Abstract
We previously reported that SM934, a water-soluble artemisinin derivative, was a viable treatment in murine lupus models. In the current study, we further investigated the therapeutic effects of a modified dosage regimen of SM934 on lupus-prone MRL/lpr mice and explored its effects on B cell responses, a central pathogenic event in systemic lupus erythematosus (SLE). When orally administered twice-daily, SM934 significantly prolonged the life-span of MRL/lpr mice, ameliorated the lymphadenopathy symptoms and decreased the levels of serum anti-nuclear antibodies (ANAs) and of the pathogenic cytokines IL-6, IL-10 and IL-21. Furthermore, SM934 treatment restored the B-cell compartment in the spleen of MRL/lpr mice by increasing quiescent B cell numbers, maintaining germinal center B-cell numbers, decreasing activated B cell numbers and reducing plasma cell (PC) numbers. Ex vivo, SM934 suppressed the Toll-like receptor (TLR)-triggered activation and proliferation of B cells, as well as antibody secretion. Moreover, the present study demonstrated that SM934 interfered with the B-cell intrinsic pathway by downregulating TLR7/9 mRNA expression, MyD88 protein expression and NF-κB phosphorylation. In human peripheral blood mononuclear cells (PBMCs), consistent with the results in MRL/lpr mice, SM934 inhibited TLR-associated B-cell activation and PC differentiation. In conclusion, a twice daily dosing regimen of SM934 had therapeutic effects on lupus-prone MRL/lpr mice by suppressing B cell activation and plasma cell formation.
Keywords: B cell, plasma cell, SM934, systemic lupus erythematosus, Toll-like receptor
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease with significant morbidity and mortality. Female MRL/lpr mice, a classic animal model of SLE, spontaneously develop autoimmune syndromes characterized by lupus nephritis, hematological changes, massive lymphadenopathy, splenomegaly and autoantibody formation, among which lupus nephritis is the key factor that leads to death.1
B lymphocytes are central players in the adaptive immune response. In response to antigen encounters, they undergo activation and differentiate into plasma or memory cells in the germinal center. Two transcriptional regulators are the main controllers of B-cell destiny: Bcl-6 and Blimp-1. Bcl-6 regulates a panel of genes involved in maintaining B-cell identity and germinal center (GC) reactions, whereas Blimp-1, a direct target of Bcl-6, is the master regulator of plasma cell differentiation. The reciprocal feedback loop between Bcl-6 and Blimp-1 ensures that B cells have two mutually exclusive fates: to enter the germinal center or the plasma cell pathway.2,3
Production of autoantibodies is the cardinal feature of SLE.4 Disease-related autoantibodies in SLE are directed to particular targets, including DNA-containing antigens, such as double-stranded DNA (dsDNA), and RNA-containing antigens.5,6 The expression of Toll-like receptors (TLRs) by B cells provides a cell-intrinsic mechanism by which innate signals regulate adaptive immune responses.7 B cells contribute to SLE pathology through BCR recognition of endogenous DNA- or RNA-associated autoantigens, as well as through the delivery of these self-constituents to endosomal TLR9 or TLR7, respectively.7,8,9 B-cell activation via these pathways leads to the production of class-switched DNA- or RNA-reactive autoantibodies. The autoantibodies then form immune complexes that can accumulate in the kidneys and other tissues, contributing to an inflammatory amplification loop.
MyD88, originally isolated as a myeloid differentiation primary response gene, is now defined as a pivotal adaptor in TLR signaling. The death domain of MyD88 mediates interactions with the IRAK complex, triggering a signaling cascade that includes the activation of IKK, leading to the degradation of IκB, which normally maintains NF-κB in an inactive state by sequestering it in the cytoplasm. Recently, Teichmann demonstrated that the MyD88-dependent TLR signaling pathway in B cells is required for anti-nuclear antibody (ANA) formation in MRL/lpr mice.10 Additionally, plasma cell generation and class switch recombination also critically depend on this pathway in B cells.10
Abnormal cytokine production has also been involved in SLE pathogenesis. IL-6 is a B-cell stimulatory cytokine that induces autoantibody production and maintains the survival of long-lived plasma cells in the bone marrow. The serum IL-6 level is significantly elevated in patients with active SLE and is correlated with the SLE activity index, erythrocyte sedimentation rate and C-reactive protein.11 IL-10, the levels of which are increased both in patients with active disease and in murine models of SLE, plays a role in B lymphocyte hyperactivity and differentiation. In vivo administration of rIL-10 accelerates lupus, whereas a monoclonal anti-IL-10 Ab delays the onset of anti-dsDNA autoantibody production, GN, and proteinuria and decreases mortality in NZB/W F1 mice.12 IL-21, a well-known signature factor of follicular helper T cells, is a pleiotropic cytokine that can influence the activation, differentiation, and expansion of germinal center B cells and is critically involved in the pathogenesis of SLE.13,14,15 It has been reported that MRL/lpr mice develop lupus-like disease in an IL-21-dependent manner.16 Collectively, IL-6, IL-10 and IL-21, which are pro-inflammatory mediators in humoral immunity, are regarded as biomarkers of SLE.17
SM934, a water-soluble artemisinin derivative, possesses higher bioavailability and better immunosuppressive activity than traditional artemisinin derivatives, which have shown curative benefits in SLE both clinically and experimentally.18,19,20,21 In our previous study, SM934 exhibited protective effects in two mouse models of SLE, MRL/lpr and NZB/W F1 mice, partly by suppressing pathogenic T-cell development.22,23 Because of its relatively short biological half-life (approximately 0.5 h tested on rats and dogs), we optimized the dose regimen of SM934 to a twice daily administration and reduced the drug doses compared to previous strategies.22 As a result, this administration strategy dramatically improved the therapeutic effects of SM934 in MRL/lpr mice, in a dose-dependent manner, as manifested by a more persistent and stable efficacy over a longer period of time. Furthermore, the current study also highlighted another therapeutic mechanism of SM934: suppression of autoreactive B-cell activation and plasma cell generation by autoantigens in lupus.
Materials and methods
Mice
Female MRL/lpr mice were obtained from the SLRC Laboratory (Shanghai, China). All mice were housed under specific pathogen-free conditions and kept in a 12 h light/dark cycle with controlled humidity (60%–80%) and temperature (22±1 °C). Food and water were freely available. All experiments were performed according to the institutional ethical guidelines on animal care and were approved by the Institute Animal Care and Use Committee at the Shanghai Institute of Materia Medica.
Experimental design
SM934 was synthesized at Shanghai Institute of Materia Medica (Shanghai, China).
For therapy, 54 female MRL/lpr mice were randomly divided into five groups according to proteinuria level, as follows: vehicle (ddH2O, n=11), prednisolone (PNS) 2 mg/kg (n=10), SM934 5 mg/kg (n=11), SM934 2.5 mg/kg (n=11) and SM934 1.25 mg/kg (n=11). Mice were treated with a twice-daily dosing regimen by oral intragastric administration from 9 weeks to 27 weeks of age. The dosage (mg/kg) listed is the dose per administration, and the doses were administered each day at 9:00 a.m. and 5:00 p.m. SM934 was dissolved and PNS was dispersed in ddH2O. At the end of the experiment, mice were anaesthetized for serum collection. Lymph nodes and spleens were isolated and weighed, photographed and lymphocytes were prepared. Both kidneys were also excised for section analysis.
For the in vitro mechanism study, 8-week-old female MRL/lpr mice were used.
To explore the pathogenesis of lupus, female MRL/lpr mice of different ages (4-week, 8-week, 12-week, 16-week, 20-week, 24-week and 27-week included) were used. All of the mice were euthanized at the same time, regardless of their age. Lymph nodes and spleens were isolated and weighed, and photographed, and lymphocytes were prepared.
Evaluation of renal injury
Urine from individual mice was collected weekly by the ‘bladder massage' method as previously described,24,25 and the concentration of urinary protein was detected by a Coomassie brilliant blue dye-binding assay. To guarantee the accuracy of the experiment, urine samples were collected at the same time from all mice and assayed immediately following sample collection. For mice that died before the termination of the study, the last known value of urinary protein was carried forward in subsequent proteinuria analysis.26,27,28
Serum albumin and blood urea nitrogen levels were determined using a HITACHI-7080 automatic biochemical analyzer (Hitachi High Technologies Corporation, Tokyo, Japan) to evaluate the renal function.
For renal histopathology assessment, left kidneys were embedded in paraffin and 4-µm sections were stained with hematoxylin and eosin. The sections were scored by two professional renal pathologists (from Drug Safety Evaluation and Research Center, SIMM, CAS) for glomerular, interstitial, and vascular lesions according to reported criteria.29
For the immunofluorescence evaluation of IgG deposits in the kidneys, right kidneys were embedded in an OCT compound, and 6-µm frozen sections were stained with FITC-conjugated goat anti-mouse IgG. The IgG deposits were semiquantitatively analyzed by Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA).
ELISA
Cytokines in sera or culture supernatants were assayed by using mouse IL-6 and IL-10 ELISA kits (BD Pharmingen, San Diego, CA, USA) and an IL-21 ELISA kit (R&D systems, Minneapolis, MN, USA) according to manufacturer's instructions. The abundance of ANA in the sera (diluted 1∶100) was detected using the mouse ANA ELISA kit (Alpha Diagnostic International, San Antonio, TX, USA) according to the manufacturer's protocol. The detection of serum anti-dsDNA IgG and IgM antibodies was performed as previously described.30 To test IgG and IgM concentrations in culture supernatants of mouse purified splenic B cells, goat anti-mouse Ig-UNLB (Southern Biotech, Birmingham, AL, USA) was used as the primary antibody, and HRP-conjugated rabbit anti-mouse IgG (H+L) (Invitrogen, Carlsbad, CA, USA) or HRP-conjugated goat anti-mouse IgM (Southern Biotech) followed as the second antibody. The reagents used for the detection of antibody production in culture supernatants of human peripheral blood mononuclear cells (PBMCs) were purchased from BD Pharmingen (San Diego, CA, USA).
Flow cytometry
Cells were blocked with 2.4G2 (eBioscience, San Diego, CA, USA) and then stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin chlorophyll protein (PerCP)-Cy5.5-, allophycocyanin (APC)- and Brilliant Violet (BV) 421-labeled antibodies. All immunofluorescent Abs used in this study were from BD Biosciences (Franklin Lakes, NJ, USA). Flow cytometric analysis was performed on BD LSRFortessa, and the data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
B cell purification and ex vivo stimulation
At the study termination, the splenic B cells from the mice in the vehicle group and SM934 5 mg/kg group were purified for ex vivo stimulation. To acquire polyclonal B cells, mAb cocktails were added to an immunomagnetic negative selection system to deplete CD4+ cells, CD8+ cells and CD11b+ cells from the splenocytes. The remaining cells were then incubated with biotin-conjugated Rat anti-Mouse CD19 (BD Pharmingen, San Diego, CA, USA) and anti-Biotin MicroBeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and positively selected by a magnetic cell-sorting protocol (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The purity of the resultant cell population was determined by flow cytometric analysis and was consistently >98%. Purified B cells from the two groups were then cultured with medium alone or with the TLR7 ligand R848 (5 µg/ml; Invivogen, San Diego, CA, USA) or the TLR9 ligand ODN 1668 (1 µM; Invivogen). After incubation, for 24-well plates, supernatants were collected to determine the cytokine levels, and the cells were assayed on flow cytometry for B-cell activation at 48 h. For 96-well plates, the cultures were pulsed with 0.5 µCi [3H] thymidine per well for 12 h before harvesting and were assessed for [3H] thymidine incorporation at 48 h. For plasma cell formation and antibody secretion assays, cells were cultured for 6 days.
In vitro stimulation of splenic B cells
Splenic B cells were purified from unmanipulated 8-week-old MRL/lpr mice according to above instructions. Purified B cells were then cultured under the stimulation of TLR ligands or anti-IgM (20 µg/ml; Jackson ImmunoResearch Lab, West Grove, PA, USA), with or without SM934 (10 µM, without cytotoxicity to splenic B cells) added simultaneously. At 48 h, the proliferation of B cell was measured by [3H] thymidine incorporation and the activation of B cell was detected by FACS. After 72-h incubation, cells were collected for protein extraction for western blotting.
In vitro stimulation of human PBMCs
PBMCs from several healthy donors (n=4) were supplied by the Shanghai Blood Center (informed consent was obtained by the blood center) and approved by the Ethics Committee of Shanghai Blood Management Office. Human PBMCs were cultured under stimulation of R848 (3 µg/ml) or ODN2336 (1 µM; Invivogen) with or without SM934 (60 µM, without cytotoxicity to PBMCs) simultaneously added. After incubation, supernatants were collected at day 6 for the detection of antibody concentrations, and cultured cells were harvested for FACS analysis at 48 h or on day 6. In addition, proliferation of PBMCs was measured by [3H] thymidine incorporation at 48 h.
Western blotting
Primary splenic cells or stimulated B cells were collected and lysed in sodium dodecyl sulfate sample buffer and boiled for 10 min at 100 °C. Proteins were resolved by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK). The membranes were incubated with anti-phospho-NF-κB, anti-IκB, anti-MyD88 anti-Bcl-6 and anti-Blimp-1 rabbit mAbs (all from Cell Signaling Technology, Beverly, MA, USA) using the recommended antibody dilutions according to manufacturer's instructions. Signals were detected with a HRP-conjugated anti-rabbit IgG (1∶20 000; Bio-Rad, Richmond, CA, USA) using an ECL system (Amersham Biosciences, Buckinghamshire, UK).
Real-time PCR
To quantify the expression of TLRs, mRNA was isolated from the splenocytes of tested mice with a Total RNA kit (Tiangen, Shanghai, China) and reverse transcribed with an RT Master Mix kit (Takara, Dalian, China). QRT-PCR was performed with the SYBR Premix Ex Taq kit (Takara) on an Applied Biosystems 7500 Fast Real-Time PCR System. Mouse β-actin was used as a housekeeping gene. The primer sequences used were: mouse β-actin, forward, 5′-GGC TAT ATT CCC CTC CAT CG-3′ and reverse, 5′-CCA GTT GGT AAC AAT GCC ATG T-3′ mouse tlr7, forward, 5′-ATG TGG ACA CGG AAG AGA CAA-3′ and reverse, 5′-GGT AAG GGT AAG ATT GGT G-3′ mouse tlr9, forward, 5′- ATG GTT CTC CGT CGA AGG ACT-3′ and reverse 5′-GAG GCT TCA GCT CAC AGG G-3′.
Statistical analysis
Analysis of survival curves was performed with the log-rank test. Proteinuria data were analyzed using one-way analysis of variance for repeated measures, corrected with Dunnett's post-test (for assessment of proteinuria; a last observation carried forward approach was used for the mice that died before the end of the study).26,27,28 Student's t-test was used to assess significant differences between two groups. One-way analysis of variance with Dunnett's post-test was used to test the significant effects among multiple group comparisons. Statistical analyses were conducted using GraphPad Prism 5.0 software. Data are presented as the mean±s.e.m.
Results
SM934 treatment protects MRL/lpr mice from fatal lupus nephritis
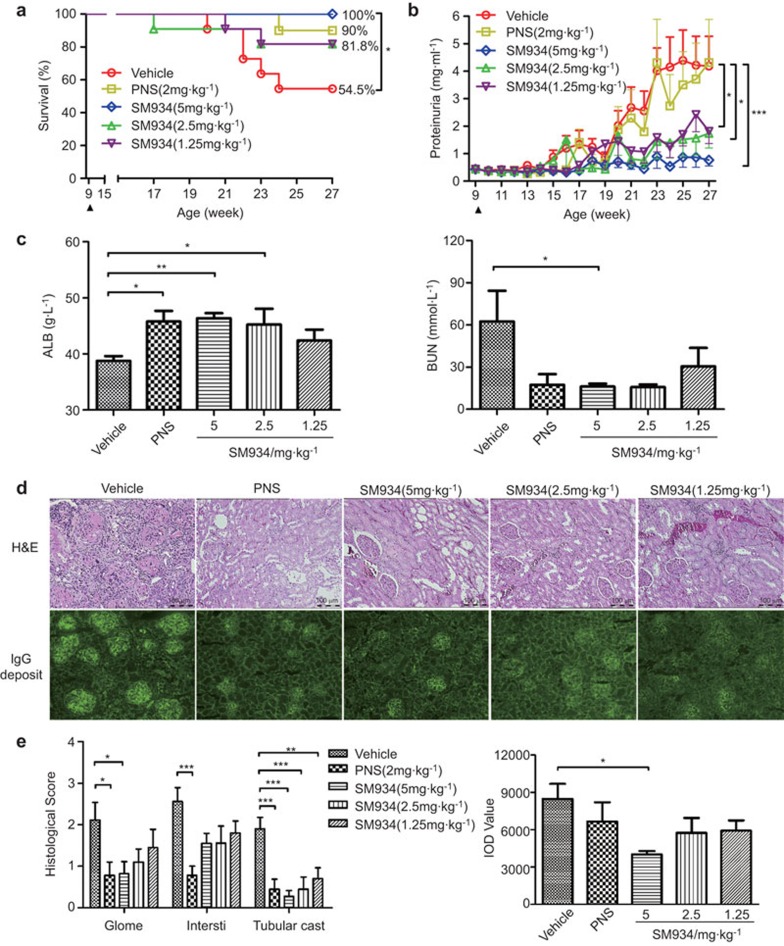
SLE is an autoimmune disease characterized by fatal nephritis. It has been reported that 50% of MRL/lpr mice die by 24 weeks of age, primarily because of renal failure.1 In our study, treatment with 5 mg/kg SM934 completely eliminated mortality, and treatment with 2.5 mg/kg or 1.25 mg/kg SM934 also greatly reduced mortality (Figure 1a). Proteinuria, one of the main functional measures of lupus, was kept at a low level during SM934 administration, in contrast to in vehicle-only treated mice (Figure 1b). Additionally, SM934 prevented the worsening of renal function, as represented by significant increase in albumin (ALB) levels and decrease in blood urea nitrogen (BUN) levels in serum (Figure 1c). A kidney histopathology assay further confirmed that the mice in the vehicle group exhibited severe renal injury, which was characterized by glomerulosclerosis, crescent formation, tubular cast deposition, increased mesangial matrix, and diffuse perivascular and interstitial mononuclear cell infiltration. However, all of these pathological features were ameliorated in SM934-treated mice (Figure 1d top panel and e left panel). Renal cryostat sections were stained to detect IgG deposition. Pronounced scattered granular IgG deposits were observed in kidneys from vehicle group mice. In contrast, mice that were administered SM934 exhibited significantly less IgG deposition (Figure 1d bottom-panel and e right-panel).
Figure 1.
SM934 ameliorates lupus nephritis and maintains survival in MRL/lpr mice. Nine-week-old female MRL/lpr mice were orally administered ddH2O (vehicle, n=11), PNS (positive control, n=10) or one of three different doses of SM934 (n=11 per group) twice daily until they were 27 weeks old. (a) Cumulative survival rate of MRL/lpr mice from 9 weeks to 27 weeks of age. (b) The proteinuria level was measured every week. Black triangles indicate the initiation of administration. (c) The serum levels of ALB and BUN were detected at the end of study. (d) Representative kidney sections stained with H&E (top panel, original magnification, ×200) and frozen sections stained for IgG deposits (bottom panel, original magnification ×100). (e) Histological score for glomerulonephritis (Glome), interstitial nephritis (Intersti) and tubular casts, according to H&E staining (left-panel) and the IOD value of IgG deposits in the frozen kidney sections (right-panel). Graphs show the mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 versus vehicle. ALB, albumin; BUN, blood urea nitrogen; H&E, hematoxylin and eosin; IOD, integrated optical density; PNS, prednisolone.
SM934 treatment alleviates splenomegaly and the lymphadenopathy symptoms of MRL/lpr mice
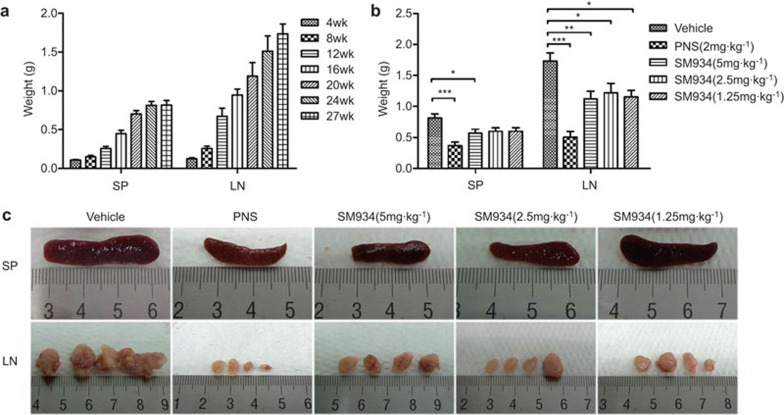
Splenomegaly and lymphadenopathy are known manifestations of fas deficiency and are considered to be clinical markers of lupus patients and MRL/lpr mice. In this mechanistic study, we showed that the weights of the spleen and lymph nodes increased with disease progression in this spontaneous lupus model (Figure 2a). In a drug efficacy study, the weights of both tissues were reduced after treatment with SM934 compared to vehicle-only treatment (Figure 2b). Additionally, the enlargement and heteromorphism of the spleen and lymph nodes observed in vehicle group mice were suppressed by SM934 treatment (Figure 2c).
Figure 2.
SM934 prevents disease progression by alleviating splenomegaly and lymphadenopathy in MRL/lpr mice. (a) For the disease mechanism study, the weights of the SP) and LN of unmanipulated MRL/lpr mice aged from 4 weeks to 27 weeks were recorded. For each age, n=5 to 7. (b) For the drug action study, the SP and LN weights of treated MRL/lpr were also examined upon termination of the experiment. (c) Representative images of SP (top panel) and LN (bottom panel) of MRL/lpr in treated groups. LN refers, in particular, to inguinal lymph nodes and axillary lymph nodes. The graphs show the mean values with s.e.m. *P<0.05, **P<0.01, ***P<0.001 versus vehicle. LN, lymph nodes; SP, spleens.
SM934 treatment lowers the serum level of pathogenic cytokines and autoantibodies
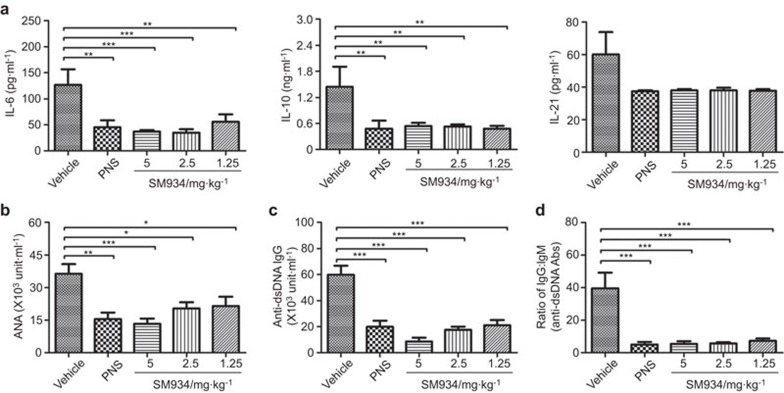
IL-6, IL-10 and IL-21 are, to some extent, catalysts for humoral responses by driving B-cell activation or differentiation, which play crucial pathological roles in the course of lupus disease. As shown, SM934 treatment significantly decreased the serum levels of IL-6 and IL-10. Although the difference was not significant (owing to obvious individual differences within the vehicle group), SM934 and PNS treatment did cause a decrease in IL-21 production (Figure 3a). In addition, activation of the immune system by aberrant self-nucleic acid has emerged as another fundamental mechanism in the pathogenesis of SLE. The serum level of ANAs increases along with age in MRL/lpr mice (data not shown). After treatment from 9 to 27 weeks with 5 mg/kg SM934, the ANA level was approximately 60% lower than that of the vehicle group at 27 weeks (Figure 3b). To quantitate more specific autoantibodies, we measured anti-dsDNA IgG, which is the hallmark of SLE and plays important pathogenic roles in lupus nephritis. Strikingly, SM934 treatment decreased the concentration of the serum anti-dsDNA antibody (Figure 3c). Class switching, measured by the ratio of IgG to IgM, markedly declined in SM934-treated mice (Figure 3d). It has been reported that ANA formation is entirely dependent on (and that class switching is largely dependent on) B cell-intrinsic MyD88-dependent TLR signaling in MRL/lpr mice.10,31,32,33 Thus, we hypothesized that SM934 may have a therapeutic effect in lupus model mice by regulating the response of autoreactive B cells to endogenous TLR ligands.
Figure 3.
SM934 modulates humoral immunity related inflammatory cytokines and reduces autoantibody levels in the serum of MRL/lpr mice. (a) The levels of IL-6, IL-10 and IL-21 in the serum were detected by ELISA. (b) The serum level of total ANAs in drug-treated MRL/lpr mice was measured. (c) The level of the IgG anti-dsDNA antibody, one subclass of ANA, was also detected. (d) The ratio of the IgG isotype to the IgM isotype of anti-dsDNA antibodies is shown. Bar graphs show the mean values with SEM. * P<0.05, ** P<0.01, ***P<0.001 versus vehicle. ANA, anti-nuclear antibody; dsDNA, double-stranded DNA.
SM934 treatment regulates the splenic B cell compartment in MRL/lpr mice
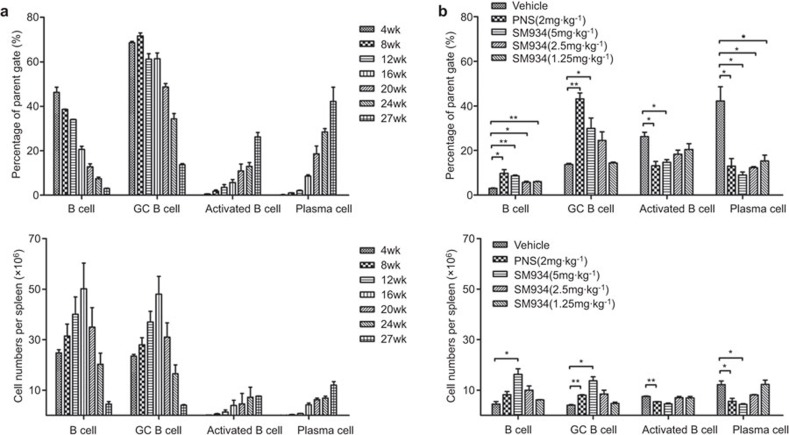
B lymphocytopenia is a common feature of lupus patients with active disease and is also found in aged MRL/lpr mice.34 In the present study, we observed that the percentage of B cells (CD3−CD19+) dropped from 47.9% (4-week-old) to 3.12% (27-week-old), confirming the B lymphocytopenia in the spleens of MRL/lpr mice. Consistent with literature reports, GC B cells (B220+GL-7+ gated on CD3−) typically decreased in number, from 69% to 14.1%, as the lupus-prone mice aged. In contrast, activated CD69+ B cells and antibody-secreting plasma cells (PCs) accumulated in large numbers in aged MRL/lpr mice (Figure 4a, top panel). We then surveyed the exact cell numbers of B cells, GC B cells, activated B cells and plasma cells per spleen in MRL/lpr mice of different ages. The trend in the cell numbers was almost consistent with that in the cell proportions, except for the early increase in the numbers of B cells and GC B cells from 4 weeks to 16 weeks owing to the physiological increase in the total cell number (Figure 4a bottom panel). During the earlier period, the spleen size increased from 6.7×107 per spleen at 4 weeks to 3.2×108 per spleen at 16 weeks, and the total cell number reached a plateau at approximately 3.2×108–3.5×108 per spleen after 16 weeks in MRL/lpr mice (data not shown).
Figure 4.
SM934 normalizes the B-cell compartment in the spleen of MRL/lpr mice. Splenic lymphocytes of MRL/lpr mice were stained with specific fluorescently labeled antibodies to identify B lymphocytes (CD3−CD19+ gated on FSC-SSC), GC B cells (B220+GL-7+ gated on CD3−), activated B cells (CD69+ gated on CD3−B220+) and plasma cells (CD19−CD138+ gated on CD3−B220+) by FACS. (a) The percentage in the parent gate (top panel) and cell number per spleen (bottom panel) of B lymphocytes, GC B cells, activated B cells and plasma cells in untreated MRL/lpr mice from 4 to 27 weeks of age. (b) Same as (a) but for drug-tested mice at the termination of the experiment (mice 27 weeks old). Bar graphs show the mean values with SEM. *P<0.05, **P<0.01, ***P<0.001 versus vehicle. GC, germinal center.
Notably, SM934 treatment increased the splenic B cells by two- to three-fold compared to vehicle mice. Interestingly, SM934 treatment also greatly elevated the GC B-cell percentage. Spleens obtained from 27 week-old MRL/lpr mice contained a relatively large proportion of activated B cells (27.6%). Treatment with SM934 profoundly suppressed the spontaneous activation of B cells in a dose-dependent manner, as the cell proportion of activated B cell reduced to 13.9% in 5 mg/kg group, 19.6% in 2.5 mg/kg group and 22.3% in 1.25 mg/kg group, respectively. Remarkably, the proportion of PCs in SM934 treated mice was 2–5-fold lower than in vehicle group mice, with the change being dose-dependent (Figure 4b top panel). As for the cell numbers of each B cell subset in drug-treated mice, in brief, SM934 increased the number of total B cells and GC B cells and diminished the number of activated B cells and plasma cells (Figure 4b bottom panel).
SM934 treatment inhibits TLR-triggered B-cell activation and plasma cell generation in MRL/lpr mice
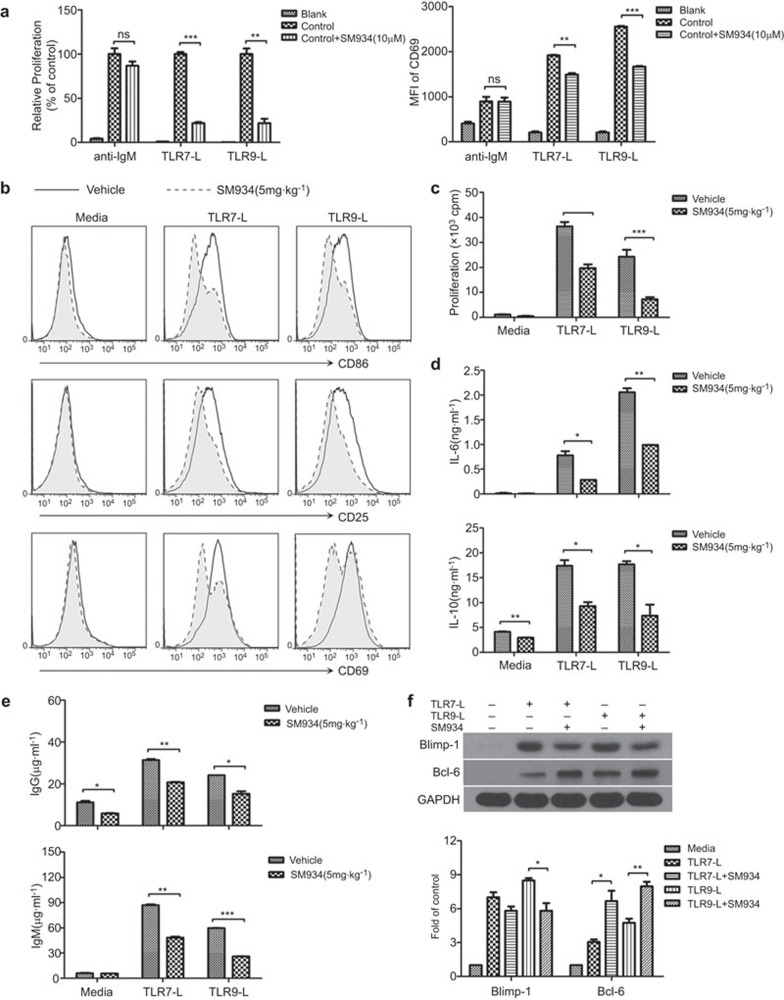
It has been reported that nuclear antigens activate B cells by dual engagement of IgM and TLR7/9.8,9 To examine whether the effects of SM934 on B cells were through a BCR- or TLR-system, we incubated purified B cells with various stimuli in vitro. We found that SM934 inhibited TLR7/9 ligand-induced B cell proliferation and activation, whereas it exerted no effects on anti-IgM-triggered B-cell proliferation and activation (Figure 5a).
Figure 5.
SM934 inhibits TLR7/9-induced B-cell activation and plasma cell differentiation in MRL/lpr mice. In vitro, splenic B cells from MRL/lpr mice were cultured with anti-IgM or TLR7-L (R848) or TLR9-L (ODN1668). (a) The proliferation (left panel) and CD69 expression (right panel) of B cells were detected after 48 h incubation. Ex vivo, purified splenic B cells from vehicle and SM934 (5 mg/kg) treated mice were cultured under TLR7/9 ligand stimulation. At 48 h, (b) the expression of CD86, CD25 and CD69 on B cells was detected by FACS; (c) B-cell proliferation was also measured; (d) the culture supernatants were collected and measured for IL-6 and IL-10 production; (e) IgG (top panel) and IgM (bottom panel) secreted in the culture supernatant were measured by ELISA on day 6. In vitro, purified B cells from untreated MRL/lpr mice were cultured under TLR7/9 ligand stimulation with or without SM934. (f) At 72 h, protein extracts were assessed for Blimp-1 and Bcl-6 expression by western blotting, and the values are the mean±s.e.m. of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. TLR, Toll-like receptor.
Ex vivo, we stimulated splenic B cells from the vehicle and 5 mg/kg SM934-treated mice with TLR7/9 agonists to further study the therapeutic mechanism of SM934. As expected, B cells from vehicle-treated mice were strongly activated by TLR7/9 agonists, with high expression of CD86, CD25 and CD69. However, B cells from SM934-treated mice were only slightly activated, with a relatively low expression of surface activation markers (Figure 5b). Similarly, TLR ligands led to robust B cell proliferation in the vehicle group but not in the SM934 treated group (Figure 5c). SM934 also prevented TLR7/9 ligand-induced production of IL-6 and IL-10 in B cells (Figure 5d).
We next sought to clarify the effects of SM934 on B cell differentiation. We measured IgG and IgM antibodies in the culture supernatant of B cells to evaluate the generation of plasma cells. Consistent with the changes in the plasma cell numbers shown in Figure 4B, the levels of both IgG and IgM were significantly diminished by SM934 (Figure 5e). Additionally, SM934 reduced TLR7/9 agonist-induced Blimp-1 expression in B cells, confirming that SM934 restricts plasma cell generation (Figure 5f). The decrease in Blimp-1 expression could partly be due to the increase in Bcl-6, which targets Blimp-1 and thereby prevents B cells from differentiating into PCs and arrests B cells in the GC (Figure 5f). Hence, the increase in Bcl-6 might also account for the up-regulation of GC B cells in SM934-treated mice (Figure 4b).
SM934 retards autoreactive B-cell development by interfering with the MyD88-dependent TLR signaling pathways
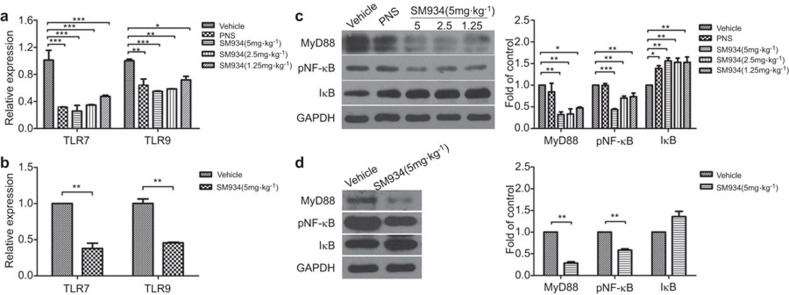
To understand how SM934 interferes with the SLE pathogenic processes, we measured the mRNA levels of TLR7 and TLR9 in the total splenocyte populations from treated MRL/lpr mice. Both the expression of TLR7 and TLR9 were reduced by SM934 treatment (Figure 6a). Other than B lymphocytes, however, some other lymphocyte subsets, such as dendritic cells, also express TLRs. Thus, we further assessed the expression of TLRs in isolated B cells from vehicle and SM934 (5 mg/kg) groups. Compared to the vehicle group, the expression of TLR7 was reduced by almost 70% and of TLR9 by approximately 55% in the B cells from the SM934 (5 mg/kg) treated group (Figure 6b). To follow this SLE-modified signaling pathway of B cells, we examined the expression of related signaling proteins in splenic lymphocytes. The results demonstrated that SM934 clearly reduced the level of the signaling adaptor MyD88, which plays a crucial role in the development of lupus nephritis.35 In addition, SM934 promoted the expression of IκB and inhibited the phosphorylation of NF-κB downstream in TLR pathways, disrupting further inflammatory responses (Figure 6c). Similarly, B lymphocytes might be the major contributor to the effects of SM934 on MyD88/IκB/NF-κB signaling (Figure 6d).
Figure 6.
SM934 interferes with MyD88-dependent TLR signaling pathways. (a) The relative levels of TLR7/9 mRNA in the total spleen lymphocyte population from drug-treated MRL/lpr mice were determined by real-time PCR. The obtained values were normalized to the mean expression of TLR7/9 in vehicle group mice. (b) As in (a) but for purified B cells isolated from vehicle group mice and 5 mg·kg−1 SM934 group mice. (c) Immunoblot analysis of cytoplasmic extracts prepared from the total lymphocytes of each group was performed using Abs specific for MyD88, p-P65, IκB and GAPDH, and the values are the mean ± s.e.m. of three independent experiments. (d) Same as (c) but for purified B cells. * P<0.05, ** P<0.01, *** P<0.001 versus vehicle. TLR, Toll-like receptor.
SM934 inhibits the TLR-associated activation of B cells and the formation of plasma cells in human PBMCs
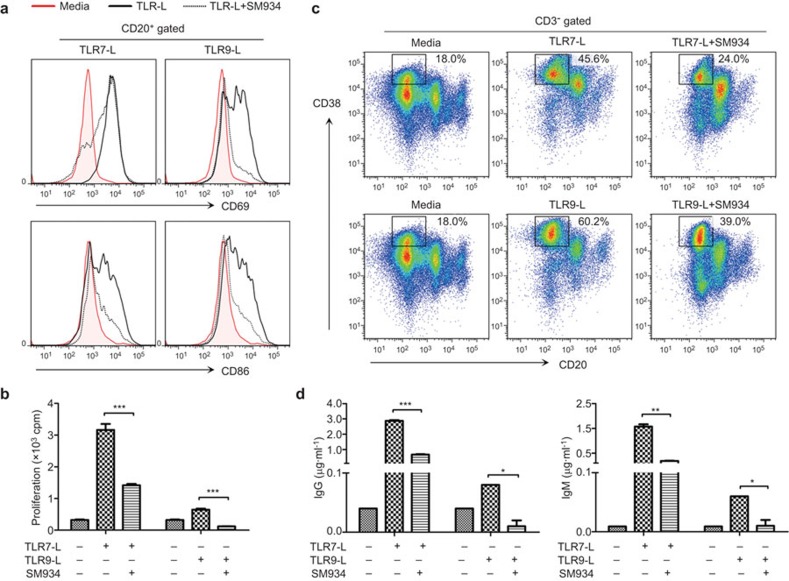
Having demonstrated that SM934 can correct the abnormal development of autoreactive B cells following TLR engagement in lupus-prone MRL/lpr mice, we sought to further define whether SM934 can affect human B cells. PBMCs from healthy donors were triggered by TLR7/9 agonists to assess the effects of SM934 on B cells. The results indicate that SM934 can inhibit the activation and proliferation of B cells in PBMCs by a synthetic ligand of TLR7 (R848) or TLR9 (ODN2336) (Figure 7a and b). Furthermore, SM934 prevented the differentiation of PCs and reduced their secretion of Abs (Figure 7c and d).
Figure 7.
The effects of SM934 on human PBMCs during stimulation with TLR agonists. Human PBMCs were cultured under stimulation with TLR7-L (R848) or TLR9-L (ODN2336) with or without SM934. (a) At 48 h, the activation of B cells (CD20+) in the PBMCs was determined by measuring CD69 and CD86 expression. (b) The proliferation of B cells was measured in the presence of the specific stimulators at 48 h. (c) The percentages of plasma cells (CD20−CD38+, gated on CD3−) were analyzed by FACS at 6 days. (d) The levels of Abs in the culture supernatants were measured by ELISA at 6 days. Similar results were obtained from four independent donors. Bar graphs show the mean values with SEM. *P<0.05, **P<0.01, ***P<0.001 compared to positive control (TLR ligand alone). PBMC, peripheral blood mononuclear cell; TLR, Toll-like receptor.
Discussion
In the present study, we demonstrated that SM934 administered at short intervals has dramatic pharmacological effects, alleviating lupus syndrome in MRL/lpr mice. Moreover, our results also uncover a new therapeutic mechanism of SM934: inhibition of B cell activation triggered by MyD88-depedent TLR signaling.
In terms of drug efficacy, the renal protective effects of SM934 in MRL/lpr mice that were described in our previous report were fully recapitulated in this study.22 Twice-daily administration of SM934 (5 mg/kg) had a better potential to maintain the survival of MRL/lpr mice than in a previous study.20 With this optimized dose regimen, we also observed more persistent and more prominent therapeutic effects of SM934 on proteinuria, serum biochemical indices and kidney pathology. In addition, using the twice-daily regimen, SM934 showed better dose dependence than the once-daily regimen. Therefore, this administration strategy might be useful in the future clinical application of SM934 for lupus treatment (the preclinical study of SM934 for SLE is now complete).
Our previous studies demonstrated that SM934 exerted comprehensive therapeutic effects in lupus models, as characterized by increasing the percentage of Tregs and by decreasing Th1 and Th17 responses.22,23,40 As for the role of artemisinin-family compounds on B cells, several studies have been reported. In 2002, Tu et al.36 found that dihydroartemisinin treatment can suppress the proliferation of B cells in lupus-prone BXSB mice. Later, in 2009, Jin et al.19 indicated that the level of BAFF, the major B-cell activation factor, was decreased in artesunate-treated MRL/lpr mice. Most recently, Hou et al.37 reported that artesunate prevented the development of arthritis in K/BxN mice by preferentially inhibiting germinal center B cells and the production of autoantibodies. Similarly, in the current study, we demonstrated that SM934 can inhibit the proliferation and activation of B cells and reduce the generation of plasma cells. However, unlike artesunate, our results showed that SM934 did not suppress the germinal center B cells in MRL/lpr mice. We speculated the following reasons might be responsible for this discrepancy. (i) Differences between mouse strains. K/BxN mice express the KRN T-cell receptor transgene and the MHC class II molecule antigen 7, leading to a robust germinal center reaction and the production of pathogenic autoantibodies against glucose-6-phosphate isomerase.38 Overall, B-cell development is relatively normal in K/BxN mice. However, in mice with an MRL background, there are defects of tolerance during B-cell development, both at the bone marrow stage and the transitional stages in the periphery. Additionally, in both MRL/lpr mice and lupus patients, in situ formation of the germinal center is common.39 (ii) Differences between autoantigens. In K/BxN mice, the autoantigen is relative clear and has been established to be the protein glucose-6-phosphate isomerase. However, in MRL/lpr mice, the autoantigens are heterogeneous, and both dsDNA and RNA are major autoantigens and are capable of directly activating TLR7/9 in B cells. Thus, the B cell activation status differs between K/BxN mice and MRL/lpr mice. (iii) Dose of artesunate and SM934. In the K/BxN study, mice were treated with artesunate at an extremely high dose of 100 mg/kg twice per day. However, in our study, we treated MRL/lpr mice with SM934 at only 5 mg/kg twice per day. We propose that the extremely high dose of artesunate might be responsible for diminishing the germinal center B-cell responses. However, when a low dose of SM934 is administered, the accumulation of plasma cells may be preferentially targeted.
SM934 might exert its effects through synergistic crosstalk involving T cells, B cells and macrophages.22,23,40 The NF-κB signaling pathway seemed to be a hub for crosstalk. It has been demonstrated that artemisinin prevents the LPS-induced proteolytic degradation of IκB and the consequent activation and translocation of NF-κB, which would otherwise lead to the promotion of iNOS transcription.40 Tu et al.41 have also shown that dihydroartemisinin ameliorated lupus symptoms in BXSB mice by blocking NF-κB translocation. In the present study, we observed similar changes in IκB and NF-κB after SM934 treatment (Figure 6c and d), and also established TLR7/9 and MyD88 as the critical regulation points of SM934 upstream of the NF-κB activating pathway (Figure 6). However, these changes were observed in both total splenocytes and isolated B cells. Whether SM934 acts specifically on the B cell-intrinsic TLR pathway or influences other TLR-expressing lymphocyte subsets is unknown and requires further study.
As a disease pathogenesis study, this article described the change in cell numbers in the B-cell compartment in the spleen of MRL/lpr mice with increasing age. Figure 4a shows that a high plasma cell number paradoxically coincided with considerable B-cell lymphopenia and a reduction in GC B-cell numbers in aged MRL/lpr mice, and similar phenomena have been reported in patients.34,42 It has long been presumed that GCs are critical for autoantibody production because they are the sites that are generally thought to sustain a high rate of somatic hypermutation. However, studies have found that autoreactive splenic B cells in autoimmune MRL/lpr mice proliferate and undergo active somatic hypermutation at the T zone–red pulp border rather than in GCs.39,43,44 Similarly, our results also indicate that B cells do not activate in the GC in MRL/lpr mice. Intriguingly, Teichmann claimed that the deletion of MyD88 in DCs led to the preservation of B cell follicles and the development of GC-like structures in the spleens of MRL/lpr mice as a result of reduced IFN-γ induction.10 The relationship between DC-intrinsic MyD88 signaling, IFN-γ, and spleen architecture and function also warrants further investigation.
Routinely, IL-10 is considered to be a regulatory cytokine.45 However, it has been reported that patients with lupus produce large amount of IL-10.46,47 Administration of an anti-IL-10 mAb to human patients with active lupus has led to the amelioration of disease activity. Additionally, serum IL-10 levels correlate with the titer of anti-dsDNA antibodies in these patients.46 The result observed in the present study, that the serum level of IL-10 in MRL/lpr mice decreased with SM934 administration, is different from that obtained in NZB/W F1 mice in a previous study, revealing a distinct pathogenetic mechanism in different animal models and a distinct curative mechanism for SM934 in these two models.
In conclusion, this study confirmed the therapeutic efficacy of SM934 in lupus-prone MRL/lpr mice and provided further evidence in support of conducting clinical trials for SLE treatment. This study is also a pilot study in that it revealed the regulatory effects of SM934 on B cells, which suggests an important clue about the mechanism of action of artemisinin analogs, and demonstrated that the MyD88-dependent TLR pathway might be a novel target for lupus treatment.
Acknowledgments
This work was supported by grants from the National Science Fair Committee (NSFC), China (No. 81273524, 81322049), National Science & Technology Major Project ‘New Drug Creation and Manufacturing Program', China (2014ZX09101002) and National Key Basic Research Programme (973 Programme, 2014CB541906). We are also grateful to Dr Hou Lifei who kindly contributed to the manuscript revision.
The authors declare no conflicts of interest.
References
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol 1985; 37: 269–390. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 2002; 17: 51–62. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 Represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 2000; 13: 199–212. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25: 1271–1277. [DOI] [PubMed] [Google Scholar]
- Egner W. The use of laboratory tests in the diagnosis of SLE. J Clin Pathol 2000; 53: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro Y. Antinuclear antibodies. Autoimmunity 2005; 38: 3–9. [DOI] [PubMed] [Google Scholar]
- Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol 2012; 10: 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002; 416: 603–607. [DOI] [PubMed] [Google Scholar]
- Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med 2005; 202: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity 2013; 38: 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak E, Sysa-Jedrzejowska A, Stepien H, Robak T. Circulating interleukin-6 type cytokines in patients with systemic lupus erythematosus. Eur Cytokine Network 1997; 8: 281–286. [PubMed] [Google Scholar]
- Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med 1995; 181: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R. Fc reduces disease progression. J Immunol 2007; 178: 3822–3830. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004; 173: 5361–5371. [DOI] [PubMed] [Google Scholar]
- Deng XM, Yan SX, Wei W. IL-21 acts as a promising therapeutic target in systemic lupus erythematosus by regulating plasma cell differentiation. Cell Mol Immunol 2015; 21: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y et al. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Faslpr/lpr/J mice. J Immunol 2012; 188: 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 2007; 27: 461–466. [DOI] [PubMed] [Google Scholar]
- Lu L. [Study on effect of Cordyceps sinensis and artemisinin in preventing recurrence of lupus nephritis]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2002; 22: 169–171. Chinese. [PubMed] [Google Scholar]
- Jin O, Zhang H, Gu Z, Zhao S, Xu T, Zhou K et al. A pilot study of the therapeutic efficacy and mechanism of artesunate in the MRL/lpr murine model of systemic lupus erythematosus. Cell Mol Immunol 2009; 6: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang W, Shi X, An P, Sun W, Wang Z. Therapeutic effect of artemisinin on lupus nephritis mice and its mechanisms. Acta Biochim Biophys Sin 2010; 42: 916–923. [DOI] [PubMed] [Google Scholar]
- Hou LF, He SJ, Wang JX, Yang Y, Zhu FH, Zhou Y et al. SM934, a water-soluble derivative of arteminisin, exerts immunosuppressive functions in vitro and in vivo. Int Immunopharmacol 2009; 9: 1509–1517. [DOI] [PubMed] [Google Scholar]
- Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y et al. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum 2011; 63: 2445–2455. [DOI] [PubMed] [Google Scholar]
- Hou LF, He SJ, Li X, Wan CP, Yang Y, Zhang XH et al. SM934 treated lupus-prone NZB×NZW F1 mice by enhancing macrophage interleukin-10 production and suppressing pathogenic T cell development. PLoS One 2012; 7: e32424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan E, Yamazaki K. An improved urine collection technique for laboratory mice: the bladder massage method. Lab Animal (USA) 1993; 38–39.
- Yang LY, Chen A, Kuo YC, Lin CY. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J Lab Clin Med 1999; 134: 492–500. [DOI] [PubMed] [Google Scholar]
- Tao X, Fan F, Hoffmann V, Longo NS, Lipsky PE. Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. Arthritis Res Ther 2006; 8: R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Fan F, Hoffmann V, Gao CY, Longo NS, Zerfas P et al. Effective therapy for nephritis in (NZB×NZW) F1 mice with triptolide and tripdiolide, the principal active components of the Chinese herbal remedy Tripterygium wilfordii Hook F. Arthritis Rheum 2008; 58: 1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahjat FR, Pine PR, Reitsma A, Cassafer G, Baluom M, Grillo S et al. An orally bioavailable spleen tyrosine kinase inhibitor delays disease progression and prolongs survival in murine lupus. Arthritis Rheum 2008; 58: 1433–1444. [DOI] [PubMed] [Google Scholar]
- Tan X, Fan F, Hoffman V, Longo NS, Lipsky PE. Therapeutic impact of the ethyl acetate extract of Tripterygium wilfordii Hook F on nephritis in NZB/W F1 mice. FASEB J 2006; 20: A1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SJ, Lin ZM, Wu YW, Bai BX, Yang XQ, He PL et al. Therapeutic effects of DZ2002, a reversible SAHH inhibitor, on lupus-prone NZB×NZW F1 mice via interference with TLR-mediated APC response. Acta Pharmacol Sin 2014; 35: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos AM, Busconi L, Marshak-Rothstein A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity 2010; 43: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell Richard A, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 2006; 25: 417–428. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol 2006; 6: 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol 2000; 165: 5970–5979. [DOI] [PubMed] [Google Scholar]
- Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T et al. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum 2007; 56: 1618–1628. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen X, Tu Y. Efect of hydroartemisinin on lupus BXSB mice. Chin J Dermatovenerol Integr Tradit West Med 2002; 1: 19–20. [Google Scholar]
- Hou L, Block KE, Huang H. Artesunate abolishes germinal center B cells and inhibits autoimmune arthritis. PLoS One 2014; 9: e104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzel HJ. The K/BxN mouse: a model of human inflammatory arthritis. Trends Mol Med 2004; 10: 40–45. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science 2002; 297: 2066–2070. [DOI] [PubMed] [Google Scholar]
- Aldieri E, Atragene D, Bergandi L, Riganti C, Costamagna C, Bosia A et al. Artemisinin inhibits inducible nitric oxide synthase and nuclear factor NF-κB activation. FEBS Lett 2003; 552: 141–144. [DOI] [PubMed] [Google Scholar]
- Li WD, Dong YJ, Tu YY, Lin ZB. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int Immunopharmacol 2006; 6: 1243–1250. [DOI] [PubMed] [Google Scholar]
- Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center b cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol 2001; 167: 2361–2369. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Euler CW, Christensen SC, William J. Activation of rheumatoid factor (RF) B cells and somatic hypermutation outside of germinal centers in autoimmune-prone MRL/lpr mice. Ann NY Acad Sci 2003; 987: 38–50. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Shlomchik MJ. Short-lived plasmablasts dominate the early spontaneous rheumatoid factor response: differentiation pathways, hypermutating cell types, and affinity maturation outside the germinal center. J Immunol 2005; 174: 6879–6887. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001; 19: 683–765. [DOI] [PubMed] [Google Scholar]
- Gröndal G, Gunnarsson I, Rönnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 1999; 18: 565–570. [PubMed] [Google Scholar]
- Park Y, Lee S, Kim D, Lee J, Lee C, Song C. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 1997; 16: 283–288. [PubMed] [Google Scholar]