Abstract
The incidence of Alzheimer's disease (AD) is growing every day and finding an effective treatment is becoming more vital. Amyloid-β (Aβ) has been the focus of research for several decades. The recent shift in the Aβ cascade hypothesis from all Aβ to small soluble oligomeric intermediates is directing the search for therapeutics towards the toxic mediators of the disease. Targeting the most toxic oligomers may prove to be an effective treatment by preventing their spread. Specific targeting of oligomers has been shown to protect cognition in rodent models. Additionally, the heterogeneity of research on Aβ oligomers may seem contradictory until size and conformation are taken into account. In this review, we will discuss Aβ oligomers and their toxicity in relation to size and conformation as well as their influence on inflammation and the potential of Aβ oligomer immunotherapy.
Keywords: Amyloid-β, Oligomers, Size, Toxicity, Inflammation, Immunotherapy
Highlights
-
•
An inverse correlation exists between oligomer size and toxicity after a critical mass is reached.
-
•
Protein aggregates propagate through prion-like mechanisms and preventing this propagation is possible through immunotherapy.
-
•
There may be an interplay between inflammation and oligomer induced toxicity and formation.
1. Introduction
According to the World Alzheimer Report 2015, it was estimated that 46.8 million people worldwide were living with dementia in 2015 and this number is expected to double every 20 years (Alzheimer's disease International: World Alzheimer Report). Thus, dementia can be considered a global threat which imposes a tremendous burden on society and the economy of the world. According to the Alzheimer's Association, Alzheimer's disease (AD) is the most common cause of dementia accounting for almost 60 to 80% of dementia cases and is the sixth leading cause of death in the United States. In 2015, the national cost of AD was expected to be $220 million which by 2050 could reach up to $1 trillion (Alzheimer's, 2015). The very thin line delineating normal aging processes and AD have led to the consideration of another category, mild cognitive impairment (MCI), since both are associated with memory loss (Mucke, 2009). Neuronal degeneration starts much earlier than the manifestation of the symptoms and clinical diagnosis. The National Institute on Aging and the Alzheimer's Association has revised the criteria and guidelines which will better identify the disease progression from its preclinical stage to the clinical stage (Sperling et al., 2011). AD is an irreversible, progressive degeneration of brain cells associated with deterioration of memory that hampers the affected person's day to day life. The molecular basis of this disease pathology is highly debated. However, the abnormal accumulation of amyloid-β (Aβ) into senile plaques and tau proteins into hyperphosphorylated neurofibrillary tangles has been pathologically identified and unequivocally accepted as the two major hallmarks of this disease. There are many risk factors for developing AD pathology with age being the greatest one. Diabetes, hypertension, and inflammation are also risk factors for AD (Shinohara et al., 2014). Additionally, there are genetic risk factors for AD including the APOE ɛ4 allele (Bertram et al., 2010). An ever-growing incidence of AD has led researchers and clinicians to search for a cure. Studies performed over decades have come to a consensus that the generation of toxic Aβ is a key event driving AD pathogenesis. Aβ is a 38 to 43 amino acid long peptide generated by the sequential proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretases (Chow et al., 2010). Even though the exact physiological function of APP is not currently identified, it is well established that the over-production of Aβ generated from APP plays a role in AD development. Apart from the over-production, there were two other pathways shown to regulate Aβ levels in the AD brain: clearance or degradation of the Aβ produced and its re-entry into the brain. Therefore, any imbalance in the dynamic equilibrium maintained by these three pathways would result in the accumulation of Aβ (Yoon and Jo, 2012, Mucke, 2009). Mutations in genes coding for key proteins also pose as a risk factor for the development of AD pathology leading to the formation of amyloids. Almost 25 autosomal dominant mutations in the APP gene have been found to be pathogenic in familial AD (Jonsson et al., 2012). Two other important genes, preselinin-1 and preselinin-2, were found to be associated with cases of familial AD. Mutations in these two genes alter the proteolytic cleavage of APP, thereby producing a more aggregation-prone isoform of Aβ (De Strooper et al., 2012).
For a considerable period of time, research in the AD field mainly encompassed the study of senile plaques composed primarily of Aβ which is one of the characteristic hallmarks of AD and explained by an earlier version of the amyloid cascade hypothesis. It relied on the fact that Aβ is released in the extracellular space where it accumulates into senile plaques leading to the formation of neurofibrillary tangles of tau protein and causing vascular damage, cell loss, and dementia (Hardy and Higgins, 1992). But researchers were intrigued by the lack of correlation between the manifestation of the disease and the plaque burden. Neuronal death also occurred in brain regions devoid of plaques. It was then discovered that Aβ plaques were present in cognitively normal individuals (Erten-Lyons et al., 2009, Sloane et al., 1997). The existence of non-demented individuals with advanced AD neuropathology demonstrates that plaque burden does not correspond to cognition or degeneration. These individuals had significant plaque burden but no memory impairments or changes in brain volume (Erten-Lyons et al., 2009). Larger insoluble aggregates, such as Aβ plaques, did not induce memory impairment in the absence of oligomers in Tg2576 mice that develop plaque pathology and behavioral deficits at 9–10 months old. In fact, a reduction in oligomer levels corresponded to improved memory in these mice (Lesné et al., 2008, Hsiao et al., 1996). Soluble Aβ oligomers, on the other hand, have been shown to produce cognitive deficits in the absence of plaques (Gandy et al., 2010). Moreover, there are individuals who appear to have AD but show no pathological changes in their brains demonstrating that larger aggregates are not essential to cognitive impairment (Petersen et al., 2013). These results suggest that larger aggregates are not responsible for neurodegeneration and that the smaller soluble oligomers are the toxic species of Aβ. It was then that the focus of research shifted to an alternate entity, soluble oligomers of Aβ. Electron microscopy and atomic force microscopy revealed that the toxic soluble oligomers are spherical in shape ranging from about 3 to 10 nm. These spheroidal structures come together forming strings of beads, termed as protofibrils which also possess toxic effects (Glabe, 2006). In addition, Aβ1–42 protofibrils but not fibrils were shown to stimulate microglial production of tumor necrosis factor α suggesting a role for soluble Aβ aggregates in stimulating inflammatory responses and toxicity (Paranjape et al., 2012). Oligomers of different proteins were reported to take a common sequence-independent conformation which suggests that a similar mechanism of toxicity would exist for all the amyloid diseases (Kayed and Glabe, 2006, Chiti and Dobson, 2006, Campioni et al., 2010). Aβ oligomers exert their toxicity through a variety of mechanisms including receptor and direct membrane interactions, reviewed by Kayed and Lasagna-Reeves (2013). At the same time, many amyloidogenic proteins, such as tau and α-synuclein (α-syn), also shifted from larger aggregates to oligomers as the toxic species suggesting a universal mechanism of toxicity for amyloid proteins such as tau (Gerson and Kayed, 2013), α-syn (Sengupta et al., 2015), and TAR DNA-binding protein 43 (TDP-43) (Choksi et al., 2014, Fang et al., 2014). We have previously demonstrated that apart from their individual oligomeric assemblies, different pathogenic proteins also co-aggregate or form hybrid oligomers including tau with α-syn and TDP-43 with Aβ, α-syn, and cellular prion protein (PrPc) in AD patients (Sengupta et al., 2015, Guerrero-Munoz et al., 2014b). For the purpose of this review we define oligomers as trimers of Aβ or larger with a conformation distinct from fibrils. In this review we will discuss oligomer size and conformation in relation to the current literature on toxicity, inflammation, and immunotherapy with a focus on Aβ but extending to other amyloid oligomers where the mechanisms seem universal. We will emphasize the role of spreading oligomers in the progression of AD.
2. Size, Stability, and Toxicity
A substantial number of investigations aiming to unravel the mechanism of the disease have accepted an intermediate structure of aggregated Aβ which is formed earlier than plaques and has been linked to the pathogenesis (Kayed and Lasagna-Reeves, 2013, Klein, 2013, Hardy and Selkoe, 2002). Despite various isoforms of Aβ, with differing propensities for aggregation, three major groups of Aβ assemblies exist. These three assemblies of Aβ are comprised of monomers, soluble oligomers, and insoluble fibrils which have been termed as ‘Aβ pools’. Each pool again encompasses multiple structures of Aβ aggregation based on various organizations (reviewed by Goure et al. (2014)). Soluble Aβ oligomers have been reported in AD to be organized into different structures ranging from dimers (Walsh et al., 2000), trimers (Walsh et al., 2000, Chen and Glabe, 2006), tetramers (Walsh et al., 2000, Chen and Glabe, 2006), pentamers and decamers (Ahmed et al., 2010), Aβ-derived diffusible ligands (ADDLs) (Hepler et al., 2006, Lambert et al., 1998), dodecamers, and Aβ*56 (Lesne et al., 2006).
Toxic soluble oligomers are distinct from the monomers or higher aggregates, such as fibrils, and were identified in AD brains (Kayed et al., 2003). Based on the published data, we suggest that there is an inverse correlation between the size of Aβ assemblies and the potency of their exerted toxicity. As the size of the oligomeric assembly increases, its deleterious effects decrease (Fig. 1). Aβ dimers have been shown to assemble forming a more stable structure of higher molecular weight, termed protofibrils which are neurotoxic. Thus, dimeric units of Aβ have been considered to be an important entity providing the building blocks for the toxic aggregates (O'nuallain et al., 2010, Mc Donald et al., 2015, Garzon-Rodriguez et al., 2000). On the contrary, in vivo micro-dialysis of mice that display age dependent plaque pathology did not detect the presence of the dimers at any age (Hong et al., 2011). It was also noticed that oligomers can form from secondary nucleation (Cohen et al., 2013). Recent studies have shown that there are at least 2 types of oligomers with type 1 being relatively more toxic than type 2 (Liu et al., 2015a), but there could be many more species of Aβ oligomers. Dodecamers and Aβ*56 appear to be diffuse throughout the tissue and exert toxic effects in cultures. Aβ*56 oligomers contain mainly replicates of Aβ trimers and were identified in the brains Tg2576 mice (Lesne et al., 2006). This soluble aggregate of Aβ appeared to impair memory function in these animals, irrespective of neuronal loss and led to synaptic dysfunction in humans (Lesne et al., 2013, Lesne et al., 2006, Lesné et al., 2008). Consistent with its toxic effects, these Aβ*56 oligomers were also found to correlate with markers of synapse dysfunction and levels of hyperphosphorylated tau (Zahs and Ashe, 2013). This suggests that toxicity is dependent on the size, aggregation state, and diffusion of Aβ oligomers. However, while Aβ*56 has been shown to be toxic it has not been compared to other oligomers for relative toxicity. It is important to identify these toxic oligomers and determine which are the most pathologically relevant to disease in order to target the most potent oligomers in treatment.
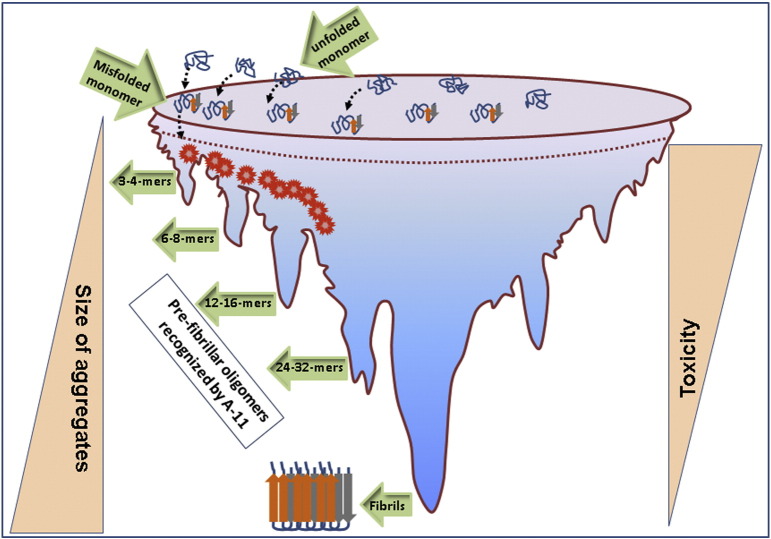
Fig. 1.
The relationship between the size of Aβ assemblies and their toxic effects. Over produced native Aβ peptides undergo misfolding and form aggregates of different sizes and shapes. As the size of the assemblies increases, the potency of their toxic effects decreases maintaining an inverse correlation. For Aβ, toxic oligomers likely range from 8-24 mers while α-syn oligomers are 6-18 mers and tau oligomers are 3-15 mers. These n-mers cause neuronal toxicity either directly or by building up the higher aggregates. As these aggregates mature more into fibrils (bottom part of the funnel), they become less potent in their toxicity.
The role of different cleavage variants, Aβ1–40 and Aβ1–42, has been under investigation for some time. They are the 2 dominant Aβ peptides produced by β-secretase and γ-secretase. In vitro, Aβ1–40 tends to be more stable in isolation and remains in the monomer stage longer before it aggregates to form fibrils. The authors also found that Aβ1–42 tends to remain in a mix of monomer, trimer, and tetramer until it aggregates into fibrils (Chang and Chen, 2014). It is notable that when the study mixed Aβ1–40 and Aβ1–42 in equimolar ratio the oligomers that formed were spherical and were the most toxic oligomers when applied to culture (Chang and Chen, 2014). Another group confirmed and extended these findings by applying Aβ1–40, Aβ1–42, and mixed Aβ oligomers onto cultured neurons and observed that the mixed Aβ1–40 and Aβ1–42 formed smaller oligomers on neurites than either pure peptide alone and that larger aggregates were observed in vitro on glass slides suggesting that the aggregation pathway is different in cell free applications versus culture (Johnson et al., 2013). These findings and others like them suggest an important role for the ratio of Aβ1–42 to Aβ1–40 in toxicity and that they interact to produce smaller, more stable, and more toxic structures. Since Aβ1–42 to Aβ1–40 ratio is known to be elevated in familial AD it could prove to be relevant in therapeutic interventions. Our laboratory recently developed and characterized a new antibody known as VIA which recognizes only Aβ1–42 oligomers. This antibody does not bind monomeric or fibrillar Aβ1–42 or any form of Aβ1–40. VIA has applications in helping us elucidate the role of Aβ1–42 oligomers in disease pathogenesis and may have potential as an immunotherapy (Bodani et al., 2015). Studies like these also suggest that Aβ oligomers form different strains, or structures depending on the specific length of the Aβ peptide and how it folds.
Membrane disruption and ion dysregulation are known components of Aβ toxicity. Aβ oligomers have also been shown to directly interact with membranes forming pores and disrupting the proper permeability of membranes (Kayed and Lasagna-Reeves, 2013). A distinct pore structured oligomer termed an annular protofibril may be responsible to this ion dysregulation. Our group showed that these annular protofibrils are associated with activated astrocytes and escape fibrillization in vitro and in vivo (Lasagna-Reeves et al., 2011, Lasagna-Reeves and Kayed, 2011). Another study used simulations to investigate oligomer insertion into a lipid bilayer and suggests that a U-shaped trimer is the smallest oligomer capable of inserting into the membrane while maintaining its structure and then assembling into pores (Jang et al., 2013). The study also suggests that monomers and dimers may insert into the membrane but require interaction with other Aβ oligomers to form the toxic pores (Jang et al., 2013). Other studies showed that Aβ oligomers exert their toxic effects through certain receptors. Data obtained from combined studies of electrophysiology, biochemistry, and immunohistochemistry showed that soluble Aβ oligomers, even at very low molecular concentration, inhibit LTP by increasing NMDA response through NR-2B containing NMDA-receptors (Li et al., 2011). These oligomers were also shown to bind to the PrPc and lead to a series of events disrupting synaptic function in hippocampal neurons (Freir et al., 2011, Lauren et al., 2009, Um et al., 2012). Another group recently showed that small Aβ1–42 oligomers cause rapid depolarization of neurons leading to the release of glutamate and excitotoxicity due to Ca2 + entry. Microglia were also shown to be depolarized by Aβ1–42 and were interestingly prevented by NMDA receptor antagonist MK801. The study also showed that Aβ oligomers may sensitize the mitochondrial permeability transition pore to Ca2 + resulting in mitochondrial dysfunction and cell death (Morkuniene et al., 2015). The mitochondria are implicated in Aβ induced toxicity through many mechanisms ranging from Ca2 + dysregulation to Aβ induced signaling and metabolism disruption (reviewed by Kaminsky et al. (2015)). These studies suggest that Aβ can interact with the membrane in potentially different ways indicating that targeting one conformation may not be enough to stop AD. Additionally, blocking Aβ oligomerization protected cells from toxicity while compounds that simply block fibril formation did not protect cells in culture (De Felice et al., 2004). Thioflavin-T (ThT) is a compound which fluoresces when it binds to Aβ fibrils and can be used to monitor fibril formation. When ThT positive fibrils were used to seed monomeric Aβ, the monomers formed fibrils quickly rather than higher order oligomers. When these ThT positive fibrils were added to a culture with monomeric Aβ, they were less toxic than when monomeric Aβ was applied alone. The authors suggested that this was due to decreased formation of oligomers (Wu et al., 2013). However, ThT-positive inert fibrils form smaller toxic aggregates by sonication (discussed later). These oligomeric aggregates are bis-ANS-positive, another widely used fluorescent dye for hydrophobicity (Guerrero-Munoz et al., 2013). Therefore, ThT-positive fibrils are less toxic compared to bis-ANS-positive oligomers (illustrated in Fig. 2). Finally, the existence of individuals who are non-demented with AD neuropathology indicates that fibrils and plaques are not toxic forms of Aβ. It has been suggested that these individuals may be resistant to cognitive decline due to a lack of Aβ oligomers binding to the synapse (Bjorklund et al., 2012). Furthermore, these studies provide further support for the strain hypothesis mentioned previously by indicating that there are different structures formed by Aβ oligomers which have differing toxicities.
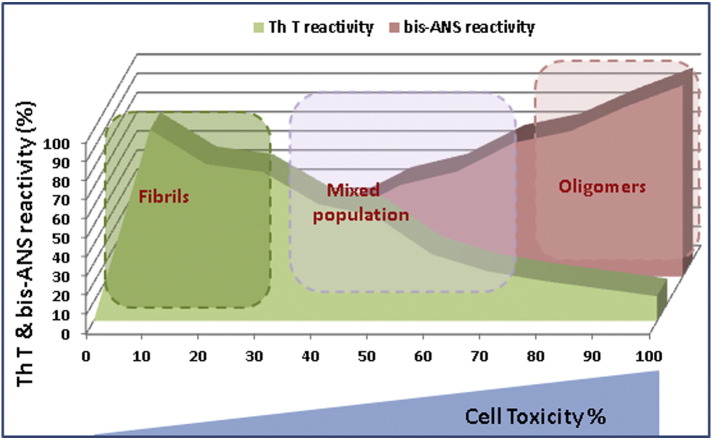
Fig. 2.
The relationship between the Aβ assemblies and their toxicity based upon binding with fluorescent probes. As bis-ANS binding decreases, ThT binding increases indicating fibril formation and decreased toxicity (in percentage). ThT and bis-ANS binding to proteins are mostly used to confirm their fibrillar or oligomeric nature, respectively (more hydrophobic). Even though ThT reacts with the prefibrillar oligomers, its strongest association occurs only in the presence of mature fibrils. Amyloid oligomers show maximum toxicity, whereas, more homogeneous fibril species (~ 100% fibrils) show less toxicity. On the other hand, bis-ANS interacts most strongly (~ 100%) with the oligomers which show the highest toxicity. As the oligomers develop into larger and more stable aggregates, bis-ANS binding diminishes and so does the toxicity.
Studies using conformational antibodies are indicating that these strains play important roles in toxicity. Sebollela et al. (2014) have shown that NU4, a conformational monoclonal antibody, protected cultured cells from Aβ induced reactive oxygen species production. The study also found that NU4 recognized a single oligomer strain at 80 kD suggesting that it was one of the toxic species of Aβ (Sebollela et al., 2014). However, the toxicity of the 80kD oligomer has not been compared to other oligomers to determine its relative toxicity. It is important to conduct these studies to determine the interrelationship between the size and the conformation in toxicity.
3. Propagation of Aβ Oligomers
Insoluble fibrils seeded larger Congo red-positive deposits which were distributed unevenly throughout the injection site, whereas, smaller soluble assemblies develop more uniformly distributed Congo red-negative deposits (reviewed by Langer et al. (2011)). These studies are in accordance with the observation that sonicated prion fibrils caused more potent seeding than unsonicated ones (Silveira et al., 2005). The observation that fibril-induced deposits are minimal compared to the more spread out development of Aβ aggregates seeded by soluble oligomeric assemblies is summarized in Fig. 3. The role of amyloid spreading in disease pathogenesis was first discovered for the neurodegenerative disease caused by the misfolding of PrPc which generated an infectious prion protein called scrapie (PrPsc). Recently, it has been shown that there are numerous species of Aβ oligomers with different propagation rates and distinct toxicity in AD pathogenesis (Musiek and Holtzman, 2015). It is suggested that the spreading of AD pathology in different regions of the brain follows a prion-like seeding mechanism for proteinaceous aggregates of Aβ and tau mediated by neurons (Walker and Jucker, 2015). Like aggregated Aβ variants, heterogeneous tau aggregates exist in different tauopathies. It was also shown that tau assemblies seed from neuron to neuron and thus the pathology was spread to multiple brain regions (Clavaguera et al., 2015). Tau oligomers, not the neurofibrillary tangles, seed and propagate in various tauopathies (Gerson and Kayed, 2013). Additionally, tau trimers were shown to be the minimal seeding unit for propagation which is also neurotoxic (Mirbaha et al., 2015). Similarly, aggregates of α-syn, the prime pathognomonic protein assemblies in synucleinopathies, seed and propagate in a prion-like manner. It holds the same for other neurodegenerative disorders (Walker and Jucker, 2015). We have previously shown that Aβ oligomers act as seeds for other proteins, α-syn, PrPc, and TDP-43 to form pathogenic oligomeric aggregates (Guerrero-Munoz et al., 2014b).
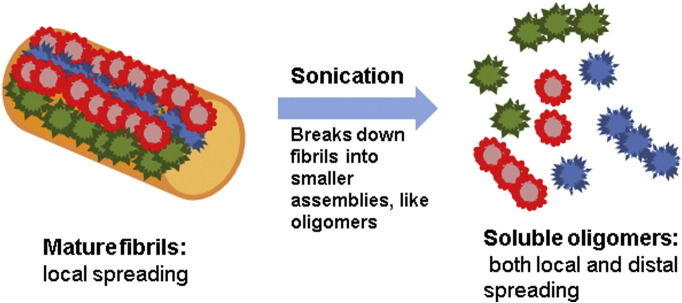
Fig. 3.
Smaller aggregates are effective and potent seeds for the spreading of Aβ. Intact mature fibrils might induce larger aggregates which are localized to the induction site. Sonicated fibrils form smaller assemblies with larger numbers of nucleation sites that seed more potently in local regions and can travel between anatomically connected regions, thus inducing aggregates in distal regions.
4. Amyloid-β Oligomers and Inflammation
Inflammation has been a known component of AD for years, however, until the last 10 years it was largely ignored as a byproduct of the disease that did not contribute to its progression. Inflammation has been shown to be anything but trivial and Aβ oligomers have been implicated in initiating the inflammatory process (Minter et al., 2015). Aβ1–42 oligomers have been suggested to promote the maturation of infiltrating peripheral monocytes into phagocytic microglia while monomeric Aβ1–42 or fibrillar Aβ1–42 did not (Crouse et al., 2009). Aβ oligomers have been associated with many membrane proteins in the synapse (Dinamarca et al., 2012) and in astrocytes and microglia (Walker et al., 2015). Many of the synaptic receptors increase the Ca2 + concentration in the post-synapse leading to inflammation and cell death. These receptors include NMDA and mGluR receptors (Dinamarca et al., 2012). In addition to aberrant Ca2 + concentrations, Aβ oligomers have been linked to the receptor for advanced glycation end products (RAGEs) which is a microglial receptor leading to the activation of pro-inflammatory pathways and was found to be elevated in AD (Walker et al., 2015). RAGE is also implicated in hypertension, cardiovascular diseases, diabetes, stroke, and traumatic brain injury which are all known risk factors for the development of AD. These risk factors are all linked to RAGE and Aβ production through its many ligands (reviewed by Matrone et al. (2015)). Furthermore, phase II clinical trials with a RAGE inhibitor called TTP448 are having beneficial effects with low toxicity in AD patients (Walker et al., 2015). Together, these studies suggest that inhibiting RAGE may have therapeutic potential in order to decrease or prevent Aβ induced inflammatory damage to the brain.
It is interesting to note that most of the current research indicates that Aβ oligomers are inducing inflammation through several receptors such as RAGE, Toll-like receptors, nod-like receptors, and formyl peptide receptors (Minter et al., 2015, He et al., 2012) but there are still conflicting reports on which strain of Aβ is inducing the inflammation (Ferrera et al., 2014). Inflammation appears prior to the formation of larger aggregates suggesting that the smaller oligomers, which form first, are initiating this inflammation. Conflicting reports about Aβ fibrils or oligomers inducing inflammation could easily be the result of different methods. Studies on in vitro microglia cultures implicate Aβ fibrils (Ferrera et al., 2014) while, in vivo mouse injections implicate Aβ oligomers in causing inflammation (He et al., 2012). Or it could be the difference in acute responses versus prolonged responses in the aforementioned studies.
Inflammation is one of the earliest signs of AD beginning prior to the formation of Aβ plaques as mentioned above. While there is evidence that Aβ is inducing the inflammation through many mechanisms such as receptor mediated or direct interaction with the membrane, it is still debated which came first: oligomer or inflammation. Understanding the sequence of events at this stage could provide insight into the mechanisms by which pathology spreads. For example, does chronic inflammation lead to the initial formation of Aβ oligomer seeds and the spread of pathology? Or perhaps as the Aβ oligomers spread and seed aggregation in new regions, they induce inflammation and accelerate degeneration. These questions are not only relevant for Aβ oligomers but other amyloids as well, especially tau. Tau oligomers are known to spread locally and via synaptic connections (Boluda et al., 2015, Ahmed et al., 2014, Asai et al., 2015). Since most amyloids are known to spread and propagate over the course of disease, the question of inflammation preceding amyloid oligomers or oligomers preceding inflammation is a relevant topic for all amyloidogenic proteins.
5. Immunotherapy
Aβ immunotherapy has been a hot topic in recent years with the failure of several passive immunotherapies against Aβ. Many of these antibodies do not discriminate between the different Aβ sizes and conformations. Some prefer monomers but still react to larger aggregates while others have targeted the plaques but also react to smaller species. First, Solanezumab is an immunotherapy which, to date, uniquely targets monomers and oligomers but not fibrils. However, Solanezumab binds the monomer with higher affinity which means that the dose required to bind oligomers is much higher (Goure et al., 2014). Preferential binding of the monomer may explain why clinical trials failed to show improvement due to the fact that they used a lower dosage which may have been saturated by monomers (Goure et al., 2014, Doody et al., 2014). A similar reason was cited for the lack of efficacy of Bapineuzumab which binds monomer and oligomers with similar affinity but since oligomers are much less prevalent the dosage used may not have been adequate to see improvements (Goure et al., 2014). Bapineuzumab and most of the other immunotherapies tested recognize fibril Aβ as well as the smaller species. Additionally, antibodies that selectively recognize Aβ1–40 or Aβ1–42, Ponezumab and BiiB037 respectively, but do not discriminate between sizes did not prove to be effective (Goure et al., 2014). Ponezumab decreased plaque burden in 20 month old Tg2576 mice indicating that it may act by disaggregating plaques which may not be beneficial (discussed later) (Carty et al., 2006). BiiB037 binds fibrils and oligomers with high affinity (Goure et al., 2014). These studies may indicate that specific targeting of one peptide, Aβ1–40 or Aβ1–42, may not be an effective target for immunotherapy or they may suffer from the same problem as other immunotherapies which are their lack of specificity for oligomeric Aβ1–40 or Aβ1–42. For a complete review on the targets of the major clinical trials see Goure et al. (2014). These trials and studies have shown that unspecific targeting or the targeting of plaques is not effective in the clinical setting. Solanezumab and Bapineuzumab indicate that targeting oligomers requires an antibody with lower affinity for the monomer, while Ponezumab and BiiB037 indicate that the size of the aggregate may be more important than the peptide isoform. This lack of specificity for the oligomer has stimulated research into more selective antibodies. With the lack of effective or safe immunotherapies, there has been a push to improve specificity with the idea that side effects can be minimized. Many researchers have managed to create monomer or fibril specific antibodies. Antibodies which target the N-terminal of Aβ can bind to fibrils and have been shown to increase the concentration of oligomers and thus increased toxicity through disaggregation (Liu et al., 2015b). Liu et al. (2015b) did not find the same increase in oligomer concentration when using C-terminal or mid-peptide recognizing antibodies. Another group tested a monomer specific antibody which was a precursor to Solanezumab. In J20 mice the Aβ concentration bound to the antibody in the blood increased but the oligomeric concentration in the cortex did not change nor did cognition improve (Mably et al., 2015). The study also tested a fibril antibody 1C22 which increased free Aβ in the blood but also did not influence cerebral oligomer levels or cognition (Mably et al., 2015). Additionally, 20% of the J20 mice immunized with either antibody died during the course of the study while control and PDAPP mice did not show the same mortality. These results indicate that extensive safety studies need to be pursued but Mably et al. (2015) did not look into the cause of death for these mice. These studies suggest that a specific oligomer antibody may be required to effectively improve cognition and decrease side effects. In 2014, a study was published which indicated the use of an oligomer specific conformation antibody eliminated synapse loss distant from plaques in Tg2576 mice and attenuated synapse loss in proximity to the plaques (Dorostkar et al., 2014). This group did not conduct behavior tests but it suggests that cognition could be improved with these antibodies. Additionally, Dorostkar et al. (2014) did not find significant changes with the IgG or pan-Aβ antibodies used. Oligomer specific antibodies need to be investigated more thoroughly to prove their efficacy and safety.
While most studies support the idea for the use of Aβ oligomer specific antibodies as therapeutics, there are some researchers arguing that these antibodies are still toxic and may increase the toxicity of Aβ oligomers. One study aiming to explain the meningoencephalitis seen in clinical trials looked at several antibodies which bind different forms of Aβ: Aβ1–40 and Aβ1–42 (Morkuniene et al., 2013). In the presence of microglia the antibody #11E12, in complex with either Aβ1–40 or Aβ1–42 oligomers, was more toxic than when oligomers were applied alone. The authors suggest that the microglia FcγR receptor was mediating this interaction (Morkuniene et al., 2013). However, it is important to note that the antibody used in this study recognized the N-terminal which has been shown to increase toxicity by disaggregating fibrillar Aβ into oligomers and they did not investigate other antibodies that recognize the C-terminal or middle sections of Aβ. The use of N-terminal antibodies could be disastrous if these mechanisms act synergistically by increasing oligomer and antibody–antigen complex concentration and increasing oligomer concentration through the disaggregation of fibrillar Aβ. However, there is another potential reason for the increased toxicity of these antibodies when complexed with oligomers. The peripheral sink hypothesis states that the antibody–antigen complex may be cleared from the brain through the blood creating a decrease, or sink, in the Aβ levels in the brain. This mechanism of clearance cannot be tested in culture since the Aβ-antibody complex remains in the media. To test these concepts, it would be interesting to see if these results can be validated in vivo through the use of microglia suppressors as well as investigating the peripheral sink hypothesis through serum analysis. Further research into immunotherapies is warranted with an emphasis on oligomer specific antibodies but many obstacles remain. Immunotherapy studies need to start including inflammation markers to better predict complications in humans. Antibodies targeting a region of the peptide sequence are not proving to be effective and cross react with too many forms of Aβ. Perhaps conformational antibodies are the key to targeting the oligomers specifically. It was suggested several years ago that the sequence may not be as important as the conformation in producing toxicity. A generic amyloid oligomer antibody proved effective at reducing plaque burden with decreased microhemorrhaging (Rasool et al., 2012). It is important to point out that this antibody selectively recognized oligomers but did not differentiate between amyloid proteins such as Aβ and tau. Rasool et al. (2012) also conducted active immunization prior to the appearance of plaques in the Tg2576 mouse model and the random sequence oligomer antigen produced less autoimmune response than active immunization with Aβ. The random sequence oligomer antigen was made from a random 20mer sequence of 8 amino acids, had low homology to human proteins, and strongly reacted with A11 oligomer specific antibody (Rasool et al., 2012). This study was followed by a report that monoclonal amyloid oligomer antibodies, made from this random sequence oligomer antigen, administered by passive immunization reduced plaque burden, reduced hyperphosphorylated tau, and improved cognition in 3xTg-AD mice (Rasool et al., 2013). To our knowledge, these studies have not been extended further in the last few years but warrant further investigation due to their efficacy and decreased inflammatory responses. Passive immunotherapy targeting oligomeric conformations is still the most promising avenue towards treating AD. Targeting toxic oligomers from different proteins with a single antibody which recognizes conformational similarities would likely be therapeutically beneficial since many disease including AD are characterized by aggregates of multiple proteins (Guerrero-Munoz et al., 2014a, Rasool et al., 2013, Kayed and Glabe, 2006, Kayed et al., 2003, Kayed and Lasagna-Reeves, 2013). Oligomer specific immunotherapy is proving to be effective in rodent and culture studies but needs to be extended into clinical trials.
Removing plaques from the brains of AD patients does not appear to be effective and the targeting of oligomeric forms of Aβ seems to be the most promising therapeutic in development. The mechanism by which these antibodies work is thought to be through the binding of extracellular oligomers preventing the spread of the pathology and they do not bind to plaques. These antibodies do not need to be able to get inside cells but can instead reduce or eliminate the cell to cell spread of Aβ oligomers preventing oligomers from seeding pathology in new regions or initiating inflammation. Other amyloids such as α-syn and tau are also showing reduced spreading and degeneration using immunotherapy suggesting that Aβ oligomers could be similar. Several studies support this idea that the antibodies work by binding extracellular amyloids and prevent the spread of the seeds into new regions (Valera and Masliah, 2015, Sankaranarayanan et al., 2015, Valera et al., 2015). Thus, these antibodies prevent the progression of the pathology. Another study showed that an antibody to α-syn protected cells in culture by binding to it and preventing it from entering the cell and seeding the endogenous α-syn (Tran et al., 2014). Preventing the spread of pathology could be key in treating AD. Additionally, combination therapies targeting Aβ oligomers along with inflammation or other amyloid oligomers could provide the edge that many of these therapies lack on their own. Such treatments have already been called for by the α-syn field and may apply to other amyloids such as Aβ (Valera and Masliah, 2015). Removing Aβ oligomers while inhibiting inflammation and reducing other amyloid oligomers could work synergistically by targeting key problem areas in this multifactorial disease.
6. Outstanding Questions
While we know more about AD and Aβ oligomers than ever before, there are still some outstanding questions that need to be answered. For example, how important are conformation, size, and their interplay in determining the toxicity of Aβ oligomers. The development of conformation specific, sequence independent antibodies is facilitating this search. Additionally, which comes first, inflammation or oligomers? Answering this question may prove to be difficult due to the potential for positive feedback in the inflammatory pathway but could have a profound impact on how we view AD. Furthermore, is inhibiting the spread of Aβ oligomers, or a subset of Aβ oligomers, sufficient to delay or stop the progression of AD or do we need to investigate multifactorial treatments. Immunotherapy targeting Aβ oligomers specifically is effective in rodents but human trials need to be conducted to determine their safety and efficacy. However, multifactorial treatments appear to be the most promising as it has recently been shown that multiple protein species spread in neurodegenerative diseases. Furthermore, immunotherapy against multiple amyloid oligomers or in combination with anti-inflammatory treatments has the potential to work synergistically. It is essential that we answer these questions in order to advance our understanding of AD and therapeutic development. Oligomer specific antibodies may be the treatment for AD we have been searching for or we may need to refine our theories further, but we cannot move forward without answering these questions. There is a current push for anti-oligomer treatments with promising results seen in animal studies but we are at the critical test for this hypothesis: will they work alone or in combination with other therapies in humans?
7. Search Strategy and Selection Criteria
Articles used for this review were identified from PubMed searches using keywords such as “amyloid-β”, “oligomers”, “conformations”, “inflammation”, “immunotherapy”, “spreading”, and “Alzheimer's disease”. Peer reviewed sources written in English were used, and news and reports were excluded.
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Urmi Sengupta, Ashley N Nilson, and Rakez Kayed contributed to the manuscript, literature review and figures.
Acknowledgments
This review is supported by the Mitchell Center for Neurodegenerative Diseases and John Sealy Memorial Fund. We are grateful to the Kayed lab members for their helpful suggestions.
References
- Ahmed M., Davis J., Aucoin D., Sato T., Ahuja S., Aimoto S., Elliott J.I., Van Nostrand W.E., Smith S.O. Structural conversion of neurotoxic amyloid-beta(1-42) oligomers to fibrils. Nat. Struct. Mol. Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Cooper J., Murray T., Garn K., Mcnaughton E., Clarke H., Parhizkar S., Ward M., Cavallini A., Jackson S., Bose S., Clavaguera F., Tolnay M., Lavenir I., Goedert M., Hutton M., O'neill M. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol. 2014;127:667–683. doi: 10.1007/s00401-014-1254-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's A. Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T., Wolozin B., Butovsky O., Kugler S., Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L., Lill C.M., Tanzi R.E. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Bjorklund N.L., Reese L.C., Sadagoparamanujam V.M., Ghirardi V., Woltjer R.L., Taglialatela G. Absence of amyloid β oligomers at the postsynapse and regulated synaptic Zn(2 +) in cognitively intact aged individuals with Alzheimer's disease neuropathology. Mol. Neurodegener. 2012;7:23-23. doi: 10.1186/1750-1326-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodani R.U., Sengupta U., Castillo-Carranza D.L., Guerrero-Munoz M.J., Gerson J.E., Rudra J., Kayed R. Antibody against small aggregated peptide specifically recognizes toxic abeta-42 oligomers in Alzheimer's disease. ACS Chem. Neurosci. 2015 doi: 10.1021/acschemneuro.5b00231. [DOI] [PubMed] [Google Scholar]
- Boluda S., Iba M., Zhang B., Raible K., Lee V.-Y., Trojanowski J. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer's disease or corticobasal degeneration brains. Acta Neuropathol. 2015;129:221–237. doi: 10.1007/s00401-014-1373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni S., Mannini B., Zampagni M., Pensalfini A., Parrini C., Evangelisti E., Relini A., Stefani M., Dobson C.M., Cecchi C., Chiti F. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 2010;6:140–147. doi: 10.1038/nchembio.283. [DOI] [PubMed] [Google Scholar]
- Carty N.C., Wilcock D.M., Rosenthal A., Grimm J., Pons J., Ronan V., Gottschall P.E., Gordon M.N., Morgan D. Intracranial administration of deglycosylated C-terminal-specific anti-Abeta antibody efficiently clears amyloid plaques without activating microglia in amyloid-depositing transgenic mice. J. Neuroinflammation. 2006;3:11. doi: 10.1186/1742-2094-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.J., Chen Y.R. The coexistence of an equal amount of Alzheimer's amyloid-beta 40 and 42 forms structurally stable and toxic oligomers through a distinct pathway. FEBS J. 2014;281:2674–2687. doi: 10.1111/febs.12813. [DOI] [PubMed] [Google Scholar]
- Chen Y.R., Glabe C.G. Distinct early folding and aggregation properties of Alzheimer amyloid-beta peptides Abeta40 and Abeta42: stable trimer or tetramer formation by Abeta42. J. Biol. Chem. 2006;281:24414–24422. doi: 10.1074/jbc.M602363200. [DOI] [PubMed] [Google Scholar]
- Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Choksi D.K., Roy B., Chatterjee S., Yusuff T., Bakhoum M.F., Sengupta U., Ambegaokar S., Kayed R., Jackson G.R. TDP-43 phosphorylation by casein kinase Iepsilon promotes oligomerization and enhances toxicity in vivo. Hum. Mol. Genet. 2014;23:1025–1035. doi: 10.1093/hmg/ddt498. [DOI] [PubMed] [Google Scholar]
- Chow V.W., Mattson M.P., Wong P.C., Gleichmann M. An overview of APP processing enzymes and products. Neruomol. Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Hench J., Goedert M., Tolnay M. Invited review: prion-like transmission and spreading of tau pathology. Neuropathol. Appl. Neurobiol. 2015;41:47–58. doi: 10.1111/nan.12197. [DOI] [PubMed] [Google Scholar]
- Cohen S.I., Linse S., Luheshi L.M., Hellstrand E., White D.A., Rajah L., Otzen D.E., Vendruscolo M., Dobson C.M., Knowles T.P. Proliferation of amyloid-beta42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9758–9763. doi: 10.1073/pnas.1218402110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse N.R., Ajit D., Udan M.L.D., Nichols M.R. Oligomeric amyloid-β(1–42) induces THP-1 human monocyte adhesion and maturation. Brain Res. 2009;1254:109–119. doi: 10.1016/j.brainres.2008.11.093. [DOI] [PubMed] [Google Scholar]
- De Felice F.G., Vieira M.N.N., Saraiva L.M., Figueroa-Villar J.D., Garcia-Abreu J., Liu R.O.Y., Chang L.E.I., Klein W.L., Ferreira S.T. Targeting the neurotoxic species in Alzheimer's disease: inhibitors of Aβ oligomerization. FASEB J. 2004;18:1366–1372. doi: 10.1096/fj.04-1764com. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Iwatsubo T., Wolfe M.S. Presenilins and gamma-secretase: structure, function, and role in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinamarca M.C., Rios J.A., Inestrosa N.C. Postsynaptic receptors for amyloid-beta oligomers as mediators of neuronal damage in Alzheimer's disease. Front. Physiol. 2012;3:464. doi: 10.3389/fphys.2012.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., Raman R., Sun X., Aisen P.S., Siemers E., Liu-Seifert H., Mohs R. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N. Engl. J. Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Dorostkar M.M., Burgold S., Filser S., Barghorn S., Schmidt B., Anumala U.R., Hillen H., Klein C., Herms J. Immunotherapy alleviates amyloid-associated synaptic pathology in an Alzheimer's disease mouse model. Brain. 2014;137:3319–3326. doi: 10.1093/brain/awu280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erten-Lyons D., Woltjer R.L., Dodge H., Nixon R., Vorobik R., Calvert J.F., Leahy M., Montine T., Kaye J. Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurology. 2009;72:354–360. doi: 10.1212/01.wnl.0000341273.18141.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y.S., Tsai K.J., Chang Y.J., Kao P., Woods R., Kuo P.H., Wu C.C., Liao J.Y., Chou S.C., Lin V., Jin L.W., Yuan H.S., Cheng I.H., Tu P.H., Chen Y.R. Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia-TDP patients. Nat. Commun. 2014;5:4824. doi: 10.1038/ncomms5824. [DOI] [PubMed] [Google Scholar]
- Ferrera D., Mazzaro N., Canale C., Gasparini L. Resting microglia react to Abeta42 fibrils but do not detect oligomers or oligomer-induced neuronal damage. Neurobiol. Aging. 2014;35:2444–2457. doi: 10.1016/j.neurobiolaging.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Freir D.B., Nicoll A.J., Klyubin I., Panico S., Mc Donald J.M., Risse E., Asante E.A., Farrow M.A., Sessions R.B., Saibil H.R., Clarke A.R., Rowan M.J., Walsh D.M., Collinge J. Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nat. Commun. 2011;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S., Simon A.J., Steele J.W., Lublin A.L., Lah J.J., Walker L.C., Levey A.I., Krafft G.A., Levy E., Checler F., Glabe C., Bilker W., Abel T., Schmeidler J., Ehrlich M.E. Days-to-criterion as an indicator of toxicity associated with human Alzheimer amyloid-β oligomers. Ann. Neurol. 2010;68:220–230. doi: 10.1002/ana.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon-Rodriguez W., Vega A., Sepulveda-Becerra M., Milton S., Johnson D.A., Yatsimirsky A.K., Glabe C.G. A conformation change in the carboxyl terminus of Alzheimer's Abeta (1-40) accompanies the transition from dimer to fibril as revealed by fluorescence quenching analysis. J. Biol. Chem. 2000;275:22645–22649. doi: 10.1074/jbc.M000756200. [DOI] [PubMed] [Google Scholar]
- Gerson J.E., Kayed R. Formation and propagation of tau oligomeric seeds. Front. Neurol. 2013;4:93. doi: 10.3389/fneur.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C.G. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol. Aging. 2006;27:570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Goure W.F., Krafft G.A., Jerecic J., Hefti F. Targeting the proper amyloid-beta neuronal toxins: a path forward for Alzheimer's disease immunotherapeutics. Alzheimers Res. Ther. 2014;6:42. doi: 10.1186/alzrt272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Munoz M.J., Castillo-Carranza D.L., Sengupta U., White M.A., Kayed R. Design of metastable beta-sheet oligomers from natively unstructured peptide. ACS Chem. Neurosci. 2013;4:1520–1523. doi: 10.1021/cn4001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Munoz M.J., Castillo-Carranza D.L., Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem. Pharmacol. 2014;88:468–478. doi: 10.1016/j.bcp.2013.12.023. [DOI] [PubMed] [Google Scholar]
- Guerrero-Munoz M.J., Castillo-Carranza D.L., Krishnamurthy S., Paulucci-Holthauzen A.A., Sengupta U., Lasagna-Reeves C.A., Ahmad Y., Jackson G.R., Kayed R. Amyloid-beta oligomers as a template for secondary amyloidosis in Alzheimer's disease. Neurobiol. Dis. 2014;71:14–23. doi: 10.1016/j.nbd.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- He Y., Zheng M.M., Ma Y., Han X.J., Ma X.Q., Qu C.Q., Du Y.F. Soluble oligomers and fibrillar species of amyloid beta-peptide differentially affect cognitive functions and hippocampal inflammatory response. Biochem. Biophys. Res. Commun. 2012;429:125–130. doi: 10.1016/j.bbrc.2012.10.129. [DOI] [PubMed] [Google Scholar]
- Hepler R.W., Grimm K.M., Nahas D.D., Breese R., Dodson E.C., Acton P., Keller P.M., Yeager M., Wang H., Shughrue P., Kinney G., Joyce J.G. Solution state characterization of amyloid beta-derived diffusible ligands. Biochemistry. 2006;45:15157–15167. doi: 10.1021/bi061850f. [DOI] [PubMed] [Google Scholar]
- Hong S., Quintero-Monzon O., Ostaszewski B.L., Podlisny D.R., Cavanaugh W.T., Yang T., Holtzman D.M., Cirrito J.R., Selkoe D.J. Dynamic analysis of amyloid beta-protein in behaving mice reveals opposing changes in ISF versus parenchymal Abeta during age-related plaque formation. J. Neurosci. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jang H., Connelly L., Arce F.T., Ramachandran S., Kagan B.L., Lal R., Nussinov R. Mechanisms for the insertion of toxic, fibril-like beta-amyloid oligomers into the membrane. J. Chem. Theory Comput. 2013;9:822–833. doi: 10.1021/ct300916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.D., Schauerte J.A., Chang C.C., Wisser K.C., Althaus J.C., Carruthers C.J., Sutton M.A., Steel D.G., Gafni A. Single-molecule imaging reveals abeta42:abeta40 ratio-dependent oligomer growth on neuronal processes. Biophys. J. 2013;104:894–903. doi: 10.1016/j.bpj.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T., Atwal J.K., Steinberg S., Snaedal J., Jonsson P.V., Bjornsson S., Stefansson H., Sulem P., Gudbjartsson D., Maloney J., Hoyte K., Gustafson A., Liu Y., Lu Y., Bhangale T., Graham R.R., Huttenlocher J., Bjornsdottir G., Andreassen O.A., Jonsson E.G., Palotie A., Behrens T.W., Magnusson O.T., Kong A., Thorsteinsdottir U., Watts R.J., Stefansson K. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Kaminsky Y.G., Tikhonova L.A., Kosenko E.A. Critical analysis of Alzheimer's amyloid-beta toxicity to mitochondria. Front. Biosci. (Landmark Ed) 2015;20:173–197. doi: 10.2741/4304. [DOI] [PubMed] [Google Scholar]
- Kayed R., Glabe C.G. Conformation-dependent anti-amyloid oligomer antibodies. Methods Enzymol. 2006;413:326–344. doi: 10.1016/S0076-6879(06)13017-7. [DOI] [PubMed] [Google Scholar]
- Kayed R., Lasagna-Reeves C.A. Molecular mechanisms of amyloid oligomers toxicity. J. Alzheimers Dis. 2013;33(Suppl. 1):S67–S78. doi: 10.3233/JAD-2012-129001. [DOI] [PubMed] [Google Scholar]
- Kayed R., Head E., Thompson J.L., Mcintire T.M., Milton S.C., Cotman C.W., Glabe C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Klein W.L. Synaptotoxic amyloid-beta oligomers: a molecular basis for the cause, diagnosis, and treatment of Alzheimer's disease? J. Alzheimers Dis. 2013;33(Suppl. 1):S49–S65. doi: 10.3233/JAD-2012-129039. [DOI] [PubMed] [Google Scholar]
- Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., Wals P., Zhang C., Finch C.E., Krafft G.A., Klein W.L. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F., Eisele Y.S., Fritschi S.K., Staufenbiel M., Walker L.C., Jucker M. Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J. Neurosci. 2011;31:14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves C.A., Kayed R. Astrocytes contain amyloid-beta annular protofibrils in Alzheimer's disease brains. FEBS Lett. 2011;585:3052–3057. doi: 10.1016/j.febslet.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves C.A., Glabe C.G., Kayed R. Amyloid-beta annular protofibrils evade fibrillar fate in Alzheimer disease brain. J. Biol. Chem. 2011;286:22122–22130. doi: 10.1074/jbc.M111.236257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J., Gimbel D.A., Nygaard H.B., Gilbert J.W., Strittmatter S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S., Koh M.T., Kotilinek L., Kayed R., Glabe C.G., Yang A., Gallagher M., Ashe K.H. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lesné S., Kotilinek L., Ashe K.H. Plaque-bearing mice with reduced levels of oligomeric amyloid-β assemblies have intact memory function. Neuroscience. 2008;151:745–749. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S.E., Sherman M.A., Grant M., Kuskowski M., Schneider J.A., Bennett D.A., Ashe K.H. Brain amyloid-beta oligomers in ageing and Alzheimer's disease. Brain. 2013;136:1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Jin M., Koeglsperger T., Shepardson N.E., Shankar G.M., Selkoe D.J. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Reed M.N., Kotilinek L.A., Grant M.K., Forster C.L., Qiang W., Shapiro S.L., Reichl J.H., Chiang A.C., Jankowsky J.L., Wilmot C.M., Cleary J.P., Zahs K.R., Ashe K.H. Quaternary structure defines a large class of amyloid-beta oligomers neutralized by sequestration. Cell Rep. 2015;11:1760–1771. doi: 10.1016/j.celrep.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.H., Bu X.L., Liang C.R., Wang Y.R., Zhang T., Jiao S.S., Zeng F., Yao X.Q., Zhou H.D., Deng J., Wang Y.J. An N-terminal antibody promotes the transformation of amyloid fibrils into oligomers and enhances the neurotoxicity of amyloid-beta: the dust-raising effect. J. Neuroinflammation. 2015;12:153. doi: 10.1186/s12974-015-0379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mably A.J., Liu W., Mc Donald J.M., Dodart J.C., Bard F., Lemere C.A., O'nuallain B., Walsh D.M. Anti-Abeta antibodies incapable of reducing cerebral Abeta oligomers fail to attenuate spatial reference memory deficits in J20 mice. Neurobiol. Dis. 2015;82:372–384. doi: 10.1016/j.nbd.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrone C., Djelloul M., Taglialatela G., Perrone L. Inflammatory risk factors and pathologies promoting Alzheimer's disease progression: is RAGE the key? Histol. Histopathol. 2015;30:125–139. doi: 10.14670/HH-30.125. [DOI] [PubMed] [Google Scholar]
- Mc Donald J.M., O'malley T.T., Liu W., Mably A.J., Brinkmalm G., Portelius E., Wittbold W.M., Frosch M.P., Walsh D.M. The aqueous phase of Alzheimer's disease brain contains assemblies built from approximately 4 and approximately 7 kDa Abeta species. Alzheimers Dement. 2015;11:1286–1305. doi: 10.1016/j.jalz.2015.01.005. 3RD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter M.R., Taylor J.M., Crack P.J. The contribution of neuro-inflammation to amyloid toxicity in Alzheimer's disease. J. Neurochem. 2015 doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- Mirbaha H., Holmes B.B., Sanders D.W., Bieschke J., Diamond M.I. Tau trimers are the minimal propagation unit spontaneously internalized to seed intracellular aggregation. J. Biol. Chem. 2015;290:14893–14903. doi: 10.1074/jbc.M115.652693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkuniene R., Zvirbliene A., Dalgediene I., Cizas P., Jankeviciute S., Baliutyte G., Jokubka R., Jankunec M., Valincius G., Borutaite V. Antibodies bound to Aβ oligomers potentiate the neurotoxicity of Aβ by activating microglia. J. Neurochem. 2013;126:604–615. doi: 10.1111/jnc.12332. [DOI] [PubMed] [Google Scholar]
- Morkuniene R., Cizas P., Jankeviciute S., Petrolis R., Arandarcikaite O., Krisciukaitis A., Borutaite V. Small Abeta1-42 oligomer-induced membrane depolarization of neuronal and microglial cells: role of N-methyl-d-aspartate receptors. J. Neurosci. Res. 2015;93:475–486. doi: 10.1002/jnr.23510. [DOI] [PubMed] [Google Scholar]
- Mucke L. Neuroscience: Alzheimer's disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat. Neurosci. 2015:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'nuallain B., Freir D.B., Nicoll A.J., Risse E., Ferguson N., Herron C.E., Collinge J., Walsh D.M. Amyloid beta-protein dimers rapidly form stable synaptotoxic protofibrils. J. Neurosci. 2010;30:14411–14419. doi: 10.1523/JNEUROSCI.3537-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape G.S., Gouwens L.K., Osborn D.C., Nichols M.R. Isolated amyloid-β(1–42) protofibrils, but not isolated fibrils, are robust stimulators of microglia. ACS Chem. Neurosci. 2012;3:302–311. doi: 10.1021/cn2001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R.C., Aisen P., Boeve B.F., Geda Y.E., Ivnik R.J., Knopman D.S., Mielke M., Pankratz V.S., Roberts R., Rocca W.A., Weigand S., Weiner M., Wiste H., Jack C.R. Criteria for mild cognitive impairment due to Alzheimer's disease in the community. Ann. Neurol. 2013;74:199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool S., Albay R., Martinez-Coria H., Breydo L., Wu J., Milton S., Misra S., Tran A., Pensalfini A., Laferla F., Kayed R., Glabe C.G. Vaccination with a non-human random sequence amyloid oligomer mimic results in improved cognitive function and reduced plaque deposition and micro hemorrhage in Tg2576 mice. Mol. Neurodegener. 2012;7:37. doi: 10.1186/1750-1326-7-37. 3RD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool S., Martinez-Coria H., Wu J.W., Laferla F., Glabe C.G. Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Abeta deposition and tau pathology in 3xTg-AD mice. J. Neurochem. 2013;126:473–482. doi: 10.1111/jnc.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S., Barten D.M., Vana L., Devidze N., Yang L., Cadelina G., Hoque N., Decarr L., Keenan S., Lin A., Cao Y., Snyder B., Zhang B., Nitla M., Hirschfeld G., Barrezueta N., Polson C., Wes P., Rangan V.S., Cacace A., Albright C.F., Meredith J., Trojanowski J.Q., Lee V.M.Y., Brunden K.R., Ahlijanian M. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PLoS One. 2015;10:e0125614. doi: 10.1371/journal.pone.0125614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebollela A., Mustata G.M., Luo K., Velasco P.T., Viola K.L., Cline E.N., Shekhawat G.S., Wilcox K.C., Dravid V.P., Klein W.L. Elucidating molecular mass and shape of a neurotoxic Abeta oligomer. ACS Chem. Neurosci. 2014;5:1238–1245. doi: 10.1021/cn500156r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta U., Guerrero-Munoz M.J., Castillo-Carranza D.L., Lasagna-Reeves C.A., Gerson J.E., Paulucci-Holthauzen A.A., Krishnamurthy S., Farhed M., Jackson G.R., Kayed R. Pathological interface between oligomeric alpha-synuclein and tau in synucleinopathies. Biol. Psychiatry. 2015;78:672–683. doi: 10.1016/j.biopsych.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Shinohara M., Sato N., Shimamura M., Kurinami H., Hamasaki T., Chatterjee A., Rakugi H., Morishita R. Possible modification of Alzheimer's disease by statins in midlife: interactions with genetic and non-genetic risk factors. Front. Aging Neurosci. 2014;6:71. doi: 10.3389/fnagi.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira J.R., Raymond G.J., Hughson A.G., Race R.E., Sim V.L., Hayes S.F., Caughey B. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane J.A., Pietropaolo M.F., Rosene D.L., Moss M.B., Peters A., Kemper T., Abraham C.R. Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol. 1997;94:471–478. doi: 10.1007/s004010050735. [DOI] [PubMed] [Google Scholar]
- Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Kaye J., Jr., Montine T.J., Park D.C., Reiman E.M., Rowe C.C., Siemers E., Stern Y., Yaffe K., Carrillo M.C., Thies B., Morrison-Bogorad M., Wagster M.V., Phelps C.H. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H.T., Chung C.H.-Y., Iba M., Zhang B., Trojanowski J.Q., Luk K.C., Lee V.M.Y. α-Synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J.W., Nygaard H.B., Heiss J.K., Kostylev M.A., Stagi M., Vortmeyer A., Wisniewski T., Gunther E.C., Strittmatter S.M. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 2012;15:1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E., Masliah E. Combination therapies: the next logical Step for the treatment of synucleinopathies? Mov. Disord. 2015 doi: 10.1002/mds.26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E., Spencer B., Masliah E. Immunotherapeutic approaches targeting amyloid-beta, alpha-synuclein, and tau for the treatment of neurodegenerative disorders. Neurotherapeutics. 2015 doi: 10.1007/s13311-015-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.C., Jucker M. Neurodegenerative diseases: expanding the prion concept. Annu. Rev. Neurosci. 2015;38:87–103. doi: 10.1146/annurev-neuro-071714-033828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D., Lue L.F., Paul G., Patel A., Sabbagh M.N. Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer's disease. Expert Opin. Investig. Drugs. 2015;24:393–399. doi: 10.1517/13543784.2015.1001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D.M., Tseng B.P., Rydel R.E., Podlisny M.B., Selkoe D.J. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- Wu W.H., Liu Q., Sun X., Yu J.S., Zhao D.S., Yu Y.P., Luo J.J., Hu J., Yu Z.W., Zhao Y.F., Li Y.M. Fibrillar seeds alleviate amyloid-beta cytotoxicity by omitting formation of higher-molecular-weight oligomers. Biochem. Biophys. Res. Commun. 2013;439:321–326. doi: 10.1016/j.bbrc.2013.08.088. [DOI] [PubMed] [Google Scholar]
- Yoon S.S., Jo S.A. Mechanisms of amyloid-beta peptide clearance: potential therapeutic targets for Alzheimer's disease. Biomol. Ther. (Seoul) 2012;20:245–255. doi: 10.4062/biomolther.2012.20.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahs K.R., Ashe K.H. beta-Amyloid oligomers in aging and Alzheimer's disease. Front. Aging Neurosci. 2013;5(28) doi: 10.3389/fnagi.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]