Graphical abstract
Keywords: Chromium, Alfa fetoprotein, COMET, Micronucleated RBCs, Oxidative stress, Radish
Abstract
To study the impact of radish oil on the possible genotoxic and hepatotoxic effects of hexavalent chromium, male rats were divided into 4 groups. Group 1 served as control, group 2 received radish oil at the recommended human therapeutic dose (0.07 mL/kg) by gavage, group 3 received sodium dichromate dihydrate (SDD) 520 mg/L in drinking water, and group 4 received both SDD and radish oil as previously mentioned in groups 2 and 3. All treatments were continued for six months. The results revealed that chromium exposure promoted oxidative stress with a consequently marked hepatic histopathological alterations, increased serum alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities, alfa fetoprotein (AFP) levels, and micronucleated erythrocytes (MNE) % in peripheral blood. Moreover, COMET assay of hepatic DNA revealed that SDD exposure significantly decreased the intact cells %, head diameter, and head DNA % compared to control, indicating DNA damage. However, radish oil co-administration with SDD resulted in marked amendment in the altered parameters as detected by improved liver function markers (ALT and ALP) and AFP level, decreased lipid peroxidation, increased antioxidant markers, inhibited hepatic DNA damage and restored the hepatic histology by preventing the appearance of the altered hepatocytes’ foci and decreasing chromium induced histopathological lesions. It could be concluded that radish oil was able to provide a convergent complete protection against the geno- and hepatotoxicity of chromium by its potent antioxidant effect.
Introduction
Chromium is a naturally occurring and widely distributed element in the environment. It could be found in rocks, soil, animals, plants and volcanic dust and gases. Chromium predominates in the environment in one of two valence states: the trivalent, which occurs naturally and is an essential nutrient, and the hexavalent state which is commonly produced by industrial processes [1].
Chromium emission to the environment takes place through several ways including anthropogenic sources (e.g. combustion of fuels, metallic industries such as manufacture of dyes and pigments, leather and wood preservation, and treatment of cooling tower water) and natural sources (e.g., forest fires) [1]. The trivalent chromium is one of the essential nutrients that involved in regulation of carbohydrate, lipid, and protein metabolism through enhancement of insulin action [2]; hence, it is widely used in diabetics and appetite control medicines. The reason for mammals’ need for chromium is to keep balanced glucose metabolism and for its anabolic function [3]. It had been known that the exposure to high concentrations of hexavalent chromium could result in severe multisystem damage such as respiratory, cardiovascular, gastrointestinal, hepatic and renal damage and potentially death [4]. In addition, prolonged exposure to trivalent chromium had been reported to result in weight loss, anemia, liver dysfunction and renal failure [5].
Recently, much alertness has been paid to the role of antioxidants in protecting the cells against chromium induced injuries such as DNA damage, lipid peroxidation, enzyme inhibition, cytotoxicity and mutations [5]. Radish (Raphanus sativus L.) is a cruciferous vegetable. It is not only a vegetable crop but also an important source of medicinal compounds and from antiquity, it has been used in folk medicine against many toxicants [6]. The unique chemical properties of the constituents in the non-traditional vegetable oils make them very important and could boost the other edible oil sources, among them is radish oil which is known to possess major antioxidants and anticarcinogenic properties against many chemically induced toxicities [7], [8]. The crude extract of radish was found to possess marked antioxidant enzyme activities and radish root could inhibit the membrane changes, affect the natural scavenging activity and protect the cell membrane against lipid peroxidation [6].
Moreover, Raphani Semen has been found to contain many active compounds such as alkaloids, glucosinolates, brassinosteroids, and flavonoids [9], [10]. In fact, most of these phytochemicals have been shown to have different bioactivities such as antioxidant, hepatoprotective, antimutagenic, anticarcinogenic and antimicrobial.
The present investigation was designed to study the possible ameliorative effect of radish (R. sativus) oil on the genotoxic and hepatotoxic effects following chronic SDD exposure in terms of antioxidant status, micronucleus assay, and hepatic DNA damage using comet assay as well as histopathological examination.
Material and methods
Animals
Forty-four adult male Sprague–Dawley rats with an average weight 150–160 g were obtained from the animal house unit belonging to Dept. of Forensic Medicine, Faculty of Vet. Med., Cairo University. Rats were maintained in stainless steel cages, at a temperature of 25 ± 5 °C, 60% humidity, suitable illumination conditions (light/dark cycle) and good ventilation. They were allowed for standard commercial rodent pellets (Kafr El-Zayat Co. for Feeds, 57031, Cairo, Egypt) and water ad libitum throughout the experiment. Animals were left for acclimatization in the laboratory conditions for one week prior to use. All experiments using animals were performed according to the protocol approved by the Institutional Animal Care and Use Committee at Cairo University (IACUC).
Chemicals
Sodium dichromate dihydrate “SDD” was obtained from BDH Chemicals Ltd Poole England (Item no is 30130).
Preparation of radish oil
Radish oil was prepared by the cold pressing technique [11] of R. sativus var niger seeds that were obtained from Gaara Quality Seeds Company, Cairo, Egypt. It was identified and authenticated in Dept. of Botany, faculty of science, Cairo University.
Experimental design
Animals were randomly divided into 4 groups, 11 animals each. Rats of group 1 (G1) were kept as control. Rats of G2 were exposed to radish oil once daily by gavage at the recommended human therapeutic dose (0.07 ml/kg) which was converted to rat therapeutic dose [13]. Rats of G3 were supplied with freshly prepared solution of SDD 520 mg/L drinking water (equivalent to 182 mg/L of Cr VI) [12]. Rats of G4 were exposed to both SDD and radish oil as previously mentioned in G2 and G3. Radish oil was given one hour prior to SDD administration. All treatments continued for six months. At the end of the experimental period, rats of all groups were sacrificed under gentle diethyl-ether anesthesia prior to which blood samples were collected from the retro-orbital venous plexus of each animal in clean sterile tubes. They were left to stand for 30 min at room temperature and then they were centrifuged at 3000 rpm for 15 min. The clean supernatant serum was collected and used for determination of serum activities of ALT, ALP, and the carcinogenicity marker; AFP level.
After scarification, liver was carefully dissected out, blotted free of blood and divided into two parts, one was kept in 10% buffered neutral formalin for histopathological examination and the other was kept at −20 °C for further investigations (Assessment of antioxidant markers, chromium residues and DNA damage).
Assessment of liver function markers and AFP level
Liver function markers ALT [14] and ALP [15] were assessed calorimetrically in serum samples of all groups using commercial kits (Bio-Diagnostic Co., Cairo, Egypt). The serum carcinogenicity marker; Oncofetal antigen; alfa fetoprotein (AFP) was determined by ELISA technique using a ready-made kits (Wkea Med Supplies Corp, China).
Evaluation of antioxidant marker in liver tissue homogenate
Briefly homogenization of liver tissue was carried out in 5–10 ml cold buffer (50 mM potassium phosphate (pH7.5) with 1 mM EDTA or 1 mM EDTA and 1 ml/l Triton X-100) per gram tissue for MDA, GSH and CAT assays respectively. All tissue homogenates were then centrifuged at 5000 rpm for 20 min at 4 °C and the supernatants were aspirated and transferred into Eppendorf tubes, and preserved at −80 °C in a deep freezer until used.
The lipid peroxidation marker; malondialdehyde level (MDA) [16], catalase activity [17] and glutathione (GSH) content [18] were assessed in liver tissue homogenates of all animals.
Estimation of chromium residues in liver tissue
Wet digestion of liver tissues followed by homogenates preparation was carried out and the obtained solutions were used for estimation of chromium residue by Atomic Absorption Spectrophotometer (AAS, unicam 969) [19]. Simply wet digestion of liver tissue specimens was carried out in a mixture of nitric acid and hydrogen peroxide (3:1) and the samples were left for 10 min and then were putted on hot plate at 60–70 °C for 1 h with addition of nitric acid till sample becomes colorless.
Genotoxicity investigations
Micronucleus (MN) assay
Fresh blood samples from each rat of the experimental as well as control groups were smeared onto clean glass slides. The slides were air-dried for 1–2 h and then fixed in absolute methanol for 10 min. The slides were then stained with aqueous Giemsa (5%) for about 10 min [20]. For each rat’s group, five microscopic slides were prepared, and 100 cells/rat were analyzed, totaling 500 erythrocytes/group. The frequencies of micronuclei in erythrocytes were detected under a Binocular microscope using a 1000× oil-immersion lens.
Assessment of DNA damage using comet assay
Hepatic tissue preparation and hepatocytes isolation were carried out according to the method adopted by Velma and Tchounwou [21]. After isolation and counting, hepatocytes were suspended in 1 ml ice-cold PBS with 1 × 105 cells/ml for use in the single cell gel electrophoresis (comet) assay as described by Velma and Tchounwou [21].
Histopathological examination
Formalin fixed liver specimens were routinely dehydrated by graded series of alcohol, cleared in xylol and finally embedded in paraffin. Paraffin blocks were serially sectioned at 4–5 μm thickness and stained with H&E [22]. Azan stain was used on need [22]. Four point numerical scoring system has been used to express the degree of severity of the observed histopathological lesions (where 0 indicates no change and 1, 2, and 3 indicate mild, moderate and severe changes respectively), while the grading was determined by percentage according to Arsad et al. [23] as follows: changes less than 30% (<30%) indicating mild changes, changes less than 30–50% (<30–50%) indicating moderate changes and changes more than 50% (>50%) indicating severe changes.
Statistical analysis
A statistical analysis was performed using the statistical software SPSS16 (SPPS Inc. Chicago, IL, USA). Statistical significance of SDD and/or radish oil treatment effects between the different groups was tested using one-way ANOVA followed by Tukey’s post hoc test. The obtained data are expressed as mean ± SE.
Results
Effect on liver function markers and oncogene marker; AFP
Radish oil exposed rats showed non-significant difference in their ALT, ALP activities and AFP levels when compared with control sets. However, exposure of rats to SDD alone for six months resulted in significant (P < 0.05) increase in serum ALT, ALP activities (Fig. 1) and AFP levels (Fig. 2) compared with that of control rats. The later effect was significantly reversed by the co-administration of radish oil with SDD as evidenced by significant (P < 0.05) decrement in serum ALT, ALP activities and AFP levels when compared with the sole exposure to SDD.
Fig. 1.
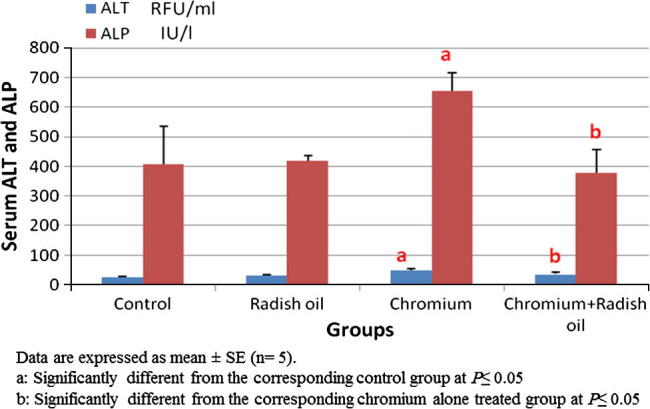
The effect of SDD, radish oil and their co administration on serum levels of liver function markers; ALT and ALP of all groups.
Fig. 2.
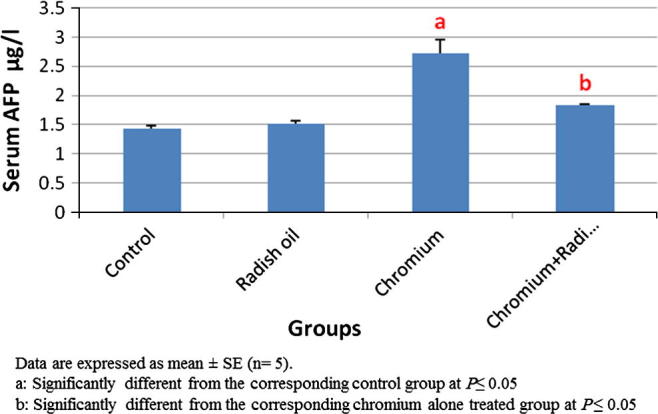
The effect of SDD, radish oil and their co administration on serum AFP level of all groups.
Effect on oxidative stress markers
Treatment of rats with radish oil alone exhibited non-significant changes in their hepatic tissue MDA level and GSH contents as well as catalase activity when compared with control group. On contrary, significant (P < 0.05) elevation of hepatic MDA content (Fig. 3) accompanied with significant (P < 0.05) reduction in hepatic GSH content and catalase activity (Fig. 4) were observed in SDD treated rats. The co-administration of radish oil with SDD exhibited significant (P < 0.05) recuperation of that altered oxidative markers by significant decrease in the elevated MDA level and significant (P < 0.05) increase in the hepatic GSH content and catalase activity.
Fig. 3.
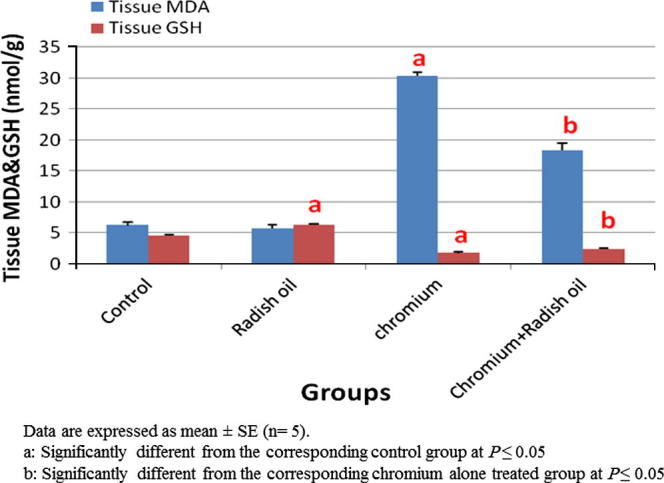
The effect of SDD, radish oil and their co administration on hepatic MDA and GSH contents in all groups.
Fig. 4.
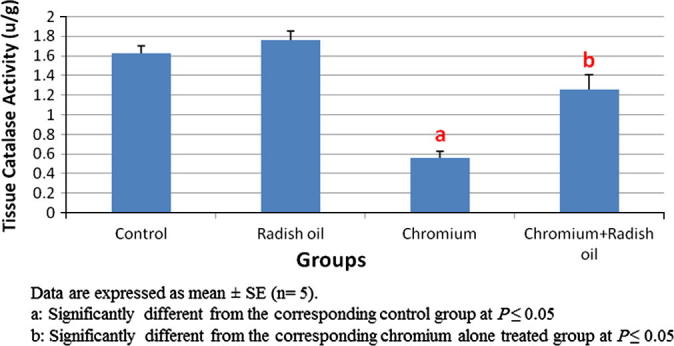
The effect of SDD, radish oil and their co administration on hepatic catalase activity of all groups.
Effect on hepatic chromium content
Atomic absorption spectrophotometer assay of Cr residue in hepatic tissue homogenates of all groups’ revealed significant (P < 0.05) increase in Cr content in SDD-treated rats compared with that of controls. Both of the control and radish oil treated rats showed traces of Cr in their hepatic tissue homogenates. However, the co-treatment with radish oil significantly (P < 0.05) decreased that elevated Cr residues in hepatic tissue (Fig. 5).
Fig. 5.
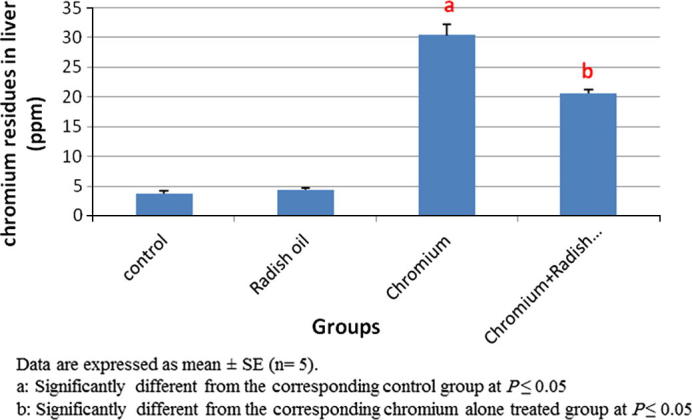
The effect of SDD, radish oil and their co administration on chromium content in hepatic tissue of all groups.
Micronucleus assay
The observation of small, non-refractive, circular or ovoid chromatin bodies in the erythrocytes (E) was considered as MN. It was noticed that there was a significant (P < 0.05) increase in the incidence of micronucleated erythrocytes (MNE) of the sole SDD-exposed rats when compared with both control and radish oil-treated rats (Table 1). The co-treatment of SDD and radish oil together ameliorated this effect and significantly lowered the incidence of MNE in SDD + radish oil-treated group compared to SDD alone-exposed group.
Table 1.
Effects of SDD and/or Radish oil on the incidence of micro nucleated erythrocytes (MNE) in peripheral blood of exposed groups.
| Group | Parameter |
|
|---|---|---|
| Total MNE/100 | MNE % | |
| Control | 0 | 0 |
| Radish oil | 0 | 0 |
| SDD | 4 ± 0.003a | 4a |
| SDD + Radish oil | 1 ± 0.004b | 1b |
Data are presented as mean ± SE (n = 5 rats/group).
Significantly different from groups 1 and 2 at P < 0.05.
Significantly different from group 3 at P < 0.05.
Comet assay
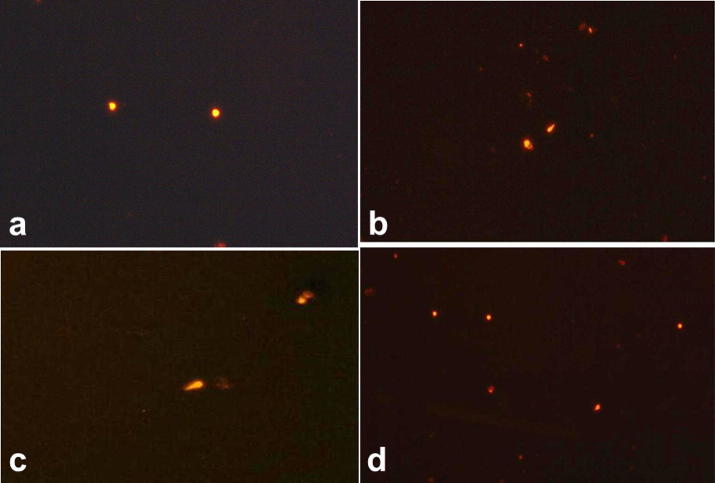
Comet assay of hepatic DNA of SDD exposed rats exhibited higher hepatocytes’ DNA damage (Observed as DNA breakage and moving of the broken part “tail” in the gel to a distance from the whole DNA “head”) than that of control (Fig. 6). Exposure to SDD induced significant (P < 0.05) decrease in intact cells %, head diameter, and head DNA % as well as significant (P < 0.05) increase in tailed cells %, tail DNA% and tail length (Table 2) indicating DNA single-strand breaks and/or alkali-labile sites. All these parameters of DNA damage were significantly reversed on the co-administration of radish oil and SDD. Radish oil alone exposure of rats resulted in non-significant DNA damage.
Fig. 6.
Comet assay image of DNA: (a) control, (b and c) SDD treated and (d) SDD + radish oil co-treated rats.
Table 2.
Comet assay of hepatic DNA of control, radish oil, SDD treated, and SDD + radish oil co-treated rats after six months.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | Radish oil | SDD | SDD + radish oil | |
| Intact cells % | 81.00 ± 1.60 | 80.29 ± 1.70 | 64.80 ± 2.60a,b | 76.33 ± 0.90 |
| Tailed cells % | 19.00 ± 1.65 | 19.81 ± 1.85 | 35.19 ± 2.45a,b | 23.67 ± 1.07 |
| Tailed cells | ||||
| Head diameter (Px) | 62.42 ± 2.33 | 61.50 ± 2.16 | 43.27 ± 2.88a,b | 58.73 ± 4.77 |
| Head DNA % | 91.33 ± 1.63 | 90.51 ± 1.55 | 69.90 ± 3.30a,b | 86.20 ± 2.19 |
| Tail DNA % | 8.67 ± 0.49 | 9.49 ± 0.69 | 30.09 ± 2.21a,b | 13.7 ± 2.19 |
| Tail length (pexel) | 18.88 ± 0.87 | 18.24 ± 0.94 | 27.08 ± 1.78a,b | 20.24 ± 2.05 |
Data are expressed as mean ± SE (n = 5).
Significantly different from corresponding control group at P ⩽ 0.05.
Significantly different from corresponding SDD + radish oil co-treated group at P ⩽ 0.05.
Histopathological examination
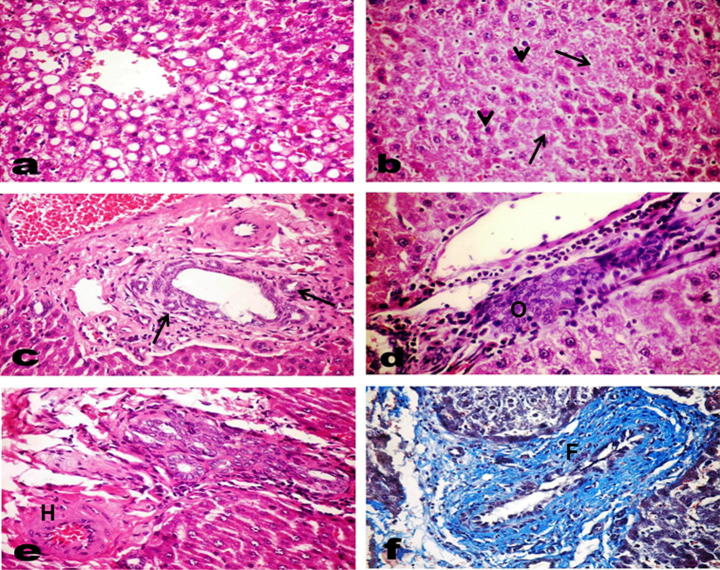
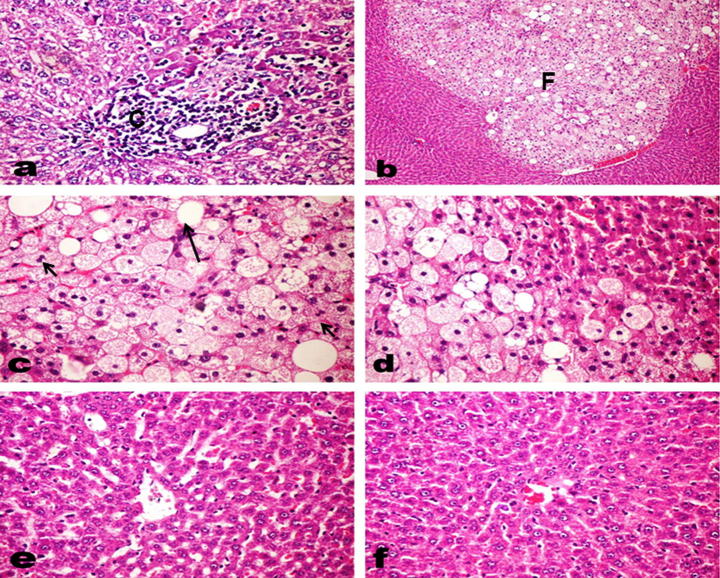
Examination of different liver sections from control and radish oil treated groups revealed normal hepatic architecture and parenchyma. However, liver of SDD-administrated rats exhibited detrimental histopathological changes which were summarized and scored in Table 3. Various degenerative changes in the hepatocytes mostly granular and vacuolar degeneration (Fig.7a), kupffer cells activation and variable degrees of hepatocellular necrosis were observed; the necrotic cells appeared either with pyknotic or karyolitic nuclei or without any nuclear structure (Fig.7b). Foci of necrotic hepatocytes replaced by mononuclear inflammatory cells were observed. Several apoptotic cells were observed. The portal triads showed vascular congestion with sometimes thickening and hyalinization of the blood vessel wall, marked bile duct hyperplasia with multiple newly formed bile ducteols (Fig.7c), oval cell hyperplasia in some cases (Fig.7d), moderate periductal fibroplasia with marked edema (Fig.7e and f) and inflammatory infiltrates of moderate intensity (Fig.8a). Interestingly, seven rats of this group showed altered hepatocellular foci of mixed type (Fig.8b). The hepatocytes in such foci appeared large, with clear and eosinophilic cytoplasm and mostly centrally located nuclei (Fig.8c and d). In contrast, liver of rats co-treated with radish oil and SDD did not show any of those altered foci and it was noticed that radish oil could markedly reduce the SDD-induced histopathological changes at which the hepatocyte appeared near to normal pattern with mild to moderate degrees of hepatocellular degeneration and few necrotic cells as well as Kupffer cell activation (Fig.8e and f). The portal triads appeared normal with no or few inflammatory infiltrates and mild proliferation of bile duct epithelium.
Table 3.
Lesion scores of liver of control, SDD treated, radish oil treated, and SDD + radish oil co-treated rats.
| Control |
Radish oil treated group |
SDD treated group |
SDD + radish oil treated group |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of animals showed the lesion | Numerical score | No. of animals showed the lesion | Numerical score | No. of animals showed the lesion | Numerical score | No. of animals showed the lesion | Numerical score | |
| A. Parenchymal changes | ||||||||
| 1. Cytoplasmic vacuolation | 0 | 0 | 2 | 0.18 ± 0.12 | 11 | 2.63 ± 0.15a | 6 | 0.55 ± 0.16b |
| 2. Hepatocellular necrosis | 0 | 0 | 2 | 0.18 ± 0.12 | 11 | 2.63 ± 0.15a | 5 | 0.54 ± 0.20b |
| 3. Kupffer cells activation | 0 | 0 | 1 | 0.09 ± 0.09 | 11 | 2.63 ± 0.15a | 7 | 0.81 ± 0.22b |
| 4. Altered hepatocellular foci | 0 | 0 | 0 | 0 | 7 | 1.63 ± 0.41a | 0 | 0 ± 0b |
| B. Portal changes | ||||||||
| 1. Inflammatory cell infiltration | 0 | 0 | 0 | 0 | 9 | 2.00 ± 0.33a | 4 | 0.36 ± 0.15b |
| 2. Bile duct hyperplasia | 0 | 0 | 0 | 0 | 9 | 1.54 ± 0.28a | 3 | 0.27 ± 0.14b |
| 3. Fibroplasia | 0 | 0 | 0 | 0 | 8 | 1.27 ± 0.27a | 0 | 0 ± 0b |
| 4. Oval cell hyperplasia | 0 | 0 | 0 | 0 | 7 | 1.09 ± 0.28a | 0 | 0 ± 0b |
Data are expressed as mean ± SE (n = 11).
Significantly different from corresponding control group at P ⩽ 0.05.
Significantly different from corresponding SDD treated group at P ⩽ 0.05.
Fig. 7.
Liver of SDD treated rats showing (a) Marked granular and vacuolar degeneration of the hepatocytes. (b) Hepatocellular necrosis; some appear with pyknotic nuclei and others without any nuclear structure (arrow), notice the appearance of apoptotic cells (arrow head). (c–f) Portal triads showing (c) marked bile duct hyperplasia with multiple newly formed bile ducteols (arrow). (d) Oval cell hyperplasia (O). (e) Moderate periductal fibroplasia, marked edema and thickening and hyalinization (H) of the blood vessel wall. (f) Marked periductul fibroplasia (F) with Azan stain.
Fig. 8.
(a–d) Liver of SDD treated rats showing (a) moderate inflammatory cell infiltration in the portal triad (C). (b) Altered hepatocellular focus of mixed type (F). (c and d) Hepatocytes in the altered focus appearing large, with clear (long arrow) and eosinophilic (short arrow) cytoplasm and mostly centrally located nuclei. (e and f) Liver of SDD and radish oil co treated rats showing moderate degrees of hepatocellular degeneration, scattered necrotic cells and kupffer cell activation.
Discussion
Liver is considered as a major target organ for many toxicants and it is a site for biotransformation of most of the xenobiotic compounds. It is well known that liver contains metabolizing enzymes that could change toxic agents to less toxic ones. In addition, it is important in oxidative stress tests because lipid peroxidation is a major cause of liver pathology. Hence, the present study was undertaken to assess the hepatocellular damage, gentotoxic effect and antioxidant markers depletion induced by hexavalent chromium. The present work investigated both ALT and ALP activities as liver function markers. ALT is well known as a sensitive indicator of acute hepatic necrosis [24], while ALP is known to be indicative of hepatobiliary diseases [25]. It was observed that the sole administration of SDD resulted in significant increase in serum ALT and ALP activities reflecting liver dysfunction, a result which is in agreement with many previous investigations [26], [27]. Those elevated levels of both enzymes markers reflect a marked hepatocellular and hepatobiliary alteration as a result of chromium toxicity. Reactive oxygen species (ROS) generated by chromium could attack the polyunsaturated fatty acids of the lipid membrane and cause lipid peroxidation and disruption of the plasma membrane leading to leak of the intracellular enzymes into the blood stream. A significant increase in hepatic MDA level accompanied with significant decrease in GSH content and CAT activity was observed in SDD-treated rats compared with control sets. This comes in harmony with previous studies [28], [29]. Once Cr (VI) is taken inside the cell, it undergoes a reduction division by the cellular reductants to generate different reactive chromium intermediates, such as Cr (V) and Cr (IV). During Cr (VI) reduction process, molecular oxygen is reduced to superoxide radical which subsequently forms hydrogen peroxide and ROS are produced. The increased ROS production due to Cr (VI) exposure may lead to generation of oxidative stress, which is responsible for many deleterious effects in the cell including DNA damage, lipid peroxidation and protein modification [30]. In addition, increased free radical generation by chromium may cause depletion of the antioxidants enzymes due to their consumption in the scavenging of free radical generated by the effect of chromium [28], [29].
Many previous literatures presented the hepatoprotective effects of radish oil and its active constituents against drugs, chemicals and xenobiotics. That protective role may be mediated through several actions: the first of which could be by prevention of excessive free radical production due to its sulphated and phenolic compound contents [31] and the second through prevention of lipid peroxidation. This is in accordance with the results of Salah-Abbes et al. [6] who found that the co-treatment of Balb/c mice with zearalenone and radish extract resulted in restoration of the antioxidant enzyme activities.
The current study showed that SDD administration to rats induced DNA damage in cells as observed by MN induction in RBCs and increased tail-DNA percent in comet assay of hepatic cells. Similar results were obtained by Ahmed et al. [32]. It has been reported that Cr (VI) itself is not reactive to DNA; however, the produced chromium metabolites (radicals) during reduction of the unstable Cr (VI) inside the body, can subsequently attack macromolecules and lead to multiform DNA damages such as strand breakage, DNA–protein cross-links, DNA–DNA cross-links and others. In particular DNA strand breaks are mainly ascribed to the ROS [28]. Moreover, replication arrest could be resulted from the response of DNA polymerase to DNA–Cr lesions and subsequently lead to inhibition of DNA synthesis and s-phase cell-cycle delay that occurs in mammalian cells treated with genotoxic hexavalent chromium [33]. All of the observed DNA damage parameters were reversed on the co-administration of radish with SDD. The possible mechanism of protection offered by radish oil against Cr-induced genotoxicity and DNA damage is its ability to inhibit the oxidative process by neutralizing reactive oxygen species and enhance the DNA-repair system or DNA synthesis [6].
The observed histopathological results of SDD treated group revealed severe hepatic alteration including hepatocellular degeneration, necrosis, portal triads’ reaction and appearance of altered hepatocellular foci of mixed type, all indicating the hepatotoxic effect of chromium. Several studies have confirmed the hepatotoxic effect of hexavalent chromium on the liver [29], [34]. Chromium-induced hepatotoxic effect may be attributed to the formation of highly reactive radicals that initiate lipid peroxidation and damage the various cellular components [34].
In spite of the fact that oxidative stress leads to mitochondrial dysfunction, decreased oxygen consumption and ATP production, alteration in calcium homeostasis, oxidation of DNA, lipids and proteins as well as permeability transition pores (PTP) opening [35], [36], the observed altered hepatocellular foci in most of SDD-treated rats could be a precarcinogenic lesion and confirmed the DNA damage. It has been reported that the altered hepatocellular foci usually precede neoplastic proliferation and they have been categorized as preneoplastic lesions although they could be reversible [37]. This SDD-induced carcinogenic potential could be confirmed by the recorded significant increase in serum AFP levels compared to control.
The observed apoptosis may be a result of oxidative stress-induced increase in permeability of the mitochondrial membrane with formation of non-specific pores in the inner membrane [38]. Those pores cause membrane depolarization, calcium release, and rupture of outer membrane with release of intermembrane components that induce apoptosis. Oval cell proliferation may be a relatively unique response of the rodent liver in case of toxicity; it is being believed to differentiate into hepatocytes or biliary cells.
On contrary, radish oil co-administration with SDD resulted in an obvious repairing of the histological architecture of the liver with the apparent absence of altered hepatocellular foci, a result which could be related to the great antioxidant effect of radish oil which could protect the cellular components against the detrimental effect of SDD and its metabolites on the cells. In addition, glucosinolate contents in R. sativus are well-known protectors against carcinogenesis [6].
The cruciferous compounds in the radish extract activate the hepatic antioxidant defense system a process which is mediated by several enzymes functioning in a concerted manner in removing peroxide and superoxide anions generated within the cell after zearalenone damage [39]. In this regard, Sipos et al. [40] demonstrated that radish root extract protected cell membrane against lipid peroxidation in rats fed on a fat-rich diet.
Conclusions
It can be concluded that chronic exposure to Sodium dichromate dihydrate resulted in oxidative stress, impairment in hepatic function markers, histopathological changes and induced cellular DNA damage observed by MN induction in RBCs and increased tail-DNA percent in comet assay of hepatic cells. Radish oil co-administration showed marked antioxidant effect and resulted in approximate complete protection in terms of oxidative stress, hepatic histology, biochemical changes and genotoxicity parameters.
Conflict of Interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Joel B. Chromium chemistry implications for environmental fate and toxicity. J Soil Contam. 1997;6(6):561–568. [Google Scholar]
- 2.Anderson R.A. Essential and toxic trace elements in human health and disease: an update. Wiley-Liss, Inc; New York: 1993. Recent advances in the clinical and biochemical effects of chromium deficiency; pp. 221–234. [PubMed] [Google Scholar]
- 3.Evans G.W. The effect of chromium picolinate on insulin controlled parameters in humans. Int J Biosoc Med Res. 1989;11:163–180. [Google Scholar]
- 4.Assem L., Zhu H. Institute of Environment and Health, Cranfield University; 2007. “Chromium, Toxicological overview”. Prepared by L Assem and H Zhu. [Google Scholar]
- 5.Sugiyama M. Role of cellular antioxidants in metal-induced damage. Cell Biol Toxicol. 1994;10:1–22. doi: 10.1007/BF00757183. [DOI] [PubMed] [Google Scholar]
- 6.Salah-Abbès J.B., Abbès S., Ouanes Z., Houas Z., Abdel-Wahhab M.A., Bacha H. Isothiocyanate from the Tunisian radish (Raphanus sativus) prevents genotoxicity of Zearalenone in vivo and in vitro. Mut Res. 2009;677:59–65. doi: 10.1016/j.mrgentox.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Ramadan M.F., Moersel J.T. Screening of antiradical action of vegetable oils. J Food Comp Anal. 2006;19:838–842. [Google Scholar]
- 8.Chung D.H., Kim S.H., Myung N., Cho K.J., Chang M.J. The antihypertensive effect of ethyl acetate extract of radish leaves in spontaneously hypertensive rats. Nutr Res Pract. 2012;6(4):308–314. doi: 10.4162/nrp.2012.6.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutíerrez R.M.P., Perez R.L. Raphanus sativus (Radish): their chemistry and biology. The Sci W J. 2004;4:811–837. doi: 10.1100/tsw.2004.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan P., Jiang H.Y., Lu W.H. A research review of Raphani Semen. J Pract Trad Chin Med. 2005;21:254–255. [Google Scholar]
- 11.Rusinek R., Rybczynski R., Tys J., Gawrysiak-Wituliska M., Nogala- Kaluska M., Siger A. The process parameters for non-typical seeds during simulated cold deep oil expression. Czech J Food Sci. 2012;30(2):126–134. [Google Scholar]
- 12.Thompson C.M., Proctor D.M., Suh M., Haws L.C., Hebert C.D., Mann J.F. Comparison of the effects of hexavalent chromium in the alimentary canal of F344 rats and B6C3F1 mice following exposure in drinking water: implications for carcinogenic modes of action. Toxicol Sci. 2012;125:79–90. doi: 10.1093/toxsci/kfr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TACTWORLD © Central Pharma Group. Radish oil, the recommended human therapeutic dose of Radidsh oil. <http://www.tactworld.com/e_oils/radish.htm>; 2010.
- 14.Reitman A., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–68. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 15.Belfield A., Goldberg D. Colorimetric determination of alkaline phosphates activity. Enzyme. 1971;12:561–568. [Google Scholar]
- 16.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chem Acta. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 17.Aebi H. Catalase in Vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 18.Beutler E., Duron O., Kellin B.M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 19.Dey S.S., Roy S. Effect of chromium on certain aspects of cellular toxicity. Iranian J Toxicol. 2009;2(4):260–267. [Google Scholar]
- 20.Palhares D., Grisolia C.K. Comparison between the micronucleus frequencies of kidney and gill erythrocytes in Tilapia fish, following Mitomycin C treatment. Genet. Mol. Biol. 2002;25:281–284. [Google Scholar]
- 21.Velma V., Tchounwou P.B. Oxidative stress and DNA damage induced by chromium in liver and kidney of goldfish, Carassius auratus. Biomark Insights. 2013;8:43–51. doi: 10.4137/BMI.S11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bancroft J.D., Gamble M. 6th ed. Elsevier; Churchill Livingstone, China: 2008. Theory and practice of histological techniques. [Google Scholar]
- 23.Arsad S.S., Esa N.M., Hamzah H. Histopathologic changes in liver and kidney tissues from male Sprague Dawley rats treated with Rhaphidophora decursiva (Roxb.) schott extract. J Cytol Histol. 2014;S4 [Google Scholar]
- 24.Abdel-Wahab M.A., Ahmed H.H., Hagazi M.M. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J Appl Toxicol. 2006;26:229–238. doi: 10.1002/jat.1127. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Wahab M.A., Nada S.A., Khalil F.A. Physiological and toxicological responses in rats fed aflatoxin-contaminated diet with or without sorbent materials. Anim Feed Sci Technol. 2002;107:401–411. [Google Scholar]
- 26.Glaser U., Hochrainer D., Steinhoff D. Investigation of irritating properties of inhaled Cr (VI) with possible influence on its carcinogenic action. In: Seemayer N.O., Hadnagy W., editors. Environmental hygiene II. Springer-Verlag; Berlin/New York: 1990. [Google Scholar]
- 27.García-Niño W., Tapia E., Zazueta C., Zatarain-Barrón Z.L., Hernández-Pando R., Vega-García C.C. Curcumin pretreatment prevents potassium dichromate-induced hepatotoxicity, oxidative stress, decreased respiratory complex I activity, and membrane permeability. Evid Based Compl Alternate Med. 2013:1–19. doi: 10.1155/2013/424692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X.F., Xing M.L., Shen Y., Xhu X., Xu L.H. Oral administration of Cr (VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicology. 2006;228(1):16–23. doi: 10.1016/j.tox.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Shati A.A. Ameliorative effect of vitamin E on potassium dichromate- induced hepatotoxicity in rats. J King Sad Univ. 2013;25(3):181–189. [Google Scholar]
- 30.Stohs J.S., Bagchi D., Hassoun E., Bagchi M. Oxidative mechanism in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol. 2001;20(2):77–88. [PubMed] [Google Scholar]
- 31.Rafatullah S., Al-Sheikh A., Alqsoumi S., Al-Yahya M., El-Tahir K., Galal A. Protective effect of fresh radish juice (Raphanus sativus L.) against carbon tetrachloride-induced hepatotoxicity. Int J Pharma. 2008;4(2):130–134. [Google Scholar]
- 32.Ahmed M.K., Kundu G.K., Al-Mamun M.H., Sarkar S.K., Akter M.S., Khan M.S. Chromium (VI) induced acute toxicity and genotoxicity in fresh water stinging catfish, Heteropneustes fossilis. Ecotoxicol Environ Safety. 2013;92:64–70. doi: 10.1016/j.ecoenv.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Bridgewater L.C., Manning F.C.R., Patierno S.R. Arrest of replication by mammalian DNA polymerases alpha and beta caused by chromium-DNA lesions. Mol Carcinog. 1998;23(4):201–206. [PubMed] [Google Scholar]
- 34.Acharya S., Mehta K., Krishnan S., Rao C.V. A subtoxic interactive toxicity study of ethanol and chromium in male Wistar rats. Alcohol. 2001;23(2):99–108. doi: 10.1016/s0741-8329(00)00139-7. [DOI] [PubMed] [Google Scholar]
- 35.Calabrese V., Lodi R., Tonon C., D’Agata V., Sapienza M., Scapagnini G. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J Neurol Sci. 2005;233(1–2):145–162. doi: 10.1016/j.jns.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira C.P., Coelho A.M., Barbeiro H.V., Lima V.M., Soriano F., Ribeiro C. Liver mitochondrial dysfunction and oxidative stess in the pathogenesis of experimental nonalcoholic fatty liver disease. Braz J Med Biol Res. 2006;39(2):189–194. doi: 10.1590/s0100-879x2006000200004. [DOI] [PubMed] [Google Scholar]
- 37.Eldridge S.R., Goldsworthy S.M. Cell proliferation rates in common cancer target tissues of B6C3F1 mice and F344 rats: effects of age, gender, and choice of marker. Fundam Appl Toxicol. 1996;32:159–167. doi: 10.1006/faat.1996.0119. [DOI] [PubMed] [Google Scholar]
- 38.Javadov S., Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007;20(1–4):1–22. doi: 10.1159/000103747. [DOI] [PubMed] [Google Scholar]
- 39.Shklar G. Mechanisms of cancer inhibition by antioxidant nutrients. Oral Oncol. 1998;34(1):24–29. doi: 10.1016/s1368-8375(97)00060-2. [DOI] [PubMed] [Google Scholar]
- 40.Sipos P., Hagymasi K., Lugasi A., Feher E., Blazovics A. Effects of black radish root (Raphanus sativus L. var niger) on the colon mucosa in rats fed a fat rich diet. Phytother Res. 2000;16(7):677–679. doi: 10.1002/ptr.950. [DOI] [PubMed] [Google Scholar]