Graphical abstract
Keywords: Prostate cancer, miR-499, miR-196a2, miR-146a, miR-149, Polymorphism
Abstract
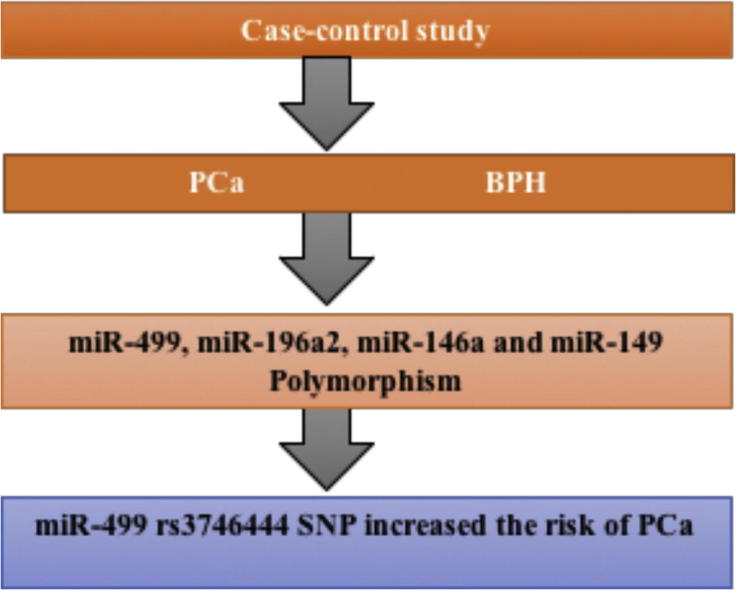
MicroRNAs (miRNAs) play an important role in regulating gene expression at the post-transcriptional level and are involved in numerous physiological processes. Accumulating evidence suggests that single-nucleotide polymorphisms (SNPs) in human miRNA genes may affect miRNA biogenesis pathway and influence the susceptibility to several diseases such as cancer. The present study aimed to evaluate the impact of miR-499 rs3746444, miR-196a2 rs11614913, miR-149 rs2292832, and miR-146a rs2910164 polymorphisms on prostate cancer (PCa) risk in a sample of Iranian population. This case-control study was done on 169 patients with pathologically confirmed PCa and 182 benign prostatic hyperplasia (BPH). The genotyping assays were done using T-ARMS-PCR or PCR-RFLP methods. The findings indicated that CC genotype of miR-499 rs3746444 polymorphism increased the risk of PCa (OR = 1.76, 95% CI = 1.12–2.79, P = 0.019) compared to TT genotype. No statistically significant association was found between miR-196a2 rs11614913, miR-149 rs2292832, and miR-146a rs2910164 polymorphisms and PCa risk. In summary, the findings indicated that miR-499 rs3746444 polymorphism increased the risk of PCa in an Iranian population. Further studies with larger sample sizes and different ethnicities are necessary to verify the findings of the present study.
Introduction
Prostate cancer (PCa) is the most malignant tumor among men in the United States [1]. The lowest incidence rate of PCa is in the Asian population [2], [3]. In Iran, the incidence rate of PCa is approximately 9.6 per 100,000 [4], [5] which is comparable to Asia–Pacific region (9.9 per 100,000), but considerably lower than the world (32.8 per 100,000) [6].However, the exact mechanisms underlying the development and progression of PCa remain generally unknown. It has been proposed that both genetic and environmental factors contribute to the development and progression of PCa [7], [8], [9]. Genetic factors have been estimated to account for over 40% of PCa risk. Single nucleotide polymorphism (SNP) is the most common type of genetic variation in human genome and has been shown to be associated with PCa risk [10], [11], [12]. Genomewide association studies (GWAS) showed that more than 100 single nucleotide polymorphisms (SNPs) involved in prostate cancer (PCa) risk. However, the molecular mechanisms are unclear for most of these SNPs [13].
MicroRNAs (miRNAs) are a class of small single-stranded noncoding RNAs usually composed of about 17–25 nucleotides. They widely exist in human cells and regulate gene expression at the posttranscriptional level via either translational repression or mRNA degradation through binding to the 3′-untranslated region (3′-UTR) of target mRNAs [14], [15], [16]. miRNAs play an important regulating role in many biological processes, including cell proliferation, differentiation, and apoptosis, and also function as tumor suppressors and oncogenes [17], [18], [19], [20].
SNPs residing within the miRNA genes could potentially alter various biological processes by influencing the miRNA biogenesis and altering target selection [21]. SNPs and mutations in miRNAs or miRNA target sites may affect the maturation process or target selection, respectively [22], [23], [24], [25]. Several studies investigated the impact of mIR polymorphisms and risk of various cancers. In a meta-analysis performed by Fan et al. [26] revealed no significant association between miR-499 rs3746444 polymorphism and cancer risk. But in subgroup analysis by cancer type, this variant was associated with an increased risk of BC. The findings of a meta-analysis did not support an association between mIR-196a2 rs11614913, mIR-146a rs2910164, and mIR-423 rs6505162 polymorphism and esophageal cancer risk [27]. The findings of a meta-analysis revealed that miR-146a rs2910164 polymorphism is associated with increased risk for cervical and skin squamous cell carcinoma (SCC), while this variant decreased the risk of nasopharyngeal and oral SCC [28].
The miR-149 rs2292832 variant may decrease the risk of digestive cancer [29]. It has been reported that miR-146a rs2910164 polymorphism marginally decreased the risk of gastric cancer [30]. The rs3746444 variant of miR-499 has been reported to be associated with susceptibility to cancer [28].
There is little and inconsistent data regarding the impact of miRNA gene polymorphisms on risk/protection of PCa [31], [32]. To the best of our knowledge, there is no report regarding the impact of miRs variants on PCa risk in Iranian population. Hence, the current study was aimed to find out the possible association between miR-499 rs3746444, miR-196a2 rs11614913, miR-146a rs2910164 and mir-149 rs2292832 variants polymorphisms and PCa in a sample of Iranian population.
Patients and methods
Patients
This case-control study was done on 169 unrelated men with histopathologically confirmed adenocarcinoma of prostate and 182 ages matched unrelated men with benign prostatic hyperplasia (BPH) with no history of any cancer. The study design and recruitment procedures were described previously [33]. The demographic and clinicopathological characteristics are shown in Table 1. Briefly, all subjects were registered from Department of Urology, Shahid Labbafinejad Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The project was approved by local Ethics Committee of Zahedan University of Medical Sciences (#7081), and written informed consent was obtained from all cases and controls. Blood samples were collected in EDTA-containing tubes and genomic DNA was extracted using salting out method as described previously [34].
Table 1.
Demographic and clinicopathological characteristics of prostate cancer (PCa) and control subjects.
| Factors | Prostate Cancer | Control |
|---|---|---|
| Age, Mean ± SD (Years) | 61.36 ± 6.61 | 62.51 ± 7.67 |
| Post-void residual, mean ± SD (mL) | 27.2 ± 25.2 | – |
| PSA at diagnosis mean ± SD (ng/mL) | 14.9 ± 14.3 | – |
| Gealson score | ||
| ⩽6 | 57 (33.7) | – |
| 7 | 73 (43.2) | – |
| >7 | 39 (23.1) | – |
| Stage | ||
| PT1 | 8 (4.7) | – |
| PT2a | 27 (16.0) | – |
| PT2b | 11 (6.5) | |
| PT2c | 76 (45.0) | – |
| PT3a | 13 (7.7) | – |
| Perineural invasion | ||
| Yes | 106 (62.7) | – |
| No | 63 (37.3) | – |
| Impotency | ||
| Yes | 26 (15.74) | – |
| No | 143 (84.6) | – |
| Loss of Libido | ||
| Yes | 24 (14.2) | |
| No | 145 (85.8) | |
| Addiction | ||
| Yes | 8 (4.7) | 4 (2.2) |
| No | 161 (95.3) | 178 (97.8) |
| Any history of smoking | ||
| Yes | 27 (16.0) | 6 (3.3) |
| No | 142 (84.0) | 176 (96.7) |
| Alcohol drinking | ||
| Yes | 7 (4.1) | 0 (0.0) |
| No | 162 (95.9) | 182 (0.0) |
| Hypertension | ||
| Yes | 23 (13.6) | 5 (2.7) |
| No | 146 (86.4) | 177 (97.3) |
Genotyping
The primers used for detection of miRs polymorphisms are shown in Table 2. Genotyping of miR-146a rs2910164, and miR-196a2 rs11614913 was performed using T-ARMS-PCR assay as described previously [35], [36]. Genotyping of miR-499 rs3746444 [37] and miR-149 rs2292832 was performed by PCR-RFLP method. PCR was done using commercially available Prime Taq premix (Genetbio, South Korea) according to the manufacturer’s recommended protocol. In each 0.20 mL reaction PCR reaction tube, 1 μL of genomic DNA (∼100 ng/mL), 1 μL of each primers (10 μM), 10 μL of 2X Prime Taq Premix and appropriate amount of ddH2O were added. The PCR conditions were set as follows: 5 min at 95 °C, followed by 30 cycles of 30 s at 95 °C, 30 s at 62 °C for rs2910164, 63 °C for rs11614913, 64 °C for rs3746444, 66 °C for rs2292832, and 72 °C for 30 s with a final extension step of 72 °C for 10 min. For detection of rs2292832 and rs3746444 variants, 10 μL of PCR product digested by restriction enzymes (Table 2). The PCR products were electrophoresed on agarose gel containing 0.5 μg/mL ethidium bromide and visualized on a UV transilluminator.
Table 2.
The primers used for miR polymorphisms genotyping.
| Polymorphism | Sequence (5′ ⩾ 3′) | Restriction enzyme | Product size (bp) |
|---|---|---|---|
| miR-146a rs2910164 G > C | FO: GGCCTGGTCTCCTCCAGATGTTTAT | – | Contol: 364 G allele: 249 C allele: 169 |
| RO: ATACCTTCAGAGCCTGAGACTCTGCC | |||
| FI (C allele): ATGGGTTGTGTCAGTGTCAGACGTC | |||
| RI (G allele): GATATCCCAGCTGAAGAACTGAATTTGAC | |||
| miR-196a2 rs11614913 C > T | FO: ACCCCCTTCCCTTCTCCTCCAGATAGAT | – | Control: 297 T allele: 199 C allele: 153 |
| RO: AAAGCAGGGTTCTCCAGACTTGTTCTGC | |||
| FI (T allele): AGTTTTGAACTCGGCAACAAGAAACGGT | |||
| RI (C allele): GACGAAAACCGACTGATGTAACTCCGG | |||
| miR-149 rs2292832 C > T | F: CTCTGGCTCCGTGTCTTCACTC | PvuII | C allele: 225 T allele: 154, 71 |
| R: CCTGCAGGTTCTGAGGGGC | |||
| miR-499 rs3746444 T > C | F: CAAAGTCTTCACTTCCCTGCCA | BclI | C allele: 146 T allele: 122, 24 |
| R: GATGTTTAACTCCTCTCCACGTGATC |
Statistical analysis
Statistical analysis was done using statistical package SPSS 20 software. Data were analyzed by independent sample t-test and χ2 test. Association between polymorphisms and PCa was calculated by computing the odds ratio (OR) and 95% confidence intervals (95% CI) from logistic regression analyses. The statistical level of significance was defined as P-value less than 0.05.
Results
The study group consists of 169 Pca patients with an average age of 61.36 ± 6.61 years and 182 benign prostatic hyperplasia (BPH) with a mean age of 62.51 ± 7.67 years. No significant difference was found between the groups concerning age using independent sample t-test (P = 0.135).
The genotypes and allele frequencies of miR polymorphisms in PCa and control subjects are shown in Table 3. The results proposed that that TC genotype of miR-499 rs3746444 polymorphism increased the risk of PCa (OR = 1.76, 95% CI = 1.12–2.79, P = 0.019) compared to TT genotype, while the minor allele frequency (C allele) of rs3746444 was not associated with PCa.
Table 3.
Genotypic and allelic frequencies of miR499 rs3746444, miR-196a2 rs11614913, miR-146a rs2910164 and miR-149 rs2292832 variants polymorphisms in prostate cancer (PCa) and control subjects.
| Polymorphism | Prostate cancer (169 subjects) n (%) |
Control (182 subjects) n (%) |
OR (95% CI) | P-value |
|---|---|---|---|---|
| miR-499 rs3746444 T > C | ||||
| Codominant | ||||
| TT | 62 (37.6) | 85 (46.7) | 1.00 | – |
| TC | 82 (48.5) | 64 (35.2) | 1.76 (1.12–2.79) | 0.019 |
| CC | 25 (14.8) | 33 (18.1) | 1.04 (0.56–1.92) | 0.897 |
| Dominant | ||||
| TT | 62 (37.6) | 85 (46.7) | 1.00 | – |
| TC + CC | 107 (62.4) | 97 (53.3) | 1.51 (0.99–2.32) | 0.066 |
| Recessive | ||||
| TT + TC | 144 (85.2) | 149 (81.9) | 1.00 | – |
| CC | 25 (14.8) | 33 (18.1) | 0.78 (0.44–1.38) | 0.472 |
| Allele | ||||
| T | 206 (60.9) | 234 (64.3) | 1.00 | – |
| C | 132 (39.1) | 130 (35.7) | 1.02 (0.85–1.57) | 0.390 |
| miR-196a2 rs11614913 C > T | ||||
| Codominant | ||||
| CC | 64 (37.9) | 77 (42.3) | 1.00 | – |
| CT | 88 (52.1) | 93 (51.1) | 1.14 (0.73–1.77) | 0.576 |
| TT | 17 (10.0) | 12 (6.6) | 1.70 (0.76–3.82) | 0.224 |
| Dominant | ||||
| CC | 64 (37.9) | 77 (42.3) | 1.00 | – |
| CT + TT | 105 (62.1) | 105 (57.7) | 1.20 (0.78–1.85) | 0.446 |
| Recessive | ||||
| CC + CT | 152 (90.0) | 170 (93.4) | 1.00 | – |
| TT | 17 (10.0) | 12 (6.6) | 1.58 (0.73–3.43) | 0.251 |
| Allele | ||||
| C | 216 (63.9) | 247 (67.9) | 1.00 | – |
| T | 122 (36.1) | 117 (32.1) | 1.19 (0.87–1.63) | 0.300 |
| miR-149 rs2292832 C > T | ||||
| Codominant | ||||
| CC | 77 (45.6) | 101 (55.5) | 1.00 | – |
| CT | 68 (40.2) | 57 (31.3) | 1.57 (0.99–2.480) | 0.062 |
| TT | 24 (14.2) | 24 (13.2) | 1.31 (0.69–2.48) | 0.418 |
| Dominant | ||||
| CC | 77 (45.6) | 101 (55.5) | 1.00 | – |
| CT + TT | 92 (54.4) | 81 (44.5) | 1.49 (0.98–2.27) | 0.069 |
| Recessive | ||||
| CC + CT | 145 (85.8) | 168 (86.8) | 1.00 | – |
| TT | 24 (14.2) | 24 (13.2) | 1.16 (0.63–2.13) | 0.645 |
| Allele | ||||
| C | 222 (65.7) | 258 (70.9) | 1.00 | – |
| T | 116 (34.3) | 106 (29.1) | 1.27 (0.92–1.75) | 0.144 |
| miR-146a rs2910164 G > C | ||||
| Codominant | ||||
| GG | 25 (14.8) | 24 (13.2) | 1.00 | – |
| GC | 131 (77.5) | 147 (80.8) | 0.86 (0.47–1.57) | 0.644 |
| CC | 13 (7.7) | 11 (6.0) | 1.14 (0.43–3.02) | 0.917 |
| Dominant | ||||
| GG | 25 (14.8) | 24 (13.2) | 1.00 | – |
| GC + CC | 144 (85.2) | 158 (86.8) | 0.87 (0.48–1.60) | 0.758 |
| Recessive | ||||
| GG + GC | 156 (92.3) | 171 (94.0) | 1.00 | – |
| CC | 13 (7.7) | 11 (6.0) | 1.29 (0.56–2.98) | 0.673 |
| Allele | ||||
| G | 181 (53.6) | 195 (53.6) | 1.00 | – |
| C | 157 (46.4) | 169 (46.4) | 1.00 (0.74–1.35) | 0.974 |
As shown in Table 3, the miR-196a2 rs11614913, miR-146a rs2910164 and Mir-149 rs2292832 variants were not associated with PCa in any inheritance models tested (co-dominant, dominant and recessive). Regarding miR-149 rs2292832 C > T the result is not strong enough to attain a P-value < 0.05 but there is a tendency toward that.
As shown in Table 4, miR-146a rs2910164 variant was significantly associated with stage of disease [contingency coefficient (CC) = 0.333, P = 0.021]. The findings showed no significant association between miR-149 rs2292832, miR-196a2 rs11614913 and miR-499 rs3746444 polymorphism and clinicopathological characteristics of the PCa patients (Table 4).
Table 4.
Association of miR polymorphisms with clinicopathologic parameters in prostate cancer (PCa) patients.
| Factors | miR-499 rs3746444 |
P | miR-196a2 rs11614913 |
P | miR-149 rs2292832 |
P | miR-146a rs2910164 |
P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TT | TC | CC | CC | CT | TT | CC | CT | TT | GG | GC | CC | |||||
| Age at diagnosis Y, n | 0.333 | 0.797 | 0.536 | 0.902 | ||||||||||||
| ⩽65 | 47 | 59 | 15 | 46 | 64 | 11 | 56 | 46 | 19 | 18 | 93 | 10 | ||||
| >65 | 15 | 23 | 10 | 18 | 24 | 6 | 21 | 22 | 5 | 7 | 38 | 3 | ||||
| Stage | 0.144 | 0.532 | 0.761 | 0.021 | ||||||||||||
| pT1 | 1 | 5 | 2 | 3 | 5 | 0 | 5 | 2 | 1 | 2 | 3 | 3 | ||||
| pT2a | 8 | 16 | 3 | 11 | 15 | 1 | 12 | 13 | 2 | 2 | 25 | 0 | ||||
| pT2b | 6 | 5 | 0 | 3 | 7 | 1 | 6 | 3 | 2 | 2 | 8 | 1 | ||||
| pT2c | 27 | 33 | 16 | 32 | 36 | 8 | 37 | 28 | 11 | 12 | 59 | 5 | ||||
| pT3a | 3 | 7 | 3 | 7 | 5 | 1 | 6 | 6 | 1 | 4 | 7 | 2 | ||||
| pT3b | 17 | 16 | 1 | 8 | 20 | 6 | 11 | 16 | 7 | 3 | 29 | 2 | ||||
| PSA at diagnosis (ng/ml), n | 0.094 | 0.061 | 0.722 | 0.545 | ||||||||||||
| ⩽4 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | ||||
| 4–10 | 24 | 43 | 17 | 32 | 44 | 8 | 41 | 32 | 11 | 14 | 61 | 9 | ||||
| >10 | 37 | 39 | 8 | 32 | 44 | 8 | 35 | 36 | 13 | 11 | 69 | 4 | ||||
| Gleason score, n | 0.334 | 0.238 | 0.998 | 0.465 | ||||||||||||
| ⩽6 | 18 | 26 | 13 | 26 | 28 | 3 | 26 | 23 | 8 | 8 | 42 | 7 | ||||
| 7 | 28 | 37 | 8 | 25 | 41 | 7 | 34 | 29 | 10 | 12 | 56 | 5 | ||||
| >7 | 16 | 19 | 4 | 13 | 19 | 7 | 17 | 16 | 6 | 5 | 33 | 1 | ||||
| Perineural invasion, n | 0.728 | 0.781 | 0.431 | 0.122 | ||||||||||||
| Positive | 41 | 49 | 16 | 38 | 57 | 11 | 52 | 41 | 13 | 18 | 83 | 5 | ||||
| Negative | 21 | 33 | 9 | 26 | 31 | 6 | 25 | 27 | 11 | 7 | 48 | 8 | ||||
| Surgical margin, n | 0.330 | 0.215 | 0.715 | 0.731 | ||||||||||||
| Positive | 28 | 32 | 7 | 20 | 39 | 8 | 30 | 29 | 8 | 11 | 52 | 4 | ||||
| Negative | 34 | 50 | 18 | 44 | 49 | 9 | 47 | 39 | 16 | 14 | 79 | 9 | ||||
The bold indicate statistically significant.
Discussion
In the current study we examined the impact of miR-146a rs2910164, miR-149 rs2292832, miR-196a2 rs11614913 and miR-499 rs3746444 polymorphisms on PCa risk in a sample of Iranian population. We found that miR-499 rs3746444 variant significantly increased the risk of PCa in the population studied. The results did not support an association between miR-146a rs2910164, mir-149 rs2292832 and miR-196a2 rs11614913 polymorphism and PCa risk. Till now, 3 studies investigated the impact of miRNA polymorphisms on PCa susceptibility. Findings of George et al. [38] study showed that heterozygous genotype in miR196a2 and miR-499, heterozygotes confers the increased risk of developing PCa in North Indian population.
Nikolic et al. [31] have found no statistically significant association between miR-499 rs3746444 and miR-196a2 rs11614913 variant and PCa risk in Serbian population. Their findings proposed that rs3746444 variant is associated with PCa aggressiveness so that the rs3746444 minor allele G confers the decreased risk of PCa progression.
Xu et al. [32] found the subjects carrying miR-146a rs2910164 CC had a 0.65-fold reduced risk (95% CI = 0.43–0.99) than those carrying GG/GC genotypes (P = 0.03), and the C allele displayed a lower prevalence of PCa compared with the G allele (OR = 0.73, 95% CI = 0.57–0.94, P = 0.01).
Wang et al. [39] performed a meta-analysis and found that miR-146a rs2910164 polymorphism increased the risk of cancer risk in dominant model when all studies were pooled into the meta-analysis. Stratified analysis revealed that significant association between rs2910164 variant and cancer susceptibility was found in Asians but not in Caucasian populations. In the subgroup analysis by cancer types, no significantly increased risk of breast, gastric, prostate or bladder cancer was found in any of the genetic models. While, another meta-analysis indicated that miR-146a rs2910164 C allele decreased PCa risk among Chinese population [40], [41].
It has been shown that the expression level of hsa-miR-155, hsa-miR-141 and hsa-miR-21 significantly elevated in PCa samples and negatively correlated with that of mismatch repair genes [42].
Previously we have investigated the impact of hsa-mir-146a rs2910164, has-miR-499 rs3746444 and Hsa-miRNA-196a2 (rs11614913 C > T and rs185070757 T > G) and risk of breast cancer. The results showed an association between miR-499 rs3746444 variant and risk of BC [43].
The miR-499 gene was mapped to 20q11.22. It lies within the 20th intron of the beta-myosin heavy chain 7B (Myh7b) gene. The miR-499 variant may influence the individual susceptibility to cancer risk by affecting MYH7B gene function as well functions of miR-499 [44], [45]. It has been shown that MDM4 oncogene contributes to cancer susceptibility and progression through its capability to negatively regulate tumor suppressor genes [46]. The rs4245739 A > C variant located in the 3′-untranslated region (UTR) has been reported to create a target site for hsa-miR-191, resulting in decreased MDM4 mRNA levels [47]. Computational calculations revealed that this variant is located within a predicted binding site for miR-191-5p, miR-887 and miR-3669. Thus the MDM4 rs4245739 A allele may be associated with increased risk of PCa [47]. MiR-143 is one major tumor suppressor miRNA. A functional rs4705342 T > C variant in miR-143 promoter has shown to be associated with PCa risk [48]. Subjects with TC/CC genotypes had significantly decreased risk of PCa compared with those with TT genotype. It has been proposed that genetic variants in miRs and miR target sites predict biochemical recurrence after radical prostatectomy in localized prostate cancer [25].
A meta-analysis performed by Ma et al. [49], investigated the association between miR-146a rs2910164, miR-196a2 rs11614913, miR-499 rs3746444, miR-149 rs2292832, and miR-27a rs895919 and the risk of cancer development. They found no significant association between rs2910164 and cancer risk in the overall group. However, in stratified analysis, they found that either the rs2910164 C allele or the CC genotype was protective against bladder cancer, prostate cancer, cervical cancer, and colorectal cancer, while it was a risk factor for papillary thyroid carcinoma and squamous cell carcinoma of the head and neck (SCCHN). In addition, rs11614913 was found to be significantly associated with decreased cancer risk, in particular, for bladder cancer, gastric cancer, and SCCHN. For miR-499, a significant association was found between the rs3746444 polymorphism and cancer risk in pooled analysis.
It has been reported that genetic variants in the miR machinery gene GEMIN4 are associated with risk of PCa in Chinese Han population [50]. It has been shown that rs1434536 variant in the 3′UTR of bone morphogenetic protein membrane receptor type IB (BMPR1B) gene affects the binding ability of miR-125b to BMPR1B mRNA and contributes to the genetic susceptibility to localized PCa as well as patients aged >70 years [51]. Prostate tumor invasion and hormone refractoriness may be caused by aberrant expression of miR-146a and miR-146b-5p [52].
The limitations of the present study are the following: (i) relatively small sample sizes, so replication with larger sample is needed. (ii) We did not determine gene-environment interactions. It has been proposed that both genetic and environmental factors may contribute to prostate cancer susceptibility.
Conclusions
In conclusion, the findings proposed that miR-499 rs3746444 polymorphism increased the risk of PCa. The results did not support an association between genetic variant of miR-196a2, miR-149, and miR-146a and the risk of developing PCa. Larger sample sizes with diverse ethnicities are needed to confirm the findings.
Funding
This project was funded by a dissertation grant (MSc thesis of NM) from Zahedan University of Medical Sciences, Zahedan, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest to disclose.
Acknowledgement
The authors thank all individuals who willingly participated the study.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hsing A.W., Devesa S.S. Trends and patterns of prostate cancer: what do they suggest? Epidemiol Rev. 2001;23:3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 3.Parkin D.M., Muir C.S. Cancer incidence in five continents. Comparability and quality of data. IARC Sci Publ. 1992:45–173. [PubMed] [Google Scholar]
- 4.Farahmand M., Khademolhosseini F., Mehrabani D. Trend of prostate cancer in Fars Province, Southern Iran, 2001–2007. J Res Med Sci. 2010;15:295–297. [PMC free article] [PubMed] [Google Scholar]
- 5.Talaiezadeh A., Tabesh H., Sattari A., Ebrahimi S. Cancer incidence in southwest of Iran: first report from khuzestan population-based cancer registry, 2002–2009. Asian Pac J Cancer Prev. 2013;14:7517–7522. doi: 10.7314/apjcp.2013.14.12.7517. [DOI] [PubMed] [Google Scholar]
- 6.Baade P.D., Youlden D.R., Cramb S.M., Dunn J., Gardiner R.A. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ntais C., Polycarpou A., Tsatsoulis A. Molecular epidemiology of prostate cancer: androgens and polymorphisms in androgen-related genes. Eur J Endocrinol. 2003;149:469–477. doi: 10.1530/eje.0.1490469. [DOI] [PubMed] [Google Scholar]
- 8.Chokkalingam A.P., Stanczyk F.Z., Reichardt J.K., Hsing A.W. Molecular epidemiology of prostate cancer: hormone-related genetic loci. Front Biosci. 2007;12:3436–3460. doi: 10.2741/2325. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X., Wei L., Jiao G., Gao W., Ying M., Wang N. The association between the APE1 Asp148Glu polymorphism and prostate cancer susceptibility: a meta-analysis based on case-control studies. Mol Genet Genom. 2015;290:281–288. doi: 10.1007/s00438-014-0916-3. [DOI] [PubMed] [Google Scholar]
- 10.Huang Q., Whitington T., Gao P., Lindberg J.F., Yang Y., Sun J. A prostate cancer susceptibility allele at 6q22 increases RFX6 expression by modulating HOXB13 chromatin binding. Nat Genet. 2014;46:126–135. doi: 10.1038/ng.2862. [DOI] [PubMed] [Google Scholar]
- 11.Hazelett D.J., Rhie S.K., Gaddis M., Yan C., Lakeland D.L., Coetzee S.G. Comprehensive functional annotation of 77 prostate cancer risk loci. PLoS Genet. 2014;10:e1004102. doi: 10.1371/journal.pgen.1004102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spisak S., Lawrenson K., Fu Y., Csabai I., Cottman R.T., Seo J.H. CAUSEL: an epigenome- and genome-editing pipeline for establishing function of noncoding GWAS variants. Nat Med. 2015;21:1357–1363. doi: 10.1038/nm.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Yu H., Wang J., Zhang Z., Gao Z., Chen Z. Systematic enrichment analysis of potentially functional regions for 103 prostate cancer risk-associated loci. Prostate. 2015;75:1264–1276. doi: 10.1002/pros.23008. [DOI] [PubMed] [Google Scholar]
- 14.Cai Y., Yu X., Hu S., Yu J. A brief review on the mechanisms of miRNA regulation. Genom Proteom Bioinform. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabian M.R., Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nat Struct Mol Biol. 2012;19:586–593. doi: 10.1038/nsmb.2296. [DOI] [PubMed] [Google Scholar]
- 16.Kloosterman W.P., Plasterk R.H. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Esquela-Kerscher A., Slack F.J. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 18.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard C.C., Cheng H.H., Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–369. doi: 10.1038/nrg3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren W., Li C., Duan W., Du S., Yang F., Zhou J. MicroRNA-613 represses prostate cancer cell proliferation and invasion through targeting Frizzled7. Biochem Biophys Res Commun. 2015 doi: 10.1016/j.bbrc.2015.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Landi D., Moreno V., Guino E., Vodicka P., Pardini B., Naccarati A. Polymorphisms affecting micro-RNA regulation and associated with the risk of dietary-related cancers: a review from the literature and new evidence for a functional role of rs17281995 (CD86) and rs1051690 (INSR), previously associated with colorectal cancer. Mutat Res. 2011;717:109–115. doi: 10.1016/j.mrfmmm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Ryan B.M., Robles A.I., Harris C.C. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwai N., Naraba H. Polymorphisms in human pre-miRNAs. Biochem Biophys Res Commun. 2005;331:1439–1444. doi: 10.1016/j.bbrc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 24.Yu Z., Li Z., Jolicoeur N., Zhang L., Fortin Y., Wang E. Aberrant allele frequencies of the SNPs located in microRNA target sites are potentially associated with human cancers. Nucl Acids Res. 2007;35:4535–4541. doi: 10.1093/nar/gkm480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S.P., Levesque E., Guillemette C., Yu C.C., Huang C.Y., Lin V.C. Genetic variants in microRNAs and microRNA target sites predict biochemical recurrence after radical prostatectomy in localized prostate cancer. Int J Cancer. 2014;135:2661–2667. doi: 10.1002/ijc.28904. [DOI] [PubMed] [Google Scholar]
- 26.Fan C., Chen C., Wu D. The association between common genetic variant of microRNA-499 and cancer susceptibility: a meta-analysis. Mol Biol Rep. 2013;40:3389–3394. doi: 10.1007/s11033-012-2416-z. [DOI] [PubMed] [Google Scholar]
- 27.Ji H.H., Hong L., Huang G.L., Yin H.X., Xu P., Luo S.Y. Association between microRNA-196a2 rs11614913, microRNA-146a rs2910164, and microRNA-423 rs6505162 polymorphisms and esophageal cancer risk: a meta-analysis. Meta Gene. 2015;3:14–25. doi: 10.1016/j.mgene.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Z., Zhang E., Duan W., Sun C., Bai S., Tan X. The association between miR-499 polymorphism and cancer susceptibility: a meta-analysis. Onco Targets Ther. 2015;8:2179–2186. doi: 10.2147/OTT.S88224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Liu T., Li Z., Zhang L., Zhang Z. The miR-149 rs2292832 T/C polymorphism may decrease digestive cancer susceptibility: an updated meta-analysis. Int J Clin Exp Med. 2015;8:15351–15361. [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y., Li L., Gao J. The association between two common polymorphisms (miR-146a rs2910164 and miR-196a2 rs11614913) and susceptibility to gastric cancer: a meta-analysis. Cancer Biomark. 2015;15:235–248. doi: 10.3233/CBM-150470. [DOI] [PubMed] [Google Scholar]
- 31.Nikolic Z., Savic Pavicevic D., Vucic N., Cidilko S., Filipovic N., Cerovic S. Assessment of association between genetic variants in microRNA genes hsa-miR-499, hsa-miR-196a2 and hsa-miR-27a and prostate cancer risk in Serbian population. Exp Mol Pathol. 2015;99:145–150. doi: 10.1016/j.yexmp.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Xu B., Feng N.H., Li P.C., Tao J., Wu D., Zhang Z.D. A functional polymorphism in Pre-miR-146a gene is associated with prostate cancer risk and mature miR-146a expression in vivo. Prostate. 2010;70:467–472. doi: 10.1002/pros.21080. [DOI] [PubMed] [Google Scholar]
- 33.Hashemi M., Shahkar G., Simforoosh N., Basiri A., Ziaee S.A., Narouie B. Association of polymorphisms in PRKCI gene and risk of prostate cancer in a sample of Iranian population. Cell Mol Biol (Noisy-le-grand) 2015;61:16–21. [PubMed] [Google Scholar]
- 34.Hashemi M., Hanafi Bojd H., Eskandari Nasab E., Bahari A., Hashemzehi N.A., Shafieipour S. Association of adiponectin rs1501299 and rs266729 gene polymorphisms with nonalcoholic fatty liver disease. Hepat Mon. 2013;13:e9527. doi: 10.5812/hepatmon.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashemi M., Eskandari-Nasab E., Zakeri Z., Atabaki M., Bahari G., Jahantigh M. Association of pre-miRNA-146a rs2910164 and pre-miRNA-499 rs3746444 polymorphisms and susceptibility to rheumatoid arthritis. Mol Med Rep. 2013;7:287–291. doi: 10.3892/mmr.2012.1176. [DOI] [PubMed] [Google Scholar]
- 36.Hasani S.S., Hashemi M., Eskandari-Nasab E., Naderi M., Omrani M., Sheybani-Nasab M. A functional polymorphism in the miR-146a gene is associated with the risk of childhood acute lymphoblastic leukemia: a preliminary report. Tumour Biol. 2014;35:219–225. doi: 10.1007/s13277-013-1027-1. [DOI] [PubMed] [Google Scholar]
- 37.Hu Z., Chen J., Tian T., Zhou X., Gu H., Xu L. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008;118:2600–2608. doi: 10.1172/JCI34934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George G.P., Gangwar R., Mandal R.K., Sankhwar S.N., Mittal R.D. Genetic variation in microRNA genes and prostate cancer risk in North Indian population. Mol Biol Rep. 2011;38:1609–1615. doi: 10.1007/s11033-010-0270-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang J., Bi J., Liu X., Li K., Di J., Wang B. Has-miR-146a polymorphism (rs2910164) and cancer risk: a meta-analysis of 19 case-control studies. Mol Biol Rep. 2012;39:4571–4579. doi: 10.1007/s11033-011-1247-7. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y., Gu L., Pan Y., Li R., Gao T., Song G. Different effects of three polymorphisms in MicroRNAs on cancer risk in Asian population: evidence from published literatures. PLoS ONE. 2013;8:e65123. doi: 10.1371/journal.pone.0065123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He B., Pan Y., Cho W.C., Xu Y., Gu L., Nie Z. The association between four genetic variants in microRNAs (rs11614913, rs2910164, rs3746444, rs2292832) and cancer risk: evidence from published studies. PLoS ONE. 2012;7:e49032. doi: 10.1371/journal.pone.0049032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basu S., Majumder S., Bhowal A., Ghosh A., Naskar S., Nandy S. A study of molecular signals deregulating mismatch repair genes in prostate cancer compared to benign prostatic hyperplasia. PLoS ONE. 2015;10:e0125560. doi: 10.1371/journal.pone.0125560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Omrani M., Hashemi M., Eskandari-Nasab E., Hasani S.S., Mashhadi M.A., Arbabi F. Hsa-mir-499 rs3746444 gene polymorphism is associated with susceptibility to breast cancer in an Iranian population. Biomark Med. 2014;8:259–267. doi: 10.2217/bmm.13.118. [DOI] [PubMed] [Google Scholar]
- 44.Loktionov A. Common gene polymorphisms, cancer progression and prognosis. Cancer Lett. 2004;208:1–33. doi: 10.1016/j.canlet.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Akkiz H., Bayram S., Bekar A., Akgollu E., Uskudar O. Genetic variation in the microRNA-499 gene and hepatocellular carcinoma risk in a Turkish population: lack of any association in a case-control study. Asian Pac J Cancer Prev. 2011;12:3107–3112. [PubMed] [Google Scholar]
- 46.Gansmo L.B., Romundstad P., Birkeland E., Hveem K., Vatten L., Knappskog S. MDM4 SNP34091 (rs4245739) and its effect on breast-, colon-, lung-, and prostate cancer risk. Cancer Med. 2015 doi: 10.1002/cam4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stegeman S., Moya L., Selth L.A., Spurdle A.B., Clements J.A., Batra J. A genetic variant of MDM4 influences regulation by multiple microRNAs in prostate cancer. Endocr Relat Cancer. 2015;22:265–276. doi: 10.1530/ERC-15-0013. [DOI] [PubMed] [Google Scholar]
- 48.Chu H., Zhong D., Tang J., Li J., Xue Y., Tong N. A functional variant in miR-143 promoter contributes to prostate cancer risk. Arch Toxicol. 2014 doi: 10.1007/s00204-014-1396-2. [DOI] [PubMed] [Google Scholar]
- 49.Ma X.P., Zhang T., Peng B., Yu L., Jiang de K. Association between microRNA polymorphisms and cancer risk based on the findings of 66 case-control studies. PLoS ONE. 2013;8:e79584. doi: 10.1371/journal.pone.0079584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J., Liu J., Wei M., He Y., Liao B., Liao G. Genetic variants in the microRNA machinery gene GEMIN4 are associated with risk of prostate cancer: a case-control study of the Chinese Han population. DNA Cell Biol. 2012;31:1296–1302. doi: 10.1089/dna.2011.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng N., Xu B., Tao J., Li P., Cheng G., Min Z. A miR-125b binding site polymorphism in bone morphogenetic protein membrane receptor type IB gene and prostate cancer risk in China. Mol Biol Rep. 2012;39:369–373. doi: 10.1007/s11033-011-0747-9. [DOI] [PubMed] [Google Scholar]
- 52.Man Y.G., Fu S.W., Liu A.J., Stojadinovic A., Izadjoo M.J., Chen L. Aberrant expression of chromogranin A, miR-146a, and miR-146b-5p in prostate structures with focally disrupted basal cell layers: an early sign of invasion and hormone-refractory cancer? Cancer Genom Proteom. 2011;8:235–244. [PubMed] [Google Scholar]



