Abstract
Recent studies have identified olfactory ecto-mesenchymal stem cells (OE-MSCs) as a new type of resident stem cell in the olfactory lamina propria. However, it remains unclear whether OE-MSCs possess any immunoregulatory functions. In this study, we found that mouse OE-MSCs expressed higher transforming growth factor-beta and interleukin-10 levels than bone marrow-derived MSCs. In culture, OE-MSCs exerted their immunosuppressive capacity via directly suppressing effector T-cell proliferation and increasing regulatory T (Treg) cell expansion. In mice with collagen-induced arthritis, adoptive transfer of OE-MSCs markedly suppressed arthritis onset and disease severity, which was accompanied by increased Treg cells and reduced Th1/Th17 cell responses in vivo. Taken together, our findings identified a novel function of OE-MSCs in regulating T-cell responses, indicating that OE-MSCs may represent a new cell therapy for the treatment of rheumatoid arthritis and other autoimmune diseases.
Keywords: CIA, olfactory ecto-mesenchymal stem cells, Treg cell
Introduction
Mesenchymal stem cells (MSCs) are multipotent mesoderm-derived stromal cells that can differentiate into various lineages of non-hematopoietic cells in connective tissues.1 MSCs usually reside in the bone marrow, cord blood, muscle, fat and other tissues, bearing surface antigens CD29, CD44, CD90, CD73, and CD105, but they lack the hematopoietic cell antigens CD34 and CD45.2 MSCs have been found to possess potent immunosuppressive functions by modulating T- and B-cell proliferation and differentiation, dendritic cell maturation as well as NK cell activity.3,4 These immunoregulatory properties make them well suited for modulating autoimmune responses and targeting autoimmune diseases. Significant progress has been made in applying MSCs for the treatment of autoimmune disorders and allograft rejection.5,6,7 To date, bone marrow-derived MSCs (BM-MSCs) are among the most commonly used MSCs in pre-clinical research for the treatment of rheumatoid arthritis (RA) and other autoimmune diseases.8,9,10 However, there are some limitations associated with BM-MSCs in clinical applications, including the technical difficulty in acquiring sufficient numbers of cells for therapy.11
Recently, olfactory ecto-mesenchymal stem cells (OE-MSCs), a new type of resident stem cell in the olfactory lamina propria, have been characterized.12 Residing in the nasal cavity, OE-MSCs are derived primarily from neural crest cells and possess a high proliferation rate, self-renewal capability, and multiple lineage differentiation capabilities.13 Several studies have reported that OE-MSC transplantation can induce neurogenesis and restore hippocampal neuronal networks in mice.14 However, it remains largely unknown whether OE-MSCs, which are similar to other types of MSCs, possess any immunoregulatory functions. Moreover, the potential utilization of OE-MSCs for the treatment of autoimmune diseases has not yet been explored.
In this study, we investigated the immunoregulatory property of OE-MSCs in modulating T-cell functions. We found that OE-MSCs exerted their immunosuppressive capacity via directly suppressing effector T-cell proliferation and increasing regulatory T (Treg) cell expansion. Using a mouse model of collagen-induced arthritis (CIA), we showed that adoptive transfer of OE-MSCs could effectively suppress arthritic progression, accompanied by reduced Th1/Th17 cell responses with increased Treg cells in vivo. Thus, our findings identified a novel, potent function of OE-MSCs in regulating T-cell functions, which suggests that OE-MSCs may represent a new cell therapy for the treatment of RA and other autoimmune diseases.
Materials and Methods
Isolation and culture of OE-MSCs and BM-MSCs
OE-MSCs were obtained from the nasal cavity of wild-type mice by delicately discarding the turbinates, as previously described.15 The retained olfactory epithelium tissue sample was cut into small pieces and cultured in flasks with the medium (Dulbecco's modified Eagle's medium (DMEM)/HAM'S F-12 supplemented with 15% fetal calf serum) (Gibco, Carlsbad, CA) for 7 days. After non-adherent cell removal, the remaining cells were trypsinized (0.05% trypsin-ethylenediaminetetraacetic acid at 37 °C for 2 min) and further expanded for 3 passages. For the culture of BM-MSCs, bone marrow cells were collected by flushing the cells out of femurs and tibiae with cold phosphate-buffered saline (PBS). The cells were cultured in the medium (DMEM supplemented with 15% fetal calf serum) (Gibco, Carlsbad, CA) for 3 days. Then, the non-adherent cells were removed and when the remaining cells reached 80% confluence in the dish, the adherent cells were trypsinized and expanded for 3 passages.
OE-MSC differentiation induction
Osteogenic and adipogenic differentiation of OE-MSCs was achieved by culturing under induction conditions. The osteogenic induction medium consisted of minimum essential medium that was supplemented with 0.1 μM dexamethasone (SigmaAldrich, St. Louis, MO), 10 mM β-glycerol phosphate (SigmaAldrich, St. Louis, MO), and 0.2 mM ascorbic acid (SigmaAldrich, St. Louis, MO). After 21 days of induction, the osteogenic differentiation was evaluated by alizarin red (Guangzhou, China) staining to detect the presence of calcium deposition into osteocytes.16 To induce adipogenic differentiation, OE-MSCs were cultured in MSC-adipogenic differentiation medium (Cyagen, Guangzhou, China) for 14 days. The cells were then subjected to oil red O staining (Cyagen, Guangzhou, China).17
Mice
DBA/1J mice (8–10 weeks old) were obtained from the Shanghai Laboratory Animal Center (Shanghai, China) and maintained in the Jiangsu University Animal Center (Jiangsu, China). All animal experiments were approved by the Jiangsu University Animal Ethics and Experimentation Committee.
CIA induction and OE-MSC treatment
For CIA induction, DBA/1J mice were immunized with 100 μg of bovine type II collagen (Chondrex, WA, USA) that was emulsified with Freund's complete adjuvant (SigmaAldrich, St. Louis, MO) via intradermal injection at the base of the tail, and a booster emulsion of CII in Freund's incomplete adjuvant (SigmaAldrich, St. Louis, MO) was administered on day 21 after the first immunization.18 To determine the effects of the OE-MSC treatment, mice were given two intravenous injections of OE-MSCs or BM-MSCs (1 × 106) on days 18 and 24 after the first immunization. The control group was administered with the same volume of PBS at the same time points. The mice were scored for signs of arthritis every 3 days after the second immunization.
Joint fixation and histopathological assessment
Mice were killed on day 42 after the first immunization, and joint specimens were fixed in 10% phosphate-buffered formalin for 3 days. Joint tissues were sectioned at 4 μm thickness and stained with hematoxylin and eosin for histopathological evaluation.19
Co-culture of OE-MSCs and T cells
OE-MSCs were co-cultured with splenic CD4+ T cells that were sorted from wild-type mice using CD4+ T-cell microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Responder cells were co-cultured with different OE-MSC ratios in U-bottomed 96-well plates (Costar, Corning, NY) in the presence of anti-CD3 (10 μg mL−1, Biolegend, San Diego, CA) and anti-CD28 (5 μg mL−1, Biolegend, San Diego, CA) monoclonal antibodies (mAbs) for 72 h, and they were pulsed with [3H]-thymidine (Pharmacia, Stockholm, Sweden, 1 μCi/well) for the last 16 h of culture. Anti-CD3 and anti-CD28 mAbs were used for activating the T cells.
Flow cytometric analysis
Single cell suspensions were stained with fluorochrome-conjugated mAbs specific for the following phenotypic markers: anti-CD29, anti-CD90, anti-CD44, anti-CD34, anti-CD45, and anti-CD11b (eBioscience, San Diego, CA). For Treg cell detection, cells were triple stained with anti-CD4, anti-CD25, and anti-Foxp3 mAbs according to the manufacturer's instructions with a Foxp3 Staining Kit (eBioscience, San Diego, CA). For intracellular cytokine staining, single cell suspensions were suspended in the medium with phorbol myristate acetate (50 ng mL–1, SigmaAldrich, St. Louis, MO), ionomycin (1 μg mL−1, Enzo, Farmingdale, NY) and monensin (2 μg mL−1, Enzo, Farmingdale, NY) for 5 h. The cells were then stained with anti-CD4 mAbs (eBioscience, San Diego, CA) followed by permeabilization and incubation with intracellular anti-interferon gamma (IFN-γ) and anti-interleukin-17 (IL-17) antibodies (eBioscience, San Diego, CA). As a control, the appropriate isotype-matched antibodies were used for each staining. The stained cells were analyzed with a fluorescence-activated cell sorting Calibur flow cytometer (Becton Dickinson, Sparks, MD).
Immunofluorescence microscopy
OE-MSCs were harvested at 90% confluence in 24-well plates, fixed with 4% paraformaldehyde, incubated for 1 h at room temperature with blocking buffer (1% bovine serum albumin, 5% goat serum, and 0.25% Triton X-100 in PBS) and stained with rabbit polyclonal anti-nestin and anti-vimentin antibodies for 90 min.
Autoantibody and cytokine detection
Serum bovine type II collagen-specific antibody levels were measured with a sandwich enzyme-linked immunosorbent assay (ELISA), as previously described.20 Culture supernatants and mouse serum levels of IL-17, transforming growth factor-beta (TGF-β), IFN-γ, and IL-10 were measured with ELISA Kits (eBioscience, San Diego, CA) following the manufacturer's protocol. Nitric oxide (NO) was detected by determining the concentration of nitrite that accumulated in the culture supernatants using the colorimetric Griess reaction (Promega, Madison, WI).21
Quantitative real-time PCR (qRT-PCR)
Cells were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA was isolated and reverse-transcribed with the ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan) according to the manufacturer's instructions. The reverse transcript PCR and qRT-PCR were performed as previously described.18 The following primers were sequences utilized: TGF-β, 5′-AACCGGCCCTTCCTGCTCCTCAT-3′ (forward), 5′-CGCCCGGGTTGTGTTGGTTGTAGA-3′ (reverse); IL-10, 5′-GGTTGCCAAGCCTTATCGGA-3′ (forward), 5′-ACCTGCTCCACTGCCTTGCT-3′ (reverse); β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ (forward), 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (reverse); inducible nitric oxide synthase (iNOS), 5′-GAGCCCTCAGCAGCATCCAT-3′ (forward), 5′-GGTGAGGGCTTGGCTGAGTG-3′ (reverse); and 18s, 5-′TCCGGAGAGGGAGCCTGAGA-3′ (forward), 5′-GCACCAGACTTGCCCTCCAA-3′ (reverse). The relative mRNA expression quantification was calculated with the comparative threshold cycle (Ct) method.
Results
Generation and characterization of OE-MSCs
The OE-MSCs were successfully isolated from the nasal cavity of normal mice for culture. After 3 passages, the OE-MSC colonies formed, among which, most cells exhibited fibroblast-like morphology (Figure 1a). A phenotypic analysis revealed that the OE-MSCs stained strongly for CD29, CD90, and CD44 but were negative for CD34, CD45, and CD11b, which are similar phenotypic MSC markers (Figure 1b). Moreover, the nestin and vimentin expression, two proteins related to the neural stem cells, in OE-MSCs was further confirmed with immunofluorescent microscopy (Figure 1c).22,23 Consistent with the most prominent characteristics of MSCs with multi-lineage differentiation capacity, OE-MSCs can differentiate into osteocytes and adipocytes when cultured under the indicated conditions (Figure 1d). Taken together, these findings suggest that OE-MSCs with similar phenotypic features and the multiple-lineage differentiation capacities of MSCs could be efficiently generated in culture.
Figure 1.
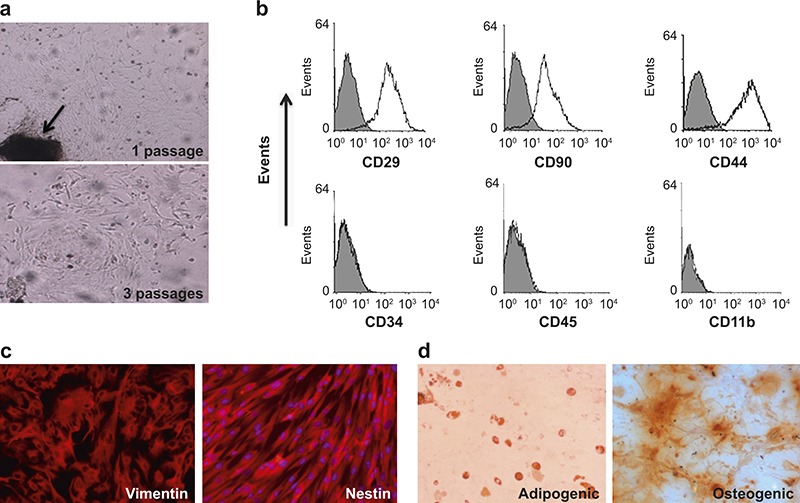
Phenotypic and functional characterization of OE-MSCs. (a) OE-MSCs generated from lamina propria explants (1 passage, arrow represents the lamina propria explants) exhibited a fibroblast-like morphology (after 3 passages). (b) Flow cytometric analysis of OE-MSC surface-marker expression, including representative MSC markers, CD29, CD90, and CD44; hematopoietic cell markers, CD34, and CD45; and the myeloid cell marker, CD11b. (c) Representative immunofluorescence staining of anti-nestin and anti-vimentin antibodies (red) in OE-MSCs. (d) OE-MSCs were cultured in adipocytes/osteogenic conditions for 14/21 days, followed by oil red O (left panel) or alizarin red staining (right panel), respectively (original magnification ×10). Results are representative of three independent experiments.
OE-MSCs possess potent immunosuppressive functions
To determine whether OE-MSCs possess any immunoregulatory function, we co-cultured splenic CD4+ T cells with OE-MSCs or BM-MSCs in the presence of anti-CD3 and anti-CD28 mAb stimulation. As shown in Figure 2a, although both MSC types efficiently suppressed CD4+ T-cell proliferation, the OE-MSCs produced more potent inhibitory effects than the BM-MSCs, suggesting much stronger immunosuppression was produced by the OE-MSCs. As TGF-β, IL-10, NO, and programmed death-ligand 1 (PD-L1) are key factors involved in MSC-mediated immunosuppression, we assessed these molecules at both the messenger RNA and protein levels in both OE-MSCs and BM-MSCs. The TGF-β, IL-10, and NO concentrations in the OE-MSC culture supernatants were significantly higher than those in the BM-MSC supernatants (Figure 2b), which was consistent with the higher TGF-β, IL-10, iNOS, and PD-L1 transcript levels observed in the OE-MSCs (Figure 2c).
Figure 2.
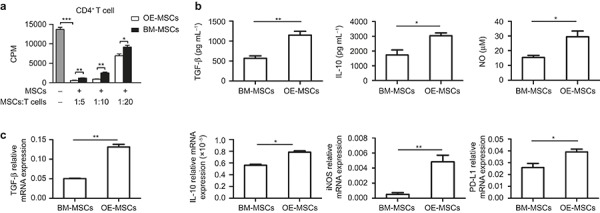
OE-MSCs inhibit CD4+ T-cell proliferation in vitro. (a) CD4+ T cells were co-cultured with BM-MSCs or OE-MSCs at the indicated ratios (MSCs: T-cell ratio of 1:5, 1:10, 1:20) in the presence of anti-CD3 mAb and anti-CD28 mAb for 72 h. T-cell proliferation was measured by [3H]-thymidine incorporation. The gray bar represents the T cell only group. (b and c) BM-MSCs or OE-MSCs were co-cultured with CD4+ T cells (MSCs: T-cell ratio of 1:10) in the presence of anti-CD3 mAb and anti-CD28 mAb for 72 h. (b) TGF-β, IL-10, and NO concentrations in the co-culture supernatant were determined with ELISAs. (c) TGF-β, IL-10, iNOS, and PD-L1 mRNA levels in OE-MSCs and BM-MSCs were determined with qRT-PCR analysis. Data are shown, as the mean ± SD of three independent experiments. ***P < 0.001, **P < 0.01, *P < 0.05.
Adoptive transfer of OE-MSCs suppresses arthritis progression in CIA mice
Next, we sought to determine the suppressive effects of OE-MSCs on autoimmune pathogenesis in vivo. We performed adoptive transfer of OE-MSCs or BM-MSCs into CIA mice and compared the therapeutic effects of OE-MSCs on arthritis onset and disease progression with those of BM-MSCs (Figure 3a). As shown in Figure 3b, the clinical scores for arthritis severity were markedly decreased in the CIA mice following adoptive transfer of both the OE-MSCs and BM-MSCs. Additionally, the OE-MSC-treated mice presented significantly reduced disease incidence, beyond what was observed in the BM-MSC-treated group. Moreover, the serum anti-CII autoantibody levels were significantly decreased in the OE-MSC-treated mice compared with the control group (Figure 3c). Furthermore, histopathological assessment showed severe fibrovascular synovial and periarticular proliferation with a high level of inflammatory cell infiltration in the CIA group. However, after the BM-MSC treatment, the joint pathology was ameliorated such that only mild fibrovascular synovial and periarticular proliferation with few infiltrated inflammatory cells was observed. Furthermore, no obvious joint damage and clear joint space and intact articular cartilage were observed in the OE-MSC-treated group. The histopathological examination showed markedly reduced joint pathology in the OE-MSC-treated mice compared with the BM-MSC-treated group (Figure 3d). Together, these data demonstrated that the OE-MSCs possess more potent immunosuppressive effects than the BM-MSCs in ameliorating autoimmune progression in CIA mice.
Figure 3.
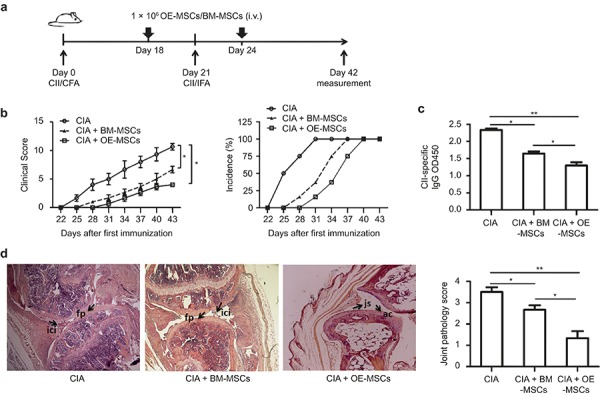
OE-MSCs prevented tissue damage in CIA. (a) A graphic scheme of CIA induction and OE-MSCs administration. DBA/1J mice were immunized with CII/CFA on day 0 and boosted with CII/IFA on day 21. The treatment groups were intravenously injected with OE-MSCs (1 × 106) on days 18 and 24 after CII/CFA immunization. Mice were killed on day 42. (b) Clinical score and incidence of arthritis development in the immunized mice that treated with OE-MSCs, BM-MSCs, or PBS were monitored every 3 days (n = 6 per group). (c) Serum levels of CII-specific autoantibodies from the OE-MSCs, BM-MSCs, or PBS-treated groups were measured with ELISAs. (d) Representative hind paw sections are shown that were stained with hematoxylin and eosin, and then assessed for histopathological joint tissue scores from OE-MSC, BM-MSC, or PBS-treated CIA mice. The CIA group had augmented fibrovascular synovial and periarticular proliferation (fp) and inflammatory cell infiltration (ici) levels. Clear joint spaces (js) and intact articular cartilage (ac) were observed in the CIA mice that were treated with OE-MSCs. Results are expressed as the mean ± SD. **P < 0.01, *P < 0.05.
OE-MSCs treatment promotes Treg cell expansion and suppresses Th1/Th17 responses in vivo
To examine the possible mechanisms underlying the immunosuppressive effects of OE-MSCs in vivo, we performed an immunophenotypic analysis of T-cell responses in draining lymph nodes (dLN). As shown in Figure 4a, OE-MSC treatment significantly increased the CD4+CD25+Foxp3+ Treg frequency in the dLNs. By contrast, the percentages of CD4+IFN-γ+ Th1 cells (Figure 4b) and CD4+IL-17+ Th17 cells (Figure 4c) upon OE-MSC treatment were decreased when compared with the control and the BM-MSC-treated groups. Moreover, the serum levels of the pro-inflammatory cytokines, IFN-γ and IL-17 were decreased whereas the TGF-β, IL-10 and NO levels were increased. As shown in Figure 4d, the OE-MSC treatment significantly reduced the pro-inflammatory cytokine levels compared with the BM-MSCs, although there was no significant difference in the serum IL-10, TGF-β, and NO levels between the two groups. Together, these data indicate that the OE-MSCs were able to suppress the inflammatory response by recalibrating the balance between anti-inflammatory Tregs and inflammatory effector T-cell subsets.
Figure 4.
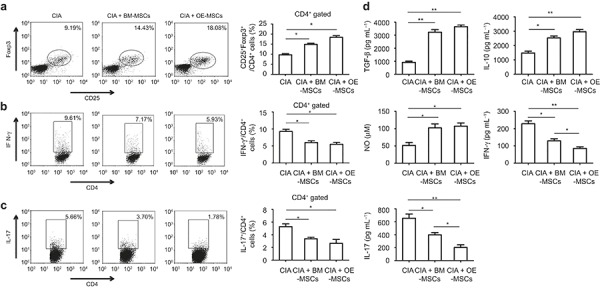
OE-MSCs enhanced Treg cell levels during CIA. (a–c). Flow cytometric analysis of CD4+ Foxp3 +CD25+ Treg cells (a), CD4+ IFN-γ+ Th1 (b) and CD4+ IL-17A+ Th17 (c) in the dLN of CIA mice that were treated with OE-MSCs, BM-MSCs, or PBS. (d) Serum TGF-β, IL-10, NO, IFN-γ, and IL-17 levels in CIA mice that were treated with OE-MSCs, BM-MSCs, or PBS were detected with ELISAs (n = 3/group). Results are expressed as the mean ± SD. **P < 0.01,*P < 0.05.
OE-MSCs promote Treg cell expansion in culture
To determine whether OE-MSCs could directly modulate Treg cell differentiation in vitro, a co-culture of naïve CD4+ T cells with OE-MSCs was performed. As shown in Figure 5a, a significantly increased frequency of CD4+CD25+Foxp3+ T cells was detected when naïve CD4+ T cells were co-cultured with OE-MSCs. Furthermore, under polarized Treg cell differentiation conditions, the OE-MSCs markedly promoted the expansion of Treg cells that were differentiated from naïve CD4+ T cells in culture (Figure 5b). Therefore, these results demonstrated that OE-MSCs could promote Treg cell expansion, which might contribute to their therapeutic effect in suppressing CIA progression in vivo.
Figure 5.
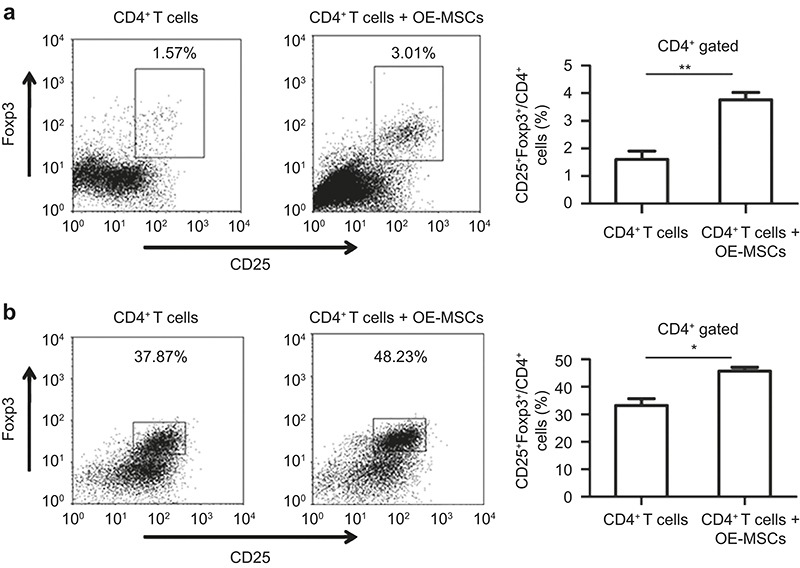
OE-MSCs induced regulatory T-cell expansion. (a) Naïve CD4+ T cells (1 × 106) isolated from C57BL/6 mouse spleens were co-cultured with or without OE-MSCs (5 × 104) in the presence of anti-CD3 and anti-CD28 mAbs. After 3 days, regulatory T cells were analyzed in the CD4+ T-cell fraction by flow cytometry. (b) Naïve CD4+ T cells (1 × 106) sorted from C57BL/6 mouse spleens and were cultured in Treg cell polarization medium with or without OE-MSCs (5 × 104). After 5 days, the proportion of regulatory T cells in the CD4+ T-cell fraction was analyzed by flow cytometry. Data are shown as the mean ± SD of three independent experiments. **P < 0.01, *P < 0.05.
Discussion
Olfactory MSCs have been reported to possess the potential for multi-lineage differentiation. Olfactory MSCs could differentiate into cells with cardiomyocyte characteristics or cells with bone-specific bioactivity.22,24 Recently, human MSCs isolated from the olfactory mucosa were found to have a promyelination effect that contributed to the repairment of spinal cord injury.23 However, it was not well characterized whether OE-MSCs possess any similar immunoregulatory properties as other MSCs and if so, whether adoptive transfer of OE-MSCs could suppress autoimmune pathogenesis in vivo.
In this study, we first showed that in vitro-expanded OE-MSCs expressed phenotypic MSC markers, including CD29, CD90, CD44, nestin, and vimentin, but they did not express CD34, CD45, and CD11b. Moreover, the OE-MSCs were capable of differentiating into mesenchymal cell types, such as adipocytes and osteocytes. Several studies have demonstrated the ability of MSCs to polarize T cells toward a regulatory phenotype; this serves as an important mechanism by which MSCs dampen inflammation reactions and tissue damage.25 Notably, we found for the first time that OE-MSCs possess a profound inhibitory capacity in suppressing effector T-cell proliferation. MSC-mediated immune modulation occurs mainly through paracrine effects by producing soluble factors, including NO, IL-10, and TGF-β, but this may also occur through cell surface-expressed inhibitory molecules, such as PD-L1, to mediate direct cell–cell interactions. Compared with BM-MSCs, OE-MSCs produced higher inhibitory factor levels, including NO,26 IL-10, TGF-β,27 and PD-L1.28 These mediators have been shown to play a critical role in effector T-cell proliferation suppression and in the induction of Treg differentiation, both in vitro and in vivo. Moreover, recent studies have demonstrated that both allogeneic BM-MSCs and human adipose-derived MSCs can treat experimental arthritis and induce immune tolerance by inhibiting autoreactive effector T-cell response and inducing Treg cell expansion.29,30 Thus, the immunoregulatory properties of OE-MSCs identified in this study may license them to suppress autoimmune responses and inflammatory reactions.
To further verify the immunoregulatory function of OE-MSCs in suppressing autoimmune progression in vivo, we performed adoptive transfer of OE-MSCs and observed significantly ameliorated synovial hyperplasia and joint tissue damage in CIA mice. Remarkably, the OE-MSC treatment resulted in a significantly increased Treg percentages and decreased Th1 and Th17 cell responses. Notably, the immunoregulatory function exerted by the OE-MSCs was much stronger than that exerted by the BM-MSCs in suppressing arthritic progression in CIA mice, which could primarily be attributed to their capacity to produce higher IL-10 and TGF-β expression levels. Consistent with these in vivo findings, we further revealed that the co-culture of naïve T cells with OE-MSCs promoted the expansion of Treg cells under a polarized condition for Treg differentiation. Although it currently remains unclear how OE-MSCs promote Treg cell differentiation, it is likely that soluble mediators produced by OE-MSCs, such as TGF-β, IL-10, and NO may contribute to the functional induction of Treg cells. In particular, it is evident that TGF-β plays a non-redundant role in the activation of Foxp3 expression and the expansion of Treg cells.
RA is a common autoimmune disease that progressively affects multiple joints with synovial hyperplasia, cartilage destruction, and bone erosion. Although the etiology of RA is still unclear, current strategies for the treatment of RA include the suppression of autoimmune inflammation using newly developed biologics, such as IL-1 receptor antagonists,31 TNF inhibitors,32 and an anti-IL-6 receptor antibody.33 Although these treatments can significantly inhibit the inflammatory response, achieving full remission of disease progression remains a challenge for many RA patients.34 Therefore, it is important to develop more effective therapies for RA.
MSCs are a type of stromal cell that are capable of differentiating into cartilage, bone, and other tissues. Other than their clinical applications in regenerative medicine, MSCs have been extensively investigated for their immunosuppressive functions. In the clinical settings, BM-MSCs have been used to treat inflammatory and autoimmune diseases by promoting tissue damage repair and tissue regeneration.35,36 Recent studies have characterized the immunoregulatory functions of MSCs in modulating immune responses that are mediated by various immune cells, such as T- and B lymphocytes and dendritic cells.37 It has been reported that BM-MSCs strongly suppress T-lymphocyte proliferation via mechanisms involved in cell–cell contact and in soluble factor production.38 However, clinical applications of BM-MSCs have encountered difficulties, including the invasive approach that is required for cell harvest and donor age-related limitations.39,40 Because the nasal lamina propria is an easily accessible tissue that can be harvested under local anesthesia,41 OE-MSCs could be collected for autologous transplantation. Moreover, OE-MSCs exhibit a higher proliferation profile than BM-MSCs and can be expanded in culture for therapeutic approaches.12 Considering the advantages of their easy accessibility and efficient expansion for adoptive transfer, OE-MSCs may serve as a possible cellular therapy for RA.
In conclusion, we have identified a novel function for OE-MSCs with immunoregulatory properties. Moreover, OE-MSCs could exert their immunosuppressive capacity in modulating T-cell responses and could significantly ameliorate disease severity in CIA mice. These findings suggest that OE-MSCs represent a new potential cell therapy for targeting autoimmune diseases.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 31470881, 81072453), the Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20133227110008), the Health Department Foundation of Jiangsu Province (Grant No. Z201312), the Graduate Student Research and the Innovation Program of Jiangsu Province (Grant No. KYLX_1074), the Jiangsu Province ‘333' Project (Grant No. BRA2015197), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the National Basic Research Program of China (2014CB541904), and the Hong Kong Croucher Foundation.
The authors declare that no conflicts of interest exist.
References
- Di Trapani M, Bassi G, Ricciardi M, Fontana E, Bifari F, Pacelli L et al. Comparative study of immune regulatory properties of stem cells derived from different tissues. Stem Cells Dev 2013; 22: 2990–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8: 315–317. [DOI] [PubMed] [Google Scholar]
- Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ 2014; 21: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110: 3499–3506. [DOI] [PubMed] [Google Scholar]
- Figueroa FE, Carrion F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res 2012; 45: 269–277. [DOI] [PubMed] [Google Scholar]
- Vina JA, El-Alami M, Gambini J, Borras C, Vina J, Penarrocha MA. Application of mesenchymal stem cells in bone regenerative procedures in oral implantology. A literature review. J Clin Exp Dent 2014; 6: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart EA, Meade S, Azad V, Yeh S, Al-Zube L, Lee YS et al. Mesenchymal stem cells accelerate bone allograft incorporation in the presence of diabetes mellitus. J Orthop Res 2010; 28: 942–949. [DOI] [PubMed] [Google Scholar]
- El-Jawhari JJ, El-Sherbiny YM, Jones EA, McGonagle D. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM 2014; 107: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S et al. Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 2009; 183: 993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Zhang H, Jin O, Feng X, Yao G, Hou Y et al. Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol 2008; 5: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty P, Cooper K, Viswanathan C. Comparison of proliferative and multilineage differentiation potentials of cord matrix, cord blood, and bone marrow mesenchymal stem cells. Asian J Transfus Sci 2010; 4: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme B, Nivet E, Gaillard J, Haupl T, Ringe J, Deveze A et al. The human nose harbors a niche of olfactory ecto-mesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev 2010; 19: 853–866. [DOI] [PubMed] [Google Scholar]
- Hauser S, Widera D, Qunneis F, Muller J, Zander C, Greiner J et al. Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev 2012; 21: 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivet E, Vignes M, Girard SD, Pierrisnard C, Baril N, Devèze A et al. Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. J Clin Invest 2011; 121: 2808–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamegna JC, Girard SD, Veron A, Sicard G, Khrestchatisky M, Feron F et al. A unique method for the isolation of nasal olfactory stem cells in living rats. Stem Cell Res 2014; 12: 673-–679. [DOI] [PubMed] [Google Scholar]
- Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater 2012; 23: 13–27. [DOI] [PubMed] [Google Scholar]
- Yang MT, Fu J, Wang YK, Desai RA, Chen CS. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat Protoc 2011; 6: 187–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Liu Y, Yang M, Wang S, Zhang M, Wang X et al. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum 2012; 64: 3564–3573. [DOI] [PubMed] [Google Scholar]
- Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc 2007; 2: 1269–1275. [DOI] [PubMed] [Google Scholar]
- Carnrot C, Prokopec KE, Rasbo K, Karlsson MC, Kleinau S. Marginal zone B cells are naturally reactive to collagen type II and are involved in the initiation of the immune response in collagen-induced arthritis. Cell Mol Immunol 2011; 8: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 2007; 43: 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SA, Liley M, Dalby MJ, Barnett SC. Comparison of human olfactory and skeletal MSCs using osteogenic nanotopography to demonstrate bone-specific bioactivity of the surfaces. Acta Biomater 2015; 13: 266–276. [DOI] [PubMed] [Google Scholar]
- Lindsay SL, Johnstone SA, Mountford JC, Sheikh S, Allan DB, Clark L et al. Human mesenchymal stem cells isolated from olfactory biopsies but not bone enhance CNS myelination in vitro. Glia 2013; 61: 368–382. [DOI] [PubMed] [Google Scholar]
- Huang Y-S, Li IH, Chueh S-H, Hueng D-Y, Tai M-C, Liang C-M et al. Mesenchymal stem cells from rat olfactory bulbs can differentiate into cells with cardiomyocyte characteristics. J Tissue Eng Regen Med 2013. [DOI] [PubMed]
- Burr SP, Dazzi F, Garden OA. Mesenchymal stromal cells and regulatory T cells: the Yin and Yang of peripheral tolerance? Immunol Cell Biol 2013; 91: 12–18. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008; 2: 141–150. [DOI] [PubMed] [Google Scholar]
- Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology 2006; 117: 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechtenstein T, Dufait I, Bricogne C, Lanna A, Pen J, Breckpot K et al. PD-L1/PD-1 co-stimulation, a brake for T cell activation and a T cell differentiation signal. J Clin Cell Immunol 2012; S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum 2007; 56: 1175–1186. [DOI] [PubMed] [Google Scholar]
- González MA, Gonzalez–Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009; 136: 978–989. [DOI] [PubMed] [Google Scholar]
- Bresnihan B. Treatment of rheumatoid arthritis with interleukin 1 receptor antagonist. Ann Rheum Dis 1999; 58: I96–I98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenauer B, Judd M, Wells G, Burls A, Cranney A, Hochberg M et al. Infliximab for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2002; 2002: CD003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004; 50: 1761–1769. [DOI] [PubMed] [Google Scholar]
- Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev 2015; 67: 280–309. [DOI] [PubMed] [Google Scholar]
- Sage EK, Loebinger MR, Polak J, Janes SM. The Role of Bone Marrow-derived Stem Cells in Lung Regeneration and Repair. Cambridge, MA: StemBook, 2008. [PubMed] [Google Scholar]
- Unsicker K. Neurotrophic molecules in the treatment of neurodegenerative disease with focus on the retina: status and perspectives. Cell Tissue Res 2013; 353: 205–218. [DOI] [PubMed] [Google Scholar]
- Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol 2013; 91: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? BioMed Res Int 2014; 2014: 216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem 2001; 82: 583–590. [DOI] [PubMed] [Google Scholar]
- Lee SY, Miwa M, Sakai Y, Kuroda R, Matsumoto T, Iwakura T et al. In vitro multipotentiality and characterization of human unfractured traumatic hemarthrosis-derived progenitor cells: a potential cell source for tissue repair. J Cell Physiol 2007; 210: 561–566. [DOI] [PubMed] [Google Scholar]
- Féron F, Perry C, Girard SD, Mackay-Sim A. Isolation of adult stem cells from the human olfactory mucosa. Methods Mol Biol 2013; 1059: 107–114 [DOI] [PubMed] [Google Scholar]


