Abstract
Mesenchymal stem/stromal cells (MSCs) possess some characteristics of immune cells, including a pro-inflammatory phenotype, an immunosuppressive phenotype, antibacterial properties and the expression of Toll-like receptor proteins. Here we show that, similar to immune cells, MSCs retain information from danger signals or environmental stimuli for a period of time. When treated with the pro-inflammatory factors lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-α), MSCs display increased expression of IL-6, IL-8 and MCP-1. Following re-plating and several rounds of cell division in the absence of stimulating factors, the expression of IL-6, IL-8 and MCP-1 remained higher than in untreated cells for over 7 days. A spike in cytokine secretion occurred when cells were exposed to a second round of stimulation. We primed MSCs with LPS and LPS-primed MSCs had better therapeutic efficacy at promoting skin flap survival in a diabetic rat model than did unprimed MSCs. Finally, we found that several microRNAs, including miR146a, miR150 and miR155, along with the modification of DNA by 5-hydroxymethylcytosine (5hmC), mediate the MSC response to LPS and TNF-α stimulation. Collectively, our data suggest that MSCs have a short-term memory of environmental signals, which may impact their therapeutic potential.
Keywords: environmental stimuli, mesenchymal stem cells, pre-conditioning, short-term memory
Introduction
Mesenchymal stem/stromal cells (MSCs) have been isolated from multiple human tissues, including bone marrow (BM-MSCs), umbilical cord, umbilical cord blood, subcutaneous adipose tissue (AD-MSCs), the placenta and dental pulp.1,2 Because these cells can home to damaged and inflamed tissues, they are useful for stem cell-based strategies for tissue repair and gene therapy. Like all stem cells, MSCs display self-renewal and multilineage differentiation potential. However, MSCs also exhibit certain characteristics of immune cells. For example, MSCs can display a pro-inflammatory, MSC1, phenotype or an immunosuppressive, MSC2, phenotype under certain conditions.3,4 MSCs also have antibacterial properties5 and express Toll-like receptors.6,7
In recent years, a number of pre-conditioning strategies have been used to boost MSC function and therapeutic potential. Such strategies include treatment with growth factors,8,9 cytokines,10,11,12 hypoxic shock,13 HDLs,14 lipopolysaccharide (LPS)15 or chemical compounds.16,17 Based on findings from these studies, pre-treatment greatly enhances cell survival and the potential to migrate to damaged or inflamed tissues, thereby markedly improving the efficacy of these cells in the treatment of certain diseases or in tissue repair in an injury-specific fashion. A notable example is that hypoxia preconditioned MSCs dramatically promote tissue repair of myocardial ischemia-reperfusion injury,18 ischemic acute kidney injury19 and experimental traumatic brain injury.20 However, it remains unclear why a short treatment of MSCs exerts a long-lasting effect and why MSCs react differently to different environmental stimuli. One possibility is that MSCs possess a ‘short-term memory' for danger signals or environmental stimuli.21
In this study, we tested the hypothesis that MSCs display a ‘short-term memory' effect when exposed to danger signals or environmental stimuli. We found that transient stimulation of AD-MSCs with tumor necrosis factor-α (TNF-α) or LPS dramatically increased the secretion of MCP-1, IL-8 and IL-6. Interestingly, all cytokine levels remained elevated, even after re-plating and allowing multiple cell divisions to occur in the absence of stimulating factor. Following a second round of stimulation, cytokines were quickly released. We found that LPS-primed MSCs display enhanced therapeutic efficacy in skin flap survival in a diabetic rat model than did unprimed MSCs. Finally, we identified several epigenetic factors mediating short-term memory in MSCs.
Materials and methods
Diabetic rat model
Male Wistar rats weighing 200–250 g (Laboratory Animal Center, Academy of Military Medical Sciences, Beijing, China) were used for modeling diabetes. The animals received humane care, and all experimental procedures were carried out with the approval of the Animal Use and Care Committee of Beijing Institute of Radiation Medicine (Beijing, China). Diabetes was induced in rats by a single intraperitoneal injection of 45 mg/kg streptozotocin (Sigma Chemical Co., St Louis, MO, USA). Plasma glucose levels were measured with a glucose analyzer (Roche, Buonas, Switzerland). Rats with glucose levels greater than 15 mmol/l on the fifth day after streptozotocin treatment were considered diabetic and were included in the study.
Preparation and culture of MSCs
In accordance with the Ethics Committee at the Beijing Institute of Radiation, adipose tissues from normal healthy donors were obtained from 301 hospitals of the Chinese People's Liberation Army.
AD-MSCs were isolated and cultured according to a collagenase digestion protocol. Briefly, adipose tissues were extensively washed with phosphate-buffered saline and mechanically minced into small pieces. Tissues were then digested with 0.075% collagenase I (Invitrogen, Carlsbad, CA, USA) at 37 °C for 30 min. Next, a high-density stromal vascular fraction was collected by centrifugation. The cell pellet was washed with phosphate-buffered saline and filtered through a 100 µm nylon mesh to remove cellular debris. The cells were then incubated overnight at 37 °C/5% CO2 in control medium (α-MEM medium containing 10% FBS).
Chemical reagent treatment
A total of 105 MSCs were plated in α-MEM containing 10% FBS in 25 cm2 culture flasks (Corning, Corning, NY, USA) and incubated for 24 h. For pre-treatment, AD-MSCs were treated with 10 ng/ml TNF-α or 100 ng/ml LPS (Sigma, St Louis, MO, USA) for 24 h, and then the cells and culture supernatants were collected.
For the long-term treatment, on day 0, AD-MSCs were stimulated with or without LPS or TNF-α for 24 h, then replaced with fresh complete medium every 24 h. The cells were harvested with 0.05% trypsin-EDTA and sub-cultured in new flasks at a density of 1×104 cells/cm2 every 3 days; on day 3, the treated AD-MSCs were sub-cultured and divided into two groups. One group was stimulated with LPS or TNF-α for the second time, and another group was left untreated. Cell culture supernatants were collected every day for Enzyme-linked immunosorbent assay (ELISA) analysis.
Immunophenotype assay
Treated and untreated MSCs were analyzed by flow cytometry using a FACS Calibur flow cytometer and the following monoclonal antibodies: CD34-FITC, CD45-PE, HLA-DR-PE, CD90-PE, CD73-PE (BD Biosciences, San Jose, CA, USA) and CD105-PE (Tianjin Sungene Biotech, Tianjin, China). A nonspecific mouse isotype (IgG1) was used as a negative control.
Differentiation assay
Adipogenic differentiation
MSCs were plated in 24-well plates at a density of 2×104 cells/well. When confluence was reached, adipogenic differentiation medium was added to the cells and changed every 3 days. After 2 weeks of culturing, differentiated cells were fixed with 4% formaldehyde and stained with oil red-O (Sigma). Adipogenic differentiation medium consists of DMEM supplemented with 10% FBS, 1 mmol/l dexamethasone (Sigma), 500 mmol/l isobutylmethylxanthine (Sigma) and 60 mmol/l indomethacin (Sigma).
Osteogenic differentiation
MSCs were plated in 24-well plates at a density of 1×104 cells/well. When confluence was reached, osteogenic differentiation medium was added and changed every 3 days. On day 21, the cells were fixed and stained with alizarin red-S. Osteogenic growth medium consists of DMEM supplemented with 10% FBS, 10 mmol/l b-glycerophosphate (Sigma), 100 nmol/l dexamethasone (Sigma) and 100 mmol/l ascorbic acid-2-phosphate (Sigma).
Real-time reverse transcription polymerase chain Reaction
The cells were harvested and rinsed twice with 1× phosphate-buffered saline. Total RNA was extracted using TRIzol reagent (Invitrogen), and first-strand cDNA was reverse-transcribed using a qPCR Reverse Transcriptase MIX Kit (Toyobo Co., Ltd, Osaka, Japan). Quantitative real-time PCR was performed with an ABI 7500 fast real-time PCR system (Applied Biosystems Inc., Foster City, CA, USA) using the Fast SYBR Green PCR Master Mix (Applied Biosystems Inc.). Primer pairs for the following human genes were used: β-actin (5′-GAA GGT GAA GGT CGG AGT CA-3′ forward, 5′-GAA GAT GGT GAT GGG ATT TC-3′ reverse); IL-6 (5′-CTA GAG TAC CTC CAG AAC AG-3′ forward, 5′-TGA CCA GAA GAA GGA ATG C-3′ reverse); IL-8 (5′-TGC AGC TCT GTG TGA AGG TG-3′ forward, 5′-AAT TTC TGT GTT GGC GCA GT-3′ reverse); and MCP-1 (5′-ATC AAT GCC CCA GTC ACC TG-3′ forward, 5′-TCT CCT TGG CCA CAA TGG TC-3′ reverse).
cDNA for microRNA (miRNA) analysis was converted from mRNA using the TaqMan miRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). The expression of miR-146a, miR-150, and miR-155 (Applied Biosystems) was measured with TaqMan qPCR assays (DBI Bioscience,Ludwigshafen, Germany) according to the manufacturer's protocol. MicroRNA levels were normalized to U6 small nuclear RNA (snRNA; Applied Biosystems). The ΔΔCt method was used for analysis.
ELISA
MSCs were treated with LPS or TNF-α for 24 h. The cell culture medium was changed every day, and supernatants were collected to evaluate the concentrations of MCP-1, IL-6, and IL-8 using the Human MCP-1, IL-6 and IL-8 ELISA Kits (Neobioscience Technology Co., Ltd, Beijing, China), respectively. The kits were used according to the manufacturer's instructions.
Skin flap surgery of diabetic rats
Twenty male rats were treated with streptozotocin by intraperitoneal injection. We chose 15 diabetic rats that had plasma glucose levels ranging from 15–25 mmol/l. The backs of the rats were completely shaved with an electric razor, and a randomly selected dorsal skin flap (8×3 cm) was elevated on the dorsal trunk of the animal according to a previous protocol.22 Each of the 15 diabetic rats was randomly placed into one of three groups. One hour after skin flap surgery, rats in the three groups were intraperitoneally injected with isotonic sodium chloride, 2×106 AD-MSCs or 2×106 LPS-treated AD-MSCs. During the postoperative period, the rats were individually housed to prevent cannibalism. Flap viability was evaluated on postoperative days 7, 9 and 11.
Dot blot analysis
DNA from MSCs was isolated using a General AllGen Kit (CW Biotech, Beijing, China). Then, 3 µg/ml DNA was denatured in TE buffer for 10 min at 100 °C and serially diluted twofold with TE buffer on ice. One hundred microliter DNA samples were spotted on a nylon membrane (Biodyne-Plus; Pall Corporation, New York, USA) pre-wet with TE buffer using a Bio-Dot apparatus (Bio-Rad, Hercules, CA, USA) connected to a vacuum. The blotted membrane was dried at 80 °C for 5 min, and then UV cross-linking was performed at 120 000 µJ/cm2. The membrane was then blocked with 5% nonfat milk in TBS containing 0.1% Tween 20 for 1 h. This was followed by incubation with mouse anti-5-methylcytosine monoclonal antibody (1∶500; Active Motif, Carlsbad, CA, USA) or rabbit anti-5-hydroxymethylcytosine (5hmC) polyclonal antibody (1∶1000; Active Motif) for 3 h at room temperature. The membrane was washed three times in TBS containing 0.1% Tween 20 and then incubated with HRP-conjugated secondary antibody and visualized using Super Signal West Pico Chemiluminescent Substrate (Millipore, Bedford, MA, USA).
Statistical analysis
The data are presented as the mean±standard deviation. The means were compared using Student's two-tailed t-test. A P value<0.05 was considered statistically significant.
Results
MCP-1 expression in AD-MSCs treated with TNF-α
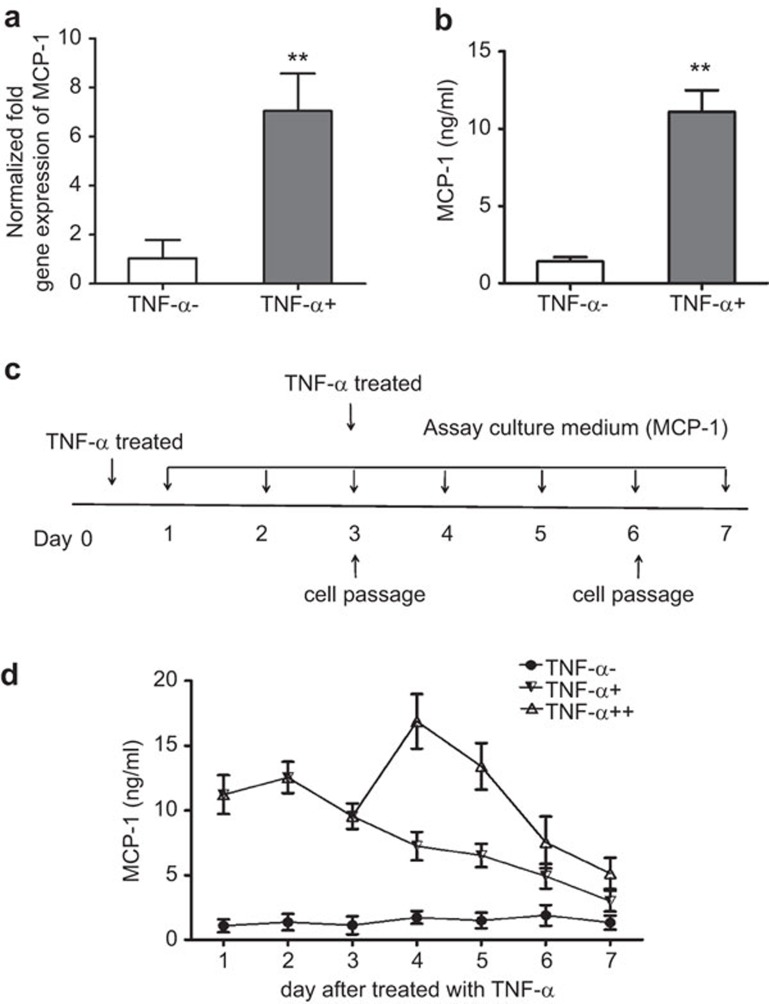
Previous work has shown that BM-MSCs treated with the pro-inflammatory factor TNF-α display increased cytokine/chemokine production. Most notably, MCP-1, the major chemokine for monocyte trafficking, was expressed at an extremely high level in TNF-α-treated MSCs.10 Consistent with these findings, we show that AD-MSCs treated with TNF-α for 24 h display strong induction of MCP-1 at both the mRNA and protein levels (Figure 1a and b). To test whether this response was transient or whether it could be maintained for a prolonged period, we collected supernatants from BM-MSC cultures every day for 7 days and measured MCP-1 expression by ELISA. Cells were treated with TNF- a for 24 h on day 0 and then stimulated with TNF-α for a second time on day 3 (after the first round of sub-culturing); a schematic diagram of the treatment program is shown in Figure 1c. Interestingly, the expression of MCP-1 remained elevated even in the absence of stimulating factor after a single treatment with TNF-α. This effect increased when cells were treated for a second time. However, the expression of MCP-1 decreased after every subculture (Figure 1d).
Figure 1.
Real-time PCR and ELISA used to analyze MCP-1 expression in AD-MSCs after TNF-α stimulation. (a) Gene expression analysis of MCP-1 in AD-MSCs incubated with TNF-α at a concentration of 20 ng/ml for 24 h by real-time PCR. The values are the means±s.d. (n=3; **P<0.01; two-tailed Student's t-test). (b) ELISA for MCP-1 expression in medium from AD-MSCs incubated as in (a) for 24 h (**P<0.01). (c) Schematic diagram of the study design. (d) ELISA for the expression of MCP-1 in culture supernatants from AD-MSCs treated with TNF-α for 7 days. Three independent experiments were carried out and yielded similar results. AD-MSC, adipose tissue mesenchymal stem/stromal cell; TNF, tumor necrosis factor; TNF-α−, AD-MSC not treated with TNF-α TNF-α+, AD-MSC treated with TNF-α once; TNF-α++, AD-MSC treated with TNF-α twice.
IL-6 and IL-8 expression in AD-MSCs treated with LPS
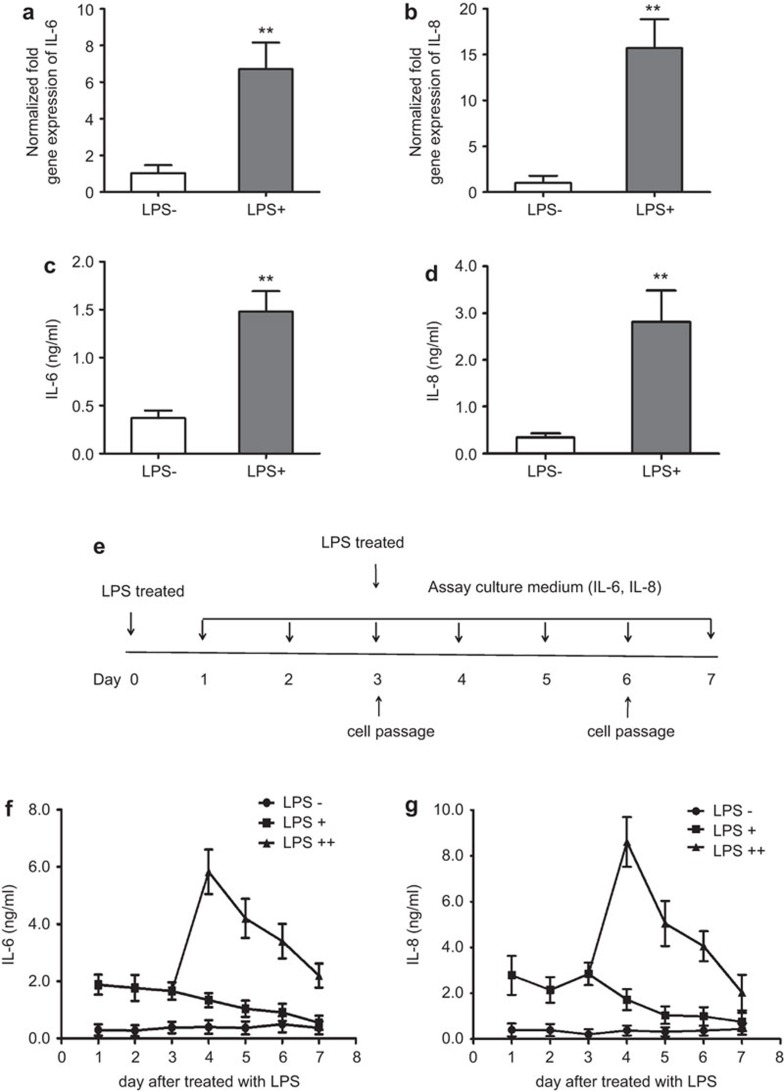
We treated AD-MSCs with another pro-inflammatory factor, LPS, and measured the expression of IL-6 and IL-8 following treatment. Consistent with earlier reports, IL-6 and IL-8 were increased at both the mRNA (Figure 2a and b) and protein (Figure 2c and d) levels following the treatment of AD-MSCs with LPS. We next tested whether IL-6 and IL-8 remained elevated for a prolonged period of time. Supernatants from AD-MSC cultures were collected every day, and the cells were sub-cultured every 3 days for 7 days. One of the AD-MSC groups was treated a second time with LPS during the first round of sub-culturing (Figure 2e). The expression of IL-6 and IL-8 remained high even in the absence of stimulating factor (Figure 2f). The levels were further increased after secondary stimulation (Figure 2g and h).
Figure 2.
Real-time PCR and ELISA used to analyze IL-6 and IL-8 expression in AD-MSCs after LPS treatment. (a and b) Gene expression analysis by real-time PCR of IL-6 and IL-8 in AD-MSCs treated with LPS at a concentration of 100 ng/ml for 24 h. The values are the means±s.d. (n=3; **P<0.01; two-tailed Student's t-test). (c and d) ELISA for IL-6 and IL-8 in medium from AD-MSCs incubated as in (a and b) for 24 h (**P<0.01). (e) Schematic diagram of the study design. (f and g) ELISA for the expression of IL-6 and IL-8 in culture supernatants from AD-MSCs treated with LPS for 7 days. Three independent experiments were carried out and yielded similar results. AD-MSC, adipose tissue mesenchymal stem/stromal cell; LPS, lipopolysaccharide; LPS−, AD-MSC not treated with LPS; LPS+, AD-MSC treated with LPS once; LPS++, AD-MSC treated with LPS twice.
Immunophenotype, differentiation potential and morphology of AD-MSCs treated with LPS/TNF-α
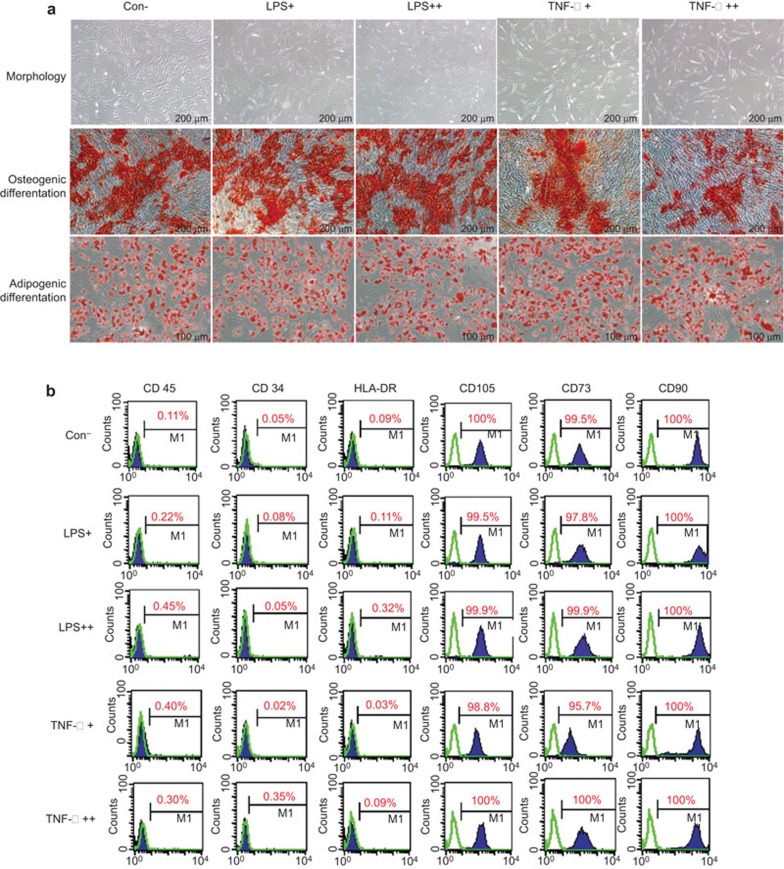
MSCs can ‘remember' transient stimulation by pro-inflammatory factors (TNF-α and LPS). Thus, we next determined whether these pro-inflammatory stimuli affect the morphology, immunophenotype and differentiation potential of MSCs. The immunophenotypes of AD-MSCs were analyzed by flow cytometry. Osteogenic and adipogenic differentiation were assessed by alizarin red-S and oil red-O staining, respectively.
The morphology of AD-MSCs was observed under an inverted light microscope. TNF-α-primed AD-MSCs were slightly longer in shape and bigger in size. In contrast, AD-MSCs did not show marked morphological changes upon treatment with LPS (Figure 3a). Flow cytometric analysis confirmed that regardless of priming with LPS/TNF-α, AD-MSCs were positive for CD73, CD90 and CD105, but negative for CD34, CD45 and HLA-DR (Figure 3b). Moreover, AD-MSCs did not show an obvious divergence in differentiation, regardless of priming with LPS/TNF-α (Figure 3a).
Figure 3.
Phenotype, differentiation potential and growth rate of AD-MSCs treated with LPS/TNF-α. (a) The morphology, and adipogenic and osteogenic differentiation of AD-MSCs primed with LPS/TNF-α. (b) Flow cytometric analysis of the immunophenotype of AD-MSCs and BM-MSCs. AD-MSC, adipose tissue mesenchymal stem/stromal cell; BM-MSC, bone marrow mesenchymal stem/stromal cell; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
Effect of AD-MSC treatment on skin flap survival in diabetic rats
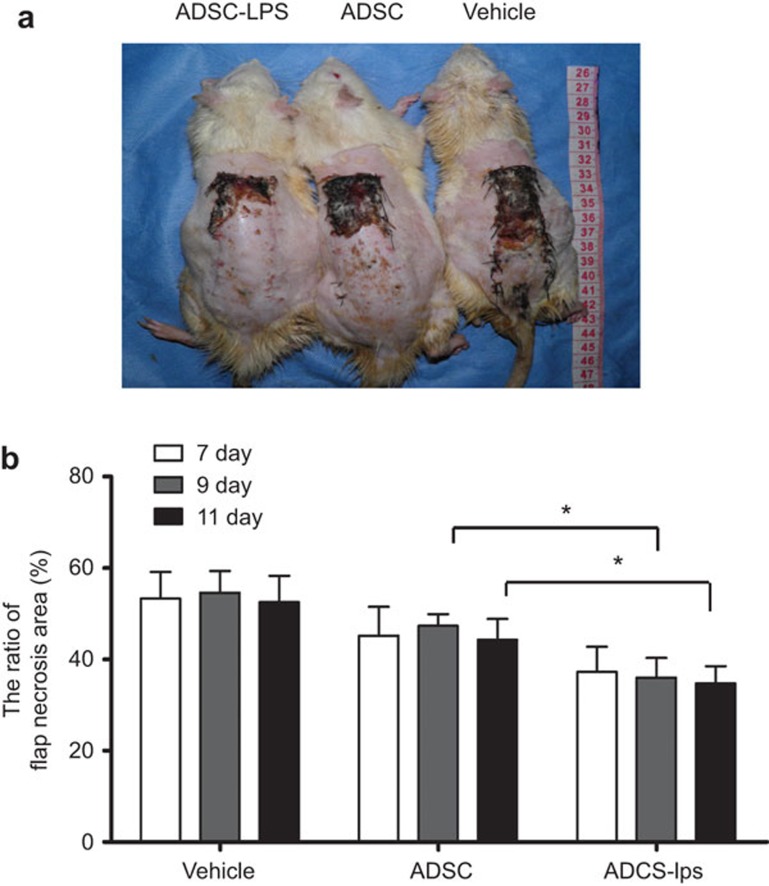
Compared to internal injuries, trauma is more susceptible to pathogenic microbes. Here, we generated a dorsal skin flap (trauma) in diabetic rats to further explore the therapeutic potential of the short-term memory of MSCs. AD-MSCs were preconditioned with the bacterial endotoxin LPS. They were then intraperitoneally administered to rats with a randomly selected dorsal skin flap (8×3 cm). The necrotic size/flap size ratio in each sample was analyzed on postoperative days 7, 9 and 11. The regions of survival and the necrotic areas were clearly demarcated in every flap because the flaps had not shrunk by postoperative day 7. The section of surviving skin appeared pink-white in color and was normal in texture. In contrast, necrotic skin was black and rigid and did not bleed. Clearly, LPS-primed AD-MSC treatment resulted in a significant decrease in skin flap necrosis compared to either vehicle-treated or unprimed AD-MSCs. There was no change in necrosis between days 7 and 11. In contrast, treatment with LPS-primed AD-MSCs displayed continuous therapeutic benefits for skin flap survival over time (Figure 4).
Figure 4.
Improved skin flap survival in diabetic rats by LPS-primed AD-MSCs. (a) A representative image indicates the survival and the necrotic areas of the skin flap in each group on the postoperative ninth day. (b) The necrotic size/flap size ratio in each diabetic rat group was analyzed on postoperative days 7, 9 and 11. The values are presented as the means±s.d. (n=3; *P<0.05). AD-MSC, adipose tissue mesenchymal stem/stromal cell.
DNA hydroxymethylation and microRNA levels are altered in AD-MSCs treated with LPS/TNF-α
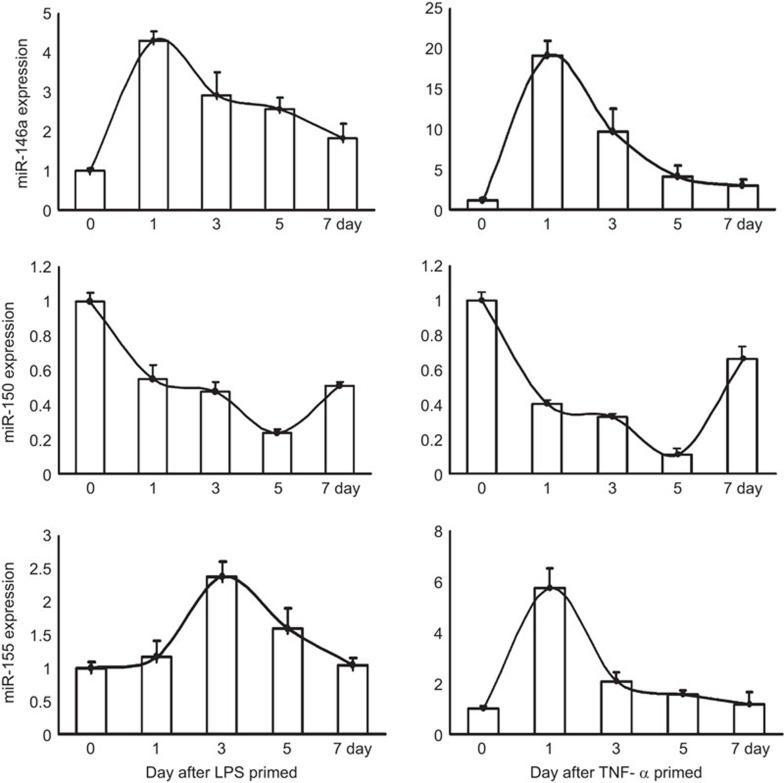
The potential mediators of short-term memory may belong to a broad class of stimulus-responsive regulatory molecules that differ from labile second messengers. A number of reports have firmly established the importance of miRNAs in development, differentiation, maturation, and activation of immune cells. Some miRNAs, such as miR146a, miR155 and miR150, also mediate the response of immune cells to danger signals or environmental stimuli. These miRNAs are likely candidates for the regulation of short-term memory in immune cells. To determine the importance of miRNAs in the short-term memory maintenance of MSCs, we analyzed the expression of miR-146a, miR-155, and miR-150 in AD-MSCs at different time points after transient treatment with LPS/TNF-α. miR-146a and miR-155 are upregulated in AD-MSCs by both LPS and TNF-α similar findings were reported in immune cells.23,24 In contrast, the expression of miR-150 was decreased. Interestingly, the time course of changes in expression of miR-146a, miR-155 and miR-150 in TNF-α-treated MSCs was similar to the changes seen in LPS-treated MSCs (Figure 5). In contrast to control cells, the expression of miR-146a and miR-155 remained high for at least 5 days following either LPS or TNF-α treatment. The decrease in miR-150 also lasted for several days. These observations indicate that some miRNAs serve as underlying regulators of short-term memory in MSCs.
Figure 5.
miR146a, 155 and 150 expression in AD-MSCs treated with LPS/TNF-α. Real-time PCR analysis of miR-146a, miR-150 and miR-155 expression in AD-MSCs on days 1, 3, 5 and 7 after LPS or TNF-α treatment. The values are presented as the means±s.d. AD-MSC, adipose tissue mesenchymal stem/stromal cell; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
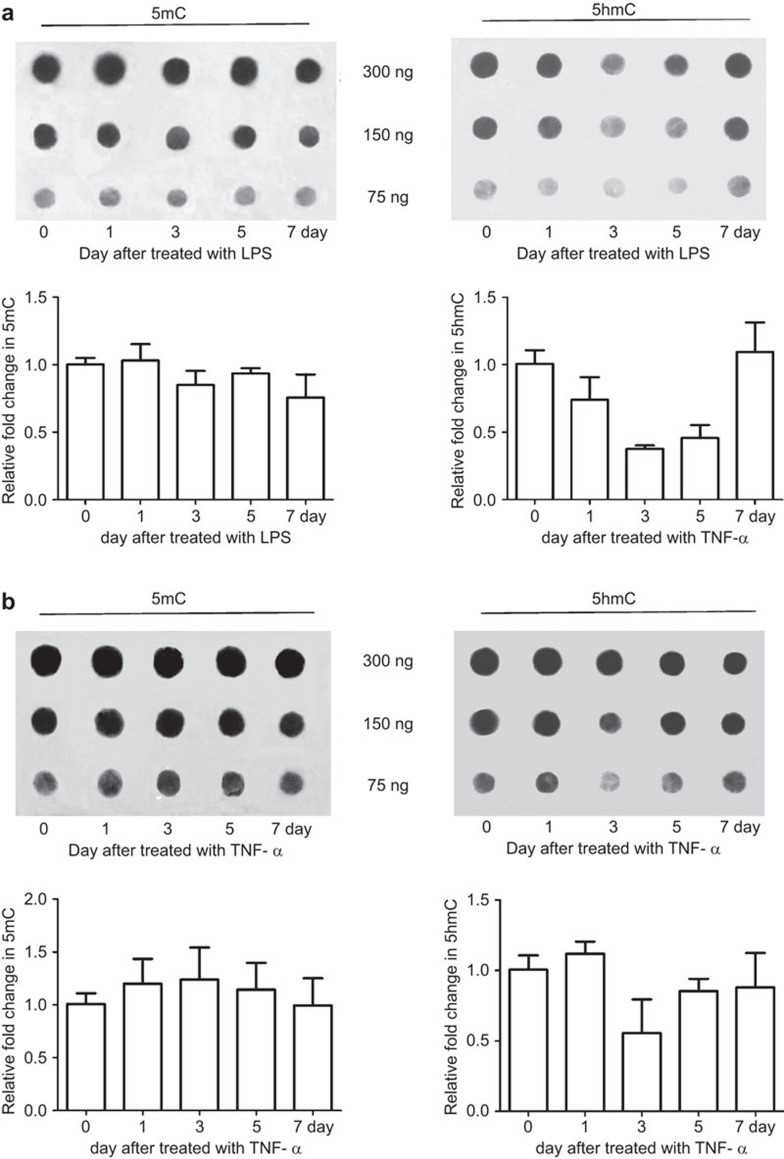
The epigenetic DNA modification 5hmC could be responsible for mediating the short-term memory of MSCs following exposure to certain stimuli. Importantly, this epigenetic mark is reversible and has a short half-life. Both TNF-α and LPS treatment resulted in a delayed decrease in 5hmC, which returned to the basal, pre-stimulus state 7 days after treatment (Figure 6). These data suggest that 5hmC may also be involved in the short-term memory of environmental stimuli in MSCs.
Figure 6.
DNA methylation/hydroxymethylation in AD-MSCs treated with LPS/TNF-α. Dot blot analysis for 5hmC/5mC on days 1, 3, 5 and 7 after LPS or TNF-α treatment. The values are presented as the means±s.d. AD-MSC, adipose tissue mesenchymal stem/stromal cell; 5hmC, 5-hydroxymethylcytosine; 5mC, 5-methylcytosine; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
Discussion
In a recently published review, Monticelli and Natoli proposed the concept that immune cells have a ‘short-term memory' of danger signals or environmental stimuli.21 Immune cells record information they receive from the environment and maintain it over a period of time. The essential features of such memory phenomena are their persistence beyond the initial stimulation and their decay over time.21 MSCs have great potential to be used for clinical applications, especially for immune-related diseases, because of their potent immunoregulatory properties.25,26 These cells are also regarded as sensors and switchers of inflammation and have many characteristics of immune cells.4 For example, they can exhibit either anti-inflammatory or pro-inflammatory effects when cultured under certain conditions; this is similar to macrophages and natural killer T cells.27,28 Here we demonstrate that MSCs display a characteristic short-term memory response, which is reflected in two ways: maintaining a biological change following a short stimulus, and responding more intensely to the same stimulus upon a second exposure.
This finding is important because transient preconditioning greatly improves the therapeutic efficacy of MSCs. It also implies that MSCs can be primed by different environmental signals in a disease-specific manner. For example, hypoxia preconditioning dramatically enhances the capacity of MSCs to repair ischemic tissue injury, such as a myocardial ischemia–reperfusion injury18 or an ischemic acute kidney injury.19 Here, we show that LPS pre-contacted AD-MSCs display better therapeutic efficacy for skin flap survival in a diabetic rat model. Recently, erythropoietin, which is primarily produced by renal cells, dramatically primes BM-MSCs for kidney-directional migration.29 This observation provides additional support for MSC memory in response to environmental signals and its potential clinical significance.
The potential mediators of short-term memory are a broad class of stimulus-responsive regulatory molecules that differ from labile second messengers.21 These mediators include some inducible regulatory molecules, such as miRNAs and a subset of newly deposited chromatin modifications, including DNA hydroxymethylation and histone acetylation or methylation. miRNAs have at least three essential properties that make them compatible with the maintenance of short-term memory. One is that some miRNAs are stable and can be maintained for several days.30,31 Second, some miRNAs can be recycled after recognizing their targets and can participate in multiple rounds of targeting. Finally, some miRNAs can be transferred from one cell to another, thereby transferring the memory state to neighboring cells.21,32 Here, we identify three miRNAs, miR146a, miR150 and miR155, which may mediate short-term memory both in cells of the immune system and in MSCs. Importantly, transient stimulation by both LPS and TNF-α results in a long-lasting (approximately 1 week) effect on these miRNAs.
In contrast to 5-methylcytosine, 5-methylcytosine is reversible and has a short half-life. Thus, this modification is potentially suitable for transient modification of gene expression and for mediating short-term memory. 5hmC is derived from 5-methylcytosine in a reaction catalyzed by the TET (‘ten-eleven-translocation') family of dioxygenases. Research on 5hmC and TET enzymes has mainly focused on embryonic stem cells.33,34 As a type of adult stem cell, MSCs have relatively high levels of 5hmC. This can be suppressed upon treatment with either LPS or TNF-α. Within 3–7 days of treatment, 5hmC levels are usually recovered (Figure 5b). Taken together, regulatory mechanisms governing short-term memory in immune cells may also be shared by MSCs.
Stem cell memory of environmental signals explains why conditioned stem cells may be better suited to clinical applications. These cells can migrate and adapt to the microenvironment of inflamed or injured tissues, and they can exhibit different phenotypes and modulate their function to establish a balance between pathogen elimination and tissue repair. This is a markedly different process from tumor metastasis. When tumor cells arrive at different tissues or organs, they maintain the characteristics of their tissue of origin. Chronic tissue damage and repeated inflammation are causative factors of tumorigenesis. Thus, tumors are considered to be wounds that do not heal.35 Chronic inflammation or viral infection may lead to dysfunction of local stem cells involved in tissue repair via the loss of their memory or adaptive ability, or the loss of stem cells that remember the microenvironment, as has been demonstrated in cells of immune system,36 and lead to tumorigenesis. From this perspective, tumor cells or tumor stem cells may be regarded as a class of stem cells that have lost their ability to ‘remember'. Our results indicate that epigenetic regulatory molecules or chromatin modifications may be the potential mediators of memory in stem cells. Intriguingly, both histone acetyltransferase inhibitors and DNA methyltransferase inhibitors have been widely used in the clinic as anticancer drugs.37,38 These drugs interfere with epigenetic modifications and may help tumor cells restore their memory of the surrounding environment. Therefore, it might be possible to develop a novel anti-tumor strategy by investigating the differences among stem cells, tumor cells, and tumor stem cells. Perhaps in the near future we can eradicate tumors by restoring their ability to ‘remember'.
In conclusion, we have shown that MSCs have short-term memory properties, which may have important therapeutic implications.
Acknowledgments
This work was supported by grants from the Foundation of National Basic Research Program of China (Nos. 2010CB529903, 2010CB833600 and 2012CB518105) and the National Science Foundation of China (Nos. 81201760 and 81270894). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- Tomic S, Djokic J, Vasilijic S, Vucevic D, Todorovic V, Supic G et al. Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by Toll-like receptor agonists. Stem Cells Dev 2011; 20: 695–708. [DOI] [PubMed] [Google Scholar]
- Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001; 98: 2396–2402. [DOI] [PubMed] [Google Scholar]
- Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One 2010; 5: e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 2013; 13: 392–402. [DOI] [PubMed] [Google Scholar]
- Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010; 28: 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells 2008; 26: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 2007; 109: 1422–1432. [DOI] [PubMed] [Google Scholar]
- Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock 2010; 33: 24–30. [DOI] [PubMed] [Google Scholar]
- Khan M, Akhtar S, Mohsin S, N Khan S, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev 2011; 20: 67–75. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell 2012; 11: 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Yoon N, Reneau JC, Prockop DJ. Preactivation of human MSCs with TNF-alpha enhances tumor-suppressive activity. Cell Stem Cell 2012; 11: 825–835. [DOI] [PubMed] [Google Scholar]
- Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 2010; 5: e9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008; 26: 2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Qian J, Xie X, Lin L, Zou Y, Fu M et al. High density lipoprotein protects mesenchymal stem cells from oxidative stress-induced apoptosis via activation of the PI3K/Akt pathway and suppression of reactive oxygen species. Int J Mol Sci 2012; 13: 17104–17120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei YB, Zhou WQ, Zhang XY, Wei XJ, Feng ZC. Lipopolysaccharides shapes the human Wharton's jelly-derived mesenchymal stem cells in vitro. Cell Physiol Biochem 2013; 32: 390–401. [DOI] [PubMed] [Google Scholar]
- Khan M, Ali F, Mohsin S, Akhtar S, Mehmood A, Choudhery MS et al. Preconditioning diabetic mesenchymal stem cells with myogenic medium increases their ability to repair diabetic heart. Stem Cell Res Ther 2013; 4: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007; 109: 228–234. [DOI] [PubMed] [Google Scholar]
- Jaussaud J, Biais M, Calderon J, Chevaleyre J, Duchez P, Ivanovic Z et al. Hypoxia-preconditioned mesenchymal stromal cells improve cardiac function in a swine model of chronic myocardial ischaemia. Eur J Cardiothorac Surg 2013; 43: 1050–1057. [DOI] [PubMed] [Google Scholar]
- Yu X, Lu C, Liu H, Rao S, Cai J, Liu S et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One 2013; 8: e62703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond) 2013; 124: 165–176. [DOI] [PubMed] [Google Scholar]
- Monticelli S, Natoli G. Short-term memory of danger signals and environmental stimuli in immune cells. Nat Immunol 2013; 14: 777–784. [DOI] [PubMed] [Google Scholar]
- Ozturk A, Firat C, Parlakpinar H, Bay-Karabulut A, Kirimlioglu H, Gurlek A. Beneficial effects of aminoguanidine on skin flap survival in diabetic rats. Exp Diabetes Res 2012; 2012: 721256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006; 103: 12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 2011; 208: 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371: 1579–1586. [DOI] [PubMed] [Google Scholar]
- Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut 2010; 59: 1662–1669. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5: 953–564. [DOI] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, Mingari MC et al. Receptors for HLA class-I molecules in human natural killer cells. Annu Rev Immunol 1996; 14: 619–648. [DOI] [PubMed] [Google Scholar]
- Liu N, Tian J, Cheng J, Zhang J. Effect of erythropoietin on the migration of bone marrow-derived mesenchymal stem cells to the acute kidney injury microenvironment. Exp Cell Res 2013; 319: 2019–2027. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 2006; 125: 1111–1124. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007; 316: 575–579. [DOI] [PubMed] [Google Scholar]
- Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood 2012; 119: 756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010; 466: 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013; 500: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–1659. [DOI] [PubMed] [Google Scholar]
- van Grevenynghe J, Cubas RA, Noto A, DaFonseca S, He Z, Peretz Y et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. J Clin Invest 2011; 121: 3877–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006; 6: 38–51. [DOI] [PubMed] [Google Scholar]
- Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist 2005; 10: 176–182. [DOI] [PubMed] [Google Scholar]