Abstract
Tissue engineering is currently exploring new and exciting avenues for the repair of soft tissue and organ defects. Adipose tissue engineering using the tissue engineering chamber (TEC) model has yielded promising results in animals; however, to date, there have been no reports on the use of this device in humans. Five female post mastectomy patients ranging from 35 to 49 years old were recruited and a pedicled thoracodorsal artery perforator fat flap ranging from 6 to 50 ml was harvested, transposed onto the chest wall and covered by an acrylic perforated dome-shaped chamber ranging from 140 to 350 cm3. Magnetic resonance evaluation was performed at three and six months after chamber implantation. Chambers were removed at six months and samples were obtained for histological analysis. In one patient, newly formed tissue to a volume of 210 ml was generated inside the chamber. One patient was unable to complete the trial and the other three failed to develop significant enlargement of the original fat flap, which, at the time of chamber explantation, was encased in a thick fibrous capsule. Our study provides evidence that generation of large well-vascularized tissue engineered constructs using the TEC is feasible in humans.
Keywords: Soft tissue reconstruction, Adipose, Adipose-derived stem cells, Inflammation, Tissue engineering, Chamber
Highlights
-
•
Tissue engineering has the potential to offer exciting alternatives for the repair of soft tissues and organ development,
-
•
The tissue-engineering chamber has been shown to support tissue growth and the generation of specialized organs in animals.
-
•
Here we report on the use of this chamber in humans for the successful generation of new adipose tissue.
Tissue engineering has traditionally relied on the combination of cells and scaffolds, which are subsequently implanted into the patient. However, such paradigm is limited to tissues that are thin enough to rely on diffusion for survival or that have a low metabolic rate. The tissue-engineering chamber represents an interesting approach to circumvent these obstacles, as it is able to support the growth of well-vascularized blocks of specialized tissues of varying kinds. This work presents the use of such chamber in five human patients, one of which generated a large three-dimensional well-vascularized piece of adipose tissue, probably the largest and thickest engineered tissue construct reported to date.
1. Introduction
To “rob Peter to pay Paul”, has been the cornerstone of reconstructive surgery since its inception. From the earliest reports of tissue rearrangements to resurface cutaneous defects to the development of sophisticated procedures based on sound anatomical knowledge and meticulous surgical technique, soft tissue reconstruction has invariably relied on flap harvest and inset. In spite of the remarkable advances witnessed during the last decades with regard to (micro)surgical technique and the description of new flaps, donor site morbidity and complications remain unresolved issues in tissue transfer (Mennie et al., 2015). While the use of alloplastic implants and fat grafting overcome donor-site-related problems, they also present some major drawbacks. Implants offer a straightforward and relatively simple alternative with potentially excellent results, but complications are not infrequent and can sometimes bring devastating consequences. Fat grafting, on the other hand, has gained increased popularity due to the relatively abundant donor tissue, ease of the procedure and low morbidity. Nevertheless, the unpredictability of long-term graft retention and the need for additional fat injections or stem cell enriched lipoaspirate to achieve or maintain the desired volume remain a concern. (Zhou et al., 2016). The prior application of a device generating an external traction force (e.g. BRAVA ®) may improve the reliability of fat grafting by priming the underlying tissues but such a technique is not yet widespread and has not been described for reconstruction of soft tissue defects other than breast (Khouri et al., 2014, Khouri et al., 2015).
Tissue engineering has traditionally targeted the regeneration of tissues and organs essentially through two approaches: 1) the fabrication of tissues using scaffolds and cells ex vivo and their subsequent implantation in vivo; and 2) the stimulation of tissue growth directly in vivo. Through the scaffold approach, scientists and surgeons have achieved important breakthroughs in the repair of a variety of tissues in humans, including bone, bladder, nasal cartilages and trachea (Atala et al., 2006, Raya-Rivera et al., 2014, Fulco et al., 2014, Olausson et al., 2012, Henkel et al., 2013). However major hurdles persist in ensuring proper vascularization of the construct following implantation (Post et al., 2013) and this has limited the success of this approach to the engineering of either thin or metabolically low-demanding tissues. In addition, the scaffold-cell concept usually implies the extraction of cells from the patient, their processing and assembly into scaffolds in theatre or more commonly in the laboratory, and subsequent surgical implantation. The safety and ethical issues related to the ex vivo manipulation are further obstacles to success. The second approach involves growing tissues directly in vivo within a chamber space. When a vascular loop or pedicle is connected to the host's circulation and inserted into the chamber spontaneous tissue grows around the loop (Tanaka et al., 2000, Mian et al., 2000, Lokmic et al., 2007). For tissue specificity this approach usually also requires cues from the implantation of cells, scaffold matrices or growth factor manipulation. The concept presented in this paper is based on the in vivo chamber model but is essentially different from the scaffold-cell paradigm as it involves the stimulation of tissue growth directly in vivo by exploiting the organism's regenerative capacity, without involving implantation of cells, extracellular matrix or exogenous growth factors, therefore eliminating concerns about ex vivo cell/tissue manipulation. In this sense, the tissue-engineering chamber works as an internal bioreactor in which tissue expands concomitantly with the development of a robust autologous vascular network originating from the vascular pedicle (artery and vein) inside the chamber. Unlike current techniques including fat grafting or cell-based therapies, this approach is not focused towards creating an environment that supports survival of cells, but rather to expand existing differentiated tissue by hypertrophy and hyperplasia, thus having the potential of up scaling the engineering of tissues to large, thick, three-dimensional, well-vascularized clinically relevant constructs. Previously, we have reported this phenomenon in animals. Through different experimental models we and others have demonstrated that when a fat flap is placed inside a non-collapsible chamber, de novo well-vascularized adipose tissue as large as 78.5 ml can be generated and remains stable several weeks after chamber removal (Cronin et al., 2004, Dolderer et al., 2007, Dolderer et al., 2011, Findlay et al., 2011, Zhan et al., 2015). We have shown that inflammation is one of the key factors driving the generation of new tissue inside the chamber (Lilja et al., 2013). In addition, the strong angiogenic sprouting from the vascular pedicle inside the space and a mechanotransduction effect elicited by the stretch of tissues after placing the chamber are very likely to be participating in the process as well (Mian et al., 2000, Liu and Lee, 2014). In light of the promising findings in animals, we hypothesized that the TEC model might have a similar effect on tissue growth in the clinical setting. Herein we present an upscale of our research into a clinical trial providing “proof-of-concept” that adipose tissue engineering using the chamber model is feasible in humans and that large clinically relevant volumes of tissue can be generated.
2. Patients and Methods
This study was conducted under strict adherence to Australian National Health and Medical Research Council ethics guidelines and was approved by the Human Ethics Committee of St. Vincent's Hospital Melbourne (Protocol # 156/08).
2.1. Study Design and Participants
To test our hypothesis in a situation of clinical relevance, women candidates for breast reconstruction were selected according to criteria listed in Table 1. Candidates were selected on the basis that autologous tissue was unavailable, were not prepared to undergo major surgery and wished to avoid silicone implants if possible. Those with a previous history of radiotherapy were excluded from the trial selection criteria. On recruitment, patients were informed that if the procedure failed to generate sufficient tissue, they would require a silicone implant. Additionally, patients were informed that as the chamber was as yet non-biodegradable, a second stage removal procedure would be required. Five patients were recruited, their details listed in Table 2. Written informed consent was obtained from each patient after detailed explanation of the whole procedure, its potential risks and benefits.
Table 1.
Inclusion and exclusion criteria.
Inclusion criteria
|
Exclusion criteria
|
Table 2.
Patients' data. m: months; y: years; NIDDM: non-insulin dependent diabetes mellitus; WLE: wide local excision; SSM: skin-sparing mastectomy; DCIS: ductal carcinoma in situ; IGAP: inferior gluteal artery perforator flap.
| Patient | Age, y | BMI (kg/m2) | Comorbidities | Time since mastectomy | Adjuvant therapy | Oncologic surgery | Side | Previous reconstruction |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 21 | None | 42 m | None | SSM. Presents with partial contour defect after reconstruction | Left | Initial immediate expander followed by implant Infected implant replaced by free IGAP |
| 2 | 47 | 23 | NIDDM (diet controlled) | 14 y | None | Simple mastectomy | Right | Tissue expander filled to 260 cm3 10 months earlier and removed at the time of chamber insertion No implant exchange. |
| 3 | 44 | 20.1 | None | 6 m | None | Simple mastectomy | Right | None |
| 4 | 49 | 26.8 | None | 11 m | Tamoxifen | Simple mastectomy | Right | Tissue expander during trial 8 weeks prior to chamber insertion |
| 5 | 48 | 22.4 | None | 12 m | None | SSM after WLE for DCIS | Right | Tissue expander during trial 8 weeks prior to chamber insertion |
2.2. Chamber Fabrication and Surgical Procedures
Dome-shaped, hollow, 3 mm thick perforated acrylic chambers were custom-made ranging from 140 to 360 ml (Anatomics Pty Ltd., Melbourne, Australia) ml to match each patient's contralateral breast volume. An open notch at one point of the chamber rim was incorporated to allow unimpeded access for the transposed vascular pedicle of the thoracodorsal artery perforator (TAP) fat flap (Fig. 1A). To allow for the newly formed tissue to have maximum contact with surrounding vascularized tissue and integrate with the chest wall, the chamber domes were multiply perforated and they had no base. Two patients had a tissue expander placed 8 weeks before to create sufficient space for the chamber. Preoperative markings of an ipsilateral TAP flap were made and the perforators located using handheld doppler (Fig. 1B). Under general anaesthesia, mastectomy scars and expanders (when present) were removed followed by capsulectomy and pocket dissection (subcutaneous or submuscular). Next, a TAP fat flap ranging from approximately 6 to 50 ml was harvested (Fig. 1C). The size was determined by taking into consideration fat availability and minimisation of the donor defect. The flap was then tunnelled subcutaneously, and transposed onto the chest wall as a nidus for tissue regeneration. The chamber dome was inserted over the flap and secured with resorbable sutures ensuring that the pedicle had easy passage through the notch (Fig. 1D). To provide adequate coverage of the device, a submuscular plane was chosen, except in patient 1 who had the chamber placed subcutaneously for partial reconstruction. Drains were inserted and wounds were closed in a standard fashion. No immediate postoperative complications were registered and patients were discharged on postoperative day 3.
Fig. 1.
Chamber and surgical procedure. (A) Acrylic 210 cm3 chamber with notch on one side to allow for pedicle passage. (B) Preoperative markings of thoracodorsal artery perforators and TAP flap design in patient 2. Tissue expander in place post right mastectomy. (C) Harvested fat flap approximately 30 ml based on thoracodorsal system. (D) Flap transposed onto chest wall with dome shaped chamber placed on top.
2.3. Postoperative Evaluation and Chamber Removal
After the initial postoperative visit, all patients were initially followed at monthly intervals and then every three months after the third month. Magnetic resonance imaging (MRI) was performed on each patient at three and six months postoperatively.
In accordance with study design, six months after placement, the chambers were removed under general anaesthesia through the previous mastectomy incision. In one patient (patient 2) in whom significant growth of tissue was observed on MRI, the chamber was left in place for another six months by mutual agreement.
2.4. Histological Analysis
Tissue samples were obtained at the time of chamber removal and fixed in paraffin. Immunostaining with perilipin and CD31 were then performed.
3. Results
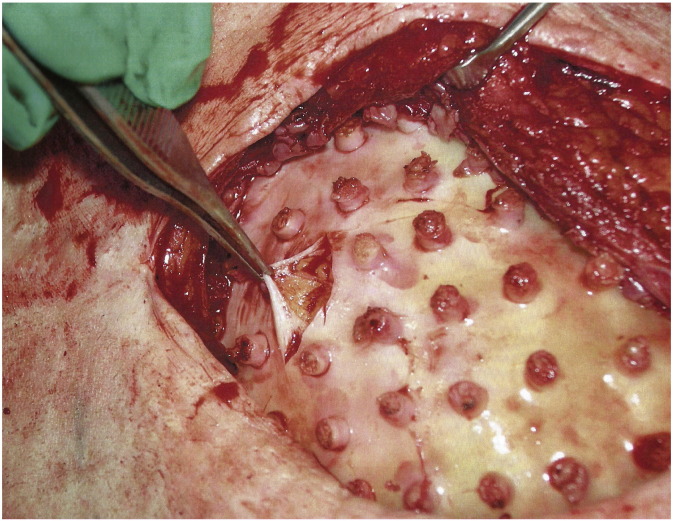
No complications such as infection, device exposure, hematoma or seroma were observed throughout the trial. All patients, except one (patient 3), tolerated the chamber adequately with early low-grade pain that resolved spontaneously without any further interventions. After chamber removal, all patients recovered uneventfully. MRI findings are summarized in Table 3. Flap and chamber details are summarized in Table 4. In patient 2, MRI at six months showed flap enlargement, so she was offered the opportunity to keep the chamber in place for another six months to allow for further growth. At 12 months, the chamber was removed and the 210 ml space was filled with tissue in its entirety (Fig. 2). While the tissue in the lateral component (i.e. next to the pedicle) had a macroscopic appearance and palpable texture very similar to that of native fat (Fig. 3), tissue in the medial aspect was firmer and looked more fibrotic. When punctured, all regions of the newly formed tissue bled, showing good vascularization of the construct. Encapsulation around the exterior of the chamber caused the flap to be embedded into a thick wall resulting in a flattening effect and reduced projection compared with the immediate post implantation appearance. Even though the final volume did not completely match the opposite breast, a decision was made not to insert an implant. At six months follow-up the tissue remained stable and had softened to a “fat-like” consistency (Fig. 4). Patient 3, who had the lowest body mass index of the cohort and consequently received the smallest chamber (140 ml), had the chamber removed at 7 weeks due to continuous pain from rubbing of the chamber rim over her rib at one point. Upon removal, the cavity was approximately half full of flimsy, haemovascular and fibrous adipose-like tissue. Once the “thread-like” tissue projections into the chamber holes were released, the tissue compacted to an estimated volume of 25 ml, which was roughly four times that of the original flap (6 ml). The piece was removed en-bloc and sent for histological analysis, showing viable fat containing perfused blood vessels (Fig. 5). A silicone implant was inserted and the postoperative course was unremarkable. Patients 1, 4 and 5 failed to grow tissue beyond the initial flap's dimensions. On exploration at six months, their chambers were densely encapsulated and embedded into the pectoral muscle. Upon removal, a thick fibrous capsule covering the flap and compressing it into a discoid flat patch was observed (Fig. 6). Histological analysis of these specimens using perilipin and CD31 staining revealed the presence of viable well-vascularized fat inside the chamber (Fig. 7). Reconstruction in these cases was carried out with silicone implants, without further complications.
Table 3.
Magnetic resonance imaging findings. N/A: not available.
| Patient | 3 months | 6 months |
|---|---|---|
| 1 | Disc-shaped fat flap lying superficial to pectoralis major with an approximate volume of 12 ml | No increase in flap growth. Evidence of well vascularized fat tissue corresponding to the flap |
| 2 | Fat flap in upper outer quadrant of chamber with an approximate volume of 20 ml. There appears to be vascularization predominantly through the holes of the chamber | Expansion of flap volume with vessels invading it via the holes of the chamber |
| 3 | N/A | N/A |
| 4 | Fat flap with an approximate volume of 27 ml. There are small vessels within the flap coming from the axilla and also vessel branching from the chest wall | Compared to previous study, there appears to have been some contraction of the fat flap with slight decrease of volume predominantly due to flattening |
| 5 | Flat discoid fat flap within the chamber with a volume of approximately 16 ml. Internal vessels are evident. | Compared to previous study, there has been little growth of the fat flap, less than 3 mm in cross sectional diameter The architecture is similar |
Table 4.
Chamber and flap details and summary of intraoperative findings upon chamber removal.
| Patient | Chamber volume (cc) | Approximate flap volume (ml) | Plane of insertion | Removal | Intraoperative findings |
|---|---|---|---|---|---|
| 1 | 150 | 12 | Subcutaneous | 6 months | No significant growth. Thick fibrous capsule covering flap |
| 2 | 210 | 30 | Submuscular | 12 months | Chamber entirely filled with tissue, which is soft and pliable in the lateral aspect and more fibrotic medially |
| 3 | 140 | 6 | Submuscular | 7 weeks | Chamber removed early because of pain. Haemovascular adipose-like tissue approximately 4 times original flap volume |
| 4 | 360 | 50 | Submuscular | 6 months | Flap encased in a thick overlying fibrous capsule |
| 5 | 250 | 30 | Submuscular | 6 months | Flap encased in a thick overlying fibrous capsule |
Fig. 2.
Intraoperative view upon chamber removal of patient 2. The 210 ml space is entirely filled with tissue. (A) Oblique lateral view. (B) Frontal view.
Fig. 3.
Detail of tissue generated in patient 2 showing adipose tissue under fibrous capsule in the lateral aspect.
Fig. 4.
Patient 2 after chamber removal. (A) Immediate postoperative result. (B) Six months follow-up. Newly grown tissue remained stable and had softened to a “fat-like” consistency.
Fig. 5.
(A) Specimen excised from patient 3 after formalin fixation. Note that in spite of early chamber removal (7 weeks), approximately a four-fold increase in tissue volume is observed. (B) Histological analysis showing viable adipose tissue as demonstrated by perilipin staining.
Fig. 6.
Intraoperative view upon chamber removal of patient 4. After removing the chamber, the space was empty, being fluid filled only, and the fat flap was seen to be encased in a thick fibrotic capsule without signs of enlargement (A). After capsulectomy, a healthy normal-looking adipose tissue flap was observed (B).
Fig. 7.
Histological analysis of encased fat excised in patient 4. (A) Perilipin staining confirms viability of adipose tissue under the fibrous capsule. (B) CD31 staining shows adipocytes surrounding perfused blood vessels.
4. Discussion
The ability to generate significant volumes of soft tissue resembling (or equal to) fat for clinical purposes would certainly have an impact on regenerative medicine. Donor site-derived issues, alloplastic-related complications and the unpredictability of fat grafting would all be addressed and eliminated, should an autologous, stable, soft, pliable, well vascularized engineered tissue become available.
The traditional paradigm of tissue engineering is based on the use of scaffolds and cells in different combinations to create tissues or enhance their growth. First-in-human studies have used this approach for the repair of tissues such as the bladder, vaginal vault, blood vessels, and nasal nostril (Atala et al., 2006, Raya-Rivera et al., 2014, Fulco et al., 2014, Olausson et al., 2012). Although attractive, we cannot rush into thinking that this approach can be readily applied to the engineering of other important tissues such as muscle or fat. The bladder wall, vaginal wall and cartilage are tissues either thin enough to rely on nutrient diffusion until neo-vascularization occurs or with a low metabolic rate that allows them to survive under hypoxic conditions. By contrast, a 210 ml volume of adipose tissue is a different challenge. Due to its thickness, dimensions and metabolic requirements, such a block of tissue needs a reliable source of blood supply from the beginning. This is achieved with the TEC: growth and expansion of tissue concomitantly with angiogenesis and the development of a reliable vasculature. This first-in-human pilot study represents an important upscale in the research and clinical application of tissue engineering, as it reports on the largest block of well differentiated vascularized tissue grown so far. Birchall and Seifalian in their Comment published in Lancet in 2014 pose the important question: Is it possible to scale up the volume of organs and tissues replaced using tissue-engineering technologies? (Birchall and Seifalian, 2014) We believe that such upscale is indeed achievable and that the TEC may hold promise for the development and growth of sizeable tissues and organs. In the current trial we have tested the chamber in a breast reconstruction setting, which is a fairly standardized model for soft tissue reconstruction. By no means the tissue-engineering chamber is specifically devised as an alternative to breast restoration but offers a solution for soft tissue defects elsewhere in the body as well as for the generation of tissues other than fat. In fact, using the TEC model, our group has been able to grow functional specialized tissues such as heart, thymus and liver, albeit with the inclusion of specific cell types (Morritt et al., 2007, Seach et al., 2010, Forster et al., 2011). Despite the unquestionable observation that human tissue did grow inside the chamber, the difference in the consistency of results compared with those obtained in animals needs further investigation. One clear difference is that small animals and pigs continue to grow throughout life and during the experiment, whilst mature human tissue is homeostatic. Additionally, wounds in animals are made de novo whereas in patients scarring from previous surgeries, might affect the tissues' vascularization and growth potential.
The exact mechanisms driving the formation of new tissue inside the chamber remain to be fully elucidated. When the chamber is placed, the tissues are inevitably stretched generating traction forces that are transmitted to the tissue inside the chamber through the shell perforations. By the phenomenon of mechanotransduction (Liu and Lee, 2014), these forces may very likely stimulate cell proliferation, migration and differentiation (Heit et al., 2012). In addition, after implantation, intra-chamber edema (fluid accumulation) ensues; previous authors have already suggested that edema stimulates adipogenesis (Brorson, 2004). Also, an angiogenic response and sprouting from the vascular pedicle is elicited by the chamber through a dual effect: by promoting an ischemic environment inside the newly created non-collapsible space and by stretching the overlying tissues. Both ischemia and tissue strain have been shown to enhance angiogenesis and result in a significant increase in blood vessel density (Huang et al., 2014). Considering that vascularization is a key aspect of tissue growth, these two phenomena surely play a fundamental role in the generation of new tissue inside the chamber. Furthermore, the surgical trauma generates an inflammatory stimulus that attracts macrophages and mesenchymal stem, which orchestrate a reparative/regenerative process. In previous experiments we have observed that inflammation, through an array of cytokines and chemoattractants including monocyte chemotactic protein-1 (MCP-1), TNF-α and Interleukin 1-ß, is an important step in adipogenesis inside the tissue-engineering chamber (Lilja et al., 2013). Lastly, the fact that patient 2 was a diabetic, might also have had an influence on the growth of tissue inside her chamber. There is evidence that diabetic patients have increased levels of circulating dickkopf-1 a potent inhibitor of the Wnt/ß-catenin signalling pathway (Lattanzio et al., 2014). Inhibition of this pathway has been regarded as an important step in the commitment of stem cells to pre-adipocytes, which eventually undergo full differentiation to functioning adipocytes (D'Alimonte et al., 2013, Ross et al., 2000).
There are several advantages of using the TEC model in humans, such as the elimination of ex vivo tissue manipulation, the avoidance of exogenous growth factors, which is paramount when dealing with cancer-related defects, and the fact that it is well tolerated and has a close resemblance to the insertion of a silicone implant. However, the fact that a significant amount of tissue was obtained in only one of the four patients who completed the trial calls for the identification of current flaws and potential improvements. One of the limiting factors for the non-successful cases seemed to be the development of a thick fibrous capsule that prevented expansion and growth of the flap. The reduction of such a capsule using antifibrotic and/or anti-inflammatory agents is therefore a potential target of improvement (DiEgidio et al., 2014). Strategies to engineer the biomaterial surfaces and/or incorporate slowly releasing anti-inflammatory molecules to reduce possible in vivo reactions to the chamber itself may also be beneficial (Go et al., 2015, Croll et al., 2006). Another hypothetical strategy that might improve the current model is the creation of a more adipogenic environment both inside and outside the chamber by: 1) enhancing the surrounding vascularity; 2) adding stromal vascular fraction (SVF) or ASCs; and 3) adding an adipogenic scaffold or matrix to stimulate flap growth (Ting et al., 2014, Poon et al., 2013).
Adequate blood supply is critical for survival of any engineered tissue. Several studies have investigated the close relationship that exists between angiogenesis and adipogenesis (Cao, 2007, Rophael et al., 2007, Hutley et al., 2001). Our previous research has shown that the vascular stimulus that drives tissue growth is derived not only from the pedicle but also from the surrounding tissues outside the chamber (Cronin et al., 2004). Hence, the presence (or the creation) of a better vascularized environment prior to chamber implantation might turn out to be more conducive of adipogenesis and tissue growth. In this sense, the BRAVA ® system described by Khouri might serve as an enhancer of our chamber model (Khouri et al., 2015). The mechanical traction generated by the device not only creates a virtual space but also elicits a profound angiogenic and adipogenic response (Lujan-Hernandez et al., 2016). Thus, it is possible that by pre-conditioning the recipient site through external mechanical traction, a more robust growth of the fat flap inside the chamber can be obtained. Multi operations in patients 1 and 5 might have reduced their tissues' vascularity and their ability to support expansion of the flap, in contrast to patient 2 and 3 who had a history of one previous surgery only. In patient 3, despite the early chamber removal, the volume of the flap was fourfold the original. Patient 4 was similar to 2 and 3 in terms of history of prior surgeries, however, at the time of chamber insertion and throughout the whole trial, she was taking tamoxifen, which has been recently shown to inhibit ASCs' proliferation and differentiation rate and is associated with decreased fat graft survival (Pike et al., 2015).
SVF and ASCs could be considered important enhancers of growth in our current model. Ex vivo expanded ASCs could potentially improve tissue growth in a way similar to that reported by Kølle et al. (Kølle et al., 2013). However, despite the reported advantages of using SVF/ASCs to enhance fat grafting, there are oncological matters that need to be further investigated before translation into human tissue engineering, as adipose-derived mesenchymal stem cells and adipocytes may promote progression and metastatic spread in breast cancer and melanoma (Rowan et al., 2014, Kwan et al., 2014).
The presence of a scaffold or matrix with an adipogenic potential able to stimulate and guide tissue growth inside the chamber could also result in a greater expansion of the flap (Ting et al., 2014, Poon et al., 2013, Wittmann et al., 2015). Additionally, a cell-homing effect for adipose and endothelial precursors by modulating the stromal-derived factor (SDF)-1/CXCR4 axis could eventually lead to a more consistent growth of the flap inside the chamber, though not without risk of cancer induction (Xu et al., 2014).
The remarkable advances in adipose tissue engineering are paving the way towards its successful clinical application. The results of this trial provide clinical evidence on the largest well-vascularized block of engineered tissue ever used in humans, along with “proof-of-concept” that generation of adipose tissue using the chamber model is clinically feasible. Undoubtedly, there are several aspects that need to be improved, and further research is warranted before definitive translation onto the clinical setting is achieved; nevertheless we believe that this report represents an important step forward on the road to human adipose tissue engineering, which is seemingly not far from reach.
Disclosure
Andrew Batty is CEO of Anatomics ®, manufacturer of the acrylic chambers.
None of the other authors has any conflicts of interest to declare.
Authors' Contribution
Wayne A. Morrison: conception and design; patient recruitment; surgical procedures; acquisition and analysis of data; preparation of manuscript.
Diego Marre: analysis of data; preparation of manuscript.
Damien Grinsell: patient recruitment and assessment; surgical procedures; revision of manuscript.
Andrew Batty: design and manufacture of chambers.
Nicholas Trost: radiological assessment; revision of manuscript.
Andrea J. O'Connor: engineering design; revision of manuscript.
Role of the Funding Source
The funding source of this study had no role in the study design, recruitment, surgical procedures or patient follow-up. Likewise, data collection, interpretation and manuscript preparation was solely the work of the authors, without any participation from the funders.
Acknowledgements
The authors would like to thank all consortium members participating in this project: O'Brien Institute, Neopec, the Department of Chemical and Biomolecular Engineering of The University of Melbourne, St. Vincent's Hospital, Anatomics and Congentum. Likewise, we would like to thank the Victorian State Government for supporting this work through the Victoria's Science Agenda.
The author would also like to thank Dr. Peter Callan, Dr. Kimberly Prince and Dr. Gillian Farrell for their help in the recruitment of patients; Dr. Angela Webb for her help with manuscript revision and as breast consultant to the Neopec board; and Dr. Heidi Debels for her contributions to the ethics preparation of this trial.
Footnotes
This work was supported in part by the Victoria's Science Agenda.
References
- Atala A., Bauer S.B., Soker S., Yoo J.J., Retik A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Birchall M.A., Seifalian A.M. Tissue engineering's green shoots of disruptive innovation. Lancet. 2014;384:288–290. doi: 10.1016/S0140-6736(14)60533-X. [DOI] [PubMed] [Google Scholar]
- Brorson H. Adipose tissue in lymphedema: the ignorance of adipose tissue in lymphedema. Lymphology. 2004;37:175–177. [PubMed] [Google Scholar]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll T.I., O'Connor A.J., Stevens G.W., Cooper-White J.J. A blank slate? Layer-by-layer deposition of hyaluronic acid and chitosan onto various surfaces. Biomacromolecules. 2006;7:1610–1622. doi: 10.1021/bm060044l. [DOI] [PubMed] [Google Scholar]
- Cronin K.J., Messina A., Knight K.R., et al. New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plast. Reconstr. Surg. 2004;113:260–269. doi: 10.1097/01.PRS.0000095942.71618.9D. [DOI] [PubMed] [Google Scholar]
- D'Alimonte I., Lannutti A., Pipino C., et al. Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. 2013;9:642–654. doi: 10.1007/s12015-013-9436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiEgidio P., Friedman H.I., Gourdie R.G., Riley A.E., Yost M.J., Goodwin R.L. Biomedical implant capsule formation: lessons learned and the road ahead. Ann. Plast. Surg. 2014;73:451–460. doi: 10.1097/SAP.0000000000000287. [DOI] [PubMed] [Google Scholar]
- Dolderer J.H., Abberton K.M., Thompson E.W., et al. Spontaneous large volume adipose tissue generation from a vascularized pedicled fat flap inside a chamber space. Tissue Eng. 2007;13:673–681. doi: 10.1089/ten.2006.0212. [DOI] [PubMed] [Google Scholar]
- Dolderer J.H., Thompson E.W., Slavin J., et al. Long-term stability of adipose tissue generated from a vascularized pedicled fat flap inside a chamber. Plast. Reconstr. Surg. 2011;127:2283–2292. doi: 10.1097/PRS.0b013e3182131c3e. [DOI] [PubMed] [Google Scholar]
- Findlay M.W., Dolderer J.H., Trost N., et al. Tissue-engineered breast reconstruction: bridging the gap toward large-volume tissue engineering in humans. Plast. Reconstr. Surg. 2011;128:1206–1215. doi: 10.1097/PRS.0b013e318230c5b2. [DOI] [PubMed] [Google Scholar]
- Forster N., Palmer J.A., Yeoh G., et al. Expansion and hepatocytic differentiation of liver progenitor cells in vivo using a vascularized tissue engineering chamber in mice. Tissue Eng. Part C Methods. 2011;17:359–366. doi: 10.1089/ten.TEC.2009.0519. [DOI] [PubMed] [Google Scholar]
- Fulco I., Miot S., Haug M.D., et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet. 2014;384:337–346. doi: 10.1016/S0140-6736(14)60544-4. [DOI] [PubMed] [Google Scholar]
- Go D.P., Palmer J.A., Mitchell G., Gras S.L., O'Connor A.J. Porous PLGA microspheres for dual delivery of biomolecules via the layer-by-layer assembly technique. J. Biomed. Mater. Res. A. 2015;103:1849–1863. doi: 10.1002/jbm.a.35319. [DOI] [PubMed] [Google Scholar]
- Heit Y.I., Lancerotto L., Mesteri I., et al. External volume expansion increases subcutaneous thickness, cell proliferation, and vascular remodeling in a murine model. Plast. Reconstr. Surg. 2012;130:541–547. doi: 10.1097/PRS.0b013e31825dc04d. [DOI] [PubMed] [Google Scholar]
- Henkel J., Woodruff M.A., Epari D.R., et al. Bone regeneration based on tissue engineering conceptions — a 21st century perspective. Bone Res. 2013;1:216–248. doi: 10.4248/BR201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Leavitt T., Bayer L.R., Orgill D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014;51:301–331. doi: 10.1067/j.cpsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Hutley L.J., Herington A.C., Shurety W., et al. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am. J. Physiol. Endocrinol. Metab. 2001;281:E1037–E1044. doi: 10.1152/ajpendo.2001.281.5.E1037. [DOI] [PubMed] [Google Scholar]
- Khouri R.K., Rigotti G., Cardoso E., Khouri R.K., Jr., Biggs T.M. Megavolume autologous fat transfer: part II. Practice and techniques. Plast. Reconstr. Surg. 2014;133:1369–1377. doi: 10.1097/PRS.0000000000000179. [DOI] [PubMed] [Google Scholar]
- Khouri R.K., Rigotti G., Khouri R.K., Jr., et al. Tissue-engineered breast reconstruction with Brava-assisted fat grafting: a 7-year, 488-patient, multicenter experience. Plast. Reconstr. Surg. 2015;135:643–658. doi: 10.1097/PRS.0000000000001039. [DOI] [PubMed] [Google Scholar]
- Kølle S.F., Fischer-Nielsen A., Mathiasen A.B., et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- Kwan H.Y., Fu X., Liu B., et al. Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J. Biol. Chem. 2014;289:30525–30537. doi: 10.1074/jbc.M114.593210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio S., Santilli F., Liani R., et al. Circulating dickkopf-1 in diabetes mellitus: association with platelet activation and effects of improved metabolic control and low-dose aspirin. J. Am. Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.001000. Jul 18. pii: e001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H.E., Morrison W.A., Han X.L., et al. An adipoinductive role of inflammation in adipose tissue engineering: key factors in the early development of engineered soft tissues. Stem Cells Dev. 2013;22:1602–1613. doi: 10.1089/scd.2012.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.S., Lee O.K. In search of the pivot point of mechanotransduction: mechanosensing of stem cells. Cell Transplant. 2014;23:1–11. doi: 10.3727/096368912X659925. [DOI] [PubMed] [Google Scholar]
- Lokmic Z., Stillaert F., Morrison W.A., Thompson E.W., Mitchell G.M. An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. FASEB J. 2007;21:511–522. doi: 10.1096/fj.06-6614com. [DOI] [PubMed] [Google Scholar]
- Lujan-Hernandez J., Lancerotto L., Nabzdyk C., et al. Induction of adipogenesis by external volume expansion. Plast. Reconstr. Surg. 2016;137:122–131. doi: 10.1097/PRS.0000000000001859. [DOI] [PubMed] [Google Scholar]
- Mennie J.C., Mohanna P.N., O'Donoghue J.M., Rainsbury R., Cromwell D.A. Donor-site hernia repair in abdominal flap breast reconstruction: a population-based cohort study of 7929 patients. Plast. Reconstr. Surg. 2015;136:1–9. doi: 10.1097/PRS.0000000000001398. [DOI] [PubMed] [Google Scholar]
- Mian R., Morrison W.A., Hurley J.V., et al. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000;6:595–603. doi: 10.1089/10763270050199541. [DOI] [PubMed] [Google Scholar]
- Morritt A.N., Bortolotto S.K., Dilley R.J., et al. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- Olausson M., Patil P.B., Kuna V.K., et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet. 2012;380:230–237. doi: 10.1016/S0140-6736(12)60633-3. [DOI] [PubMed] [Google Scholar]
- Pike S., Zhang P., Wei Z., et al. In vitro effects of tamoxifen on adipose-derived stem cells. Wound Repair Regen. 2015;23:728–736. doi: 10.1111/wrr.12322. [DOI] [PubMed] [Google Scholar]
- Poon C.J., Pereira E., Cotta M.V., Sinha S., et al. Preparation of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater. 2013;9:5609–5620. doi: 10.1016/j.actbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Post M.J., Rahimi N., Caolo V. Update on vascularization in tissue engineering. Regen. Med. 2013;8:759–770. doi: 10.2217/rme.13.74. [DOI] [PubMed] [Google Scholar]
- Raya-Rivera A.M., Esquiliano D., Fierro-Pastrana R. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;384:329–336. doi: 10.1016/S0140-6736(14)60542-0. [DOI] [PubMed] [Google Scholar]
- Rophael J.A., Craft R.O., Palmer J.A., et al. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am. J. Pathol. 2007;171:2048–2057. doi: 10.2353/ajpath.2007.070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S.E., Hemati N., Longo K.A., et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Rowan B.G., Gimble J.M., Sheng M., et al. Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seach N., Mattesich M., Abberton K., et al. Vascularized tissue engineering mouse chamber model supports thymopoiesis of ectopic thymus tissue grafts. Tissue Eng. Part C Methods. 2010;16:543–551. doi: 10.1089/ten.TEC.2009.0135. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tsutsumi A., Crowe D.M., Tajima S., Morrison W.A. Generation of an autologous tissue (matrix) flap by combining an arteriovenous shunt loop with artificial skin in rats: preliminary report. Br. J. Plast. Surg. 2000;53:51–57. doi: 10.1054/bjps.1999.3186. [DOI] [PubMed] [Google Scholar]
- Ting A.C., Craft R.O., Palmer J.A., et al. The adipogenic potential of various extracellular matrices under the influence of an angiogenic growth factor combination in a mouse tissue engineering chamber. Acta Biomater. 2014;10:1907–1918. doi: 10.1016/j.actbio.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Wittmann K., Dietl S., Ludwig N., et al. Engineering vascularized adipose tissue using the stromal-vascular fraction and fibrin hydrogels. Tissue Eng. A. 2015;21:1343–1353. doi: 10.1089/ten.TEA.2014.0299. [DOI] [PubMed] [Google Scholar]
- Xu F.T., Li H.M., Yin Q.S., et al. Human breast adipose-derived stem cells transfected with the stromal cell-derived factor-1 receptor CXCR4 exhibit enhanced viability in human autologous free fat grafts. Cell. Physiol. Biochem. 2014;34:2091–2104. doi: 10.1159/000366404. [DOI] [PubMed] [Google Scholar]
- Zhan W., Chang Q., Xiao X., et al. Self-synthesized extracellular matrix contributes to mature adipose tissue regeneration in a tissue engineering chamber. Wound Repair Regen. 2015;23:443–452. doi: 10.1111/wrr.12292. May-Jun. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang J., Li H., et al. Efficacy and safety of cell-assisted lipotransfer; a systematic review and meta-analysis. Plast. Reconstr. Surg. 2016;137:44e–57e. doi: 10.1097/PRS.0000000000001981. [DOI] [PubMed] [Google Scholar]