Abstract
Circulating tumor cells (CTCs) are independent prognostic factors in the primary and metastatic breast cancer patients and play crucial role in hematogenous tumor dissemination. The aim of this study was to correlate the presence of CTCs in peripheral blood with the expression of proteins in tumor tissue that have a putative role in regulation of cell growth and metastatic potential. This prospective study included 203 primary breast cancer patients treated by definitive surgery. CTCs were detected by quantitative real-time PCR for the expression of epithelial (CK19) or epithelial-to-mesenchymal transition–inducing transcription factor genes (TWIST1, SNAIL1, SLUG, and ZEB1). Expression of APC, ADAM23, CXCL12, E-cadherin, RASSF1, SYK, TIMP3, BRMS1, and SOCS1 proteins in primary breast tumor tissue was evaluated by immunohistochemistry. CTCs with epithelial markers were found in 17 (9.2%) patients. Their occurrence was associated with inhibition of SOCS1 expression (odds ratio [OR] = 0.07; 95% confidence interval [CI], 0.03-0.13; P < .001). CTCs with positive epithelial-to-mesenchymal transition markers were detected in 30 (15.8%) patients; however, no association with analyzed protein expressions was found. Overall, CTCs were detected in 44 (22.9%) patients. Presence of any CTC marker was significantly associated with positive CXCL12 expression (OR = 3.08; 95% CI, 1.15-8.26; P = .025) and lack of SOCS1 expression (OR = 0.10; 95% CI, 0.04-0.25; P < .001) in patient’s tumor tissues. As both CXCL12 and SOCS1 proteins are involved in cytokine signaling, our results provide support for the hypothesis that aberrant signaling cross talk between cytokine and chemokine responses could have an important role in hematogenous dissemination of tumor cells in breast cancer.
Introduction
Breast cancer (BC) is one of the most frequently diagnosed cancers in developed countries. The majority of cancer-related deaths occur as a result of metastases, not the primary tumors from which these malignancies arise [1]. During the metastatic process, cancer cells acquire migratory and invasive capabilities that allow them to detach from the primary tumor site, invade the surrounding tissues and lymphatic or blood system, disseminate throughout the body, and seed secondary tumors at distant sites [1], [2].
Circulating tumor cells (CTCs) shed from the solid tumors into the bloodstream are extremely rare cells surrounded with billions of blood cells [3]. Growing evidence suggests that the CTC count increases along with the tumor progression, especially with development of distant metastases [4]. Higher level of the CTCs was associated with unfavorable prognosis in various types of malignant tumors including lung, breast, colorectal, and prostate cancer [5], [6], [7], [8]. It has been shown that CTCs can provide an early indication of patient’s response to treatment beyond imaging studies [9]. The great potential that CTCs hold for translational oncology depends on the level of molecular characterization of the processes associated with hematological dissemination of tumor cells [10].
To create metastasis, cells shed from the solid tumors have to undergo the widespread reorganization of their cell biology, remodel cell-matrix adhesion, diminish cell-cell contacts, and acquire invasive and migratory skills. This reversible process known as the epithelial-to-mesenchymal transition (EMT) [2], [11] is a phenomenon that is currently highly investigated in the field of oncology. The EMT process is characterized by downregulation of gene expression of epithelial markers such as cytokeratin and E-cadherin and upregulation of mesenchymal markers such as vimentin and N-cadherin [12]. Cells undergoing EMT can also acquire cancer stem cell (CSC) properties including the capacity for self-renewal, redifferentiation, dormancy, active DNA repair, and drug resistance [13].
Defects in immune response, chronic inflammation involving T lymphocytes, and infiltration by Th2 and protumor-polarized innate inflammatory cells were implicated in human tumorigenesis, including metastatic spread [14], [15]. The disruption of cancer cell dissemination represents a powerful therapeutic strategy having the potential to prevent advanced metastatic diseases. Pharmacological targeting of CTCs depends primarily on the identification of suitable molecular targets that are critical for their occurrence, survival, and seeding.
In the current study, we hypothesized that aberrant expression of proteins involved in the regulation of cell growth and inhibition of invasivity and metastasizing could play a role in hematogenous dissemination of tumor cells. Therefore, we examined the association between expression of nine proteins: adenomatous polyposis coli (APC), disintegrin and metalloprotease domain 23 (ADAM23), C-X-C motif chemokine 12 (CXCL12), cadherin 1, type 1 (E-cadherin), RAS-association domain family 1 (RASSF1), spleen tyrosine kinase (SYK), tissue inhibitor of the metalloproteinases 3 (TIMP3), breast cancer metastasis suppressor 1 (BRMS1), and suppressor of cytokine signaling 1 (SOCS1), assessed previously for the effect of DNA methylation on subsequent protein expression in tumor tissues [16] and presence of CTCs in peripheral blood of BC patients.
Materials and Methods
Patients
The samples for this translational study were collected according to the Protocol TRU-SK 002 from 203 patients with stages I-III primary BC who were undergoing definitive surgery. Each patient provided peripheral blood for CTCs detection and formalin-fixed, paraffin-embedded (FFPE) tumor tissue. Each patient was given a complete diagnostic evaluation to exclude the presence of distant metastasis. Patients with concurrent malignancy other than nonmelanoma skin cancer in the previous 5 years were excluded as well. In all patients, clinicopathologic data including age, tumor stage, histology, regional lymph node involvement, hormone receptor (oestrogen and progesterone), and HER2 status were recorded.
The study was approved by the Institutional Review Board of the National Cancer Institute of Slovakia and was conducted between March 2012 and September 2013. Healthy breast tissues from the mammoplasties (N = 11) and peripheral blood samples of normal age-matched women without BC (N = 60), who were recruited and consented according to the Institutional Review Board–approved protocol, were also analyzed. Written informed consent was obtained from all individual participants included in the study.
Detection of CTCs in Peripheral Blood
Detailed description of the CTC detection method was published recently by Cierna et al. [17]. Briefly, the protocol consists of three major steps: CD45 + cell depletion, RNA extraction, and identification of EMT-inducing factors and epithelial gene transcripts in CD45-enriched subsets.
Peripheral blood mononuclear cells (PBMCs) were depleted from blood samples using RosetteSep Human CD45 Depletion Cocktail (StemCell Technologies, Vancouver, Canada) according to the manufacturer’s instructions. RNA was extracted from CD45-depleted cells with TRIzolVR LS Reagent (Invitrogen Corporation, Carlsbad, CA). RNA was analyzed for expression of EMT-inducing transcription factor gene transcripts (TWIST1, SNAIL1, SLUG, and ZEB1) and epithelial antigen (CK19) by quantitative real-time (RT) PCR. RT-PCRs were performed using TaqMan assays as described previously [17]. Expressions of the genes of interest were calibrated using the housekeeping gene GAPDH; delta-Ct method was used for quantification of target genes. The highest expression levels of the CK19 and EMT-inducing transcription factor gene transcripts relative to that of GAPDH were 3.4 × 10− 3, 2.0 × 10− 4, 1 × 10− 2, and 2.2 × 10− 2 for CK19, TWIST1, SNAIL1, and ZEB1, respectively, whereas SLUG transcripts were not detected in any of the samples from healthy donors. These values were used as “cutoff” to determine CTCs positivity. Patient samples with higher target gene expression than those of healthy donors were considered as CTC positive. Positive gene expression of any epithelial (CTC EP) and/or mesenchymal (CTC EMT) gene transcripts was described as “CTC any.”
Immunohistochemistry
Immunohistochemical analyses were performed on FFPE tumor and normal breast tissues. Immunohistochemistry was carried out for expression of nine proteins using the general procedures described previously [18]. As negative control, breast tissue was subjected to staining procedure without reaction with the primary antibody. Proteins analyzed in our study were associated with cell growth regulation, cell adhesion, invasiveness, and metastasis (APC, ADAM23, CXCL12, E-cadherin, RASSF1, SYK, TIMP3, BRMS1, SOCS1). An ImmunoReactive Score (IRS) system, also known as the German IRS, based on the proportion of positive cells and the staining intensity of the nuclei or cytoplasm was applied [19]. The scores from staining intensity and positive cells were multiplied, giving quotients ranging between 0 and 12; protein expression was stratified as negative (0) or positive with a staining intensity between 1 and 12.
Statistical Analysis
SPSS statistics 17.0 (SPSS Inc., Chicago, IL) was used for statistical analyses of the data. Univariate analyses with χ2 or by the Fisher exact test were performed to find association between protein expression and CTC status followed by multivariate logistic regression analysis. A logistic regression was used to determine the effect of the independent categorical variables on presence of CTCs in peripheral blood. This determination included the computation of the risk estimate presented as estimated odds ratio (OR) and 95% confidence interval (CI) for the OR. Each model included hormone receptor status (negative for both or positive for either with cutoff of 10%), HER-2 status (negative or overexpressed), tumor grade (1 and 2 vs 3), tumor stage (stage T1 vs > T1), N stage (stage N0 vs N +), and proteins significant in univariate analyses, respectively. Protein expression was categorized as negative (IRS = 0) or positive (IRS ≥ 1). A backward model selection was conducted, and the final fitted model is presented. A P value < .05 was considered to indicate statistical significance.
Results
In the present study, we investigated the associations between expression of the nine above-mentioned proteins in tumor tissues of primary BC patients and presence of CTCs in their peripheral blood. Clinical characteristics of enrolled patients are shown in Table 1. The age of patients ranged from 28 to 83 years, with a median age of 60 years; 76.8% of patients were older than 50 years. The majority of the patients (86.2%) were diagnosed with invasive ductal carcinoma (IDC), hormone receptor positivity (83.5%), node negativity (59.4%), and low Ki-67 staining positivity (57.4%).
Table 1.
Clinical Characteristics
| Variables | N | % |
|---|---|---|
| All | 203 | 100.0 |
| Age (y) | ||
| ≤ 50 | 47 | 23.2 |
| > 50 | 156 | 76.8 |
| T-stage | ||
| 1 | 137 | 67.5 |
| > 1 | 66 | 32.5 |
| N-stage | ||
| 0 | 120 | 59.4 |
| > 1 | 82 | 40.6 |
| Grade | ||
| 1 and 2 | 125 | 62.8 |
| 3 | 74 | 37.2 |
| Histology | ||
| IDC | 175 | 86.2 |
| Others | 28 | 13.8 |
| Hormone receptor status* | ||
| Negative | 33 | 16.5 |
| Positive | 167 | 83.5 |
| HER2 status | ||
| Negative | 171 | 84.2 |
| Amplified | 32 | 15.8 |
| Ki-67† | ||
| Low | 116 | 57.4 |
| High | 86 | 42.6 |
| Tumor multifocality | ||
| Negative | 180 | 89.1 |
| Positive | 22 | 10.9 |
| Tumor subtypes | ||
| Luminal A like | 80 | 40.2 |
| Luminal B like HER2 − | 62 | 31.2 |
| Luminal B like HER2 + | 27 | 13.5 |
| HER2 + nonluminal | 5 | 2.5 |
| Triple negative | 25 | 12.6 |
| CTC EP | ||
| Negative | 168 | 90.8 |
| Positive | 17 | 9.2 |
| CTC EMT | ||
| Negative | 160 | 84.2 |
| Positive | 30 | 15.8 |
| CTC any | ||
| Negative | 148 | 77.1 |
| Positive | 44 | 22.9 |
Negative for both or positive for either with cutoff 10%.
Cutoff 20%.
CTC Detection
Presence of CTC EP markers in peripheral blood was detected in 17 (9.2%), CTC EMT in 30 (15.8%), and CTC any in 44 (22.9%) BC patients. Both epithelial and mesenchymal markers were found in three (1.6%) patients, and coexpression of two mesenchymal markers (SLUG and TWIST1) was detected in one (0.5%) of the patients.
Relationship between Protein Expression in FFPE Tumor Tissues and CTC in Peripheral Blood
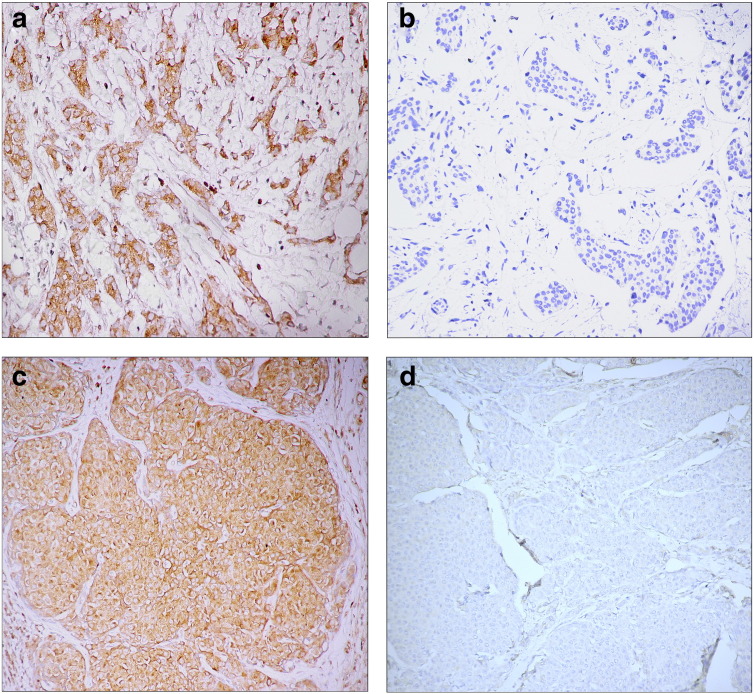
The protein expression in FFPE tumor tissues differed for particular genes. Whereas as many as half of the patients’ FFPE tumor tissues lacked expression of TIMP3, APC, E-cadherin, and SYK (50%, 44.5%, 42.6%, and 37.5%, respectively), expressions of SOCS1 and BRMS1 were comparatively high (94% and 93.4% of samples, respectively). RASSF1, ADAM23, and CXCL12 expression was inhibited in 29.8%, 24.5%, and 23.9% of the tumors. Examples of CXCL12 and SOCS1 expressions in primary tumor samples are depicted in Figure 1. Status of protein expression was compared with the presence of CTC EP, CTC EMT, or both CTC markers (Table 2).
Figure 1.
Protein expression patterns in primary breast tumors with positive and negative CXCL12 expression (a and b) and positive and negative SOCS1 expression (c and d). Magnification 350 × (B, C, D) or 400 × (A).
Table 2.
Frequencies of Protein Expression in Particular CTC Groups
| CTC EP |
CTC EMT |
CTC Any |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein Expression | Negative N (%) | Positive N (%) | P Value | Negative N (%) | Positive N (%) | P Value | Negative N (%) | Positive N (%) | P Value | |
| APC | Negative | 77 (46.7) | 9 (52.9) | .622 | 76 (48.1) | 8 (27.6) | .041 | 71 (48.6) | 15 (34.9) | .112 |
| Positive | 88 (53.3) | 8 (47.1) | 82 (51.9) | 21 (72.4) | 75 (51.4) | 28 (65.1) | ||||
| ADAM23 | Negative | 40 (24.2) | 6 (35.3) | .318 | 39 (24.8) | 8 (26.7) | .833 | 34 (23.4) | 13 (29.5) | .412 |
| Positive | 125 (75.8) | 11 (64.7) | 118 (75.2) | 22 (73.3) | 111 (76.6) | 31 (70.5) | ||||
| CXCL12 | Negative | 39 (23.5) | 3 (17.6) | .585 | 40 (25.2) | 3 (10.3) | .081 | 40 (27.2) | 5 (11.6) | .035 |
| Positive | 127 (76.5) | 14 (82.4) | 119 (74.8) | 26 (89.7) | 107 (72.8) | 38 (88.4) | ||||
| E-cadherin | Negative | 59 (39.9) | 11 (64.7) | .050 | 61 (42.4) | 11 (42.3) | .996 | 53 (40.2) | 21 (52.5) | .167 |
| Positive | 89 (60.1) | 6 (35.3) | 83 (57.6) | 15 (57.7) | 79 (59.8) | 19 (47.5) | ||||
| RASSF1* | Negative | 20 (31.7) | 3 (27.3) | .767 | 23 (32.4) | 1 (12.5) | .246 | 20 (31.3) | 4 (23.5) | .535 |
| Positive | 43 (68.3) | 8 (72.7) | 48 (67.6) | 7 (87.5) | 44 (68.8) | 13 (76.5) | ||||
| SYK | Negative | 63 (38.0) | 5 (29.4) | .488 | 58 (36.7) | 12 (40.0) | .732 | 54 (37.0) | 16 (36.4) | .940 |
| Positive | 103 (62.0) | 12 (70.6) | 100 (63.3) | 18 (60.0) | 92 (63.0) | 28 (63.6) | ||||
| TIMP3 | Negative | 79 (48.2) | 12 (75.0) | .040 | 80 (51.3) | 13 (44.8) | .523 | 71 (49.0) | 23 (54.8) | .508 |
| Positive | 85 (51.8) | 4 (25.0) | 76 (48.7) | 16 (55.2) | 74 (51.0) | 19 (45.2) | ||||
| BRMS1 | Negative | 11 (6.7) | 2 (11.8) | .447 | 14 (9.0) | 2 (6.9) | .715 | 12 (8.3) | 4 (9.3) | .842 |
| Positive | 152 (93.3) | 15 (88.2) | 142 (91.0) | 27 (93.1) | 132 (91.7) | 39 (90.7) | ||||
| SOCS1 | Negative | 5 (3.0) | 6 (35.3) | < .001 | 11 (7.0) | 1 (3.4) | .478 | 5 (3.4) | 7 (16.3) | .002 |
| Positive | 160 (97.0) | 11 (64.7) | 147 (93.0) | 28 (96.6) | 141 (96.6) | 36 (83.7) | ||||
Number of samples analyzed in the study was n = 203; only successfully analyzed samples were included in the table.
For RASSF1, protein analyses were performed on 61 samples only because of the lack of material (tumor tissue).
In the group of CTC EP–positive patients, 35.3% did not express SOCS1 protein. However, among the CTC EP–negative patient cohort, only 3% (P < .001) did not express SOCS1 protein (Table 2). Multivariate analyses confirmed an inverse association between CTC EP and SOCS1 expression; conversely, patients with positive SOCS1 expression had a decreased risk of the occurrence of positive CTC EP (OR = 0.07; 95% CI, 0.03-0.13; P < .001) (Table 3). In univariate analysis, patients with positive CTC EP had more often inhibited protein expression for E-cadherin and TIMP3 (64.7% vs 39.9%, P = .050 and 75.0% vs 48.2%, P = .040, respectively), but these results could not be confirmed by logistic regression.
Table 3.
The Risk Estimation of the Analyzed Variables for Presence of CTCs in Peripheral Blood of Patients Using Multivariate Logistic Regression
| Circulating Tumor Cells | Variables | P Value | OR | 95% CI |
|---|---|---|---|---|
| CTC EP* | Positive SOCS1 expression | < .001 | 0.07 | 0.03-0.13 |
| CTC EMT† | Grade 3 | .036 | 2.42 | 1.06-5.52 |
| Positive APC expression | .063 | 2.32 | 0.96-5.66 | |
| CTC any‡ | Positive CXCL12 expression | .025 | 3.08 | 1.15-8.26 |
| Positive SOCS1 expression | < .001 | 0.10 | 0.04-0.25 |
− 2 Log likelihood = 78.55; R2 (Cox & Snell) = 0.58; R2 (Nagelkerke) = 0.78.
− 2 Log likelihood = 147.91; R2 (Cox & Snell) = 0.04; R2 (Nagelkerke) = 0.08.
− 2 Log likelihood = 180.65; R2 (Cox & Snell) = 0.33; R2 (Nagelkerke) = 0.43.
Expression of APC protein was significantly associated with occurrence of CTC EMT markers using univariate analysis. Whereas only 27.6% of patients had inhibited APC protein expression in the CTC EMT–positive group, 48.1% of patients in CTC EMT–negative group did not express this protein (P = .041) (Table 2). However, multivariate analyses did not confirm this result, and any of the analyzed proteins showed the significant effect on CTC EMT presence with trend for inverse association between the occurrence of the CTC EMT and APC protein expression (P = .063) (Table 3).
CXCL12 and SOCS1 protein expression differed significantly according to the presence of any positive CTC marker. Inhibition of SOCS1 protein expression was present in 16.3% of CTC positive versus 3.4% of CTC-negative patients (P = .002). On the contrary, inhibition of CXCL12 expression was detected only in 11.6% of CTC-positive compared with 27.2% of CTC-negative patients (P = .035). In multivariate analysis, presence of any CTCs was significantly associated with increased CXCL12 (OR = 3.08; 95% CI, 1.15-8.26; P = .025) and inhibited SOCS1 expression (OR = 0.10; 95% CI, 0.04-0.25; P < .001) in patients’ tumor tissues (Table 3).
Discussion
The occurrence of metastases within distant organs represents a life-threatening event that is the main cause of death in BC and other malignant diseases. The early spread of tumor cells in majority of cancers may occur through either the lymphatic system and/or the blood vessels. CTCs play a crucial role in the hematogenous dissemination and could represent the independent prognostic factors for progression-free and overall survival in primary and metastatic BC [20], [21].
In the present study, we investigated the relationship between the occurrence of CTCs in peripheral blood and expression of nine proteins in the tumor tissues that have a putative role in regulation of cell growth and metastatic potential. We found the relationship between expression of cytokine signaling associated proteins, namely, CXCL12 and SOCS1, and the presence of CTCs in peripheral blood of patients.
Chemokine CXCL12, known also as stromal cell–derived factor 1, acts as a positive regulator of monocyte migration and a negative regulator of monocyte adhesion. It activates the C-X-C chemokine receptor CXCR4 and induces intracellular signaling through several divergent pathways including chemotaxis, intracellular calcium flux, cell survival, and proliferation [22]. CXCL12 expression was found to be significantly higher in plasma of BC patients than in age-matched female controls and had a significant correlation with tumor grade and epithelial subtype [23] as well as with worse prognosis and survival [24]. CXCL12 belongs to the group of chemokines that are mostly secreted by leukocytes or stromal lineages such as endothelial cells, mesenchymal fibroblasts, osteoblasts, and chondrocytes [25]. CXCL12 exhibits the peak level of the expression in organs representing the first destinations of BC metastases. Stromal CXCL12 expression might promote the survival of CXCR4-expressing cells in the circulation and cell migration into extravascular tissues, so-called homing or self-seeding, which belongs to major drivers of tumor progression [21], [26], [27]. CXCL12 activation of the specific intracellular pathways could lead to tumor progression either directly, promoting cancer cell proliferation/survival, or indirectly, recruiting stromal cells to favor tumor relapse, dissemination, and angiogenesis. The contribution of the stromal microenvironment to the development of a wide variety of cancers has been shown repeatedly [28]. Elevation of CXCL12 secretion by stromal fibroblasts increases the probability of the tumor reinfiltration by CTCs [29]. Recent studies also demonstrated that cancer cells undergoing EMT acquire the CSC phenotype [13]. Tumor self-seeding thus allows enrichment of primary neoplasm with highly aggressive cell populations that may increase its metastatic potential through the release of paracrine signals. It was shown that CSCs isolated from the human glioblastoma expressed CXCR4 and released CXCL12 in vitro [30]. The role of the CXCL12/CXCR4 signaling in CSCs maintenance, dissemination, and consequent metastatic colonization was reviewed in-depth by Cojoc et al. [31]. Although the relationship between CXCL12 expression and CTCs has not been established, our results are in accordance with previous findings showing the association between unfavorable prognosis and elevation of the CXCL12 expression. Pharmacologic inhibition of the CXCL12/CXCR4 pathway decreased cancer cell proliferation, motility, and invasion in multiple preclinical models both in vitro and in vivo [31]. Neutralization of CXCR4 receptor was shown to impair the formation of the lymph node metastases in vivo [32]. Several drugs that were already approved for treatment of hematological malignancies give therapeutic opportunities for CXCR4 blockade also in other types of cancer, including BC [33], [34], [35].
SOCS1 is a member of SOCS protein family that forms part of a classical negative feedback system that regulates cytokine signal transduction through the JAK/STAT pathway [36]. They control onset and maintenance of allergic responses mediated by T-helper type 2 cells, IL-6 signaling in vivo, and have significant roles in cancer development and progression by tumor-associated inflammation and suppression of antitumor immunity. SOCS1 contributes to the regulation of proliferation, differentiation, apoptosis, and immune surveillance in normal mammary epithelium. Abnormal expression of SOCS1 in tumor cells has been detected in various human cancers, including melanoma, chronic myeloid leukemia, and gastric and head and neck carcinoma [37], [38], [39], [40], where it was associated with dysregulation of cytokine receptor and toll-like receptor signaling and cell transformation. Silencing of the SOCS1 in BC was shown to increase epithelial proliferation and cell survival in response to cytokines and growth factors [41].
There is increasing evidence demonstrating that SOCS1 has different functions depending on the origin of the tumor. Methylation-dependent SOCS1 silencing was associated with tumor growth in vitro and in vivo in Barrett adenocarcinoma [42]. On the other hand, the constitutive expression of SOCS1 was demonstrated in peripheral blood of patients with chronic myeloid leukaemia resistant to therapy and advanced human melanoma tumor tissues [37], [38]. For BC, increased SOCS1 expression in comparison with healthy breast tissues was identified, but the number of analyzed samples was rather low (3 for healthy breast tissue, 6 for in situ ductal carcinomas, and 11 for IDC) [43]. Our results for healthy breast tissues showed the variable SOCS1 protein expression. Inhibition of SOCS1 expression was found in two healthy samples in contrast to the other eight analyzed proteins, where only positive protein expression was found [16]. Inconsistent results for different tumors brought the opposite strategies of how to reduce or restore SOCS1 expression. The comprehensive understanding of the function and the mechanisms of action of SOCS1 might facilitate its future applications as diagnostic and prognostic biomarker and therapeutic target [41]. According to our best knowledge, the increased risk of hematogenous dissemination in patients with inhibition of SOCS1 expression has not yet been reported. However, our results support the recent findings that indicate the inverse relationship between SOCS1 expression and epithelial proliferation and cell survival mediated by JAK/STAT signaling [41].
Using univariate analyses, we found the moderate significance also for TIMP3 expression and CTC EP and APC protein expression and CTC EMT; however, we did not confirm these results by multivariate analyses. The relatively small sample size and distinction of CTC EP and CTC EMT subpopulations, which was discussed previously [17], seem to be the major limitations of our study.
In conclusion, in this translational study, we observed, for the first time, association between the presence of CTCs in peripheral blood of BC patients and aberrant expression of the CXCL12 and SOCS1 proteins in tumor tissues. These proteins are both involved in cytokine signaling and immune response and could represent potential therapeutic targets to gain control over tumor dissemination and self-seeding. Our data provide support for the hypothesis that aberrant signaling cross talk between cytokine and chemokine responses could have an important role in hematogenous dissemination and tumor self-seeding. Although our current knowledge of the role of the immune system in metastatic processes remains limited, a deeper understanding of the interaction between CTCs and their microenvironment is critical for the successful control of tumor dissemination.
Conflict of Interest
None.
Acknowledgements
We acknowledge our collaborators: Gabriela Sieberova, Jan Macuch, Michal Majercik, Peter Jani, and Dusan Macak and also Gabriela Gasajova, Denisa Manasova, Emilia Klincova, and Ludovit Gaspar for their excellent technical assistance. We are grateful to all patients and controls for their participation in the study.
Footnotes
Funding: This study was supported by the Slovak Research and Development Agency (APVV) under contract no. APVV-0076-10; European Fund for Regional Development through the Operational Programme of Research and Development, project code ITMS no. 26240220058 (call code OPVaV-2009/4.2/04-SORO) and 26240220074 (call code OPVaV-2011/4.2/07-SORO); and the Scientific Grant Agency, contract nos. 1/0724/11, 1/0044/15, 2/0120/13, 2/0169/14, and 2/0092/15.
Contributor Information
Bozena Smolkova, Email: bozena.smolkova@savba.sk.
Michal Mego, Email: misomego@gmail.com.
Viera Horvathova Kajabova, Email: viera.kajabova@savba.sk.
Zuzana Cierna, Email: ciernaz@gmail.com.
Ludovit Danihel, Email: ludovit.danihel@fmed.uniba.sk.
Tatiana Sedlackova, Email: tatiana.sedlackova@gmail.com.
Gabriel Minarik, Email: gabriel.minarik@gmail.com.
Iveta Zmetakova, Email: iveta.zmetakova@savba.sk.
Tomas Krivulcik, Email: tomas.krivulcik@savba.sk.
Paulina Gronesova, Email: paulina.gronesova@savba.sk.
Marian Karaba, Email: marian.karaba@nou.sk.
Juraj Benca, Email: juraj.benca@nou.sk.
Daniel Pindak, Email: daniel.pindak@nou.sk.
Jozef Mardiak, Email: jozef.mardiak@nou.sk.
James M. Reuben, Email: jreuben@mdanderson.org.
Ivana Fridrichova, Email: ivana.fridrichova@savba.sk.
References
- 1.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1-2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 3.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, Matsumoto S, Okumura Y, Rahman S, Tsubota N, Tsujimura T. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009;15(22):6980–6986. doi: 10.1158/1078-0432.CCR-09-1095. [DOI] [PubMed] [Google Scholar]
- 5.Hofman V, Bonnetaud C, Ilie MI, Vielh P, Vignaud JM, Flejou JF, Lantuejoul S, Piaton E, Mourad N, Butori C. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17(4):827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 6.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23(7):1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ, Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 9.De Giorgi U, Valero V, Rohren E, Dawood S, Ueno NT, Miller MC, Doyle GV, Jackson S, Andreopoulou E, Handy BC. Circulating tumor cells and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for outcome prediction in metastatic breast cancer. J Clin Oncol. 2009;27(20):3303–3311. doi: 10.1200/JCO.2008.19.4423. [DOI] [PubMed] [Google Scholar]
- 10.Lowes LE, Allan AL. Recent advances in the molecular characterization of circulating tumor cells. Cancers. 2014;6(1):595–624. doi: 10.3390/cancers6010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15(6):740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 2011;13(3):R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9(4):212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat. 2005;91(2):163–171. doi: 10.1007/s10549-004-7048-0. [DOI] [PubMed] [Google Scholar]
- 16.Fridrichova I, Smolkova B, Kajabova V, Zmetakova I, Krivulcik T, Mego M, Cierna Z, Karaba M, Benca J, Pindak D. CXCL12 and ADAM23 hypermethylation are associated with advanced breast cancers. Transl Res. 2015;165(6):717–730. doi: 10.1016/j.trsl.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Cierna Z, Mego M, Janega P, Karaba M, Minarik G, Benca J, Sedlackova T, Cingelova S, Gronesova P, Manasova D. Matrix metalloproteinase 1 and circulating tumor cells in early breast cancer. BMC Cancer. 2014;14:472. doi: 10.1186/1471-2407-14-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zmetakova I, Danihel L, Smolkova B, Mego M, Kajabova V, Krivulcik T, Rusnak I, Rychly B, Danis D, Repiska V. Evaluation of protein expression and DNA methylation profiles detected by pyrosequencing in invasive breast cancer. Neoplasma. 2013;60(6):635–646. doi: 10.4149/neo_2013_082. [DOI] [PubMed] [Google Scholar]
- 19.Remmele W, Schicketanz KH. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer. Computer-assisted image analysis (QIC score) vs. subjective grading (IRS) Pathol Res Pract. 1993;189(8):862–866. doi: 10.1016/S0344-0338(11)81095-2. [DOI] [PubMed] [Google Scholar]
- 20.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Mego M, Gao H, Lee B-N, Cohen EN, Tin S, Giordano A, Wu Q, Liu P, Nieto Y, Champlin RE. Prognostic value of EMT-circulating tumor cells in metastatic breast cancer patients undergoing high-dose chemotherapy with autologous hematopoietic stem cell transplantation. J Cancer. 2012;3:369–380. doi: 10.7150/jca.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16(11):2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 23.Potter SM, Dwyer RM, Curran CE, Hennessy E, Harrington KA, Griffin DG, Kerin MJ. Systemic chemokine levels in breast cancer patients and their relationship with circulating menstrual hormones. Breast Cancer Res Treat. 2009;115(2):279–287. doi: 10.1007/s10549-008-0078-2. [DOI] [PubMed] [Google Scholar]
- 24.Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG. Stromal cell derived factor–1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005;7(4):R402–R410. doi: 10.1186/bcr1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25(2):75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrow. Exp Hematol. 2001;29(12):1439–1447. doi: 10.1016/s0301-472x(01)00741-x. [DOI] [PubMed] [Google Scholar]
- 28.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200(4):429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 29.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Gatti M, Pattarozzi A, Bajetto A, Wurth R, Daga A, Fiaschi P, Zona G, Florio T, Barbieri F. Inhibition of CXCL12/CXCR4 autocrine/paracrine loop reduces viability of human glioblastoma stem-like cells affecting self-renewal activity. Toxicology. 2013;314(2-3):209–220. doi: 10.1016/j.tox.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Cojoc M, Peitzsch C, Trautmann F, Polishchuk L, Telegeev GD, Dubrovska A. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Oncol Targets Ther. 2013;6:1347–1361. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 33.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113(24):6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epstein RJ. The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat Rev Cancer. 2004;4(11):901–909. doi: 10.1038/nrc1473. [DOI] [PubMed] [Google Scholar]
- 35.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17(8):2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ilangumaran S, Rottapel R. Regulation of cytokine receptor signaling by SOCS1. Immunol Rev. 2003;192:196–211. doi: 10.1034/j.1600-065x.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Metze D, Nashan D, Muller-Tidow C, Serve HL, Poremba C, Luger TA, Bohm M. Expression of SOCS-1, suppressor of cytokine signalling-1, in human melanoma. J Invest Dermatol. 2004;123(4):737–745. doi: 10.1111/j.0022-202X.2004.23408.x. [DOI] [PubMed] [Google Scholar]
- 38.Roman-Gomez J, Jimenez-Velasco A, Castillejo JA, Cervantes F, Barrios M, Colomer D, Heiniger A, Torres A. The suppressor of cytokine signaling-1 is constitutively expressed in chronic myeloid leukemia and correlates with poor cytogenetic response to interferon-alpha. Haematologica. 2004;89(1):42–48. [PubMed] [Google Scholar]
- 39.Oshimo Y, Kuraoka K, Nakayama H, Kitadai Y, Yoshida K, Chayama K, Yasui W. Epigenetic inactivation of SOCS-1 by CpG island hypermethylation in human gastric carcinoma. Int J Cancer. 2004;112(6):1003–1009. doi: 10.1002/ijc.20521. [DOI] [PubMed] [Google Scholar]
- 40.Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, Dietz A, Wittekind C, Tannapfel A. SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene. 2005;24(44):6699–6708. doi: 10.1038/sj.onc.1208818. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Li H, Yu JP, Wang SE, Ren XB. Role of SOCS1 in tumor progression and therapeutic application. Int J Cancer. 2012;130(9):1971–1980. doi: 10.1002/ijc.27318. [DOI] [PubMed] [Google Scholar]
- 42.Tischoff I, Hengge UR, Vieth M, Ell C, Stolte M, Weber A, Schmidt WE, Tannapfel A. Methylation of SOCS-3 and SOCS-1 in the carcinogenesis of Barrett's adenocarcinoma. Gut. 2007;56(8):1047–1053. doi: 10.1136/gut.2006.111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raccurt M, Tam SP, Lau P, Mertani HC, Lambert A, Garcia-Caballero T, Li H, Brown RJ, McGuckin MA, Morel G. Suppressor of cytokine signalling gene expression is elevated in breast carcinoma. Br J Cancer. 2003;89(3):524–532. doi: 10.1038/sj.bjc.6601115. [DOI] [PMC free article] [PubMed] [Google Scholar]