Abstract
The liver is an organ in which antigen-specific T-cell responses manifest a bias toward immune tolerance. This is clearly seen in the rejection of allogeneic liver transplants, and multiple other phenomena suggest that this effect is more general. These include tolerance toward antigens introduced via the portal vein, immune failure to several hepatotropic viruses, the lack of natural liver-stage immunity to malaria parasites, and the frequent metastasis of cancers to the liver. Here we review the mechanisms by which T cells engage with hepatocellular antigens, the context in which such encounters occur, and the mechanisms that act to suppress a full T-cell response. While many mechanisms play a role, we will argue that two important processes are the constraints on the cross-presentation of hepatocellular antigens, and the induction of negative feedback inhibition driven by interferons. The constant exposure of the liver to microbial products from the intestine may drive innate immunity, rendering the local environment unfavorable for specific T-cell responses through this mechanism. Nevertheless, tolerance toward hepatocellular antigens is not monolithic and under specific circumstances allows both effective immunity and immunopathology.
Keywords: liver, hepatocytes, immunity, tolerance
According to ancient Babylonians and in the practice of Mesopotamian medicine, the liver is metaphorically regarded as the ‘seat of the living soul' (The Evolution of Modern Medicine, William Osler). Ritualistic examination of the liver comprising assessment of its size and position, its color and the richness of its blood, was routinely interpreted as a measure of the inner invisible characteristics including emotions as well as illnesses. So while in the modern English language, the conceptualization of abstract emotion over the years has been linked to the heart, in ancient Babylonia, it all started with the liver. Pablo Neruda's magnificent ‘Ode to the Liver' is a modern manifestation of these ancient insights. Today, we know that the liver is the major organ responsible for more than 500 different functions; protein, carbohydrate and lipid metabolism, hormone production, plasma protein synthesis, decomposition of red blood cells, glycogen storage, bile production and detoxification to name a few – symbolically functioning as the organ responsible for cleansing of the soul.
Anatomically, it is interesting and unique that besides being the largest internal organ, the liver is traversed by both the hepatic artery and the portal vein, with the former carrying oxygen-rich blood from the aorta and the latter bringing in myriad antigens from the gut, spleen and pancreas and nutrients from the gastrointestinal tract. The hepatic blood exits from the sinusoids into the central vein where it is drained into the inferior vena cava. Perhaps in this antigen-rich context, it is not surprising that evolutionarily the liver has developed a tolerogenic environment in order to manage the panoply of antigens and their neo-antigenic metabolites. For tissue preservation, the liver must reduce the risk of immune activation in response to various oral and self-antigens. Importantly, the large microbial biomass found in the gut that serves as an important determinant of intestinal inflammation also directly influences the development of liver disease.1 In fact, the hyporesponsiveness to an oral antigen can be significantly reversed with the creation of a portal shunt.2 This tolerogenic aspect of the liver is consistent with the observation that liver allografts, unlike heart or kidney, can be accepted in various animal and human transplant settings in the absence of additional immunosuppression.3,4 Co-transplantation with the liver allows for protection and acceptance of other organs that normally would be rejected.5
Hepatic microvessels, known as sinusoids, slow down the blood flow and allow for the liver's biochemical functions to be enacted by specialized cells, the hepatocytes. Hepatocytes comprise ∼60% of hepatic cells and close to 90% of the liver volume. Liver sinusoidal endothelial cells (LSECs) form a fenestrated barrier separating the hepatocytes from direct blood flow and the immune cells that reside in the lumen. How does the liver balance between the need for tolerance to antigens that are metabolized in the liver and the requirement for immunity to pathogens that have developed hepatotropic propensity over the years? Are gut microbial antigens and/or neo-antigens derived from metabolic byproducts providing danger signals to induce inflammation? In addition to hepatocytes themselves controlling many aspects of inflammation, the assistance by other intrahepatic cells collaborate in managing the overall functioning of the liver. These nonparenchymal cells include lymphocytes, Kupffer cells, natural killer (NK) cells, NK-T cells, and stellate and dendritic cells (DC) that are assigned with sensing any perturbation in the liver architecture and milieu (Figure 1). The subject of this review is to delineate how immune sensing in the liver is shaped by the location, where the antigen is processed, how it is processed and to explore the influence of host innate receptors and pathogen subversion tactics as they act on hepatocytes and nonparenchymal cells in liver immunity.
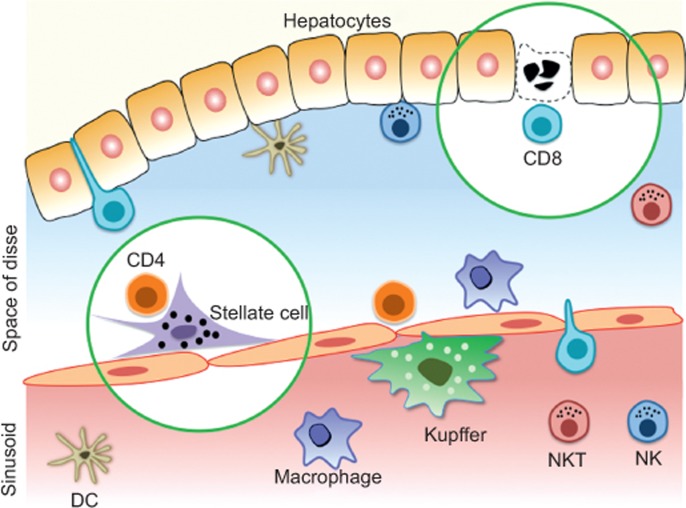
Figure 1.
Anatomy of antigen presentation in the sinusoid. Plates of hepatocytes are separated from the blood flowing in the sinusoids by liver sinusoidal endothelial cells. In the blood space are recirculating natural killer cells, natural killer T cells, monocytes–macrophages and, adherent to the endothelial wall, the Kupffer cells. To interact directly with hepatocytes, CD8+ T cells must cross the endothelial barrier and enter the space of Disse. CD4+ T cells also enter this space but cannot directly interact with hepatocytes. However, they find other interaction partners such as DCs and stellate cells.
Anatomy of antigen presentation
The presentation of antigens is controlled both by their location within the cell and by accessory signals that in turn are controlled through innate immune recognition mechanisms that sense pathogen-associated molecular patterns (PAMPs) such as viral and microbial nucleic acid motifs and distinctive glycolipids and specific conserved microbial proteins. A largely overlapping set of receptors activates similar signaling pathways in response to cellular injury through the recognition of damage-associated molecular patterns (DAMPs). Some of these receptors reside on the plasma membrane, for example Toll-like Receptor (TLR) 2 and TLR4, which recognize components of bacterial cell cells; some reside in endosomes, such as TLR3 and TLR9 that sense structural features of viral and microbial RNA and DNA respectively; and some are cytoplasmic, such as the retinoic acid-inducible–like receptors that sense viral RNA and the Nod-like receptors that sense bacterial lipids. Thus, in the liver, immune cell activation can be influenced by the cell in which the antigen is expressed, the cellular compartments in which it exists, the presence of innate immune signals that modulate antigen presentation via PAMP and DAMP receptors, and immune subversion mechanisms evolved by the pathogen to disable host defense.
Presentation of antigen to both CD4+ and CD8+ T cells depends on antigen processing, which is distinct and partitioned in different cellular compartments. Thus, viral capsid proteins and proteins that are contained within the virion can gain access to endosomes and to the processing pathway that degrades antigens and loads their peptides into major histocompatibility complex (MHC) class II molecules in a specialized compartment termed the MHC class II compartment. This route of processing is also open to virally encoded proteins that are secreted from infected cells, such as hepatitis B surface antigen in the case of hepatitis B infection. In contrast, nonstructural viral proteins are synthesized only in the infected cell, and most often in the cytoplasm. These proteins can be degraded directly by the proteasome and loaded to the MHC class I molecules via a protein transporter complex, transporter of antigenic peptides, that traffics them into membrane-bound compartments. The antigens of nonviral pathogens are also located in both membrane-bound and cytoplasmic compartments. Thus, the bacterium Listeria monocytogenes is first taken up by endocytosis, but then disrupts the membrane of the endosome through the action of an enzyme listeriolysin,6 and enters the cytoplasm where it both migrates and spreads from cell to cell by exploiting the host cell cytoskeleton.7 Therefore, this pathogen evades the classic MHC class II pathway but Listeria-encoded antigens are available for the classical MHC class I–processing pathway. While Listeria is a virulent pathogen that infects the liver, its intracellular location together with its strong activation of innate immunity conspire to render it, in healthy individuals, a potential vaccine vehicle. In fact vaccines based on attenuated Listeria organisms can induce effective anticancer immunity, making them an exciting avenue for vaccinology and immunotherapy.8,9,10
Malaria parasites enter hepatocytes by direct invasion of the cytoplasm, which appears to be mediated by a prior interaction with Kupffer cells.11,12 Thus, their antigens expressed by the invasive stage, the sporozoite, are potentially accessible to the classical MHC class I pathway, but the parasites induce the formation of a parasitophorous vacuole, the membrane of which contains both host-encoded and parasite-encoded proteins. This vacuolar membrane mediates interaction between the parasite and the infected hepatocyte, but the extent to which it controls antigen presentation is not understood. Genetically attenuated malaria parasites that can function as live vaccines may undergo developmental arrest before they form a parasitophorous vacuole;13 but late-arresting parasite variants that undergo partial differentiation within such a vacuole may also induce sterilizing immunity.14,15
Licensing the antigen-presenting cells
Many important liver pathogens infect primarily hepatocytes. These include the hepatitis viruses (hepatitis A virus [HAV], hepatitis B virus [HBV], hepatitis C virus [HCV] and other less common viruses), cytomegalovirus and the globally important malaria parasite. Since these are intracellular pathogens, host defense depends primarily on T cells and in all these infections there is strong evidence that the cytotoxic CD8+ T cells are essential for host defense. To take two among many examples: depletion of CD8+ T cells from HBV-infected chimpanzees results in a resurgence of viremia16 and similarly abrogates immunity in mice primed with radiation-attenuated malaria parasites.17,18 Once fully activated, cytotoxic CD8+ T cells undergo clonal expansion and may deliver their defensive function without support from other cell types, but for efficient primary activation, full effector function, survival and memory CD8+ T cells and the delivery of memory effector function, CD8+ T cells depend on an interaction with CD4+ T cells termed ‘help'. This interaction is mediated in several ways: through the direct delivery of supportive CD4+ T-cell–derived cytokines such as interleukin-2 (IL-2);19 through the enhanced function of specialized antigen-presenting cells (APCs) such as DCs, a mechanism termed licensing;20,21 and through a direct interaction between CD4+ and CD8+ T cells that requires the expression of CD40 on the CD8+ T cells.22
Among these mechanisms, ‘licensing' is the most efficient because it can be mediated by sequential interaction of a rare antigen-specific CD4+ T cell and subsequently a rare antigen-specific CD8+ T cell with an APC. Both the licensing interaction between the CD4+ T cell and the APC, and the licensed interaction between the APC and the CD8+ T cell, depend on MHC-restricted antigen recognition, and this in turn means that for licensing to occur, the APC must express both MHC class I and class II. Among potential liver-resident APC, trafficking DC express both classes of MHC molecules. At a lower level, so do Kupffer cells and LSECs, but hepatocytes only express MHC class I. Therefore, hepatocytes cannot be licensed by CD4+ T cells.21 Instead, the full activation of a CD8+ T cell specific for a hepatocellular antigen depends on cross-presentation by an MHC class I+ II+ cell (Figure 2).
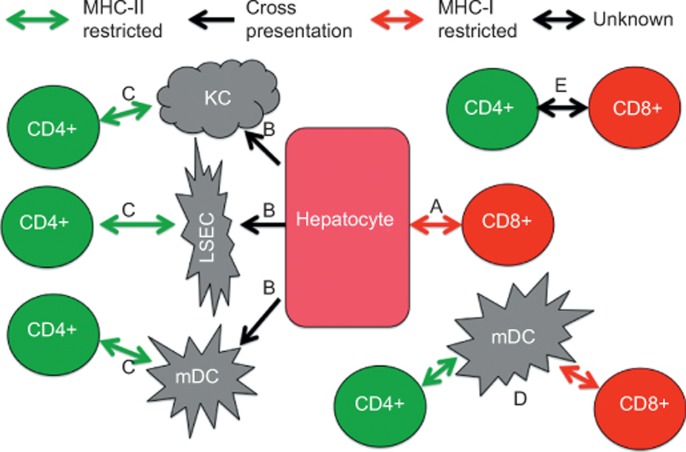
Figure 2.
Pathways of direct and cross-presentation of hepatocellular antigens. The fenestrated liver sinusoidal endothelium allows (a) direct presentation of hepatocyte antigens to CD8+ T cells, but such antigens can only engage CD4+ T cells after cross-presentation (b) via Kupffer cells (KC), liver sinusoidal endothelial cells, or myeloid dendritic cells (DCs), all of which express major histocompatibility complex class II and can activate CD4+ T cells (c). Myeloid DCs can also mediate licensing (d) in which a CD4+ T cell activates an antigen-presenting cell such as an myeloid DCs, which in turn delivers full activation signals to a CD8+ T cell. However, this straightforward model does not explain everything, as CD4+ T cells may also interact with CD8+ T cells more directly via CD40 (e).
Among liver-resident cells, the large macrophage population termed Kupffer cells would be an obvious candidate for the cross-presentation of hepatocellular antigens. However, the balance of evidence suggests that Kupffer cells are immunosuppressive. Interaction of Kupffer cells with CD8+ T cells in vitro causes proliferation,23 but they also secrete both IL-1024,25 and the immunosuppressive prostaglandin PGE2,26,27 and in vivo depletion of Kupffer cells impairs both oral tolerance28 and liver transplantation tolerance.29 It follows that there is no conflict between the abundance of Kupffer cells in HCV-infected livers and the persistence of HCV infection. If Kupffer cells were to be presenting or cross-presenting HCV-encoded antigens, tolerance would be the expected outcome.
The other liver-resident cell type that constitutively expresses MHC class II is the LSEC. These cells are very active in pinocytosis, sequester virions from the circulation30 and could readily cross-present both circulating antigens31 and hepatocellular antigens, whether these are released as soluble protein or in exosomes.23 Exosomes are membrane-bound cell fragments that transport proteins from one cell to another, and they have been implicated in the transfer of HCV-encoded proteins32 and viral RNA.33 LSECs may also cross-present cancer-derived antigens.34 However, the default outcome in the case of antigen presentation by LSECs is immune tolerance.34,35,36,37
These considerations lead to the conclusion that the potential of liver-resident cells to cross-present hepatocellular antigen does not lead to effective immunity. Instead, both LSECs and Kupffer cells may cross-present antigens, but the outcome is not ‘help' but immune suppression. This raises a fundamental biological question: in the context of liver infection, are the LSECs and the Kupffer cells resistant to licensing mechanisms that act in other contexts? Or are they licensed, but in an alternative way that enhances their immunosuppressive potential? Experiments with purified LSECs and Kupffer cells in vitro have not so far clarified this issue, because there is no way to determine whether the cells were licensed, or unlicensed (or delicensed, or alternatively licensed) already at the time of isolation.
Tolerance despite the presence of danger signals
Adaptive immune responses to pathogens occur in the context of ongoing innate immunity. The ‘Danger Model' asserts that adaptive immunity is triggered by tissue damage,38 and the current understanding of this process is that injured cells release DAMPs that engage the same classes of receptors, as do PAMPs. These receptors engage two major signaling cascades: one via the adapter molecule MyD88 that activates many innate immune genes including those encoding Tumor Necrosis Factor (TNF)-α and IL-1α/β; the other via TRIF and IRF7 activates Interferon (IFN)-α/β, and thence a large number of interferon-sensitive genes, many of which encode antiviral proteins. Both DAMP and PAMP receptors are abundantly expressed by liver cells,39 so it would be expected that the activation of innate immunity is effective in the liver. The ‘Danger Model' asserts further that these changes set the scene for adaptive T-cell immunity through increased expression of MHC class I and class II molecules, increased expression of co-stimulatory and intercellular adhesion molecules, and the switch from basal proteasomes to immunoproteasomes.38,40 All these changes can be driven by IFNs.
However, in the liver there are two confounding factors that derail this logically appealing model. First, the liver is exposed to low levels of bacterial products from the intestinal microbiota, and these include endotoxin (lipopolysaccharide, LPS) from gram-negative bacteria. While the level of LPS in the liver is increased in pathological states including alcohol toxicity, it is also detectable at baseline.41 Low-level exposure to LPS could act by inducing LPS tolerance, which is illustrated by experiments in which systemic administration of a low dose of LPS results in protection from a subsequent challenge with a lethal dose by modifying the immunobiology of liver cells.42,43 This process acts by down-regulating innate immune signaling pathways (Figure 3). Thus, the presence of low-level LPS in the liver could blunt the innate response to infection. In addition, documented effects of LPS on isolated liver cells suggest it may induce immunosuppressive molecules. Thus, exposure of Kupffer cells to low-dose LPS resulted in the secretion of IL-10, which in turn could modify the function of LSECs.24,44 In addition, liver APCs are not functionally mature in the same way as their counterparts in other organs, as identical treatments results in divergent immune responses.45 This could be either due to the presence of a large quantity of anti-inflammatory cytokines in the liver or cell intrinsic properties that make these hepatic APCs less immunostimulatory.
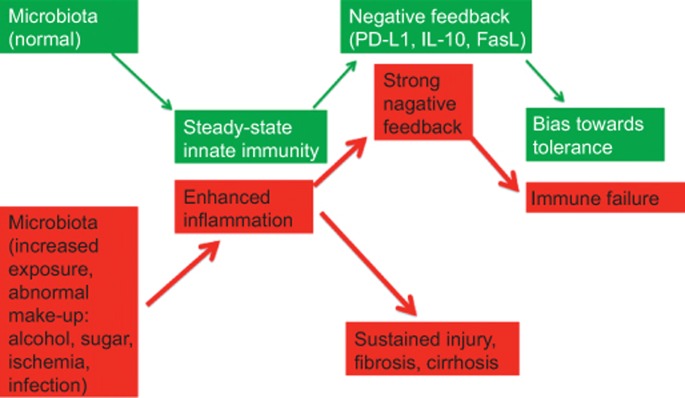
Figure 3.
Normal tolerance and pathological tolerance in the liver. In health (shown in green) the microbiota causes steady-state low-level immune activation and the negative feedback that results in bias toward immune tolerance in the liver. Disturbance of the relationship between microbiota and host, which may be caused by diverse stresses including toxic diet elements, can cause enhanced inflammation that acts through the same mechanisms, but causes more profound immune incompetence. At the same time, the inflammation itself propagates liver injury.
A second consideration is the induction of negative feedback through IFNs. Innate immunity results in the secretion of both IFN-α/β by many cell types, and IFN-γ mostly by lymphocytes including NK cells and NK-T cells. While these IFNs induce MHC I/II, co-stimulation and the immunoproteasome activation, they also induce negative feedback inhibition that may result in immune tolerance. These effects are best documented for IFN-α/β, which suppressed the CD4+ T-cell response to blood-stage malaria parasites, while mice lacking IFN-α/β signaling were relatively resistant.46 Similarly, IFN-α/β suppressed the CD8+ T-cell response in virus infection.47 In LCMV infection in mice, inhibition of IFN-α/β caused increased IFN-γ secretion and an antiviral effect.48 However, IFN-γ may also suppress effective immunity, for example in pancreatic islet transplantation into the liver.49 This type of immune suppression induced by proinflammatory cytokines in the context of cancer has been termed adaptive resistance.50 Therefore, we would argue that immune tolerance provoked by inflammation should be called adaptive tolerance.
Suppression of T-cell immunity acts via many pathways, including immunosuppressive small molecules, cytokines and cell surface ligands; many of these are induced by IFNs. IFN-β induces the enzyme IDO1, which imposes T-cell tolerance though the depletion of tryptophan and the synthesis of kynurenine.51 IFN-γ gene transduction also induces the Ido1 gene52 as well as both Fas (CD95) and Fas ligand,53 the interaction of which results in T-cell apoptosis. Some other proinflammatory cytokines have similar effects. Thus, IFN-γ along with IL-12 and IL-17 up-regulated the immunosuppressive ligand PD-L1;54 IFN-γ along with TNF-α and TLR2, 3 and 4 ligands induced galectin-9.55
There is evidence that all these immunosuppressive pathways are active in the liver. Thus, in HBV infection, IDO not only suppresses anti–hepatitis B surface antigen CD8+ T-cell activity56 but also promotes liver injury in fulminant hepatitis.57 In mice, deficiency of PD-L1 results in massive accumulation of CD8+ T cells in the liver,58 while antibody-mediated inhibition of PD-L1 signaling enhanced an anti-HBV CD8+ T-cell response.59 Similarly, in human HCV and HBV infection, dysfunctional T cells were enhanced by PD-L1 blockade.60,61 Likewise, both the Fas–FasL pathway62,63 and the galectin-9–Tim-3 pathway64,65 regulate T-cell immunity in viral hepatitis.
The IFN-induced immunosuppressive feedback pathways may actively promote T-reg activity. Thus, in HCV the interaction of galectin-9 with Tim-3 on CD4+ T cells promoted T-reg development.66 Conversely, T-reg cells in viral hepatitis express high level of PD-1.67 Most strikingly, even IFN-λ has been implicated in inducing a myeloid cell phenotype that in turn promotes the expansion of T-reg cells.68 Thus, while many liver cell types have the potential to present antigen to T cells, multiple immunosuppressive mechanisms conspire to defeat their activation.
Immunity despite the presence of tolerance
While immune failure is a common response to liver antigens, it is by no means universal. Most patients infected with HAV undergo acute hepatitis accompanied by the activation of a strong CD8+ T-cell response, and then eradicate the infection. In HBV only a subset, and in HCV infection only a minority of patients eliminate the virus, and when it occurs such self-cure is accompanied by a more diverse, sustained CD8+ T-cell and a CD4+ T-cell response.69,70 What makes HAV different? It is not the presence of diverse strategies to disable innate immune defense that makes the difference, since HAV like HCV encodes proteases that cleave host innate immune signaling proteins.71 One study of the differential roles of CD8+ and CD4+ T cells in HAV made the point that effective CD4+ T-cell function correlated with the onset of viral clearance, while CD8+ T-cell function only improved after virus was eliminated.72 This is a very provocative observation that challenges the model, argued above, that the important role of CD4+ T-cell help in HAV infection is to support and sustain the CD8+ T-cell response. Are CD4+ T cells possibly the relevant effectors in anti-HAV immunity, with the CD8+ T-cell response reduced to an unreliable biomarker? Correlation may not prove causation, but lack of correlation is a strong argument against causation.
In HCV, self-cure in chimpanzees is linked to CD8+ immunity as already noted,73 while in humans the striking correlation is with a polymorphism in the gene encoding an innate immune cytokine, IL-28B, a member of the IFN-λ family.74 The link may be that IL-28 increases the transcription factor T-bet,75 which biases CD4+ T cells toward the Th1 fate, but is also correlated with cytotoxic CD8+ T cell effector function in CD8+ T cells and NK cells.76 In summary, the conditions that lead to effective immunity against hepatitis viruses may critically involve innate immunity (IL-28B), CD4+ T cells and CD8+ T cells, but the relationships between them are not yet clear.
When inflammation overshadows tolerance
Ample evidence suggests that uncontrolled inflammation induces severe liver damage and progression to end-stage liver disease. Although the first landmark observation linking liver cirrhosis and inflammation was documented over 100 years ago, our understanding of liver disease processes has only recently evolved to include inflammation as a mechanism rather than a consequence.77 Liver lymphocyte infiltration, increased levels of circulating LPS and elevated levels of proinflammatory cytokines such as TNF-α are key hallmarks of not just alcoholic liver disease but also nonalcoholic steato-hepatitis, and cirrhosis due to viral infection. Consistent with this idea, a recent study by one of our laboratories showed surprisingly that liver recruitment and activation of DCs was a general consequence of inflammation, irrespective of viral infection status.78 In fact, the immune manifestations of alcoholic liver disease and nonalcoholic steato-hepatitis are virtually indistinguishable through current diagnostic methods, suggesting that common core pathways of inflammation-fueled cirrhosis exist that may serve useful as therapeutic targets.79
Recently, studies have alluded to the contribution of intestinal microbiota as prime suspects to promote metabolic diseases by driving low-level inflammation.80,81 In fact TLR/Nod-like receptors activation in resident Kupffer cells by low-level bacterial products (endotoxin) in the liver through TLR4 pathway is critical for liver inflammation induced by ischemia reperfusion, alcohol and viral components.82,83,84 Given the alarming correlation seen between changes in our diet to obesity, metabolic syndrome and nonalcoholic steato-hepatitis, gut permeability and elevated serum endotoxins have been shown to be critical components in promoting liver inflammation and disease progression.85 Interestingly, many of the diet-induced metabolic manifestations seem to be reversed in germ-free mice and/or in mice devoid of TLR signaling.86,87,88 In addition, gut microbiota also play an important role in the progression of liver disease to fibrosis as chemically induced treated mice with carbon tetrachloride showed increases in bacterial translocation.89 Furthermore, intestinal microbiota and signaling through TLR4 on hepatic stellate cells was shown to be critical for the development of fibrosis by modulation of TGFβ and subsequent unrestricted activation of Kupffer cells.90
In summary, the liver is an environment where antigens are readily presented by diverse liver cell types, but the prevalence of background low-level exposure to innate immune stimuli results in continuous feedback inhibition of T-cell immunity through many parallel pathways. However, the inextricable connectivity between the intestinal microbiota and the liver also selects for an inflammatory thermostat that shifts the balance from tolerance to inflammatory-induced liver disease when gut microbial antigens and/or neo-antigens provide unchecked danger signals to the liver. In Neruda's words (translated by H. Morales and W. Hochman) ‘one small cell goes astray, the pilot flies in the wrong sky, the tenor shrinks to a whisper, the astronomer loses his planet'. Such is the central role of the liver in immunity.
Acknowledgments
We acknowledge the support provided by the National Institutes of Health (PHS grants R01AI070101 and R21AI118337 to A.G.; and R01AI114630 and R21AI114827 to I.N.C.) and ORIP/OD P51OD011132 (formerly NCRR P51RR000165) to the Yerkes National Primate Research Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Figure 1 was drafted by Dr Young-Jin Seo.
References
- Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology 2014; 59: 328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callery MP, Kamei T, Flye MW. The effect of portacaval shunt on delayed-hypersensitivity responses following antigen feeding. J Surg Res 1989; 46: 391–394. [DOI] [PubMed] [Google Scholar]
- Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM et al. Induction of immunological tolerance by porcine liver allografts. Nature 1969; 223: 472–476. [DOI] [PubMed] [Google Scholar]
- Kamada N, Davies HS, Roser B. Reversal of transplantation immunity by liver grafting. Nature 1981; 292: 840–842. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation 1995; 59: 919–921. [PubMed] [Google Scholar]
- Vadia S, Arnett E, Haghighat AC, Wilson-Kubalek EM, Tweten RK, Seveau S. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Pathog 2011; 7: e1002356. doi: 10.1371/journal.ppat.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J, Ryter A, Coquis-Rondon M, Sansonetti PJ. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun 1990; 58: 1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 2001; 167: 6471–6479. [DOI] [PubMed] [Google Scholar]
- Neeson P, Pan ZK, Paterson Y. Listeriolysin O is an improved protein carrier for lymphoma immunoglobulin idiotype and provides systemic protection against 38C13 lymphoma. Cancer Immunol Immunother 2008; 57: 493–505. doi: 10.1007/s00262-007-0388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res 2009; 69: 5860–5866. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Roosevelt M, Clarkson ABJr, van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol 2007; 9: 397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- Pradel G, Frevert U. Malaria sporozoites actively enter and pass through rat Kupffer cells prior to hepatocyte invasion. Hepatology 2001; 33: 1154–1165. doi: 10.1053/jhep.2001.24237. [DOI] [PubMed] [Google Scholar]
- Labaied M, Harupa A, Dumpit RF, Coppens I, Mikolajczak SA, Kappe SH. Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection. Infect Immun 2007; 75: 3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS et al. Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 2009; 11: 506–520. doi: 10.1111/j.1462-5822.2008.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Wang R, Kappe SH. Genetically engineered, attenuated whole-cell vaccine approaches for malaria. Hum Vaccin 2010; 6: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol 2003; 77: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss WR, Sedegah M, Beaudoin RL, Miller LH, Good MF. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A 1988; 85: 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke ED, Hoffman SL. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol 1996; 157: 4061–4067. [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006; 441: 890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol 2004; 5: 1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- Crispe IN. APC licensing and CD4+T cell help in liver-stage malaria. Front Microbiol 2014; 5: 617. doi: 10.3389/fmicb.2014.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydia M, Ge Y, Quitsch U, Beckhove P. CD40L co-stimulation from CD8+ to CD4+ effector memory T cells supports CD4+ expansion. Immunol Cell Biol 2011; 89: 670–680. doi: 10.1038/icb.2010.153. [DOI] [PubMed] [Google Scholar]
- Ebrahimkhani MR, Mohar I, Crispe IN. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology 2011; 54: 1379–1387. doi: 10.1002/hep.24508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Büschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol 1995; 22: 226–229. [DOI] [PubMed] [Google Scholar]
- Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology 1998; 28: 1597–1606. [DOI] [PubMed] [Google Scholar]
- Bowers GJ, MacVittie TJ, Hirsch EF, Conklin JC, Nelson RD, Roethel RJ et al. Prostanoid production by lipopolysaccharide-stimulated Kupffer cells. J Surg Res 1985; 38: 501–508. [DOI] [PubMed] [Google Scholar]
- Ogle CK, Wu JZ, Wood S, Ogle JD, Alexander JW, Warden GD. The increased release of prostaglandin E2 by Kupffer cells from burned guinea pigs. J Burn Care Rehabil 1990; 11: 287–294. [DOI] [PubMed] [Google Scholar]
- Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 2001; 161: III–XIII, 1–151. [DOI] [PubMed]
- Chen Y, Liu Z, Liang S, Luan X, Long F, Chen J et al. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl 2008; 14: 823–836. doi: 10.1002/lt.21450. [DOI] [PubMed] [Google Scholar]
- Gramberg T, Soilleux E, Fisch T, Lalor PF, Hofmann H, Wheeldon S et al. Interactions of LSECtin and DC-SIGN/DC-SIGNR with viral ligands: differential pH dependence, internalization and virion binding. Virology 2008; 373: 189–201. doi: 10.1016/j.virol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Oppen N, Schurich A, Hegenbarth S, Stabenow D, Tolba R, Weiskirchen R et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology 2009; 49: 1664–1672. doi: 10.1002/hep.22795. [DOI] [PubMed] [Google Scholar]
- Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A et al. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015; 148: 392–402.e313. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 2012; 12: 558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochst B, Schildberg FA, Böttcher J, Metzger C, Huss S, Türler A et al. Liver sinusoidal endothelial cells contribute to CD8 T cell tolerance towards circulating carcinoembryonic antigen in mice. Hepatology 2012; 56: 1924–1933. doi: 10.1002/hep.25844. [DOI] [PubMed] [Google Scholar]
- Klugewitz K, Blumenthal-Barby F, Schrage A, Knolle PA, Hamann A, Crispe IN. Immunomodulatory effects of the liver: deletion of activated CD4+ effector cells and suppression of IFN-gamma-producing cells after intravenous protein immunization. J Immunol 2002; 169: 2407–2413. [DOI] [PubMed] [Google Scholar]
- Schildberg FA, Hegenbarth SI, Schumak B, Scholz K, Limmer A, Knolle PA. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur J Immunol 2008; 38: 957–967. doi: 10.1002/eji.200738060. [DOI] [PubMed] [Google Scholar]
- Schurich A, Berg M, Stabenow D, Böttcher J, Kern M, Schild HJ et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol 2010; 184: 4107–4114. doi: 10.4049/jimmunol.0902580. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The danger model: a renewed sense of self. Science 2002; 296: 301–305. [DOI] [PubMed] [Google Scholar]
- Bigorgne AE, Crispe IN. TLRs in hepatic cellular crosstalk. Gastroenterol Res Pract 2010; 2010: 618260. doi: 10.1155/2010/618260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol 2012; 3: 287. doi: 10.3389/fimmu.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology 1988; 8: 232–236. doi: S0270913988000333. [DOI] [PubMed] [Google Scholar]
- Scott MJ, Liu S, Shapiro RA, Vodovotz Y, Billiar TR. Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism. Hepatology 2009; 49: 1695–1708. doi: 10.1002/hep.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig A, Banafsche R, Kremer M, Hegenbarth S, Hamann A, Neurath M et al. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J Leukoc Biol 2005; 77: 626–633. doi: 10.1189/jlb.0604332. [DOI] [PubMed] [Google Scholar]
- Knolle PA, Uhrig A, Hegenbarth S, Löser E, Schmitt E, Gerken G et al. IL-10 down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells through decreased antigen uptake via the mannose receptor and lowered surface expression of accessory molecules. Clin Exp Immunol 1998; 114: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol 2005; 174: 2037–2045. [DOI] [PubMed] [Google Scholar]
- Haque A, Best SE, Ammerdorffer A, Desbarrieres L, de Oca MM, Amante FH et al. Type I interferons suppress CD4+ T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur J Immunol 2011; 41: 2688–2698. doi: 10.1002/eji.201141539. [DOI] [PubMed] [Google Scholar]
- Marshall HD, Urban SL, Welsh RM. Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. J Virol 2011; 85: 5929–5939. doi: 10.1128/JVI.02516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 2013; 340: 202–207. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HR, Chou HS, Gu X, Wang L, Brown KE, Fung JJ et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology 2009; 50: 1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Köppel A et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells 2009; 27: 909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- Watcharanurak K, Zang L, Nishikawa M, Yoshinaga K, Yamamoto Y, Takahashi Y et al. Effects of upregulated indoleamine 2, 3-dioxygenase 1 by interferon gamma gene transfer on interferon gamma-mediated antitumor activity. Gene Ther 2014; 21: 794–801. doi: 10.1038/gt.2014.54. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Yamakawa T, Yamamura K, Hino O. Induction of Fas ligand and Fas antigen mRNA expressions in interferon-gamma transgenic mouse liver. Jpn J Pharmacol 1998; 78: 233–235. [DOI] [PubMed] [Google Scholar]
- Lee SK, Seo SH, Kim BS, Kim CD, Lee JH, Kang JS et al. IFN-gamma regulates the expression of B7-H1 in dermal fibroblast cells. J Dermatol Sci 2005; 40: 95–103. doi: 10.1016/j.jdermsci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Gieseke F, Kruchen A, Tzaribachev N, Bentzien F, Dominici M, Müller I et al. Proinflammatory stimuli induce galectin-9 in human mesenchymal stromal cells to suppress T-cell proliferation. Eur J Immunol 2013; 43: 2741–2749. doi: 10.1002/eji.201343335. [DOI] [PubMed] [Google Scholar]
- Ito H, Ando T, Ando K, Ishikawa T, Saito K, Moriwaki H et al. Induction of hepatitis B virus surface antigen-specific cytotoxic T lymphocytes can be up-regulated by the inhibition of indoleamine 2, 3-dioxygenase activity. Immunology 2014; 142: 614–623. doi: 10.1111/imm.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki H, Ito H, Ando K, Ishikawa T, Hoshi M, Ando T et al. Kynurenine production mediated by indoleamine 2,3-dioxygenase aggravates liver injury in HBV-specific CTL-induced fulminant hepatitis. Biochim Biophys Acta 2014; 1842: 1464–1471. doi: 10.1016/j.bbadis.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity 2004; 20: 327–336. [DOI] [PubMed] [Google Scholar]
- Maier H, Isogawa M, Freeman GJ, Chisari FV. PD-1: PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol 2007; 178: 2714–2720. [DOI] [PubMed] [Google Scholar]
- Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 2007; 45: 588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang Z, Chen W, Zhang Z, Li Y, Shi M et al. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol 2007; 178: 6634–6641. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Govindarajan S, Okamoto S, Dennert G. Fas-mediated apoptosis causes elimination of virus-specific cytotoxic T cells in the virus-infected liver. J Immunol 2001; 166: 3035–3041. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Moscioni AD, Qian Y, Qian R, Chen Y, Wilson JM et al. Fas-Fas ligand interactions play a major role in effector functions of cytotoxic T lymphocytes after adenovirus vector-mediated gene transfer. Hum Gene Ther 1999; 10: 259–269. doi: 10.1089/10430349950019048. [DOI] [PubMed] [Google Scholar]
- Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 ameliorates Con A-induced hepatitis by inducing CD4(+)CD25(low/int) effector T-Cell apoptosis and increasing regulatory T cell number. PLoS One 2012; 7: e48379. doi: 10.1371/journal.pone.0048379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One 2010; 5: e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji XJ, Ma CJ, Wang JM, Wu XY, Niki T, Hirashima M et al. HCV-infected hepatocytes drive CD4+ CD25+ Foxp3+ regulatory T-cell development through the Tim-3/Gal-9 pathway. Eur J Immunol 2013; 43: 458–467. doi: 10.1002/eji.201242768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Zheng J, Liang H, Xu C, Chen X, Zhang T et al. Characteristics and PD-1 expression of peripheral CD4+CD127loCD25hiFoxP3+ Treg cells in chronic HCV infected-patients. Virol J 2011; 8: 279. doi: 10.1186/1743-422X-8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolganiuc A, Kodys K, Marshall C, Saha B, Zhang S, Bala S et al. Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells. PLoS One 2012; 7: e44915. doi: 10.1371/journal.pone.0044915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY et al. Analysis of a successful immune response against hepatitis C virus. Immunity 1999; 10: 439–449. doi:S1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J et al. HCV persistence and immune evasion in the absence of memory T cell help. Science 2003; 302: 659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- Qu L, Lemon SM. Hepatitis A and hepatitis C viruses: divergent infection outcomes marked by similarities in induction and evasion of interferon responses. Semin Liver Dis 2011; 30: 319–332. doi: 10.1055/s-0030-1267534. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Callendret B, Xu D, Brasky KM, Feng Z, Hensley LL et al. Dominance of the CD4(+) T helper cell response during acute resolving hepatitis A virus infection. J Exp Med 2012; 209: 1481–1492. doi: 10.1084/jem.20111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med 2003; 197: 1645–1655. doi: 10.1084/jem.20030239jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Extremera A, Muñoz-Gámez JA, Salmerón-Ruiz MA, de Rueda PM, Quiles-Pérez R, Gila-Medina A et al. Genetic variation in interleukin 28B with respect to vertical transmission of hepatitis C virus and spontaneous clearance in HCV-infected children. Hepatology 2011; 53: 1830–1838. doi: 10.1002/hep.24298. [DOI] [PubMed] [Google Scholar]
- Siebler J, Wirtz S, Weigmann B, Atreya I, Schmitt E, Kreft A et al. IL-28A is a key regulator of T-cell-mediated liver injury via the T-box transcription factor T-bet. Gastroenterology 2007; 132: 358–371. doi: 10.1053/j.gastro.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Jameson SC. Effective effector generation of CD8+ T cells and NK cells: a need for T-bet and ZEB-too. J Exp Med 2015; 212: 1990. doi: 10.1084/jem.21212insight3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr 2012; 32: 343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez VM, Hon H, Ibegbu C, Knechtle SJ, Kirk AD, Grakoui A et al. Hepatic enrichment and activation of myeloid dendritic cells during chronic hepatitis C virus infection. Hepatology 2012; 56: 2071–2081. doi: 10.1002/hep.25904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest 2013; 123: 1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012; 482: 179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010; 328: 228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology 2011; 141: 1220–1230, 1230 e1221–1223. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol 2005; 175: 7661–7668. [DOI] [PubMed] [Google Scholar]
- French SW. Mechanisms of alcoholic liver injury. Can J Gastroenterol 2000; 14: 327–332. [DOI] [PubMed] [Google Scholar]
- Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 2012; 142: 1100–1101 e1102. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 2009; 10: 249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J 2010; 24: 4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007; 56: 1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- Gomez-Hurtado I, Santacruz A, Peiró G, Zapater P, Gutiérrez A, Pérez-Mateo M et al. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PLoS One 2011; 6: e23037. doi: 10.1371/journal.pone.0023037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med 2007; 13: 1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]