Abstract
Long-term weight-loss (WL) interventions reduce insulin serum levels, protect from obesity, and postpone age-associated diseases. The impact of long-term WL on adipose-derived stromal/progenitor cells (ASCs) is unknown. We identified DIRAS3 and IGF-1 as long-term WL target genes up-regulated in ASCs in subcutaneous white adipose tissue of formerly obese donors (WLDs). We show that DIRAS3 negatively regulates Akt, mTOR and ERK1/2 signaling in ASCs undergoing adipogenesis and acts as a negative regulator of this pathway and an activator of autophagy. Studying the IGF-1–DIRAS3 interaction in ASCs of WLDs, we demonstrate that IGF-1, although strongly up-regulated in these cells, hardly activates Akt, while ERK1/2 and S6K1 phosphorylation is activated by IGF-1. Overexpression of DIRAS3 in WLD ASCs completely inhibits Akt phosphorylation also in the presence of IGF-1. Phosphorylation of ERK1/2 and S6K1 is lesser reduced under these conditions. In conclusion, our key findings are that DIRAS3 down-regulates Akt–mTOR signaling in ASCs of WLDs. Moreover, DIRAS3 inhibits adipogenesis and activates autophagy in these cells.
Keywords: Adipogenesis, Aging, Autophagy, Caloric restriction, DIRAS3, ERK1/2, Human adipose-derived stromal/progenitor cells, IGF-1, Insulin, mTOR, Akt, Obesity, Weight loss
Graphical abstract
Highlights
-
•
Long-term weight loss (WL) induces DIRAS3 and IGF-1 in ASCs of sWAT in formerly obese humans.
-
•
DIRAS3 selectively down-regulates IGF-1R-Akt–mTOR signaling in ASCs and channels the IGF-1 stimulus to the ERK1/2 branch.
-
•
DIRAS3 inhibits adipogenesis and activates autophagy in ASCs.
Long-term weight loss (WL) interventions reduce insulin serum levels, protect from obesity and postpone age-associated diseases. The impact of WL on adipose-derived stromal/progenitor cells (ASCs), stem cell-like cells in human subcutaneous white adipose tissue (sWAT), is not understood. We found that WL induced GTP-binding RAS-like 3 (DIRAS3) and insulin-like growth factor 1 (IGF-1), regulators of the IGF-1–mTOR signal transduction pathway, in ASCs in sWAT of formerly obese humans. We demonstrate that DIRAS3 selectively down-regulates IGF-1R–Akt–mTOR signaling in ASCs upon WL even in the presence of high IGF-1 level and that DIRAS3 inhibits adipogenesis and activates autophagy in these cells.
1. Introduction
Long-term weight-loss (WL) interventions, such as prolonged hypocaloric diets and bariatric surgeries, lead to reduced insulin levels, improvement in insulin sensitivity and glycemic homeostasis in formerly obese people and improve glycemic control in individuals with diabetes mellitus type 2 (T2DM) (Klein et al., 2004, Dixon et al., 2012). Although the underlying mechanisms are not precisely understood, one common key effect of these interventions is a long-term caloric restriction (CR) (Klein et al., 2004, Sjöström et al., 2004, Bradley et al., 2012, Knop and Taylor, 2013). Long-term CR, also referred to as dietary restriction (DR), defined as lessening caloric intake (typically by about 30% in rodents and monkeys) without malnutrition is the most robust intervention to extend health and maximum lifespan in most, but not all, laboratory animal models (Speakman and Mitchell, 2011, de Cabo et al., 2014). It is widely accepted that CR protects cells against oxidative damage (López-Lluch et al., 2008) and induces DNA-repair (López-Otín et al., 2013) and recycling processes such as autophagy (de Cabo et al., 2014). The underlying mechanisms are however not precisely understood. Increasing evidence suggests that reduced growth factor- and nutrient-responsive protein kinase signaling mediate beneficial effects of CR. Conserved CR-responses are reduced growth hormone (GH)/insulin-like growth factor-1 (IGF-1) and insulin signaling (Bartke et al., 2013, Kenyon, 2010). In mammals, GH produced by the pituitary gland induces production and secretion of IGF-1 in the liver, which acts as endocrine regulator. IGF-1 is also produced in peripheral organs by GH-dependent and -independent pathways, which acts locally in paracrine or autocrine fashion (Sonntag et al., 2012, Bartke et al., 2013). The impact of CR on IGF-1 signaling in the periphery is little understood. Another conserved CR-response is reduced activity of the nutrient-responsive protein kinase, mechanistic target of rapamycin (mTOR), associated with lifespan extension in invertebrates and mice (Kapahi et al., 2004, Selman et al., 2009). mTOR forms a network with insulin/IGF-1 signaling, regulating a wide range of cellular processes, such as autophagy, growth, differentiation and metabolism, which are thought to mediate effects of CR (Laplante and Sabatini, 2012). The mechanisms on how CR employs the insulin/IGF-1–mTOR signaling network to influence cellular downstream pathways are the current focus of obesity and aging research.
Adipose tissue is a main organ implicated in regulation of healthspan induced by reduced insulin/IGF-1–mTOR signaling (Broughton and Partridge, 2009). Decreased insulin sensitivity in subcutaneous white adipose tissue (sWAT) due to an age-related deterioration of sWAT is a hallmark of aging (Borkan et al., 1983). Long-term CR leads to reduced adipocyte size and remodeling of body fat composition away from visceral (v) WAT to sWAT (Huffman and Barzilai, 2010, Speakman and Mitchell, 2011). Since sWAT has rather beneficial and vWAT detrimental effects in aging and obesity this contributes to extension of healthspan. While sWAT adipocytes seem to be particularly beneficial for insulin action due to their crucial role in maintaining whole body glucose homeostasis and lipid metabolism, increasing evidence suggests that health benefits of CR exceed those directly associated with weight-loss. Adipocytes arise from adipose-derived stromal/progenitor cells (ASCs), which constitute a large pool of precursors, crucial for adipose tissue renewal, homeostasis, expansion and hence function (Berry et al., 2013, Zwierzina et al., 2015). Upon stimulation by insulin, glucocorticoids, cAMP inducers, and additional serum components ASCs enter a differentiation program, referred to as adipogenesis, to acquire their specific functions as adipocytes (Rosen and MacDougald, 2006). According to the current model adipogenesis involves growth arrest, early and terminal differentiation, including morphological changes, lipid accumulation and the expression of fat cell specific genes, such as fatty acid binding protein-4 (FABP4), perilipin and adipokines. The stages of adipogenesis are orchestrated by a transcriptional cascade involving the adipogenic key factor nuclear receptor peroxisome proliferator-activated receptor-γ2 (PPARγ2) and members of the CCAAT/enhancer-binding protein (C/EBP) family.
The impact of WL on ASCs is unknown. By comparing ASCs from abdominal sWAT of normal weight (NWD), obese (OD) and long-term weight-losing formerly obese donors (WLDs) we showed that long-term WL amongst others reduced the adipogenic activity in these cells (Mitterberger et al., 2014b). To better understand the impact of long-term WL on human ASCs, we compared gene expression in a well characterized ASC population (Mitterberger et al., 2012, Zwierzina et al., 2015) isolated from sWAT of age-matched NWDs, ODs, and WLDs using microarray gene expression analysis. Intriguingly, two strongly induced WL target genes were insulin-like growth factor 1 (IGF-1), the activator of signaling from the IGF-1-receptor, and GTP-binding RAS-like 3 (DIRAS3) (Yu et al., 1999), an imprinted tumor suppressor gene. DIRAS3 encodes a small GTPase which was shown to inhibit signaling through phosphatidylinositol-3-kinase (PI3K) and Ras/Mitogen-activated protein kinase (MAPK) in tumor cells (Luo et al., 2003) and induces a dwarf phenotype in transgenic mice (Xu et al., 2000).
2. Material and methods
2.1. Donors
Human sWAT samples were taken from persons undergoing routine abdominoplasty at the Institute for Plastic and Reconstructive Surgery (Medical University Innsbruck) (Mitterberger et al., 2010, Mitterberger et al., 2011, Mitterberger et al., 2012, Mitterberger et al., 2014a, Mitterberger et al., 2014b). The patients gave their informed written consent and had been approved by the ethical committee of Innsbruck Medical University, Austria, according to the Declaration of Helsinki. All sWAT samples were obtained from the lower abdomen. Obesity and normal weight were defined according to the World Health Organization criteria on the basis of the body mass index (BMI = weight [kg]/height [m2]). Female donors were divided into three groups according to their BMI, obese (OD) (BMI ≥ 30 kg/m2), normal weight (NWD) (BMI 19–25 kg/m2), and long-term weight losing initially obese (WLD) (former BMI ≥ 30 kg/m2 and current BMI ≤ 25 kg/m2). None of the women had diabetes, liver, renal, or other severe metabolic diseases. Long-term WL was achieved either by hypocaloric diet or laparoscopic adjustable gastric banding (LAGB). The detailed WL regimens are described in Mitterberger et al. (2010). The women were age matched. The clinical and anthropometric parameters are indicated in Table S1.
2.2. Mouse xenograft studies
Mice were treated in accordance with the guidelines of the “European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes” and the Austrian law. Animal experiments were approved by the ethics committee of the Austrian Federal Ministry of Science and Research (Application No. Zl. 188809/13). Further details are explained in the supplementary experimental procedure.
2.3. Isolation of ASC from human subcutaneous adipose tissue
ASCs were isolated as described (Mitterberger et al., 2012).
2.4. Cell culture
ASCs were cultivated as described (Mitterberger et al., 2012).
2.5. Adipogenic differentiation
Adipogenic differentiation was conducted as described (Mitterberger et al., 2012).
2.6. Retroviral gene expression system
See supplementary experimental procedures.
2.7. Laser scanning confocal indirect immunofluorescence microscopy (IF-CLSM)
IF-CLSM was performed as described (Mitterberger et al., 2012).
2.8. Affymetrix microarray gene expression analysis
See supplementary experimental procedures.
2.9. Quantitative RT-PCR analysis
Expression analysis with q-RT-PCR was performed as described (Mitterberger et al., 2012). β-actin was used for normalization. Primer sequences are listed in Table S2.
2.10. Western blot analysis
Western blot analysis was performed as described (Mitterberger et al., 2012). Antibodies used in the study are listed in the supplementary experimental procedures.
2.11. Flow cytometry analysis
Cells were incubated with Lyso-tracker red (Life Technologies) and analyzed by using FACS Canto (BD Biosciences). Data was analyzed employing FlowJo software.
2.12. Multispectral imaging flow cytometry
See supplementary experimental procedures.
2.13. Statistical analysis
Statistical analysis was performed in GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). The significance of difference between means was assessed by Student's t test or analysis of variance (ANOVA). Error bars are represented as the mean ± SEM.
3. Results
3.1. DIRAS3 expression is up-regulated upon long-term WL in human ASCs
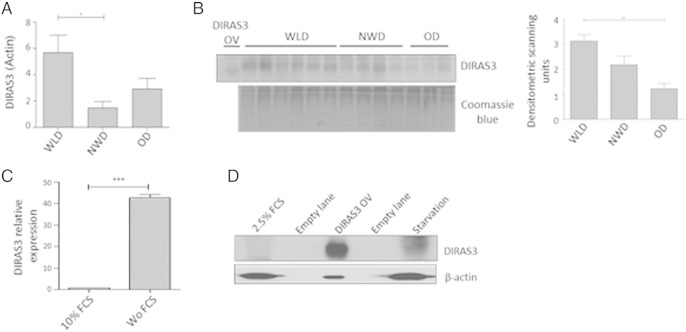
DIRAS3 was one of the WL target genes emerged from a global gene expression analysis performed on human cs (cell surface)-DLK1(PREF1)−/cs-CD34+/CD90+/CD105+ dim/α-SMA+/CD45−/CD31− ASCs freshly isolated from sWAT (Mitterberger et al., 2012). Cells from age and sex matched WLDs (hypocaloric diets and laparoscopic adjustable gastric banding (LAGB)), NWDs and ODs (Table 1A) were subjected to whole genome microarray gene expression analysis (Affymetrix Chip U133 + 2.0). Results revealed a 11.88-fold higher expression of DIRAS3 in ASCs of WLDs relative to NWDs and a 2.20-fold higher expression of DIRAS3 in ASCs from WLDs relative to ODs (Table 1B). Quantitative real time PCR (q-RT-PCR) confirmed an up-regulation of DIRAS3 expression in early passage of ASCs of the WLDs relative to NWDs and ODs (Fig. 1A) and we observed comparatively higher levels of DIRAS3 protein in adipose tissue lysates from WL donors (Fig. 1B). Although serum starvation is not an ideal model for chronic CR or long-term WL we employed this approach to analyze how DIRAS3 responds to reduced nutrient/serum levels in vitro. Cultivating ASCs in conditions of serum/growth factor starvation led to an up-regulation of DIRAS3 mRNA (Fig. 1C) and DIRAS3 protein (Fig. 1D), confirming the DIRAS3 induction upon WL in vivo. We conclude that DIRAS3 is up-regulated upon long-term WL in human ASCs.
Table 1.
(A) Characteristics of the 2 NWDs, 3 ODs and 3 WLDs (2 hypocaloric diets, 1 LAGB) employed in the global gene expression analysis. (B) IGF-1 and DIRAS3 mRNA expression based on DNA microarray expression profiling in ASCs freshly isolated from age-matched NWDs, ODs, and WLDs. Note, IGF-1 and DIRAS3 are two selected CR target genes out of a number of genes, which emerged from the mRNA screen (Mitterberger et al., unpublished results). Thresholds for identification of CR genes: Downregulated through CR (− vs. NW and OW), 50% cut-off. Upregulated through CR (+ vs. NW and OW), 2-fold cut-off. For detailed technical information, see supplementary experimental procedures.
| A. | |||||
|---|---|---|---|---|---|
| Age (years) | Procedure | BMI [kg/m2] | Delta BMI [kg/m2] | Duration CR (years) | |
| NWD | 31 ± 11 | 22 ± 3 | |||
| WLD | 36 ± 9 | 1 × LAGB, 2 × diet | 22 ± 2 | 14 ± 3 | 4 ± 2 |
| OD | 46 ± 2 | 32 ± 1 | |||
| B. | ||
|---|---|---|
| Gene | IGF-1 (fold increase) | DIRAS3 (fold increase) |
| WLD/NWD | 23.59 | 11.8 |
| WLD/OD | 4.20 | 2.20 |
| OD/NWD | 5.62 | 5.43 |
Fig. 1.
DIRAS3 expression is up-regulated upon long-term WL in human ASCs. (A) DIRAS3 expression relative to actin was analyzed by q-RT-PCR in early passage ASCs derived from WLDs (n = 4), NWDs (n = 3) and ODs (n = 3). (B) (left panel) Tissue lysates from different donors were examined for DIRAS3 expression by Western blotting. U-2OS cells overexpressing DIRAS3 served as input control (OV). Equal protein quantities were loaded on SDS PAGE based on BCA quantification. Staining of total protein served as control for equal loading. (Right panel) Densitometric band intensities of the DIRAS3 Western blot were quantified using ImageJ. (C) ASCs were cultured for 48 h in 10% FCS or in the absence of serum (Wo FCS) and relative expression of DIRAS3 mRNA was quantified by q-RT-PCR normalized to actin. (D) Detection of DIRAS3 protein in lysates from ASCs cultured in medium supplemented with 2.5% FCS and for four days without serum (starvation) by Western blotting. ASCs infected with a DIRAS3 overexpressing lentivirus (DIRAS3 OV) served as control. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001. The significance of difference between means was assessed by analysis of variance (ANOVA) (A and B) and Student's t test (C).
3.2. DIRAS3 negatively regulates Akt–mTOR pathway in human ASCs
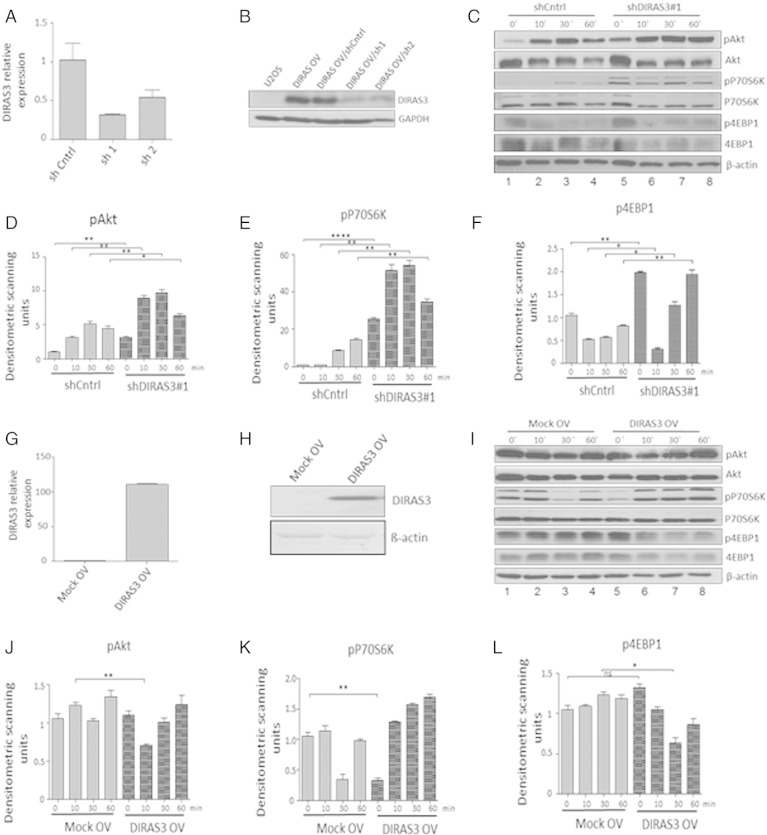
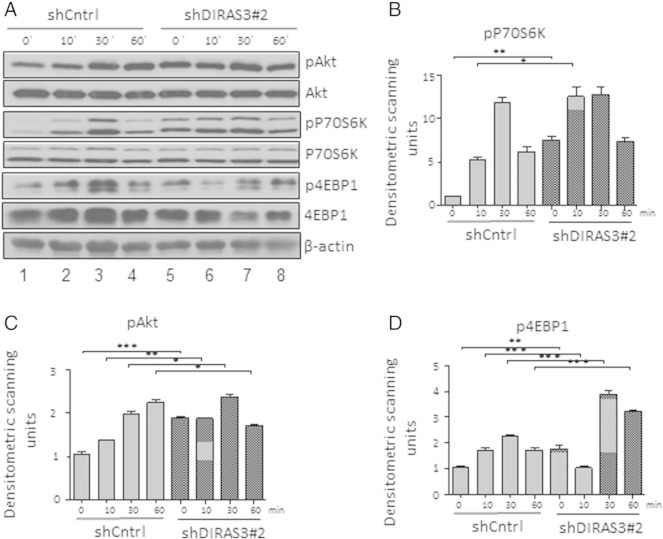
DIRAS3 can down-regulate PI3K-Akt–mTOR signaling in cancer cells by inhibition of PI3K activity (Luo et al., 2003). To assess the role of DIRAS3 in regulation of the Akt–mTOR pathway in human ASCs, we knocked down (KD) DIRAS3 using gene specific shRNAs (Fig. 2A). DIRAS3 KD was confirmed at protein level in the experimental cell line U-2OS overexpressing DIRAS3 (Fig. 2B). All further experiments were conducted in ACSs of WLDs. The effect of DIRAS3 KD was examined in serum starved ASCs and after serum re-stimulation by analyzing the phosphorylation of Akt, S6K1 and 4EBP1. Serum starvation of human ASCs down-modulated Akt and S6K1 phosphorylation (Fig. 2C; see also S. Fig. 1 comparing proliferating cells to 0 h). This down-regulation was not observed upon KD of DIRAS3, Akt–mTOR pathway remained active even in the absence of serum (Fig. 2C, compare lanes 1 and 5). Additionally, we observed a significant higher phosphorylation of Akt, S6K1 and 4EBP1 in DIRAS3 KD ASCs after serum stimulation (Fig. 2C–F). To exclude off-target effects we specifically KD DIRAS3 using a different shRNA sequence and observed similar effects on Akt–mTOR pathway activity (S. Fig. 2A–D). Similar effects were observed using ASCs from different donors. To corroborate our results, we studied the regulation of Akt–mTOR pathway in ASCs infected with lentiviruses overexpressing DIRAS3 (Fig. 2G and H). As expected, Akt–mTOR pathway down-modulation was observed at early time points upon serum re-stimulation in DIRAS3 over-expressing ASCs as shown by reduced phosphorylation of Akt, S6K1 and 4EBP1 (Fig. 2I–L). We conclude that DIRAS3 negatively regulates Akt–mTOR signaling in human ASCs.
Fig. 2.
Impact of DIRAS3 knock-down (KD) and over-expression (OV) on Akt–mTOR pathway in human ASCs. (A) Efficiency of RNA interference mediated DIRAS3 KD in ASCs was analyzed at mRNA level using q-RT-PCR employing actin as reference gene. (B) DIRAS3 KD efficacy at protein level was evaluated by Western blotting. DIRAS3 was overexpressed in the experimental cell line U-2OS, followed by KD using specific shRNA expressing lentiviruses. (C) DIRAS3 was KD in ASCs using specific shRNA, and cells were density arrested and starved by serum withdrawal for 48 h. Then a cocktail containing 2.5% FCS, insulin, 3-isobutyl-1-methylxanthine, and dexamethasone was added. Cell lysates were harvested at indicated time points. Phosphorylation of Akt (S473), S6K1 (T389) and 4EBP1 (T37/46) was examined by Western blotting. (D–F) Fold changes in densitometric band intensities, acquired by ImageJ were compared. Band intensity of shCntrl at time point 0 min was taken as 1. (G) Overexpression of DIRAS3 mRNA normalized to actin and (H) protein in ASCs infected with lentiviruses expressing DIRAS3 under control of CMV promoter. (I) Following DIRAS3 over-expression, cells were density arrested and starved by serum withdrawal for 48 h. Then a cocktail containing 2.5% FCS, insulin, 3-isobutyl-1-methylxanthine, and dexamethasone was added. Cell lysates were harvested at indicated time points. Phosphorylation of Akt (S473), S6K1 (T389) and 4EBP1 (T37/46) was examined by Western blotting from cell lysates harvested at indicated time points. (J–L) Fold changes in densitometric band intensities, acquired by ImageJ were compared. Band intensity of Mock control at time point 0 min was taken as 1. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001. The significance of difference between means was assessed by analysis of variance (ANOVA) (D, E, F, J, K and L).
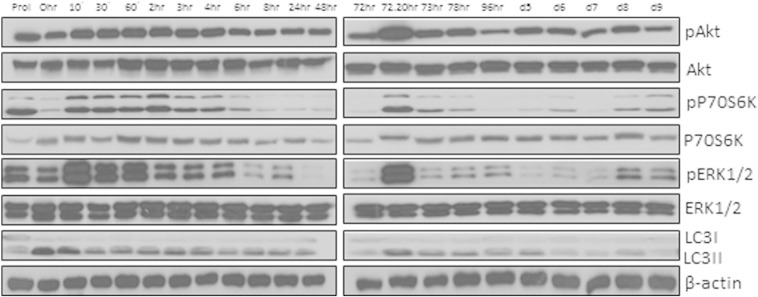
Supplementary Fig. 1.
Akt (S473), P70S6K (T389), ERK1/2 (T282/Y204) phosphorylation and LC3I to LC3II conversion pattern during the course of adipogenesis. ASCs were grown to confluence and starved for 48 h. Adipogenesis was induced using hormone cocktail. Cell lysates were collected at indicated time points and blotted for phosphorylated and total Akt, P70S6K, ERK1/2 and LC3.
Supplementary Fig. 2.
Knock-down (KD) of DIRAS3 results in up-regulation of PI3K pathway. (A) DIRAS3 was KD in human ASCs using specific shRNA, cells were density arrested and starved by serum withdrawal for 48 h and then tyrosine kinase receptor signaling was induced by a cocktail containing 2.5% FCS, insulin, 3-isobutyl-l-methylxanthine, and dexamethasone. Cell lysates were harvested at indicated time points. Phosphorylation of Akt (S473), P70S6K (T389) and 4EBP1 (T37/46) was examined by Western blotting using specific antibodies. (B–D) Fold changes in densitometric band intensities, acquired by ImageJ were compared. Band intensity of shCntrl at time point 0 min was taken as 1. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001. The significance of difference between means was assessed by analysis of variance (ANOVA) (B, C, and D).
3.3. DIRAS3 negatively regulates adipogenesis
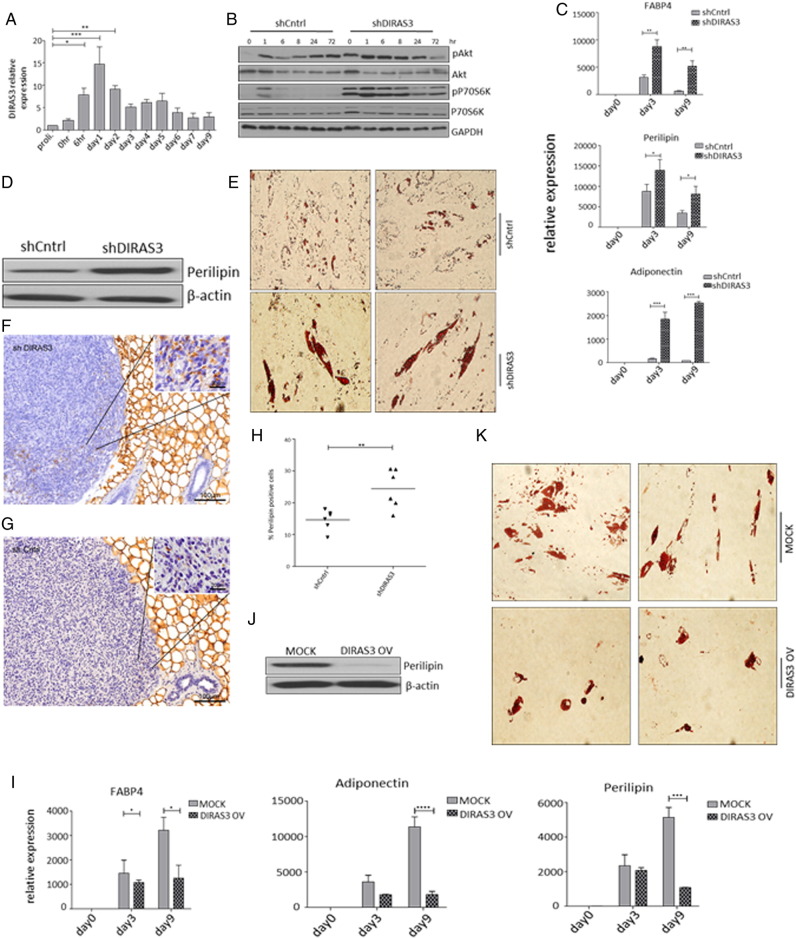
Activated insulin/IGF-1 (Boucher et al., 2010) and mTOR signaling (Laplante and Sabatini, 2012) stimulate adipogenesis. Since DIRAS3 slows Akt–mTOR signaling and long-term WL reduces adipogenic differentiation capacity of ASCs in formerly obese humans (Mitterberger et al., 2014b), we investigated whether DIRAS3 impinges on adipogenesis in ASCs. Induction of adipogenic differentiation by a hormone cocktail led to a morphological transformation of ASCs to rounded cells during first 72 h after induction, which is a hallmark of adipogenesis (S. Fig. 3). Differentiation was confirmed by staining fat droplets within the cells by Oil-Red-O (S. Fig. 3). Interestingly DIRAS3 mRNA expression was significantly up-regulated at early stages of adipogenesis, 6 h post-induction, while peak of expression was observed after 24 h followed by a gradual decrease (Fig. 3A). Up-regulation of DIRAS3 expression was dependent on the adipogenesis program, as only the complete adipogenic cocktail induced DIRAS3 expression (S. Fig. 4A). The other two members of DIRAS family, DIRAS1 and DIRAS2, were not markedly up-regulated during adipogenesis (S. Fig. 4B and C). We monitored the activity of Akt–mTOR pathway in the course of adipogenesis in human ASCs by analyzing phosphorylation of Akt and S6K1 (S. Fig. 1). Induction of adipogenesis up-regulated Akt and S6K1 phosphorylation in the density arrested cells (Fig. 3B). Phosphorylation gradually decreased to its basal level approximately 6 h post-induction. Next, we tracked the phosphorylation of Akt and S6K1 in the first 72 h after induction of adipogenesis in DIRAS3 KD ASCs and detected a marked up-regulation of Akt and S6K1 phosphorylation upon DIRAS3 knockdown (Fig. 3B). Additionally, down-regulation of DIRAS3 was associated with stronger differentiation of ASCs as revealed by a significantly increased induction of FABP4, perilipin and adiponectin (Fig. 3C), higher perilipin protein level (Fig. 3D) and increased lipid accumulation at day 9 post-induction (Fig. 3E). A homolog of DIRAS3 was not found in mice (Yu et al., 2006). To study the impact of DIRAS3 on adipogenesis in vivo, we injected DIRAS3 KD human ASCs and control ASCs committed to adipogenesis into the posterior sWAT of SCID mice. As shown in Fig. 3F and G, differentiating ASCs were predominantly found in peripheral regions of the transplants adjacent to mouse sWAT. DIRAS3 KD ASCs showed increased adipocyte differentiation in mouse sWAT relative to controls (Fig. 3H), suggesting that DIRAS3 negatively regulates adipogenesis in sWAT of mammals. In accordance with the pro-adipogenic effect of the DIRAS3 KD, overexpression of DIRAS3 in ASCs diminished adipogenesis as shown by significant decrease in mRNA level of FABP4, perilipin and adiponectin upon DIRAS3 overexpression (Fig. 3I). In addition, the perilipin protein level and lipid droplets were decreased in DIRAS3 overexpressing cells at day 9 post-adipogenesis induction (Fig. 3J and K). We conclude that DIRAS3 negatively regulates adipogenesis in human ASCs.
Supplementary Fig. 3.
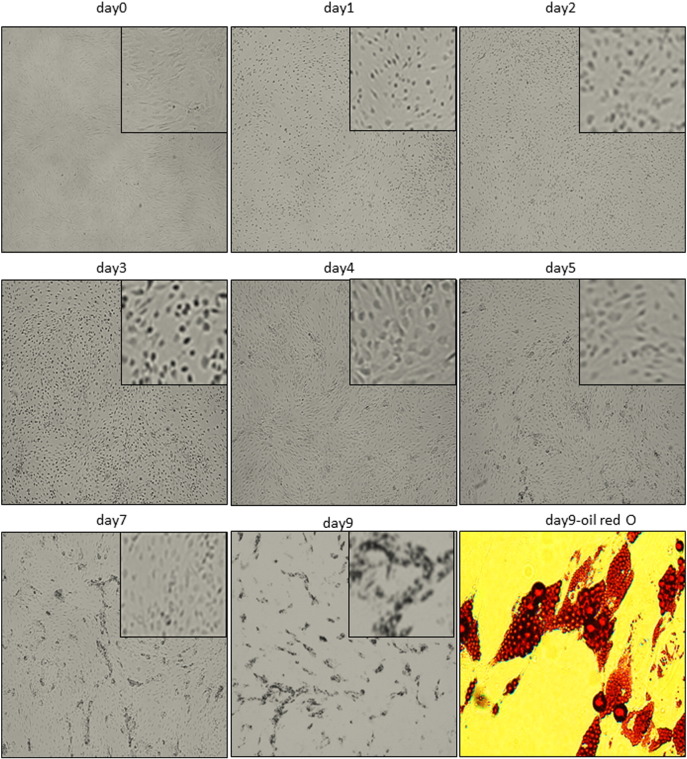
Morphological transformation of human ASCs during adipogenesis. ASCs were grown to confluence and starved for 48 h. Adipogenesis was induced using hormone cocktail. Morphology of cells was documented using bright-field microscopy. Cells were stained with Oil-Red-O to stain the lipid droplets at day 9 post-induction.
Fig. 3.
DIRAS3 is a negative regulator of adipogenesis in human ASCs. (A) DIRAS3 mRNA expression normalized to actin was investigated by q-RT-PCR during the course of adipogenesis (n = 6). (B) Akt–mTOR pathway activity pattern was monitored during the first 72 h of adipogenesis upon DIRAS3 KD. Phosphorylation of Akt (S473) and S6K1 (T389) was examined by Western blotting. (C) ASCs infected with either shCntrl or shDIRAS3 expressing lentiviruses were subjected to adipogenesis and mRNA expression of adipocyte marker genes FABP4, Perilipin and Adiponectin normalized to actin were analyzed using q-RT-PCR at the indicated time points. (D) Perilipin protein expression was analyzed by western blotting at day 9 post-adipogenesis induction in shCntrl and shDIRAS3 ASCs. (E) Adipocyte differentiation was estimated using Oil-Red-O staining at day 9 post-induction. (F and G) Representative immunohistochemical staining of xenotransplanted shDIRAS3 SCID mice (F) and shCntrl mice (G) using anti-perilipin antibodies. Region of Interest (ROI) is shown in higher magnification. (H) Margin of the transplant from each mice was imaged at 20 × magnification and perilipin positive and negative cells were counted using ImageJ cell counter plugin and shown as percentage positive cells (n = 6). (I–K) ASCs infected with either Mock or DIRAS3 overexpressing lentiviruses were subjected to adipogenesis and mRNA expression of adipocyte marker genes normalized to actin was analyzed using q-RT-PCR at the indicated time points (I). Perilipin protein analysis (J) and Oil-Red-O staining (K) were done at day 9 post-induction of adipogenesis. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001. The significance of difference between means was assessed by analysis of variance (ANOVA) (A, C and I) and Student's t test (H).
Supplementary Fig. 4.
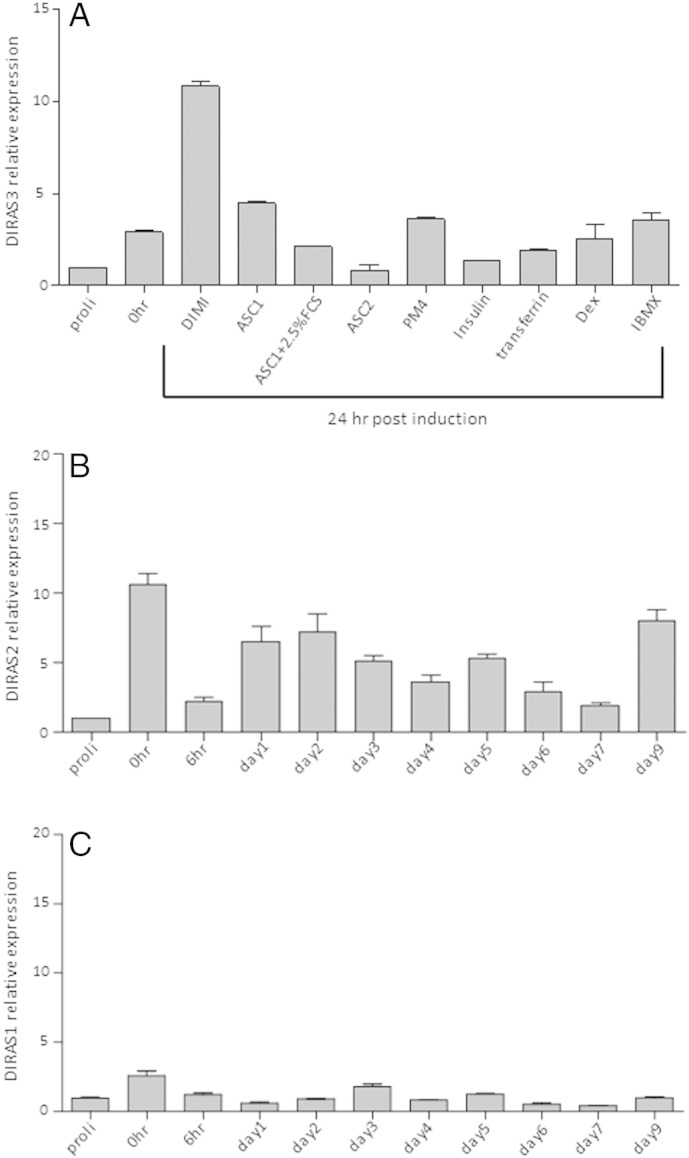
(A) Up-regulation of DIRAS3 in the course of adipogenesis depends on the adipogenic program. ASCs were grown to confluence and starved for 48 h. DIRAS3 mRNA expression was analyzed by qRT-PCR 24 h after induction either with complete hormone cocktail—DMI (DMEM-F12 HAM medium + 2.5% FCS + Dex + IBMX + insulin), or ASC1 (DMEM-F12 HAM medium without FCS) or ASC2 (DMEM-F12 HAM medium + 10% FCS) or PM4 (DMEM-F12 HAM medium + 2.5% FCS + EGF + FGF + Insulin) or specific ingredients separately as indicated. (B and C) mRNA expression was investigated in the course of adipogenesis employing q-RT-PCR for DIRAS2 (B, n = 2) and DIRAS1 (C, n = 2).
3.4. Regulation of adipogenesis by DIRAS3 is mediated by C/EBP-β and PPAR-γ2
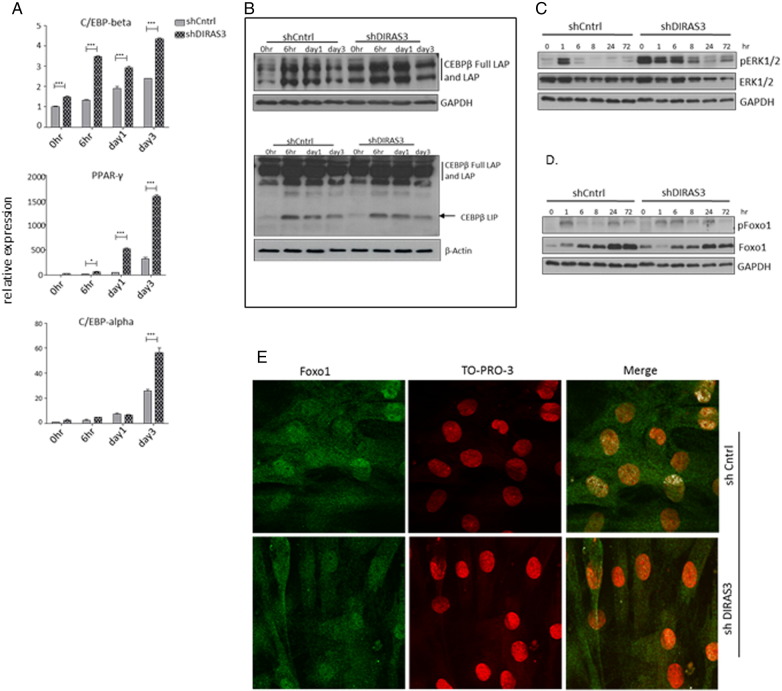
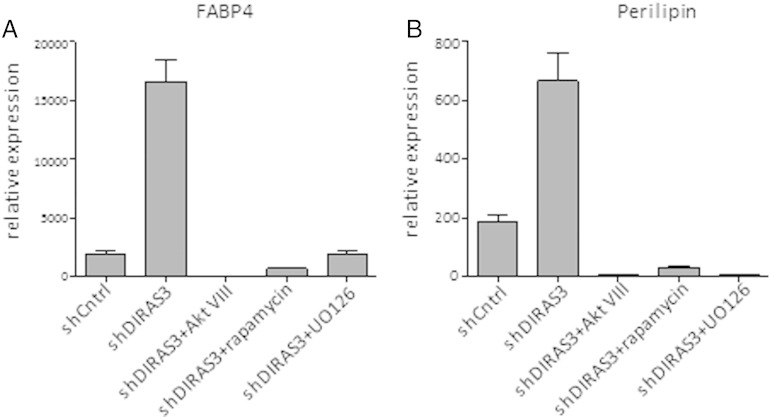
To better understand the mechanisms on how DIRAS3 regulates adipogenesis, we analyzed the expression of transcription factors C/EBP-β, C/EBP-α and PPAR-γ2, which are key regulators of adipogenesis (Rosen and MacDougald, 2006). DIRAS3 KD led to a significant up-regulation in the expression of all three genes in ASCs undergoing adipogenic differentiation (Fig. 4A). Translation of the C/EBP-β mRNA gives rise to three different isoforms, C/EBP-β Full-LAP and C/EBP-β LAP, which are activating isoforms and C/EBP-β LIP, which is an inhibitory isoform (Lechner et al., 2013). C/EBP-β induces the expression of PPAR-γ2 and C/EBP-α, which are the two key regulators of terminal adipogenesis (Park et al., 2004). We analyzed C/EBP-β protein levels and observed higher levels of C/EBP-β Full-LAP and -LAP in the first 72 h of induction upon DIRAS3 KD (Fig. 4B, left panel), while no marked difference was detected in C/EBP-β LIP protein level (Fig. 4B, right panel). Differential migration of CEBP-β Full-LAP and -LAP bands in SDS PAGE corresponds to differential phosphorylation. Activated extracellular signal regulated kinase 1/2 (ERK1/2) was shown to phosphorylate T188 residue of C/EBP-β and thus activate the transcription factor (Park et al., 2004). In accordance, Western blot analysis revealed a marked up-regulation of ERK1/2 phosphorylation during the first 72 h of adipogenesis in DIRAS3 KD ASCs (Fig. 4C). The transcription factor Foxo1 negatively controls PPAR-γ2 expression when localized within the nucleus (Nakae et al., 2003). Foxo1 is negatively regulated by Akt, as its phosphorylation by active Akt leads to its exclusion from the nucleus (Brunet et al., 1999). As DIRAS3 KD is associated with an increased phosphorylation of Akt (Figs. 2C and 3B), we analyzed changes in Foxo1 phosphorylation in ASCs. In fact, DIRAS3 KD led to an up-regulation of Foxo1 phosphorylation (Fig. 4D) and IF-CLSM showed reduced nuclear localization of Foxo1 in DIRAS3 KD ASCs relative to controls (Fig. 4E). This suggests that activated Akt induced by nuclear exclusion of Foxo1 contributes to increased PPAR-γ2 expression upon DIRAS3 KD. Inhibition of Akt and ERK by specific chemical inhibitors diminished DIRAS3 KD mediated up-regulation of adipogenesis, underscoring that active Akt and ERK1/2 execute positive regulation of adipogenesis via C/EBP-β and PPAR-γ2 respectively upon DIRAS3 KD (S. Fig. 5A and B).
Fig. 4.
DIRAS3 knock-down mediated enhancement of adipogenesis is orchestrated by up-regulation of C/EBP-β and PPAR-γ2. (A) Relative expression of CEBP-β, PPAR-γ2 and CEBP-α was analyzed normalized to actin at different time points during adipogenesis by RT-PCR. (B) CEBP-β protein level during 72 h after adipogenesis induction. For optimal separation of bands, C/EBP-β Full-LAP and C/EBP-β LAP were analyzed using a 8% PAGE (upper panel) and C/EBP-β LIP using a 12,5% PAGE (lower panel). (C and D) Phosphorylation of ERK1/2 (T282/Y204) (C) and Foxo-1(S256) (D) was monitored by Western blotting during 72 h of adipogenesis. (E) Localization of Foxo-1 within the cells upon DIRAS3 KD was visualized by IF-CLSM. Foxo-1 (green), TO-PRO3 for nuclear staining (red), Merge (white, obtained by using a co-localization mask). Co-localization mask was obtained by plotting gray scale pixels in x–y axis scatter plot, where x-axis represents green channel (Foxo1) and y-axis represents red channel (TO-PRO3). Double positive pixels are represented in co-localization mask as white. Laser Sharp 2000 software from Zeiss was employed for the image analyses. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001. The significance of difference between means was assessed by analysis of variance (ANOVA) (A).
Supplementary Fig. 5.
ERK and Akt inhibition blocks DIRAS3 knock-down mediated up-regulation of adipogenesis in human ASCs. (A and B) DIRAS3 was KD in ASCs using specific shRNA expressing lentiviruses. Cells were subjected to adipogenesis in the presence or absence of AktVIII (Akt inhibitor), rapamycin (mTOR inhibitor) and U0126 (ERK inhibitor). Adipogenesis was monitored by measuring expression of FABP4 (A) and Perilipin (B) by qRT-PCR 72 h post-induction.
3.5. DIRAS3 regulates autophagy in human ASCs
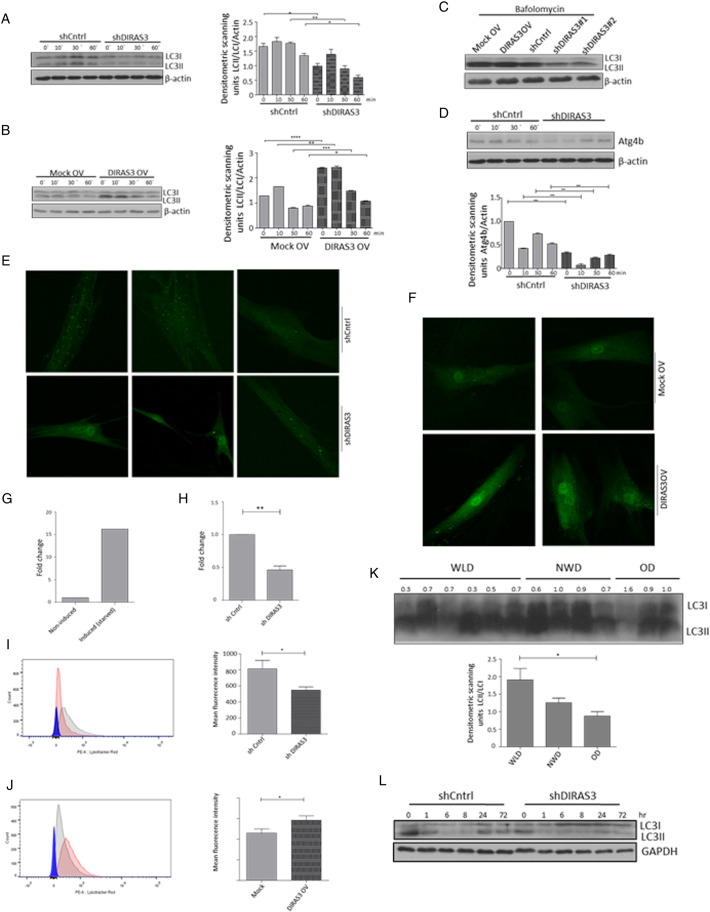
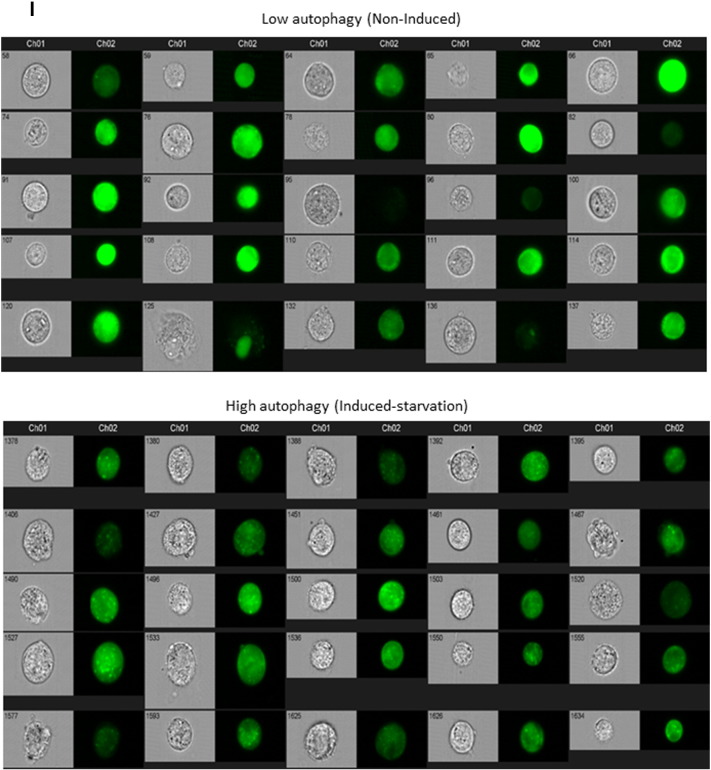
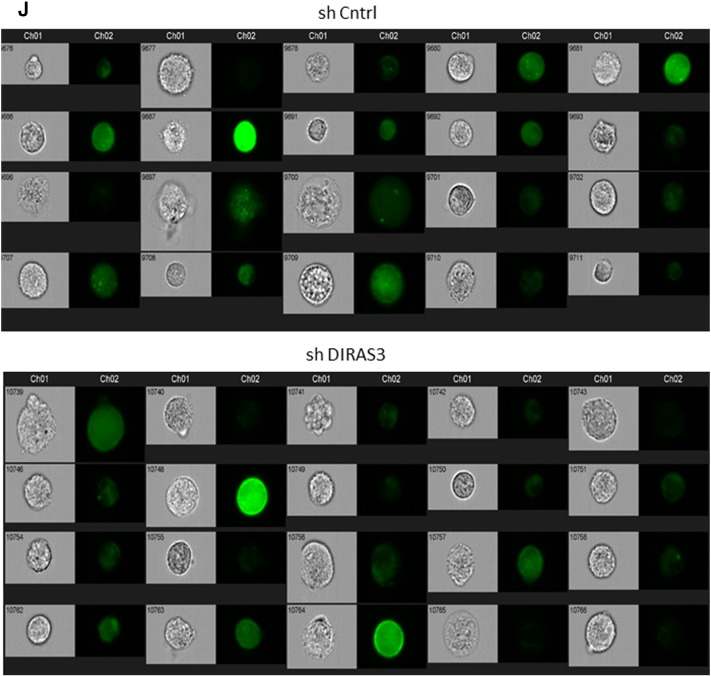
Increased autophagy is one conserved CR-response induced by reduced insulin/IGF-1–mTOR signaling (Laplante and Sabatini, 2012) and DIRAS3 was shown to induce autophagy in cancer cells (Lu et al., 2008). To test whether down-regulation of Akt–mTOR signaling by DIRAS3 stimulates autophagy in human ASCs, we initially conducted Western blot experiments in DIRAS3 KD and overexpressing cells to monitor the cleavage of LC3I to LC3II as a marker for increased autophagy (Klionsky et al., 2012). LC3I and LC3II levels were examined in serum starved ASCs and in the first hour following serum replenishment. DIRAS3 KD significantly reduced LC3I to LC3II conversion in both conditions (Fig. 5A), while over-expression of DIRAS3 significantly enhanced levels of LC3II cleaved form (Fig. 5B), suggesting a role of DIRAS3 in autophagy induction. To prove that autophagic activity includes flux through the entire system, including lysosomes, and the subsequent release of the degradation products, we analyzed autophagy flux using bafilomycin A1, an inhibitor of lysosomal degradation, resulting in accumulation of LC3II. Under these conditions we found an increased accumulation of LC3II in DIRAS3 overexpressing ASCs, while in DIRAS3 KD LC3II accumulation was markedly reduced (Fig. 5C). The cysteine protease ATG4 is necessary for the processing of LC3I to LC3II and DIRAS3 is associated with ATG4 expression in ovarian cancer cells (Lu et al., 2008). In keeping with this a significant decrease in ATG4 protein level was detected upon DIRAS3 KD (Fig. 5D), underscoring that DIRAS3 stimulates autophagy in ASCs. Moreover, visualization of autophagic punctate employing GFP-LC3 as marker for autophagosome detection, demonstrated that knock-down of DIRAS3 in GFP-LC3 expressing ASCs led to a decreased punctate formation (Fig. 5E). In contrast, DIRAS3 over-expression enhanced accumulation of punctate spots (Fig. 5F). To quantitate cells displaying high autophagic puncta we employed image stream multispectral flow cytometry (Klionsky et al., 2012). Human ASCs co-infected with lentiviruses expressing GFP-LC3 were serum-starved (positive control) or not starved (negative control) and monitored (S. Fig. 6I). We defined a cell population having more than 3 puncta as high autophagy cells. A 15-fold increase in cell number displaying high autophagy upon starvation was observed when compared to cells incubated in 10% FCS (non-induced) (Fig. 5G). Next, ASCs were co-infected with lentiviruses expressing GFP-LC3 and DIRAS3 shRNA or control shRNA. DIRAS3 KD led to a significant decrease in the number of cells displaying high autophagy (Fig. 5H and S. Fig. 6J). Another hallmark of autophagy is development of acidic vesicular organelles which can be detected by FACS using lysotracker red staining. Consistent with Western blot data (Fig. 5A–C), we observed significantly lower fluorescence intensity in DIRAS3 KD ASCs (Fig. 5I) and significant higher fluorescence intensity in DIRAS3 OV ASCs (Fig. 5J), indicating that DIRAS3 level is one of the determinants of autophagy. Furthermore, we demonstrate regulation of autophagy related genes upon DIRAS3 KD (S. Fig. 6A–D) and DIRAS3 overexpression (S. Fig. 6E–H). We conclude that DIRAS3 regulates autophagy in human ASCs. Having shown that WLDs display an increased DIRAS3 expression in their ASCs and abdominal sWAT (Fig. 1A, B and Table 1) and that increased DIRAS3 expression is linked to higher autophagy in ASCs (Fig. 5A–J), we investigated LC3I/LC3II protein level in the WAT lysates of the given donor groups. We found a higher LC3II to LC3I ratio in adipose tissue lysates from WLDs relative to NWD and OD, confirming elevated induction of autophagy (Fig. 5K). Together our results highlight a role of DIRAS3 in autophagy induction upon long-term WL in humans ASCs.
Fig. 5.
WL-induced overexpression of DIRAS3 increases autophagy. (A and B) DIRAS3 was KD (A) or overexpressed (B) in ASCs. (Left panels) Conversion of LC3I to LC3II was monitored by Western blotting at different time points after induction of adipogenesis. (Right panels) Densitometric band intensities were quantified using ImageJ. (C) Autophagy flux was evaluated in DIRAS3 KD and DIRAS3OV ASCs by starving these cells for 3 h with HBSS in the presence of 400 nM bafilomycin A1. Conversion of LC3I to LC3II was monitored by Western blotting. (D) Western blot analysis of ATG4b expression upon DIRAS3 KD (left panel). Band intensities were quantified using ImageJ (right panel). (E and F) ASCs were co-infected with GFP-LC3 and either shDIRAS3 (E) or DIRAS3 overexpressing lentiviruses (F). Cells were starved for 3 h and LC3 punctate formation was visualized by IF-CLSM. 400 × magnification. (G–H) ASCs were co-infected with GFP-LC3 and shControl or shDIRAS3 lentiviruses. Cells were analyzed by image stream multispectral flow cytometer. (G) Serum starved cells were taken as a positive control for autophagy induction and a fold change in autophagy high cells was calculated taking the number of autophagy high cells in the presence of serum as 1. (H) Number of autophagy high cells in shcontrol settings was taken as 1 and a fold change was calculated upon DIRAS3 KD. (I and J) Lysosomal acidity was monitored as a read-out of autophagy in ASCs upon DIRAS3 KD (J) or DIRAS3 OV (K) using Lyso-tracker red dye. (K) WAT lysates from WLDs, NWDs and ODs were analyzed by Western blotting to evaluate LC3I conversion to LC3II (upper panel). Densitometric band intensities were quantified using ImageJ (lower panel). (L) Conversion of LC3I to LC3II was monitored by Western blotting during adipogenesis in DIRAS3 KD and control ASCs. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001. The significance of difference between means was assessed by analysis of variance (ANOVA) (A, B, D and K) and Student's t test (H, I and J).
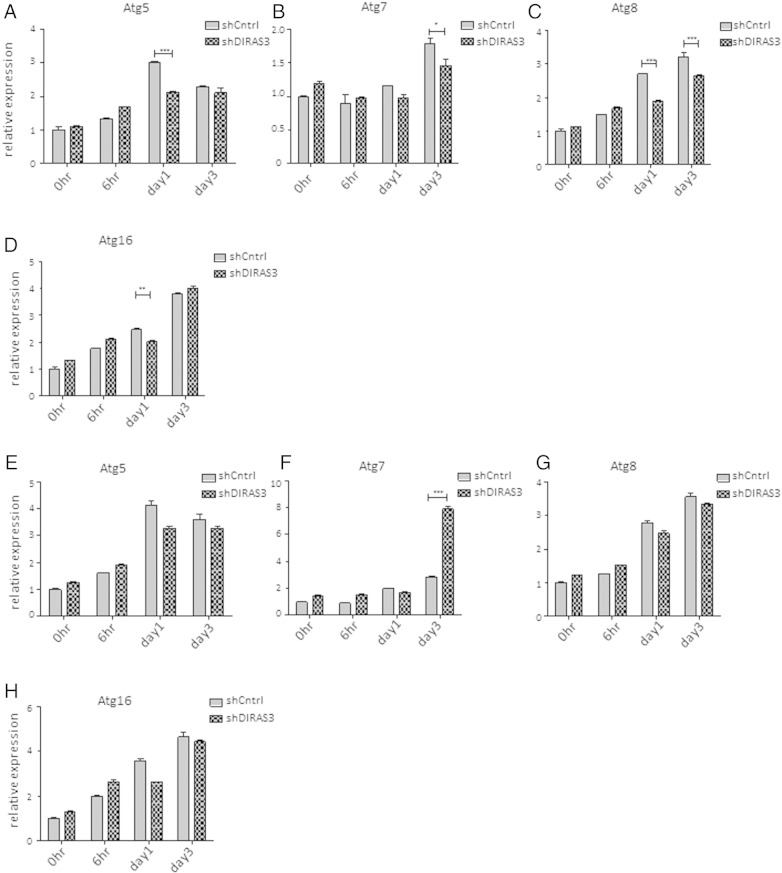
Supplementary Fig. 6.
Expression of autophagy related genes during adipogenesis in human ASCs and after knockdown or overexpression of DIRAS3. (A–D) Expression of Atg5 (A), Atg7 (B), Atg8 (C) and Atg16 (D) was analyzed by q-RT-PCR during adipogenesis, comparing shCntrl and shDIRAS3 expressing lentiviruses infected ASCs. (E–H) Q-RT-PCR was employed to monitor the expression of Atg5 (E), Atg7 (F), Atg8 (G) and Atg16 (H) during adipogenesis, comparing Mock and DIRAS3 over-expressing lentiviruses infected ASCs. All genes were normalized to actin. (I) ASCs were co-infected with GFP-LC3 lentiviruses. Serum starved cells were taken as a positive control for autophagy induction and non-serum starved cells were used as negative control. Cells were analyzed by image stream multispectral flow cytometer. Representative low autophagic (upper panel) and high autophagic cells (lower panel) are shown. (J) ASCs were co-infected with GFP-LC3 and shControl (shCntrl) or shDIRAS3 lentiviruses. Cells were analyzed by image stream multispectral flow cytometer. (J) Representative shCntrl (upper panel) and shDIRAS3 (lower panel) cells are shown. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001. The significance of difference between means was assessed by analysis of variance (ANOVA) (A–H).
Impaired adipogenesis in ATG5 and ATG7 knock-out mice indicates that autophagy is essential for adipogenesis (Baerga et al., 2009, Zhang et al., 2009). IGF-1–mTOR signaling plays an important role in the regulation of both adipogenesis and autophagy; the inhibition of IGF-1–mTOR pathway induces autophagy but reduces adipogenesis (Laplante and Sabatini, 2012; this study). To better understand the interaction between IGF-1–mTOR and autophagy activity in adipogenesis, we monitored the conversion of LC3I to LC3II in human ASC during differentiation. We found a consistent induction level of autophagy in the course of adipogenesis, demonstrating a general role of autophagy in this process (S. Fig. 1). However, upon DIRAS3 KD we detected a decrease in autophagic activity (Fig. 5L) incidental to up-regulated Akt–mTOR signaling (Fig. 3B) and increased adipogenesis (Fig. 3C–E). Thus, although autophagy is essential for adipogenesis in ASCs an activated Akt–mTOR pathway can obviously drive this process at reduced but sufficient autophagic activity.
3.6. Long-term WL induces IGF-1 expression in human ASCs
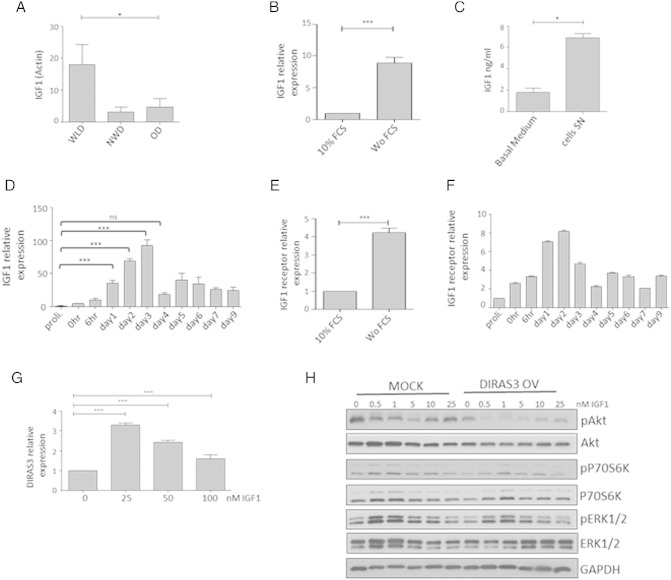
Another WL target gene which emerged from our microarray analysis was IGF-1, the activating ligand of the IGF-1 receptor (IGF-1-R). IGF-1 showed a 23.59-fold up-regulation in ASCs of WLDs versus NWDs and a 4.20-fold higher expression in ASCs from WLDs relative to ODs (Table 1B). These results were confirmed in early passage ASCs from WLD, OD and NWD donors by q-RT-PCR (Fig. 6A). The further experiments were conducted in ASCs from WLDs. Serum-starvation of proliferating ASCs induced IGF-1 mRNA expression (Fig. 6B), supporting our in vivo observed IGF-1 up-regulation upon WL. Furthermore, we corroborated that ASCs derived from sWAT secrete IGF-1 in culture (Fig. 6C). These findings were intriguing, because reduced IGF-1 serum levels in rodents (Breese et al., 1991, Sonntag et al., 1999) and reduced insulin/IGF-1 signaling in invertebrates (Kenyon, 2010) are considered as conserved CR responses but IGF-1 serum levels are not altered following long-term CR (> 1 year) in humans (Fontana et al., 2008). There is also precedence for IGF-1 up-regulation in peripheral tissues of animal longevity models, including the fat body of Drosophila (Broughton and Partridge, 2009). Since IGF-1 stimulates differentiation of preadipocytes (Boucher et al., 2010, Poulos et al., 2010), its expression during adipogenesis was investigated in human ASCs. We found a significant up-regulation of IGF-1 between days 1 and 3 post-induction of adipogenesis (Fig. 6D). A sharp decrease in the IGF-1 expression was observed at day 4 after exclusion of IMBX from the medium, suggesting that the adipogenesis program drives the increase in IGF-1 expression (Fig. 6D). Concomitant with increased IGF-1 mRNA level, we found significantly increased IGF-1-R expression upon serum starving ASCs (Fig. 6E) and during adipogenesis (Fig. 6F). The induction of DIRAS3 in adipogenesis followed a similar time course (Fig. 3A). We also found that exogenous addition of IGF-1 leads to a slight induction of DIRAS3 in WLD ASCs (Fig. 6G). This suggests that positive and negative regulators of IGF-1 signaling are induced during adipogenesis and obviously also in normal ASCs. In the course of adipogenesis, in pro-adipogenic medium, both the MAPK (Fig. 4C) and the PI3K–mTOR pathways (Figs. 2C and 3C) are strongly activated and DIRAS3 can inhibit both of them. To better understand the interaction between IGF-1 and DIRAS3 in ASCs from WLDs, we studied the biochemical interaction between the two proteins in regulating Akt, ERK1/2 and mTOR pathway. To do this, we added solely IGF-1 in concentrations at 0.5 × 10− 9 M–2.5 × 10− 8 M, which are in the physiologically range for the stimulation of human preadipocytes (Bäck and Arnqvist, 2009), to WLD ASCs infected with mock or DIRAS3 overexpression lentiviruses. Although IGF-1 is strongly up-regulated in ASCs of WLDs (Table 1B, Fig. 6A), IGF-1 could hardly induce phosphorylation of Akt in mock WLD ASCs (Fig. 6H, left panels). Phosphorylation of S6K1 and ERK1/2 was induced by IGF-1 in these cells (Fig. 6H, left panels). Overexpression of DIRAS3 in WLD ASCs completely inhibited phosphorylation of Akt also in the presence of IGF-1 (Fig. 6H, right panels). Phosphorylation of ERK1/2 was less reduced under these conditions and S6K1 remained also active to some extent.
Fig. 6.
Long-term WL induces IGF-1 expression in human ASCs. IGF-1 is up-regulated during adipogenesis and IGF-1-induced Akt–mTOR activation is counteracted by DIRAS3. (A) IGF-1 expression was studied by q-RT-PCR in ASCs derived from WLDs (n = 4), NWDs (n = 3) and ODs (n = 3) normalized to actin. (B) IGF-1 expression was analyzed upon serum starvation of ASCs in reference to actin gene. (C) IGF-1 concentration in supernatant (SN) of ASCs derived from sWAT of WLDs after 3 days of cultivation in basal medium (containing 10% FCS) relative to basal medium (containing 10% FCS) was determined by human IGF-1 ELISA kit (Ref: E20, Lot: 120115, Mediagnost, Germany). (D) IGF-1 expression was investigated in the course of adipogenesis using q-RT-PCR normalized to actin. (E) IGF-1-R expression was analyzed upon serum starvation of ASCs using actin as reference gene. (F) IGF-1R expression normalized to actin was investigated in the course of adipogenesis using q-RT-PCR. (G) DIRAS3 expression in ASCs at 6 h after addition of IGF-1 was quantified by q-RT PCR employing actin as reference gene. (H) ASCs were infected with mock or DIRAS3 overexpression lentiviruses. Cells were starved for 48 h followed by addition of increasing concentrations of IGF-1 for 10 min. Phosphorylation of Akt (S473), S6K1 (T389) and ERK1/2 (T282/Y204) was examined by Western blotting. All error bars represent the means ± SEM. p values * = p < 0.05, ** = p < 0.001 and *** = p < .0001 (number of donors = 3). The significance of difference between means was assessed by analysis of variance (ANOVA) (A, D and G) and Student's t test (B, C and E).
4. Discussion
Transgenic mice overexpressing the human DIRAS3 gene show decreased body size, reduced development in multiple organs and are proportionally smaller than nontransgenic littermates (Xu et al., 2000). This phenotype is similar to calorically restricted mice, which showed reduced insulin and IGF-1 serum levels (Speakman and Mitchell, 2011). In the present study, we identified DIRAS3 as WL target gene up-regulated in ASCs of formerly obese humans upon long-term WL and demonstrated that DIRAS3 reduces adipogenic differentiation in these cells. Under pro-adipogenic conditions DIRAS3 strongly counteracted activation of Akt and mTOR signaling. Moreover, although IGF-1 expression was up-regulated in the course of adipogenesis DIRAS3 reduced adipogenic differentiation. Thus, DIRAS3 counteracted insulin and IGF-1 activity during adipogenesis. Additionally, DIRAS3 decreased adipocyte differentiation product levels (FABP4, perilipin and adiponectin) in immature adipocytes. These data suggest that DIRAS3 activity down-modulates adipogenesis and anabolic processes in sWAT of WLDs. This is in accordance with the previously shown reduced adipogenic capacity of ASCs explanted from sWAT of WLDs (long-term restricted by hypocaloric diet or bariatric surgery) (Mitterberger et al., 2014b). Insulin levels in serum of WLDs are significantly lower than those in serum of ODs and NWDs (Mitterberger et al., 2010, Mitterberger et al., 2011). Given the importance of insulin as a potent positive regulator of adipogenesis, it is likely that increased DIRAS3 levels further add to a reduced adipogenic activity in ASCs of sWAT of formerly obese donors. Both insulin and IGF-1 can in principle stimulate adipogenesis in adipose progenitor cells, which express the highly homologous insulin receptors (IR) and IGF-1 receptors (IGF-1R) at their surface (Bäck and Arnqvist, 2009, Boucher et al., 2010). While insulin and IGF-1 bind preferentially to their cognate receptors the two hormones can induce adipogenesis via activation of both the IR and IGF-1R in preadipocytes. To clarify the importance of insulin in obesity, WL and CR it should be noted that insulin serum levels are more dynamically regulated than IGF-1 serum levels in both conditions. Moreover, both IR and IGF-1R are present at adipocytes, with considerably more IR on these cells, leading to a lipodystrophic phenotype in IR knockouts (KOs) in adipose (Blüher et al., 2002), while IGF-1R KO in adipose seem to show mild expansion of adipose (Klöting et al., 2008). Thus WL or CR-induced, reduced insulin serum concentrations count also strongly for beneficial metabolic effects at the adipocyte level, such as reduced lipid depots. When the IR (Blüher et al., 2002) or IGF-1R (Klöting et al., 2008) is inactivated in adipose tissue in mice these pathways can however at least in part compensate for each other.
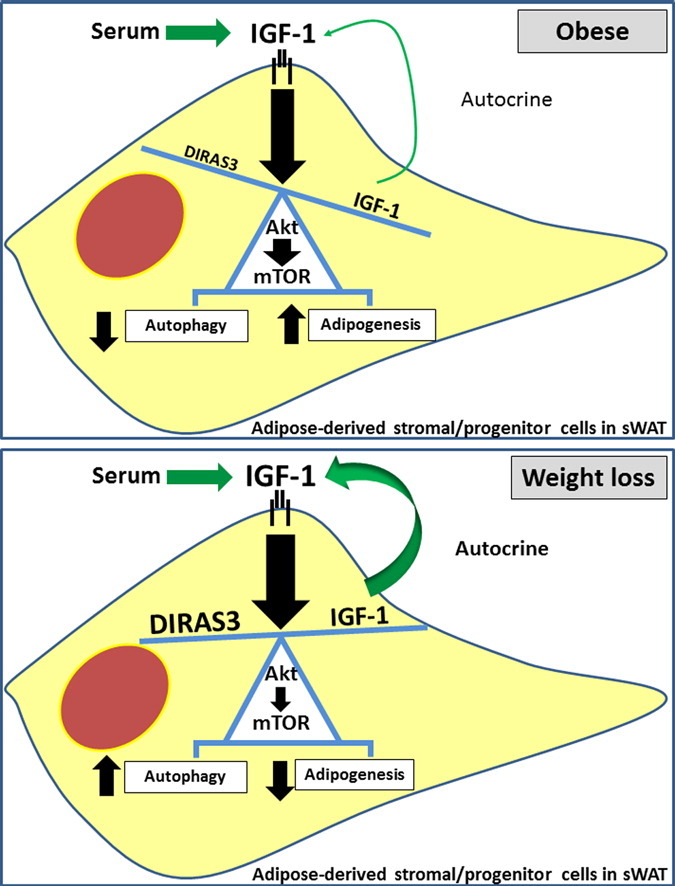
Intriguingly, in the present study, we found that in addition to DIRAS3, IGF-1, the activating ligand of the IGF-1R, was considerably induced in ASCs from WLDs. We showed however that addition of IGF-1 to WLD ASCs hardly stimulated Akt phosphorylation. Moreover, DIRAS3 overexpression completely inhibited Akt phosphorylation also in the presence of high levels of IGF-1. IGF-1 stimulated ERK1/2 phosphorylation in the WLD ASCs and S6K1 was also phosphorylated to some extent. In DIRAS3 overexpressing cells ERK1/2 and S6K1 phosphorylation was reduced also in the presence of IGF-1 but both (ERK1/2 and S6K1) remained active under these conditions. Our data suggest that DIRAS3 dominantly down-regulates the IGF-1R–Akt–mTOR branch in WLD ASCs. Moreover, it is conceivable that the functional interaction between IGF-1 and DIRAS3 channels the IGF-1 stimulus away from Akt more to the ERK1/2 branch. A minimum of IGF-1/IGF-1-R signaling is most likely important to balance down-modulated anabolic processes along with growth, differentiation and survival of ASCs. In fact, it has been shown that extreme low IGF-1 signaling is incompatible with life and supplementation of IGF-1 can ameliorate premature aging in progeria mice with very low levels of IGF-1 (Mariño et al., 2010). Further, in contrast to laboratory animal models, long-term CR does not reduce circulating IGF-1 levels and IGF-1:IGFBP-3 ratio in non-obese humans (Fontana et al., 2008). Similarly, we have shown that total IGF-1 serum levels, free IGF-1 and the molar IGF-1:IGFBP-3 ratio are not significantly lower in WLDs than in ODs (Mitterberger et al., 2011). The molar ratio between IGF-1:IGFBP-3 differed also not between WLDs and NWDs. IGF-1 serum levels are however lower in WLDs probably due to the former obesity phenotype of these donors. On one hand, excessive growth and differentiation of stem and progenitor cells can be deleterious due to accelerated exhaustion of stem cell niches, a hallmark of aging. On the contrary, excessive autophagy, a diminished growth, differentiation and survival of these cells is a detrimental factor for long-term maintenance of the organism, because of its deleterious effects on regeneration and renewal of tissues (Jones and Rando, 2011). For this reason, the maintenance of a correct balance between the two situations could represent a crucial node in ASC homeostasis. Hereby, we demonstrate a simultaneous up-regulation of IGF-1 and DIRAS3 expression upon WL/CR in ASCs, in combination with reduced insulin serum levels, and almost unaltered serum IGF-1 levels, which supports the hypothesis that WL/CR optimizes the insulin/IGF-1–mTOR signaling system in ASCs of sWAT, to achieve a protecting resting-state. As a consequence, ASCs in sWAT of weight losing or calorically restricted formerly obese individuals will present a higher renewal rate and readiness to grow and differentiate in permissive conditions. This may contribute to maintenance of sWAT in conditions of CR, as shown previously in mouse models (Huffman and Barzilai, 2010), and contribute to postponement of sWAT aging.
Our findings contribute to a better understanding of the role of the insulin/IGF-1 system in CR. In lower eukaryotes disruption of IGF-1/insulin signaling increases lifespan (Kenyon, 2010, Johnson, 2013) and knockouts of genes coding for several components of the GH/IGF-1–mTOR signaling network extend lifespan in rodents (Coschigano et al., 2000, Holzenberger et al., 2003, Taguchi et al., 2007, Selman et al., 2009). Furthermore, CR induced longevity in rodents correlates with decreased IGF-1 serum levels (Sonntag et al., 2012) and a decline in the GH/IGF-1 axis in mice and humans is associated with decreased incidence of cancer (Bartke et al., 2013). In contrast, reduced activity of the IGF-1 pathway, which has diverse physiological functions, leads often to detrimental effects. In fact, reduced IGF-1 serum levels correlate with higher incidence of T2DM, cardiovascular diseases and declining cognitive functions, suggesting that IGF-1 deficiency contributes to the pathology of aging (Broughton and Partridge, 2009, Sonntag et al., 2012). Supporting the notion, circulating IGF-1 levels decrease with age in humans and animals and a reduction in serum IGF-1 levels during aging impairs health span in mice (Gong et al., 2014). The dual, beneficial and deleterious actions of IGF-1 led to the current concept, that IGF-1 signaling is a complex endocrine, paracrine and autocrine network affecting organ development, homeostasis and function throughout life (Broughton and Partridge, 2009, Sonntag et al., 2012). Our study supports the hypothesis that the extent, tissue and cell type specific IGF-1 activity are determinants of the effect on health and lifespan.
IGF-1 is produced by the liver as endocrine hormone and in peripheral tissues in a paracrine/autocrine fashion. Adipose tissue contains more IGF-1 than any other tissues except the liver (Stratikopoulos et al., 2008). It is secreted in WAT in a GH-dependent and -independent manner (Bartke et al., 2013) and by isolated ASCs and adipocytes (Wabitsch et al., 2000, Poulos et al., 2010; this study). Supporting our data that IGF-1 is highly expressed in ASCs of sWAT from WLDs it was demonstrated that IGF-1 levels are up-regulated in peripheral tissues of Ames dwarf mice, which show the typical longevity phenotype of chronically low IGF-1 serum levels (Bartke et al., 2013). In these mice, local production of IGF-1 was demonstrated in the hippocampus (Sun et al., 2005) correlating with neuroprotection (Schrag et al., 2008). Moreover, signaling by insulin-like peptides (ILPs) is beneficial in neurons of flies and worms, despite the paradox that lowered insulin/IGF-1 activity has the potential to both compromise the integrity of the CNS and extend lifespan (Broughton and Partridge, 2009). Furthermore, DR results in reduced expression of Drosophila ILP (DLIP) 5 in neurosecretory cells in the brain. Deletion of the genes DILP 2, 3 and 5 in these cells, which extends lifespan, is however accompanied by up-regulation of DLIP 6 in the fly fat body (Partridge et al., 2011). Thus, similar to our findings in human ASCs, other studies show increased IGF-1 levels in tissues of mammals and invertebrates in response to either CR/DR or mutations reducing insulin/IGF-1 signaling, which could reflect a compensatory response.
Long-lived mutant flies with reduced DLIP signaling show increased loss of germline stem cells (Hsu and Drummond-Barbosa, 2009) while DR induces longevity and enhances germline stem cell maintenance in flies with age (Mair et al., 2010). The different mechanisms by which these apparently related interventions have opposite effects on stem cell maintenance are not precisely understood. Our data suggest that WL/CR optimizes the activity of the IGF-1–mTOR system with beneficial effects on ASCs and sWAT homeostasis.
We demonstrated increased autophagy in sWAT of WLDs and showed that negative regulation of Akt–mTOR signaling by DIRAS3 in human ASCs increases autophagy, a hallmark of CR (de Cabo et al., 2014). This establishes a mechanism how WL/CR employs IGF-1/DIRAS3-mTOR signaling to induce autophagy. Increased autophagy leads to higher turn-over of cellular material and recycles misfolded or damaged cellular components. Thus, DIRAS3-induced autophagy in ASCs might facilitate homeostasis of cellular macromolecules, providing new cellular building blocks and energy for renewal and survival of these cells. According to the current model, nutrient overload in obesity stimulates mTOR, thereby attenuating autophagy (Stienstra et al., 2014). Increasing oxidative stress, hypoxia and inflammation induce however insulin resistance in adipose tissues of obese people, leading to inhibition of mTOR and consequently stimulation of autophagy. Studies showing increased autophagy in adipose tissue of obese people suggest that stimulatory effects for autophagy prevail over putative inhibitory effects of mTORC1 activation by overeating (Stienstra et al., 2014). Hereby, we have shown that activation of autophagy in sWAT is a response to long-term WL/CR in formerly obese people. Thus, autophagy in fat tissue is induced in both conditions, obesity and WL/CR, and should affect adipose tissue mass and homeostasis; however, most likely by different mechanisms and intensity.
We have shown that long-term WL/CR modulates adipocyte differentiation of ASCs in human sWAT by inducing both positive and negative regulators of the IGF-1 system at early stages of adipogenesis. Importantly, we identified DIRAS3 as novel negative regulator of adipogenesis, which slows down this process by inactivating Akt–mTOR signaling. Congruent with our data, it was shown in other cell systems that mTOR-induced adipogenesis is mediated partly by 4E-BP1 via regulation of the translation of PPARγ2 and by S6K1, which regulates the expression of early adipogenic transcription factors including C/EBPβ (Laplante and Sabatini, 2012). Moreover, in line with data showing that activated ERK can phosphorylate and thereby activate C/EBP-β (Park et al., 2004), we showed that DIRAS3 inhibits ERK phosphorylation in ASCs and this correlates with reduced C/EBPβ activation. Pro-adipogenic effects of IGF-1 have been shown in previous studies (Bäck and Arnqvist, 2009, Boucher et al., 2010). We extended this knowledge showing that IGF-1 can act as positive regulator of adipogenesis in primary human ASCs and simultaneously induces IGF-1R and DIRAS3, indicating an interplay between IGF-1 and DIRAS3 in modulating adipogenesis.
In conclusion, our results suggest that long-term WL-induced up-regulation of DIRAS3 in ASCs in sWAT of formerly obese humans leads to down-regulation of Akt–mTOR signaling in these cells. Moreover, our data suggest that increased DIRAS3 levels in ASCs in sWAT of WLDs contribute to the reduced adipogenic activity found in ASCs explanted from sWAT of these donors. Furthermore, our findings suggest that increased DIRAS3 levels activate autophagy in ASCs. This study is at the interface between aging and obesity research. The translational value of the paper is in its contribution to a better understanding of the molecular mechanisms underlying the effects of long-term WL/CR on adipose tissue in formerly obese people.
The following are the supplementary data related to this article.
Supplementary material.
Characteristics of the subjects in the NWD, WLD and OD groups. Clinical and anthropometric parameters are indicated for age matched women which were divided into three groups according to their BMI, obese (OD) (BMI ≥ 30 kg/m2), normal weight (NWD) (BMI 19–25 kg/m2), and long-term weight-losing initially obese (WLD) (former BMI ≥ 30 kg/m2 and current BMI ≤ 25). The WLD group contains 9 hypocaloric diets and 5 LAGBs. ASCs used in the cell culture experiments shown in Figs. 1C–Fig. 5, Fig. 6B–H were isolates from WLDs.
Author contributions
WZ, AE and MCM designed the experiments. AE performed most of the experiments and contributed to writing of the manuscript. MCM, MM, MEZ, SH, HPV, AK, AM and UR performed the experiments. ZL, MM, SK, GP and RCB participated in the design of the study and contributed biopsies and/or reagents. WZ conceived the study and wrote the manuscript. All authors read and approved the manuscript.
Acknowledgments
This work was supported by intramural funding from the University of Innsbruck. The authors declare no conflict of interests.
References
- Bäck K., Arnqvist H.J. Changes in insulin and IGF-I receptor expression during differentiation of human preadipocytes. Growth Hormon. IGF Res. 2009;19:101–111. doi: 10.1016/j.ghir.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Baerga R., Zhang Y., Chen P.H., Goldman S., Jin S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5:1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A., Sun L.Y., Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev. 2013;93:571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D.C., Stenesen D., Zeve D., Graff J.M. The developmental origins of adipose tissue. Development. 2013;140:3939–3949. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M., Michael M.D., Peroni O.D., Ueki K., Carter N., Kahn B.B., Kahn C.R. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Borkan G.A., Hults D.E., Gerzof S.G., Robbins A.H., Silbert C.K. Age changes in body composition revealed by computed tomography. J. Gerontol. 1983;38:673–677. doi: 10.1093/geronj/38.6.673. [DOI] [PubMed] [Google Scholar]
- Boucher J., Tseng Y.H., Kahn C.R. Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J. Biol. Chem. 2010;285:17235–17245. doi: 10.1074/jbc.M110.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D., Magkos F., Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143:897–912. doi: 10.1053/j.gastro.2012.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese C.R., Ingram R.L., Sonntag W.E. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-I (IGF-I), IGF-I gene expression, and IGF-I binding proteins. J. Gerontol. A Biol. Sci. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- Broughton S., Partridge L. Insulin/IGF-like signaling, the central nervous system and aging. Biochem. J. 2009;418:1–12. doi: 10.1042/BJ20082102. [DOI] [PubMed] [Google Scholar]
- Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Coschigano K.T., Clemmons D., Bellush L.L., Kopchick J.J. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- de Cabo R., Carmona-Gutierrez D., Bernier M., Hall M.N., Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J.B., le Roux C.W., Rubino F., Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–2311. doi: 10.1016/S0140-6736(12)60401-2. [DOI] [PubMed] [Google Scholar]
- Fontana L., Weiss E.P., Villareal D.T., Klein S., Holloszy J.O. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z., Kennedy O., Sun H., Wu Y., Williams G.A., Klein L., Cardoso L., Matheny R.W., Jr., Hubbard G.B., Ikeno Y., et al. Reductions in serum IGF-1 during aging impair health span. Aging Cell. 2014;13:408–418. doi: 10.1111/acel.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M., Dupont J., Ducos B., Leneuve P., Géloën A., Even P.C., Cervera P., Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hsu H.J., Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman D.M., Barzilai N. Contribution of adipose tissue to health span and longevity. Interdiscip. Top. Gerontol. 2010;37:1–19. doi: 10.1159/000319991. [DOI] [PubMed] [Google Scholar]
- Johnson T.E. 25 years after age-1: genes, interventions and the revolution in aging research. Exp. Gerontol. 2013;48:640–643. doi: 10.1016/j.exger.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.L., Rando T.A. Emerging models and paradigms for stem cell ageing. Nat. Cell Biol. 2011;13:506–512. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P., Zid B.M., Harper T., Koslover D., Sapin V., Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C.J. The genetics of ageing. Nature. 2010;646:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Klein S., Sheard N.F., Pi-Sunyer X., Daly A., Wylie-Rosett J., Kulkarni K., Clark N.G., American Diabetes Association, North American Association for the Study of Obesity; American Society for Clinical Nutrition Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the study of obesity, and the American Society for clinical nutrition. Diabetes Care. 2004;27:2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K., Agholme L., Agnello M., Agostinis P., Aguirre-Ghiso J.A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöting N., Koch L., Wunderlich T., Kern M., Ruschke K., Krone W., Brüning J.C., Blüher M. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes. 2008;57:2074–2082. doi: 10.2337/db07-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop F.K., Taylor R. Mechanism of metabolic advantages after bariatric surgery: it's all gastrointestinal factors versus it's all food restriction. Diabetes Care. 2013;36(S2):287–291. doi: 10.2337/dcS13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner S., Mitterberger M.C., Mattesich M., Zwerschke W. Role of C/EBPβ-LAP and C/EBPβ-LIP in early adipogenic differentiation of human white adipose-derived progenitors and at later stages in immature adipocytes. Differentiation. 2013;85:20–31. doi: 10.1016/j.diff.2012.11.001. [DOI] [PubMed] [Google Scholar]
- López-Lluch G., Irusta P.M., Navas P., de Cabo R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Luo R.Z., Lu Y., Zhang X., Yu Q., Khare S., Kondo S., Kondo Y., Yu Y., Mills G.B., et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R.Z., Fang X., Marquez R., Liu S.Y., Mills G.B., Liao W.S., Yu Y., Bast R.C., Jr. ARHI is a Ras-related small G-protein with a novel N terminal extension that inhibits growth of ovarian and breast cancers. Oncogene. 2003;22:2897–2909. doi: 10.1038/sj.onc.1206380. [DOI] [PubMed] [Google Scholar]
- Mair W., McLeod C.J., Wang L., Jones D.L. Dietary restriction enhances germline stem cell maintenance. Aging Cell. 2010;9:916–918. doi: 10.1111/j.1474-9726.2010.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño G., Ugalde A.P., Fernández A.F., Osorio F.G., Fueyo A., Freije J.M., López-Otín C. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16268–16273. doi: 10.1073/pnas.1002696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterberger M.C., Mattesich M., Klaver E., Lechner S., Engelhardt T., Larcher L., Pierer G., Piza-Katzer H., Zwerschke W. Adipokine profile and insulin sensitivity in formerly obese women subjected to bariatric surgery or diet-induced long-term caloric restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:915–923. doi: 10.1093/gerona/glq107. [DOI] [PubMed] [Google Scholar]
- Mitterberger M.C., Mattesich M., Klaver E., Piza-Katzer H., Zwerschke W. Reduced insulin-like growth factor-I serum levels in formerly obese women subjected to laparoscopic-adjustable gastric banding or diet-induced long-term caloric restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:1169–1177. doi: 10.1093/gerona/glr149. [DOI] [PubMed] [Google Scholar]
- Mitterberger M.C., Lechner S., Mattesich M., Kaiser A., Probst D., Wenger N., Pierer G., Zwerschke W. DLK1(PREF1) is a negative regulator of adipogenesis in CD105(+)/CD90(+)/CD34(+)/CD31(−)/FABP4(−) adipose-derived stromal cells from subcutaneous abdominal fat pats of adult women. Stem Cell Res. 2012;9:35–48. doi: 10.1016/j.scr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Mitterberger M.C., Lechner S., Mattesich M., Zwerschke W. Adipogenic differentiation is impaired in replicative senescent human subcutaneous adipose-derived stromal/progenitor cells. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:13–24. doi: 10.1093/gerona/glt043. [DOI] [PubMed] [Google Scholar]
- Mitterberger M.C., Mattesich M., Zwerschke W. Bariatric surgery and diet-induced long-term caloric restriction protects subcutaneous adipose derived stromal/progenitor cells and prolongs their life span in formerly obese humans. Exp. Gerontol. 2014;56:106–113. doi: 10.1016/j.exger.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Nakae J., Kitamura T., Kitamura Y., Biggs W.H., III, Arden K.C., Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev. Cell. 2003;24:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- Park B.H., Qiang L., Farmer S.R. Phosphorylation of C/EBPbeta at a consensus extracellular signal regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol. Cell. Biol. 2004;24:8671–8680. doi: 10.1128/MCB.24.19.8671-8680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L., Alic N., Bjedov I., Piper M.D. Ageing in Drosophila: the role of the insulin/Igf and TOR signaling network. Exp. Gerontol. 2011;46:376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos S.P., Hausman D.B., Hausman G.J. The development and endocrine functions of adipose tissue. Mol. Cell. Endocrinol. 2010;323:20–34. doi: 10.1016/j.mce.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Rosen E.D., MacDougald O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Schrag M., Sharma S., Brown-Borg H., Ghribi O. Hippocampus of Ames dwarf mice is resistant to beta-amyloid-induced tau hyperphosphorylation and changes in apoptosis-regulatory protein levels. Hippocampus. 2008;18:239–244. doi: 10.1002/hipo.20387. [DOI] [PubMed] [Google Scholar]
- Selman C., Tullet J.M., Wieser D., Irvine E., Lingard S.J., Choudhury A.I., Claret M., Al-Qassab H., Carmignac D., Ramadani F., et al. Ribosomal protein S6 kinase 1 signalling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström L., Lindroos A.K., Peltonen M., Torgerson J., Bouchard C., Carlsson B., Dahlgren S., Larsson B., Narbro K., Sjöström C.D., et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- Sonntag W.E., Lynch C.D., Cefalu W.T., Ingram R.L., Bennett S.A., Thornton P.L., Khan A.S. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-I on biological aging: inferences from moderate caloric-restricted animals. J. Gerontol A Biol. Sci. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Sonntag W.E., Csiszar A., deCabo R., Ferrucci L., Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:587–598. doi: 10.1093/gerona/gls115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R., Mitchell S.E. Caloric restriction. Mol. Asp. Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Stienstra R., Haim Y., Riahi Y., Netea M., Rudich A., Leibowitz G. Autophagy in adipose tissue and the beta cell: implications for obesity and diabetes. Diabetologia. 2014;57:1505–1516. doi: 10.1007/s00125-014-3255-3. [DOI] [PubMed] [Google Scholar]
- Stratikopoulos E., Szabolcs M., Dragatsis I., Klinakis A., Efstratiadis A. The hormonal action of IGF1 in postnatal mouse growth. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19378–19383. doi: 10.1073/pnas.0809223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.Y., Al-Regaiey K., Masternak M.M., Wang J., Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol. Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Taguchi A., Wartschow L.M., White M.F. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Wabitsch M., Heinze E., Debatin K.M., Blum W.F. IGF-I- and IGFBP-3-expression in cultured human preadipocytes and adipocytes. Horm. Metab. Res. 2000;32:555–559. doi: 10.1055/s-2007-978685. [DOI] [PubMed] [Google Scholar]
- Xu F., Xia W., Luo R.Z., Peng H., Zhao S., Dai J., Long Y., Zou L., Le W., Liu J., et al. The human ARHI tumor suppressor gene inhibits lactation and growth in transgenic mice. Cancer Res. 2000;60:4913–4920. [PubMed] [Google Scholar]
- Yu Y., Xu F., Peng H., Fang X., Zhao S., Li Y., Cuevas B., Kuo W.L., Gray J.W., Siciliano M., et al. NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc. Natl. Acad. Sci. U. S. A. 1999;96:214–219. doi: 10.1073/pnas.96.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Luo R., Lu Z., Wei Feng W., Badgwell D., Issa J.P., Rosen D.G., Liu J., Bast R.C., Jr. Biochemistry and biology of ARHI (DIRAS3), an imprinted tumor suppressor gene whose expression is lost in ovarian and breast cancers. Methods Enzymol. 2006;407:455–468. doi: 10.1016/S0076-6879(05)07037-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Goldman S., Baerga R., Zhao Y., Komatsu M., Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwierzina M.E., Ejaz A., Bitsche M., Blumer M.J.F., Mitterberger M.C., Mattesich M., Amann A., Kaiser A., Pechriggl E.J., Hörl S., Rostek U., Pierer G., Fritsch H., Zwerschke W. Characterization of DLK1(PREF1)+/CD34+ cells in vascular stroma of human white adipose tissue. Stem Cell Res. 2015;15:403–418. doi: 10.1016/j.scr.2015.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Characteristics of the subjects in the NWD, WLD and OD groups. Clinical and anthropometric parameters are indicated for age matched women which were divided into three groups according to their BMI, obese (OD) (BMI ≥ 30 kg/m2), normal weight (NWD) (BMI 19–25 kg/m2), and long-term weight-losing initially obese (WLD) (former BMI ≥ 30 kg/m2 and current BMI ≤ 25). The WLD group contains 9 hypocaloric diets and 5 LAGBs. ASCs used in the cell culture experiments shown in Figs. 1C–Fig. 5, Fig. 6B–H were isolates from WLDs.