ding E.L., Song Y., Manson J.E., et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneen S.F., Gerstein H.C. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch. Intern. Med. 1997;157:1413–1418. doi: 10.1001/archinte.1997.00440340025002. [DOI] [PubMed] [Google Scholar]
- Fall T., Hägg S., Mägi R., et al. The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med. 2013;10:e1001474. doi: 10.1371/journal.pmed.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstromer M., Oja P., Sjostrom M. The International Physical Activity Questionnaire (IPAQ). A study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- Hughes K., Flynn T., de Zoysa J., et al. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int. 2014;85:344–351. doi: 10.1038/ki.2013.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inker L.A., Astor B.C., Fox C.H., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- Jansen H., Samani N.J., Schunkert H. Mendelian randomization studies in coronary artery disease. Eur. Heart J. 2014;35:1917–1924. doi: 10.1093/eurheartj/ehu208. [DOI] [PubMed] [Google Scholar]
- Jensen M.K., Bartz T.M., Djousse L., et al. Genetically elevated fetuin-A levels, fasting glucose levels, and risk of type 2 diabetes: the cardiovascular health study. Diabetes Care. 2013;36:3121–3127. doi: 10.2337/dc12-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerums G., Panagiotopoulos S., Premaratne E., et al. Integrating albuminuria and GFR in the assessment of diabetic nephropathy. Nat. Rev. Nephrol. 2009;5:397–406. doi: 10.1038/nrneph.2009.91. [DOI] [PubMed] [Google Scholar]
- K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- Kato N. Insights into the genetic basis of type 2 diabetes. J. Diabetes Investig. 2013;4:233–244. doi: 10.1111/jdi.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.A., Harbord R.M., Sterne J.A., et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- Liu S., Song Y. Building genetic scores to predict risk of complex diseases in humans: is it possible? Diabetes. 2010;59:2729–2731. doi: 10.2337/db10-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.C., Zuo L., Chen J.H., et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Ning G., Reaction Study Group Risk evaluation of cancers in Chinese diabetic individuals. A longitudinal (REACTION) study. J. Diabetes. 2012;4:172–173. doi: 10.1111/j.1753-0407.2012.00182.x. [DOI] [PubMed] [Google Scholar]
- Palmer T.M., Lawlor D.A., Harbord R.M., et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012;21:223–242. doi: 10.1177/0962280210394459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L. Mendelian randomization in nutritional epidemiology. Nutr. Rev. 2009;67:439–450. doi: 10.1111/j.1753-4887.2009.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen-Torvik L.J., Li M., Kao W.H., et al. Association of a fasting glucose genetic risk score with subclinical atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes. 2011;60:331–335. doi: 10.2337/db10-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutens A.T. Epidemiology of diabetic kidney disease. Med. Clin. North Am. 2013;97:1–18. doi: 10.1016/j.mcna.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Ross S., Gerstein H.C., Eikelboom J., et al. Mendelian randomization analysis supports the causal role of dysglycaemia and diabetes in the risk of coronary artery disease. Eur. Heart J. 2015;36:1454–1562. doi: 10.1093/eurheartj/ehv083. [DOI] [PubMed] [Google Scholar]
- So W.Y., Kong A.P., Ma R.C., et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29:2046–2052. doi: 10.2337/dc06-0248. [DOI] [PubMed] [Google Scholar]
- Viberti G.C., Hill R.D., Jarrett R.J., et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–1432. doi: 10.1016/s0140-6736(82)92450-3. http://dx.doi.org/10.1016/S0140-6736(82)92450-3 [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang F., Wang L., et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.