Abstract
Development of a dysregulated immune response discriminates sepsis from uncomplicated infection. Currently used biomarkers fail to describe simultaneously occurring pro- and anti-inflammatory responses potentially amenable to therapy.
Marker candidates were screened by microarray and, after transfer to a platform allowing point-of-care testing, validated in a confirmation set of 246 medical and surgical patients. We identified up-regulated pathways reflecting innate effector mechanisms, while down-regulated pathways related to adaptive lymphocyte functions. A panel of markers composed of three up- (Toll-like receptor 5; Protectin; Clusterin) and 4 down-regulated transcripts (Fibrinogen-like 2; Interleukin-7 receptor; Major histocompatibility complex class II, DP alpha1; Carboxypeptidase, vitellogenic-like) described the magnitude of immune alterations. The created gene expression score was significantly greater in patients with definite as well as with possible/probable infection than with no infection (median (Q25/Q75): 80 (60/101)) and 81 (58/97 vs. 49 (27/66), AUC-ROC = 0.812 (95%-CI 0.755–0.869), p < 0.0001). Down-regulated lymphocyte markers were associated with prognosis with good sensitivity but limited specificity.
Quantifying systemic inflammation by assessment of both pro- and anti-inflammatory innate and adaptive immune responses provides a novel option to identify patients-at-risk and may facilitate immune interventions in sepsis.
Keywords: Host response, Transcriptomic profiling, Adaptive immunity, Point-of-care, RT-qPCR, Clinical utility
Highlights
-
•
Pro- and anti-inflammatory signaling occurs simultaneously in the host response to infection.
-
•
This response is currently monitored using biomarkers restricted to the pro-inflammatory component of innate immunity.
-
•
We developed a biomarker panel consisting of 7 transcripts that can assess both facets at the point of care.
The concept that a selective, overwhelming systemic inflammation, the “Systemic Inflammatory Response Syndrome (SIRS)”, triggers organ failure subsequent to infection has lately been abandoned as it neglects parallel occurring anti-inflammatory responses or defects in the adaptive immune system.
The present findings suggest that a compound panel of nucleic acid biomarkers that was developed in independent training and verification cohorts and transferred to a point-of-care platform can more comprehensively describe the host response. Quantification of an enhanced innate immunity might inform studies of anti-inflammatory therapies, while measurement of derangements in specific immunity might guide strategies to restore immune effector functions.
1. Introduction
Severe sepsis and shock are among the leading causes of death globally, accounting for more than 210,000 deaths annually in the United States and more than 15 million cases worldwide (Angus et al., 2001, Kumar et al., 2011, Adhikari et al., 2010). Sepsis results from a dysregulated response to invasive infection reflected in damage to the host's tissues and organs (Singer et al., 2016). Monitoring of that response may, therefore, provide diagnostic and prognostic information. Multiple circulating proteins have been studied as biomarkers (Pierrakos and Vincent, 2010), based on the assumption that changes in their expression, may reflect eradication or propagation of pathogens. However, none of these is widely accepted or used.
An increasing body of evidence suggests that sepsis with organ failure is associated with an impaired adaptive immune response in which circulating monocytes secrete reduced amounts of pro-inflammatory cytokines (Adib-Conquy and Cavaillon, 2009), antigen-presentation fails, and apoptosis of lymphocytes predominates (Hotchkiss et al., 2013, Giamarellos-Bourboulis and Raftogiannis, 2012). These complex changes require high dimensional approaches, such as functional genomics to describe the differing aspects of the host response (Feezor et al., 2005, Desai et al., 2011).
We used a three-stage transcriptomic approach to develop a quantitative real-time polymerase chain reaction (PCR) assay of individual genes to characterize immune alterations associated with sepsis. The objective of this strategy was to assess i) infectious origin, ii) severity of systemic inflammation and iii) its association with outcome. First, patients with extreme disease phenotypes were subjected to transcriptomic analysis to identify transcripts that differentiate non-infectious systemic inflammation from bacterial infection with organ failure or shock. Results were evaluated in a second cohort of subjects representing a continuum from health to high-grade inflammation, identifying clusters of up- and down-regulated pathways that increased with disease severity. Having established a final biomarker panel and a corresponding composite score, we validated the tool regarding identification of infection and prediction of outcome in a pragmatic study in two independent patient cohorts from Germany and Greece covering a broad spectrum of medical and surgical patients with diverse comorbidities in differing health care systems.
2. Patients and Methods
Patients and healthy controls were enrolled at eight investigational sites in four countries (Appendix, Text S1). All study protocols were approved by the respective institutional review boards and written informed consent was provided by patients or their legal representatives.
Gender, age, underlying infections, reason for ICU admission, isolated pathogen, white blood cell count, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential (Sepsis-related) Organ Failure Assessment (SOFA) score, and mortality, respectively, were recorded as pertinent clinical information. For the initial training set, the verification set and the German cohort of the confirmation set C-Reactive Protein (CRP) and procalcitonin (PCT) were recorded as well.
For transcriptomic analyses, blood was sampled and collected into PaxGene tubes (PreAnalytiX, Becton Dickinson, Cockeysville, Md), and stored at − 80 °C until assayed. Cases and samples were grouped into cohorts by medical experts and data analysts by pre-specified criteria and definitions (Appendix, Text S2). We excluded patients with immunodeficiency disorders (Appendix, Text S2).
2.1. Study Design
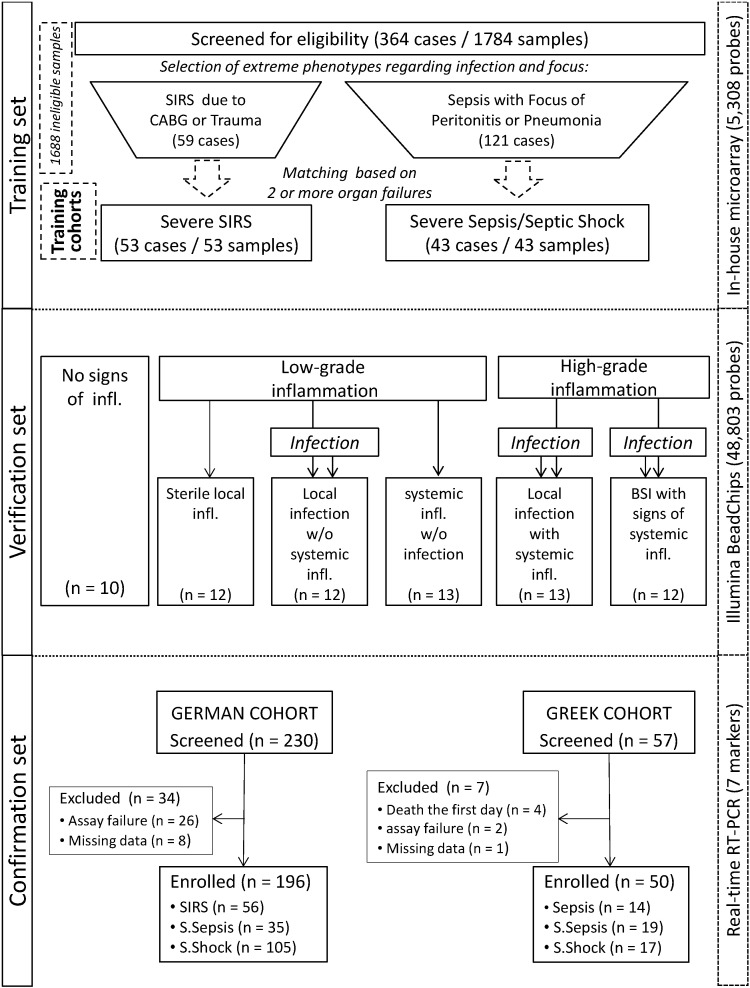
The study was designed in three stages consisting of a training set, a verification set and a confirmation set after transfer of the marker set to a RT-qPCR platform possibly to facilitate its use at the point-of-care (Fig. 1).
Fig. 1.
Study flow chart. Patients were enrolled in independent training, verification, and confirmation cohorts. The confirmation cohort was analyzed applying a limited set of 7 transcripts after platform change from microarray to RT-qPCR. BSI: bloodstream infection; SIRS: Systemic Inflammatory Response Syndrome.
The training set identified differences in the transcriptomic profile between patients with extreme phenotypes; i.e. systemic inflammation and organ failure/shock in the absence or presence of infection. Systemic inflammation was diagnosed based on the presence of at least two of four SIRS criteria. In a cohort of 364 patients hospitalized in the ICU of the Jena University Hospital (JUH) between 2002 and 2007 blood sampling was done within the first 24 h after presenting signs of systemic inflammation. Then patients were screened for eligibility. An adjudication committee of two ICU experts selected patients according to pre-specified criteria (Appendix, Text S2). Ninety-six patients met these criteria and their samples were used in order to select qualitative molecular marker candidates and to develop an appropriate classification function, which discriminated cases with and without infection. Demographic characteristics are summarized in Table S1 (Appendix).
Results of the training set were reevaluated in a verification set to validate the marker candidates on a broad spectrum of phenotypes representing a continuum from health to high-grade systemic inflammation and to characterize its suitability to quantify inflammation.
In this sub-study, patients representing six clinical phenotypes were enrolled, i) subjects and preoperative patients for scheduled operations with no signs of infection and no signs of inflammation (controls); ii) patients with local sterile inflammation, iii) patients with local infection but absent signs of systemic inflammation, iv) patients presenting signs of systemic inflammation but without evidence of infection, v) patients with local infection simultaneously fulfilling criteria for systemic inflammation, and vi) patients with bloodstream infection (BSI)-associated severe sepsis/septic shock. Samples were collected before initiation of anti-infective therapy for patients of groups v) and vi) and for patients of group iv) within the first 24 h of presentation of signs of inflammation. The demographic characteristics of the cohorts are summarized in Supplementary Table S2 (Appendix).
The confirmation set comprised two prospectively enrolled cohorts in which a subset of 7 transcripts suitable to assessing the host response to infection was tested after transfer to a RT-qPCR platform. The German cohort was enrolled between May 2009 and October 2010 from the ICU of JUH. Inclusion criteria were systemic inflammation and/or severe sepsis/septic shock with infection ruled out for patients with uncomplicated systemic inflammation and confirmed for patients with severe sepsis/septic shock according to standard definitions at time of enrolment (Levy et al., 2001, Calandra and Cohen, 2005). The Greek cohort was enrolled between October 2012 and January 2013 in three departments of the Hellenic Sepsis Study Group. Inclusion criteria were: a) diagnosis of severe sepsis or septic shock based on standard definitions; (Levy et al., 2003) b) diagnosis of acute pyelonephritis, community-acquired or ventilator-associated pneumonia (CAP or VAP), intra-abdominal infection (IAI) or BSI. For patients enrolled in the confirmation cohort sequential blood samples were obtained; the first on the day of diagnosis and the second 24 h later; in the German cohort sampling was continued on a daily basis until ICU-discharge or death for a maximum of ten days.
For the confirmation of the genomic score, patients were independently classified according to the current clinical gold standard into three groups: ‘no infection’, ‘possible/probable infection’ and ‘definite infection’ (Calandra and Cohen, 2005). In both cohorts, patients were followed up to assess 100-day mortality. Demographic data are presented in Tables S3 and S4 (Appendix).
In all patient sets, classification according to degree of inflammation or presence of infection status was made independently of the genomic score or the use of serum biomarkers.
For a detailed description of the used laboratory techniques see Appendix (Text S3).
2.2. Statistical Analysis
The study consisted of two microarray experiments with a marker screening and of a RT-qPCR evaluation for the marker confirmation. For the design and evaluation of the microarray trials specific methods were employed, including data preprocessing and transformation.
For the training set, 96 RNA samples from 96 ICU patients were hybridized against the in-house research microarray addressing 5308 transcripts. For the classification of cases with and without infection the linear discriminant analysis (LDA) was applied with up to 100 transcripts as classification markers, selected by p-values and estimates of Wilcoxon test. Marker candidates were chosen corresponding to the best concordance between molecular and clinical classification.
For the verification set, 72 RNA samples from 72 cases were hybridized against a genome-wide microarray addressing ca. 50,000 transcripts. One-way analysis of variance with 6 groups was applied gene by gene and evaluated by the estimation of the false discovery rate. The gene expression pattern of 4761 selected transcripts was visualized by a heatmap and quantified by a genomic score (GES), developed for this approach.
In the confirmation set, 7 transcripts, representing an overlap of signatures obtained in the training and verification sets were used to assess the host response to infection in 246 patients, after transfer to a RT-qPCR platform. The GES and its components of up- and down-regulation were determined for each sample and evaluated depending on the patient status regarding presence of infection and mortality.
For a more detailed description of the statistical analysis see Appendix (Text S4).
2.3. Funding and Role of the Funding Source
The study was funded by German Federal Ministry of Education and Research, Thuringian Ministry of Education, Science and Art, Thüringer Aufbaubank and Hellenic Institute for the Study of Sepsis. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Training Set
The training set served to specify marker candidates, which distinguished between critically ill patients with signs of systemic inflammation in the absence or presence of infection. Using linear discriminant analysis, these groups were discriminated with mean power of 90% correct classifications as obtained by cross-validation. This initial result was obtained applying 50 transcripts out of the whole evaluated number of 5308 transcripts. However the classification power remained stable when the number of transcripts was reduced (Appendix, Fig. S1). More precisely, the least classification error was obtained with more than 10 and fewer than 20 transcripts, where the groups were discriminated with mean sensitivity of 95.5% (95%-CI 84.5%, 98.7%) and specificity of 94.5% (95%-CI 84.6%, 98.1%). In the next step, we evaluated these 20 transcripts, which provided the most sensitive and specific information for the inflammatory status of the host.
3.2. Verification Set
Patients were divided into three groups representing graded intensity of the inflammatory response: absence of inflammation, low grade systemic inflammation and high grade systemic inflammation in the absence or presence of infection (Fig. 1).
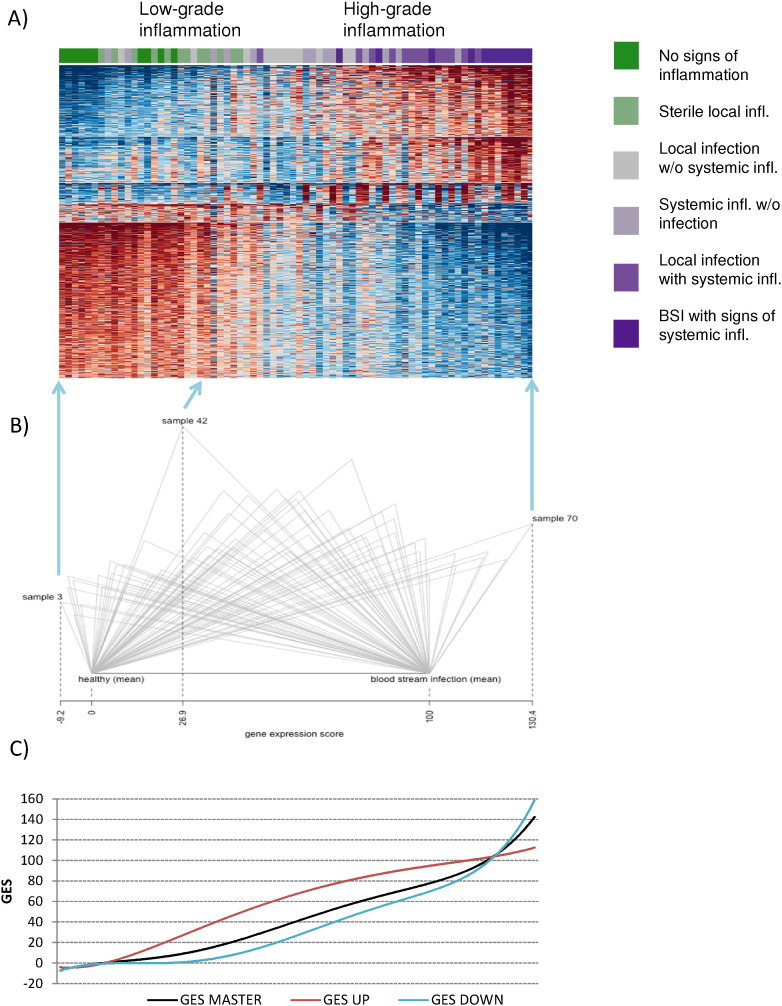
Investigations of the verification set resulted in the characterization of the molecular pattern of the inflammatory-infectious process as visualized in Fig. 2 by a heat-map for a broad spectrum of phenotypes representing the entirety from health to high-grade systemic inflammation. The continuous shift of the transcriptome supports the concept that the transcriptomic profile of patients can quantify the intensity of the inflammatory response. The expression of gene ontologies differed with increasing severity of a clinical inflammatory response; expression was up-regulated with progression from low to high grade inflammation for 36% of gene ontologies whereas 64% were down-regulated during that course.
Fig. 2.
Generation of a genomic score to quantify the host response from the gene expression pattern. A) Heat-map reflecting the gene expression pattern in the verification set: each column reflects a sample of an individual patient with a color code on the top covering the continuum from green (healthy) to magenta (BSI with signs of systemic inflammation); each row reflects an individual transcript. Samples were arranged by GES reflecting the individual host response. Thus, the shift in gene expression depending on disease severity is reflected in the heat-map, with blue indicating low expression and red indicating high expression levels compared to the mean of each individual transcript. B) Scheme of the computation of GES: each triangle is derived from one sample. Its horizontal side is the Euclid distance between the mean healthy pattern and mean pattern in BSI-associated severe sepsis/septic shock; mean healthy value was set to zero and the mean value in BSI with systemic inflammation was set to 100. Its left side corresponds with the distance between the mean healthy pattern and the individual patient pattern, and its right side with the distance between the individual patient pattern and the mean BSI pattern. The GES is computed as the projection of the upper tip of triangle on the base side (for GES the height of the triangle is not relevant). C) Potential of GES (black line) and its components GES UP and GES DOWN (red and blue lines) to predict a given patient's immune state as reflected by a pangenomic assessment of the blood transcriptome. Down-regulated adaptive immune functions (GES DOWN) are impacted particularly by disease severity. In contrast, up-regulated transcripts encoding innate immune functions (GES UP) reached a plateau.
The whole set of 4761 selected transcripts was included in the computation of the gene expression score (GES), to order the patterns of the heat-map. Potential of GES and its components GES UP and GES DOWN to predict a given patient's immune state as reflected by a pangenomic assessment of the blood transcriptome are represented by Fig. 2C. While down-regulated adaptive immune functions (GES DOWN) were impacted particularly by disease severity, up-regulated transcripts encoding innate immune functions (GES UP) reached a plateau.
In the next step, we then tried to identify a reduced subset of representative genes with highest phenotypic separation capacity to determine marker candidates. Through multiple in silico simulations, no unique set of preferable marker candidates, but marker combinations were determined reflecting this course with equivalent capability. The analysis identified that a subset of seven genes used to differentiate presence of infection in the training set – i.e. TLR5, CD59, CLU, FGL2, IL7R, HLA-DPA1 and CPVL – can be applied to represent the whole genomic version of GES with appropriate power (for description of the genes see Table 1). Albeit these 7 genes predicted the position of a given patient's transcriptomic response in the continuum reflected in the heat map, the substantial heterogeneity of individual patients regarding the 7 transcripts lends support to the need to individualize therapeutic interventions (Fig. 3A).
Table 1.
Characteristics of the seven genes used for the generation of the genomic score.
| Gene | Description | Function | Implicated pathway | Expression in severe inflammation |
|---|---|---|---|---|
| TLR 5 | Toll-like receptor 5 (flagellin) | Pathogen recognition | Toll-like receptor signaling | Up-regulation |
| CD59 | Protectin | Complement regulatory protein | Fcy receptor mediated phagocytosis | Up-regulation |
| CLU | Clusterin | Complement lysis inhibitor | Integrin signaling | Up-regulation |
| FGL2 | Fibrinogen-like 2 | Immune regulator, prothrombinase | Nur77 signaling in T lymphocytes | Down-regulation |
| IL7R | Interleukin-7 receptor | Lymphocyte development | CD28 signaling in T helper cells | Down-regulation |
| HLA-DPA1 | Major histocompatibility complex class II, DP alpha1 | Antigen presentation | Allograft rejection signaling | Down-regulation |
| CPVL | Carboxypeptidase, vitellogenic-like | Macrophages, inflammatory protease cascade, phagocytosis | Calcium-induced T lymphocyte apoptosis | Down-regulation |
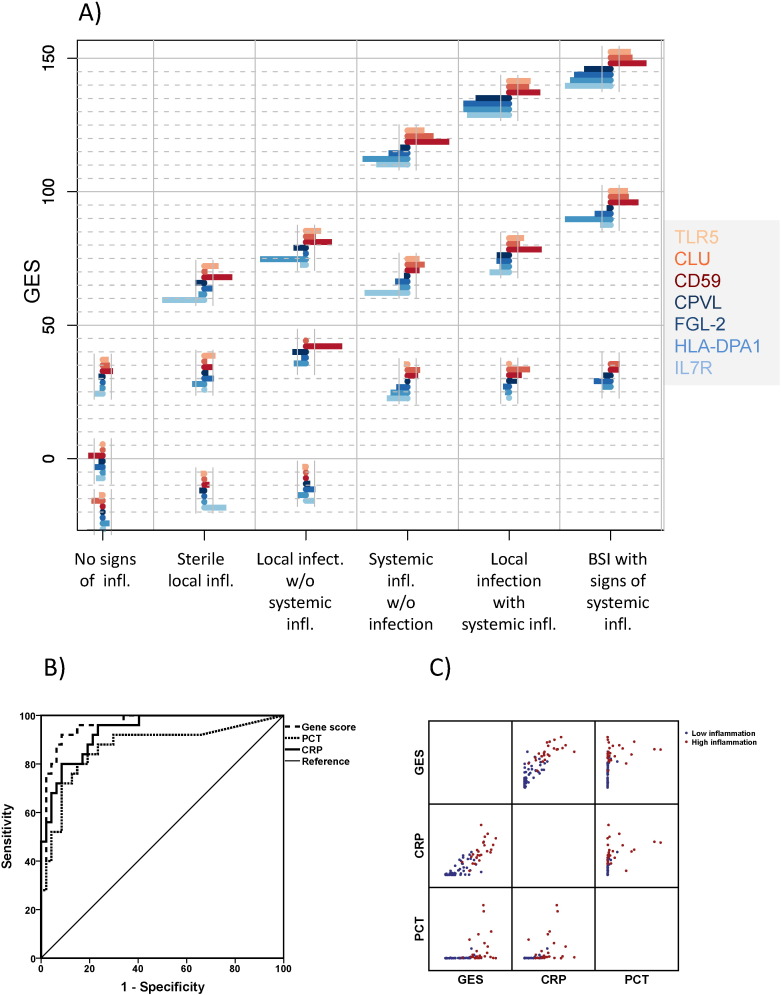
Fig. 3.
Individual expression of the selected transcripts of the marker set within the verification cohort. A) A bar chart represents the deviation of each marker from corresponding mean value obtained in healthy controls, consisting of markers reflecting up-regulated effector functions of innate and down-regulated functions of adaptive immunity. Each chart is plotted with the value of the corresponding gene expression score (GES) on the vertical axis and the individual deflection of the 7 transcripts on the horizontal axis for the three individual patients shown in each group, namely those belonging to the 10th, 50th, and 90th percentiles of the corresponding GES values. This presentation reflects the heterogeneity of the response of the various pro- and anti-inflammatory compounds underlying the overall value of the obtained score in individual patients. B) Receiver Operator Characteristics (ROCs) for the score compared with procalcitonin (PCT) and C-Reactive Protein (CRP) to differentiate a state of high grade systemic inflammation from a state of low grade systemic inflammation (AUCROC: GES: 0.963 (95% CIs 0.923–1.000); PCT: 0.869 (95% CIs 0.771–0.967); and CRP: 0.935 (95% CIs 0.882–0.988). AUCs GES vs PCT p = 0.020). C) Individual Correlation of the genomic score with PCT and CRP.
The identification of a parsimonious subset of 7 genes supported the feasibility of a diagnostic test based on a limited number of transcripts that describe both up-regulation of innate immune functions and impaired specific immunity in sepsis. The individual change of the expression of each gene is scored. The three genes TLR5, CD59 and CLU that are up-regulated comprise the UP-genomic score; and the four genes FGL2, IL7R, HLA-DPA1 and CPVL that are down-regulated comprise the DOWN-genomic score. Combining both scores results in an overall gene expression score (GES) for systemic immune dysregulation in sepsis (Fig. 3A).
ROC analysis was performed to evaluate whether the GES or its subscores can distinguish a state of high grade inflammation from a state of low grade inflammation (Fig. 3B). The area under the ROC curve of the GES was significantly greater than for PCT and tended to be greater than for CRP (Fig. 3B). Twenty-five of the 72 patients enrolled in this verification set were classified according to the predefined clinical criteria as patients with a state of high grade inflammation. Correlation statistics within these patients failed to identify a positive correlation between GES and PCT showing that the clinical information provided by GES was different from PCT (rank correlation coefficient between GES and PCT 0.58, p = 0.116), while it correlated significantly with CRP (rank correlation coefficient between GES and CRP 0.77, p < 0.001) (Fig. 3C).
3.3. Confirmation Set
The pool of seven genes that reflected the host response, both, with respect to the infectious nature (‘training set’) and the magnitude of the host inflammatory response (‘verification set’) were selected for prospective validation as component biomarkers in the ‘confirmation set’.
The primary endpoint of this sub-study was to confirm the validity of the genomic instrument to differentiate states of inflammation of infectious or non-infectious origin. The secondary endpoint was to evaluate the importance of changes of the score over time as a surrogate marker for outcome. Two independent cohorts were used, one enrolled in Germany with 196 patients from a mixed but predominantly surgical ICU and another from Greece with 50 medical patients (for characteristics see Tables S3 and S4).
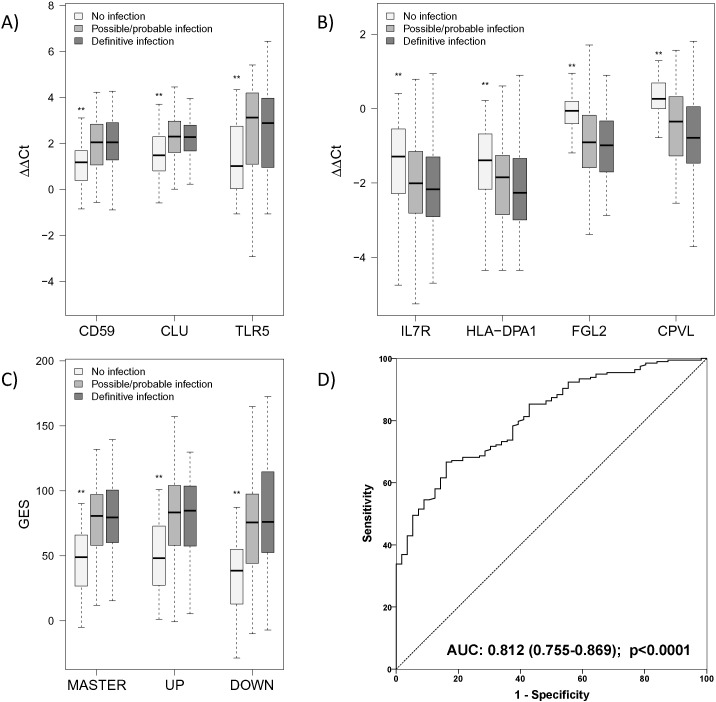
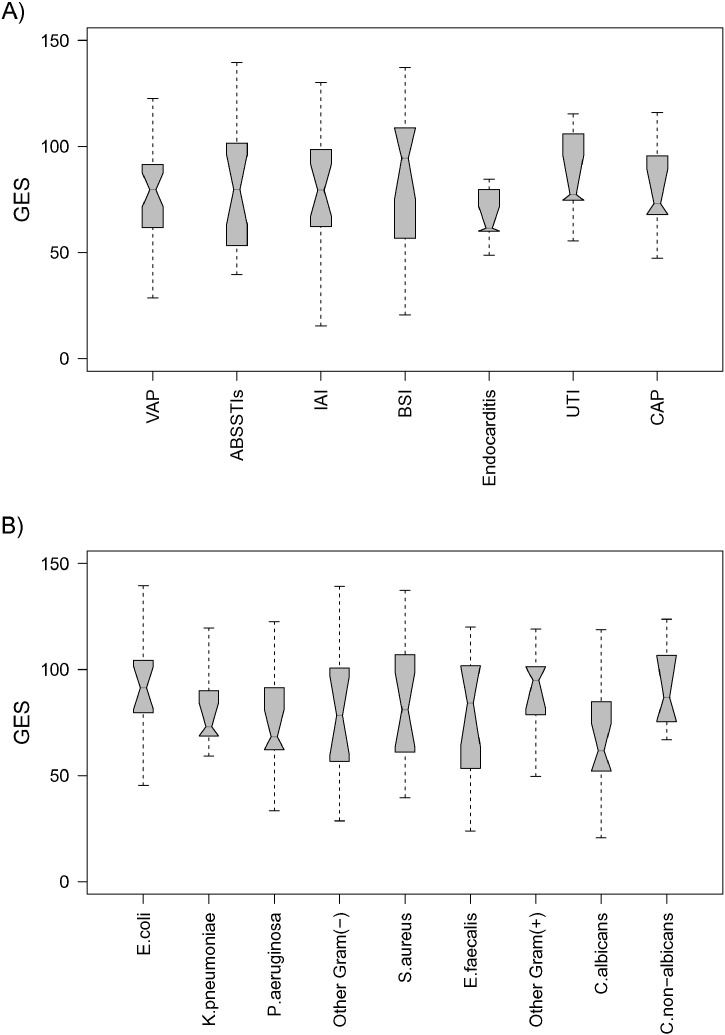
All three genes of the UP-genomic score were expressed at significantly greater levels among patients with possible/probable and definitive infections than among patients with no infection. The score did not differ between patients with possible/probable infection and definitive infection (Fig. 4A). In a similar fashion, all four genes of the DOWN-genomic score were expressed at significantly lower levels among patients with possible/probable and definitive infections than among patients with no infection (Fig. 4B). As expected, the overall genomic score was significantly greater in patients with definite as well as with possible/probable infection than with no infection (median (Q25/Q75): 80 (60/101) and 81 (58/97) vs. 49 (27/66) with no difference as to whether or not a microbiological confirmation of an alleged pathogen was achieved (median difference [95%-CI] of definitive infection vs. no infection: 34·9 [25·9, 44·0], possible/probable vs. no infection: 33.0 [22.7, 42.7] and possible/probable vs. definitive infection: − 1.7 [− 10.3, 6.9]) (Fig. 4C). ROC analysis showed good sensitivity and reasonable specificity for GES to identify presence of infection (Fig. 4D, Table 2). Corresponding data are provided for CRP and PCT (Fig. S3, Table S5) but are only available for the German cohort. Sub-analysis conducted among patients with definitive infections showed that the genomic score did not differ between different types of infections (Fig. 5A) and between different pathogens (Fig. 5B).
Fig. 4.
Expression characteristics of the individual transcripts of the genomic score and its sub-scores for patients enrolled in the confirmation cohorts. A) Differences to the mean value of a control cohort expressed as ΔΔCt values (Kenneth and Thomas, 2001) (i.e., deviation from group mean of healthy volunteers) for each of the three up-regulated genes forming the UP score (TLR5: Toll-like receptor 5; CD59: Protectin; CLU: Clusterin); B) ΔΔCt values for each of the four down-regulated genes forming the DOWN score (FGL2: Fibrinogen-like 2; HLA-DPA1: Major histocompatibility complex class II, DP alpha1; CPVL: Carboxypeptidase, vitellogenic-like; IL7R: Interleukin-7 receptor); ΔΔCts represent differences of the time to reaction between patients and healthy controls, both normalized to internal reference genes. C) Values of the calculated genomic master score including both aspects of the host response. Biological functions of the individual transcripts are summarized in Table 1. For all markers and scores, significant differences compared with the “no infection” group were confirmed (**p < 0.01), however no significant differences were observed between possible/probable and definitive infection groups (results of the post-hoc pairwise comparison after Kruskal–Wallis test). D) Receiver Operator Characteristics (ROCs) for the genomic master score to differentiate definite and possible/probable infection from no infection.
Table 2.
Classification of German cohort regarding the state of infection and the GES level of more and less than 55, thus adjusting for a sensitivity of 80% (95%-CIs are provided in addition).
| Definitive/possible/probable infection | No infection | Total | ||
|---|---|---|---|---|
| GES ≥ 55 | 151 (61%) | 23 (9%) | 174 (71%) | PPV = 87% (80.9–91.3) |
| GES < 55 | 39 (16%) | 33 (13%) | 72 (29%) | NPV = 46% (34.8–57.3) |
| Total | 190 (77%) | 56 (23%) | 246 (100%) | |
| Sensitivity = 80% (73.2–84.6%) |
Specificity = 59% (45.9–70.8%) |
Fig. 5.
Independence of the developed genomic score from the type of infection and from the implicated pathogens. A) The genomic score is presented stratified according the type of underlying infection/focus of patients enrolled in the two cohorts of the confirmation set, where p-value of one-way-ANOVA between type of infection is 0.749. ABSSTI: acute bacterial skin and soft tissue infection; IAI: intra-abdominal infection; BSI: bloodstream infection; UTI: urinary tract infection; CAP: community-acquired pneumonia; B) the genomic score is presented in relation with the isolated microorganism from patients enrolled in the two cohorts of the confirmation set, where p-value of one-way-ANOVA between the various pathogens is 0.307.
Significant differences between infected and non-infected patients were also observed for PCT, CRP (Fig. S3) and SOFA scores; however, a constant and statistically significant correlation between any pair of features could not be detected within any of the three studied subgroups (i.e. no infection, possible/probable infection, definitive infection). Furthermore, GES and its UP- and DOWN-scores did not differ between patients admitted with infections to the ICU and patients with ICU-acquired infections (Fig. S2).
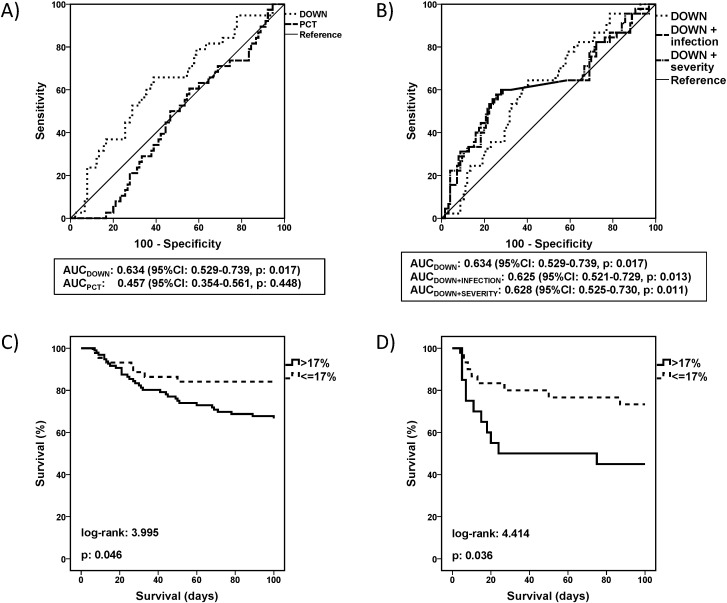
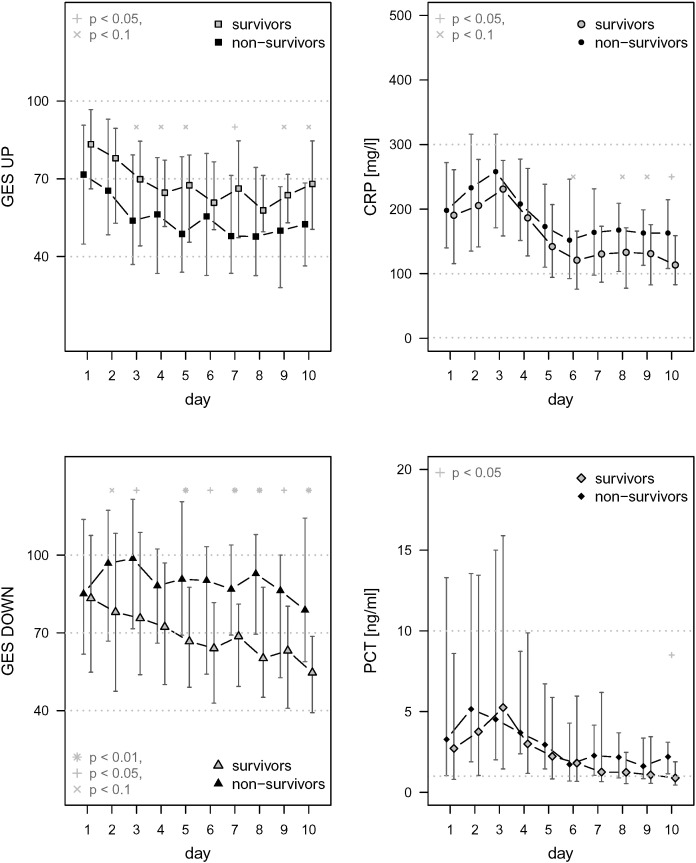
Early changes of the expression of the seven transcripts were measured among patients of the German cohort. ROC analysis was used to identify a cut-off of an early change of the genomic score that can inform about the risk to die. No cut-off could be found for the UP-score and neither for PCT and CRP as the corresponding AUC-values were not significantly different from 50%. However, it was found that any decrease of the DOWN-score by more than 17% within the first 24 h had a sensitivity of 80.0% (95%-CI 66.2, 89.1%) and a specificity of 37.0% (95%-CI 29.1, 45.7%) to predict death (Fig. 6A, Table 3). This would allow to rule in a high-risk population e.g. in interventional trials. The cut-off was identified for the German cohort and verified for the Greek cohort. In order to test whether a change of the DOWN-score was an independent factor associated with outcome, a logistic regression analysis was performed. This revealed two independent factors associated with mortality, i.e. early decrease of the DOWN-score and the presence of severe sepsis/septic shock (Table 4). However, ROC analysis where sepsis severity and the change of the DOWN score were considered together did not improve the area under the curve of prediction, confirming that early change of the DOWN score was a dynamic independent predictor of mortality (Fig. 6B and C). Once the importance of the change of the DOWN score to predict mortality was fully established in the German cohort, its significance was further confirmed by survival analysis of patients enrolled in the cohort from Greece (Fig. 6D). Time course of the DOWN score in the German cohort differed between survivors and non-survivors with higher values in non-survivors from day two to day ten, while PCT and CRP values did not differ (Fig. 7).
Fig. 6.
Early changes of the DOWN-genomic score as an independent prognostic factor. A) Receiver Operator Characteristic curves of the change of the DOWN genomic score and of procalcitonin (PCT) within the first 24 h for the prediction of mortality. Areas under the curve (AUCs) and p-values for the ROC analysis are provided. B) Comparative ROCs of the DOWN score with the presence of infection and with the presence of organ failure to predict mortality. AUCs and p-values for the ROC analysis are provided. C) Survival of patients enrolled in the confirmation cohort from Germany are divided into subgroups with and without early decrease of the down-genomic score by 17%. Those with less than 17% early decrease have prolonged survival. D) The prognostic value of this early decrease of the down-genomic score is confirmed in an independent cohort from Greece. p values of the log-rank tests are provided.
Table 3.
Classification of German cohort into subgroups with and without early decrease of the down-genomic score by 17% in relation to 100-day mortality.
| Non-survivors | Survivors | Total | |||
|---|---|---|---|---|---|
| GES DOWN | Decrease ≤ 17% | 36 (21%) | 80 (47%) | 116 (67%) | PPV = 31% (23.3–39.9%) |
| Decrease > 17% | 9 (5%) | 47 (27%) | 56 (33%) | NPV = 84% (72.2–91.3%) |
|
| Total | 45 (26%) | 127 (74%) | 172 (100%) | ||
| Sensitivity = 80% (66.2–89.1%) |
Specificity = 37% (29.1–45.7%) |
||||
The threshold, adjusted to the sensitivity of 80% to predict a mortality, finally resulted in the negative predictive value NPV = 84%. The adjustment was at expense of specificity (37%) and positive predictive value (PPV = 31%). Analysis involved 172 of the initially enrolled patients because 24 patients were discharged from ICU or died before the second sampling.
Table 4.
Logistic regression analysis of factors related with 100-day mortality among patients enrolled in the German cohort of the confirmation study set.
| Unadjusted |
Adjusteda |
|||||
|---|---|---|---|---|---|---|
| Factors | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
| APACHE II score | 1.00 | 0.95–1.05 | 0.985 | – | ||
| Presence of severe sepsis/septic shock | 7.87 | 1.64–37.92 | 0.010 | 7.92 | 1.80–34.77 | 0.006 |
| Less than 17% decrease of the DOWN-score within the first 24 h | 0.40 | 0.17–0.93 | 0.033 | 0.40 | 0.17–0.93 | 0.032 |
Forward step-wise analysis.
Fig. 7.
Time course of GES UP and DOWN, CRP and PCT in survivors and non-survivors of the German cohort. For these patients RNA samples were collected on a daily basis until ICU-discharge or death for a maximum of ten days. Time courses of GES UP and DOWN are presented for survivors and non-survivors, and in comparison to CRP and PCT (each as median and Q25/Q75). The time course of GES DOWN displayed differences primarily for transcripts coding for adaptive immune functions while such differences were observed neither for GES UP nor the single protein biomarkers depending on 100-day mortality.
4. Discussion
The need for better diagnostic tests has been voiced for infectious diseases with special emphasis on sepsis (Cohen et al., 2015). We have shown that a compound biomarker panel that consists of 7 transcripts and can be used at the point-of-care provides additional information on the host response compared to conventional biomarkers: Unlike CRP and PCT, the transcriptomic panel covers both, pro- and anti-inflammatory aspects of the host response. Down-regulated transcripts reflecting effector functions of the specific immune system, a currently neglected aspect in the monitoring of sepsis exclusively prognosticated outcome with good sensitivity but limited specificity.
Two main strengths of the study can be recognized: a) the development of the biomarker in a three-step approach; and b) the confirmation of the provided information for diagnosis and prognosis in a completely independent cohort of patients. The Greek cohort is geographically and genetically far different than the German cohort presenting also a different pattern of comorbidities and microbial pathogens confirming the robustness of the transcriptomic signature.
One limitation of the present study is the exclusion of patients with preexisting immunodeficiency; further studies are needed whether the present or a modified signature may help to guide decision making in this high risk patient population. In addition, the lack of sensitivity and specificity of using the SIRS criteria to identify patients who are likely to be infected is well-recognized (Kaukonen et al., 2015), and their use is on the decline and no longer recommended in the new definitions for sepsis (Singer et al., 2016). However, alternate clinical criteria are currently not available or validated. Furthermore, the relatively small number of community-acquired infections could be considered as another limitation. However, the diagnostic uncertainty of absence or presence of infection in particular in the critically ill is a main driver for antibiotic overuse in intensive care (Opal and Calandra, 2009, Vincent et al., 2009), requiring biomarkers to distinguish between infectious and non-infectious origin of systemic inflammation. The limited specificity to separate states of systemic inflammation of non-infectious origin from sepsis even when applying a pangenomic assessment of the host response points towards biological restrictions, e.g. release of mitochondrial components reflecting evolutionary endosymbionts in particular in the most severely ill patients (Zhang et al., 2010).
The bias towards hospital-acquired infection in our cohort could lead to over-emphasis of the immuno-suppressive aspect of the host response as this has been considered a late occurring event. A most recent study confirmed two of the three biomarkers of our DOWN-score, namely IL7R and HLA-DPA1, in a cohort comprising exclusively community-acquired pneumonia (Scicluna et al., 2015). Similarly, use of HLA-DR expression on the surface of peripheral blood mononuclear cells suggested early occurrence of immunosuppression in severe either community- or hospital-acquired infections complicated by organ dysfunction (Gomez et al., 2014).
A multi-biomarker based outcome stratification model has also been advocated by Wong et al. that reliably estimated probability of mortality based on inflammatory markers along with conventional clinical and demographic data. Similar to our approach the sensitivity is much better than the specificity which would qualify these approaches to enroll patients at risk e.g. into sepsis trials (Wong et al., 2014).
Sepsis results from a dysregulated host inflammatory response to ‘pathogen associated molecular patterns’ (PAMPs). PAMPs, such as endo- or exotoxins are recognized by pattern recognition receptors (PRRs) leading to a signal-specific transcriptomic fingerprint (Calvano et al., 2005). potentially providing information to guide therapeutic decisions (Giamarellos-Bourboulis and Raftogiannis, 2012). Translation of these principles into clinical application has become feasible with the advent of ‘omics’-technologies. Limitations in the critically ill are, however, many, including an alternate binding of the ‘Danger associated molecular patterns’ family of ligands, genetic variability of the inflammatory response, and absorption of PAMPs from the gastrointestinal tract (Zhang et al., 2010). These confounding factors and technical hurdles associated with array technology, have prevented its broader use in particular at the point-of-care.
Moreover, an ever increasing body of evidence has led to questioning of the concept of SIRS proposed as an exaggerated immune response driving the course of disease in the critically ill. An increasingly better defined failure of central immune functions has been recognized (Hotchkiss et al., 2013), which is further modulated by metabolic adaptations (Medzhitov et al., 2012). Thus, novel biomarkers of sepsis should also take into account these facets of the disease. Consistently, our data lend support to the notion that including biomarkers of the anti-inflammatory or immunosuppressive facet of the host response to infection improves clinical utility to identify infection and prognosticate outcome. These currently available data are restricted to the transcriptome and introduce the need to corroborate them in the context of proteomic and metabolomic data which might require analysis on pivotal public databases and interdisciplinary knowledge platforms (Montague et al., 2014).
Immune dysfunction affects antigen-presenting cells (APC) and T-lymphocytes although the contribution of this phenomenon to the prolonged morbidity and mortality in survivors of sepsis remains ill-defined. Surrogate parameters to describe the phenotype of immune effector cells include decreased expression of HLA-DR on antigen-presenting cells, reduced stimulated ex vivo cytokine production, and impaired T-cell function, such as increased production of programmed death 1 (PD-1), cytotoxic T-lymphocyte-associated antigen 4, and B and T-lymphocyte attenuator molecules (Boomer et al., 2011).
While clinical studies applying immunostimulatory agents, such as γ-interferon or colony-stimulating factors in sepsis are still in their infancy, patient populations potentially benefitting from such strategies might exist (Bo et al., 2011, Boomer et al., 2014). A prerequisite to personalizing care is to describe inter-individual and population-to-population variability in health intervention outcomes using diagnostic tests to customize the type and extent of intervention (Ozdemir et al., 2006). As a result, the need to individualize immunomodulatory strategies in sepsis is increasingly acknowledged and is supported by the substantial intra-individual and inter-group variability of the marker genes measured in the present study (Fig. 3A) making such a biomarker in the first place a promising tool for interventional studies.
Various clinical studies have analyzed the transcriptomic profile of sepsis. The main difficulty in these studies is how to reach quantification of the results in a way that would allow using this information in the clinical context after transfer onto a feasible platform at the point-of-care. Our results confirm the observed parallel occurrence of pro- and anti-inflammatory changes (Opal and Calandra, 2009, Xiao et al., 2011, Parnell et al., 2013, Tang et al., 2007) and extend this concept to suggest a composite biomarker set that might be used in clinical utility studies. Vice versa our data also indicate that the conventional biomarkers CRP and PCT are significantly impacted by disease severity assuming that a pangenomic analysis of gene expression patterns describes the host response appropriately.
Two other studies assessed the transcriptomic profile during the early hours of sepsis in whole blood (Lissauer et al., 2009, Johnson et al., 2007). Results of both studies revealed increased expression of TLR5 as well as mediators of apoptosis similar to the present study. Overall, the increasing number of proof-of-concept studies suggests that transcriptomic profiling is technically feasible and leads to reproducible findings indicative of sub-categories of the host response that are not accessible to clinical diagnosis or conventional single-protein biomarkers. Furthermore, a most recent study confirms the potential of limited numbers of transcripts as biomarkers to classify patients with systemic inflammation on the ICU (McHugh et al., 2015).
While measurements of proteins in plasma can rely on established platforms, the hurdles to establish multiplexed transcriptome-based biomarkers are significant.
The present findings based on a three-step approach and comprising confirmation in two independent cohorts introduce a new genomic biomarker that can separate critically ill patients with infection from those without infection. The biomarker behaves similarly among patients with clinical signs of infection with or without confirmatory microbiology; these are used as a positive infection group to simulate the everyday clinical scenario where microbiology findings either fail or delay considerably. One of its basic components, namely the DOWN-score, changes very early i.e. within the first 24 h indicating prognosis, allowing to identify a population-at-risk with a good sensitivity but limited specificity that might be included in studies aiming to improve mortality. Such strategies are increasingly recognized as an option to avoid doing harm in patients ultimately surviving without immunomodulatory interventions when aiming to attenuate a principally adaptive response to infection (Singer and Glynne, 2005).
5. Conclusions
Multiplexed transcriptome-based biomarkers provide additional information compared to currently available single protein biomarkers of the host response. Quantification of impaired innate immunity can potentially guide studies of anti-inflammatory therapies, while measurement of derangements in specific immunity may inform the design of studies of strategies to restore immune effector functions.
Funding
The works were supported by the BMBF within the framework of the “MetaZIK”/ZIK Septomics Jena (03Z2J521), the Thuringian Ministry of Education, Science and Art (projects B-309-00014 and A 309-04002), and by Thüringer Aufbaubank (“RNA Score 2007 FE 014”). Part of the study was funded by the Hellenic Institute for the Study of Sepsis.
Competing Interest
Patents have been filed and obtained by SIRS-Lab GmbH and Analytik Jena AG for the use of transcriptomic biomarkers for the diagnosis of sepsis.
Ethics Statement
All study protocols were approved by the respective responsible institutional review boards, i.e. the ethics committees of Friedrich-Schiller-University Jena, Geneva University Hospital and Pitié-Salpétrière Hospital Paris, and the institutional review boards of ATTIKON University hospital, Korinthos General Hospital and Alexandra General Hospital. Written informed consent was provided by patients or their legal representatives.
Author Contributions
MB, EM, SR and KR were responsible for study conception, design and/or supervision.
MB, EJGB, AK, EM, KF, AR, MK, BW, SR, and KR analyzed and/or interpreted data.
KF, AR and BW designed assays and performed laboratory experiments.
EM, MK and EJGB conducted statistical analyses.
MB, EJGB, JMC, OGL, OR, SR, and KR contributed reagents, materials and/or clinical samples.
MB, EJGB, AK, EM, and JCM drafted the manuscript.
All authors revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- APACHE
Acute Physiology and Chronic Health Evaluation
- AUC
area under curve
- BSI
bloodstream infection
- CAP
community-acquired pneumonia
- CD59
Protectin
- CLU
Clusterin
- CPVL
Carboxypeptidase, vitellogenic-like
- CRP
C-Reactive Protein
- Ct
cycle threshold
- FGL2
Fibrinogen-like 2
- GES
gene expression score
- HLA-DPA1
Major histocompatibility complex class II, DP alpha1
- IAI
intraabdominal infection
- ICU
intensive care unit
- IL
Interleukin
- IL7R
Interleukin-7 receptor
- IQR
interquartile range
- JUH
Jena University Hospital
- LPS
lipopolysaccharide
- MAPK
mitogen-associated kinase
- OR
odds ratio
- PAMP
pathogen associated molecular pattern
- PCR
polymerase chain reaction
- PCT
procalcitonin
- PRR
pattern recognition receptor
- RT-qPCR
reverse transcription quantitative real-time polymerase chain reaction
- ROCs
receiver operating characteristics
- SIRS
Systemic Inflammatory Response Syndrome
- SOFA
Sequential (Sepsis-related) Organ Failure Assessment
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- TLR5
Toll-like receptor 5
- VAP
ventilator-associated pneumonia
Acknowledgment
The authors gratefully acknowledge the team of Elke Grunow, Vera Hanemann, Regina Heinze, Kristina Wunsch, and Cristina Guillen Rovira (Analytik-Jena AG and/or SIRS-Lab GmbH) as well as the lab of Ralf Claus (Jena University Hospital) for conduction of transcriptomic analyses. The support of Frank Bloos and Alessandro Romualdi is acknowledged for documentation and confirming plausibility of the clinical and microarray data.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.03.006.
Appendix A. Supplementary data
Supplementary material 1.
Supplementary material 2.
References
- Adhikari N.K., Fowler R.A., Bhagwanjee S., Rubenfeld G.D. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adib-Conquy M., Cavaillon J.M. Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;9:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Bo L., Wang F., Zhu J., Li J., Deng X. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit. Care. 2011;15:R58. doi: 10.1186/cc10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomer J.S., To K, Chang K.C., et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomer J.S., Green J.M., Hotchkiss R.S. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence. 2014;5:45–56. doi: 10.4161/viru.26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T., Cohen J. The International Sepsis Forum Consensus definitions of infections in the intensive care unit. Crit. Care Med. 2005;33:1639–1648. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- Calvano S.E., Xiao W., Richards D.R., et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- Cohen J., Vincent J.L., Adhikari N.K., et al. Sepsis: a roadmap for future research. Lancet Infect. Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- Desai K.H., Tan C.S., Leek J.T., Maier R.V., Tompkins R.G., Storey J.D. Dissecting inflammatory complications in critically injured patients by within-patient gene expression changes: a longitudinal clinical genomics study. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feezor R.J., Cheng A., Paddock H.N., Baker H.V., Moldawer L.L. Functional genomics and gene expression profiling in sepsis: beyond class prediction. Clin. Infect. Dis. 2005;41:S427–S435. doi: 10.1086/431993. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Raftogiannis M. The immune response to severe bacterial infections: consequences for therapy. Exp. Rev. Anti-Infect. Ther. 2012;10:369–380. doi: 10.1586/eri.12.2. [DOI] [PubMed] [Google Scholar]
- Gomez H.G., Gonzalez S.M., Londoño J.M., et al. Immunological characterization of compensatory anti-inflammatory response syndrome in patients with severe sepsis: a longitudinal study. Crit. Care Med. 2014;42:771–780. doi: 10.1097/CCM.0000000000000100. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R.S., Monneret G., Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.B., Lissauer M., Bochiccchio G.V., et al. Gene expression profiles differentiate between sterile SIRS and early sepsis. Ann. Surg. 2007;245:611–621. doi: 10.1097/01.sla.0000251619.10648.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukonen K.M., Bailey M., Pilcher D., Cooper D.J., Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015;372:1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- Kenneth J.L., Thomas D.S. Analysis of relative gene expression data using real-time quantitative PCR and the ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Kumar G., Kumar A., Taneja A., et al. Nationwide trends of severe sepsis in the 21st century (2000–2007) Chest. 2011;140:122331. doi: 10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- Levy M., Fink M.P., Marshall J.C., et al. SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 2001;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. (2003) [DOI] [PubMed] [Google Scholar]
- Lissauer M.E., Johnson S.B., Bochicchio G.V., et al. Differential expression of Toll-like receptor genes: sepsis compared with sterile inflammation 1 day before sepsis diagnosis. Shock. 2009;31:238–244. doi: 10.1097/SHK.0b013e3181834991. [DOI] [PubMed] [Google Scholar]
- McHugh L., Seldon T.A., Brandon R.A., et al. A molecular host response assay to discriminate between sepsis and infection-negative systemic inflammation in critically ill patients: discovery and validation in independent cohorts. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague E., Stanberry L., Higdon R., et al. MOPED 2.5—an integrated multi-omics resource: multi-omics profiling expression database now includes transcriptomics data. OMICS. 2014;18:335–343. doi: 10.1089/omi.2014.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal S.M., Calandra T. Antibiotic usage and resistance: gaining or losing ground on infections in critically ill patients? JAMA. 2009;302:2367–2368. doi: 10.1001/jama.2009.1774. [DOI] [PubMed] [Google Scholar]
- Ozdemir V., Williams-Jones B., Glatt S.J., et al. Shifting emphasis from pharmacogenomics to theragnostics. Nat. Biotechnol. 2006;24:942–946. doi: 10.1038/nbt0806-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell G.P., Tang B.M., Nalos M., et al. Identifying key regulatory genes in the whole blood of septic patients to monitor underlying immune dysfunctions. Shock. 2013;40:166–174. doi: 10.1097/SHK.0b013e31829ee604. [DOI] [PubMed] [Google Scholar]
- Pierrakos C., Vincent J.L. Sepsis biomarkers: a review. Crit. Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicluna B.P., Klein Klouwenberg P.M., van Vught L.A., et al. A molecular biomarker to diagnose community-acquired pneumonia on intensive care unit admission. Am. J. Respir. Crit. Care Med. 2015;192:826–835. doi: 10.1164/rccm.201502-0355OC. [DOI] [PubMed] [Google Scholar]
- Singer M., Glynne P. Treating critical illness: the importance of first doing no harm. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.M., McLean A.S., Dawes I.W., Huang S.J., Lin R.C. The use of gene-expression profiling to identify candidate genes in human sepsis. Am. J. Respir. Crit. Care Med. 2007;176:676–684. doi: 10.1164/rccm.200612-1819OC. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Rello J., Marshall J., et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- Wong H.R., Lindsell C.J., Pettilä V., et al. A multibiomarker-based outcome risk stratification model for adult septic shock. Crit. Care Med. 2014;42:781–789. doi: 10.1097/CCM.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Mindrinos M.N., Seok J., et al. A genomic storm in critically injured humans. J. Exp. Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Raoof M., Chen Y., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1.
Supplementary material 2.