Abstract
The high recurrence rate remains a major problem that strongly influenced the prognosis of hepatocellular carcinoma (HCC) patients who received hepatectomy. The presence of microvascular invasion (MVI) is regarded as the most important risk factor that contributes to the postoperative recurrence. Our previous study has hinted that serum microRNA-125b (miR-125b) was associated with MVI. The aim of the present study was to identify whether serum miR-125b can serve as a biomarker to reliably predict microvascular invasion (MVI) preoperatively. MiR-125b was quantified in 108 HCC patients’ serum before they received surgery by quantitative real-time PCR (qRT-PCR). Our results revealed that MVI was associated with relapse free survival (RFS) of postoperative HCC patients; surgical margin width was associated with postoperative RFS in MVI present patients, but not in the patients without MVI. Multivariate analysis revealed that miR-125b, tumor size and AFP were the independent predictive factors associated with MVI in this cohort (P = .001, .001, .003, respectively). The probability of the predictive accuracy of miR-125b was 76.95% (51.32% specificity and 87.50% sensitivity), which was almost equal to the classifier established by combination of AFP and tumor size (78.82% probability, 65.63% specificity and 84.21% sensitivity). Furthermore, the combination of tumor size, AFP and miR-125b yielded a ROC curve area of 86.68% (72.37% specificity and 84.38% sensitivity). Our study indicated that serum miR-125b can be used to predict MVI of HCC patients before they received hepatic resection. Therefore, miR-125b can potentially guide individualized treatment, which helps HCC patients, with or without MVI, to benefit from different surgical approach.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the second most common cause of cancer mortality. An estimated 782,000 new liver cancer cases and 745,000 cancer deaths occurred worldwide [1]. Liver resection (LR) and orthotopic liver transplantation (OLT) are the best radical treatments and well perceived as a curative treatment for HCC in cirrhotic patients with good functional liver reserves [2]. However, the high recurrence rate of HCC in the remnant liver, which reach an incidence of more than 20% at 1 year and 70% at 5 years [3], remains one major obstacle that is strongly influenced the long-term survival of patients with HCC who have undergone hepatectomy. The presence of microvascular invasion (MVI) is regarded as the most important risk factor that is significantly associated with recurrence within two years after surgery [4], [5]. Unfortunately, MVI can currently be detected only by postoperative histological examination, which greatly limits the usefulness for preoperative assessment of prognosis [6], let alone when patients are given nonsurgical treatments, such as radiofrequency ablation, ethanol injection or transcatheter arterial chemoembolization. And, the incidence of MVI is greater than 20% in resected HCC patients [7]. Currently, there are no effective predictors with the ability to predict MVI effectively before hepatic resection. Certain serum factors, such as AFP [8] and PON1 [9], have been identified as predictors of MVI preoperatively, but the effectiveness or convenience was far from satisfaction. Identification preoperative predictors of MVI were able to provide satisfactory reference information for clinicians to select appropriate surgical and/or therapeutic strategies for patients with HCC.
MicroRNAs (miRNA) are small non-coding RNAs and have crucial functions in human diseases, including cancer. Due to its high stability and detectability in blood plasma or serum, miRNAs constitute a novel class of non-invasive biomarkers [10]. In recent years, there are many studies investigating the possible ability of miRNAs serve as diagnostic or prognostic biomarkers in human cancers, including HCC [11], [12]. miR-125b has been demonstrated to suppress the proliferation and metastasis of human liver cancer cell [13], [14]. However, only a few studies have focused on identifying the predictive value of circulating miR-125b in the serum of patients with HCC [15]. Our earlier study hinted that the expression of serum miR-125b was associated with microvascular invasion [16], however, additional studies are needed to reaffirm. In this study, we detected the level of miR-125b in a cohort of 108 HCC patients’ serum in order to validate its value of predicting MVI and its ability to serve as a biomarker. Our study suggested that preoperative serum miR-125b can serve as a useful biomarker that helps to reliably predict the presence of MVI before HCC patients received surgery.
Materials and Methods
Patients With HCC
One hundred eight patients with HCC who underwent hepatectomy from April 2012 to October 2013 were included in this study. The including criteria were as follow: (1) did not receive any preoperative treatment, such as ablation, TACE, radiotherapy, chemotherapy or targeted therapy, (2) preoperative liver function was Child-Pugh A degree, (3) treated with curative surgical liver resection (microscopically surgical margin free of tumor), (4) intraoperative blood loss was less than 600 ml and no intraoperative or postoperative blood transfusion, (5) single lesion or synchronism multiple primary lesion and no satellite nodules were proven by postoperative pathology, (6) liver failure or death did not happened within 30 days postoperatively.
All serum samples were collected before the patients had received surgery at Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CAMS), between April 2012 and October 2013 and stored at − 80 °C refrigerator. This study was approved by ethics committee approval from cancer hospital CAMS, and all the participants signed written informed consent forms.
miRNA-Specific Quantitative Real-Time RT-PCR
Serum samples from 108 HCC patients were analyzed by using miRNA-specific quantitative real-time RT-PCR. miRNA was isolated using a mirVana PARIS kit (Ambion). Megaplex RT reactions and pre-amplification reactions were run according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA, USA). Let-7d was used as an internal control for normalization [17]. Real-time PCR was performed using the StepOne Plus Real-time system (Applied Biosystems, Foster City, CA, USA) and fold changes in gene expression were calculated using the 2-ΔΔCt method [18]. The mean miRNA level from three real-time quantitative PCR experiments was calculated for each case.
Follow-Up
Patients were followed-up at 3-month intervals for the first 2 years and at 6-month intervals thereafter. During the follow-up visit, alpha-fetoprotein (AFP), liver function, chest x-ray, enhanced computed tomography (CT) and/or enhanced magnetic resonance imaging (MRI) were performed. If inner-hepatic-recurrence was suspected, the lesion was confirmed by hepatic digital subtraction angiography (DSA) and/or contrast-enhanced ultrasound (CEUS). The recurrence status was determined as described before [16]. Relapse free survival was defined as the time interval from the date of surgery to the time of initially detected recurrence/progression or censored on the last follow-up. The last follow-up was May 2015. Until then, 55 patients developed recurrence.
Survival Analysis
Based on MVI and surgical margin width, the Kaplan-Meier estimator was used to calculate the median survival time of the relapse free survival (RFS) and to describe the survival curve. A comparison of survival analysis was performed and the P value was calculated by the log-rank test. According to pathologically proven MVI status, the patients were divided into 2 groups: MVI present group and MVI absent group. The surgical margin was divided into the following 2 categories as the subgroups: wide surgical margin group (WSM group) with a negative surgical margin historically measured as greater more than 10 mm in width, narrow surgical margin group (NSM group) with a negative surgical margin historically measured less than 10 mm in width.
Statistical Analysis
SPSS 19.0 software was used for the statistical analysis. The P value was bilaterally tested, and values less than 0.05 were regarded as statistically significant. The patients were divided into groups based on the median values of continuous variables or discrete variables. The significance of differences between each pair of groups that created was assessed by the chi-square test and fisher exact test. Student t test was used to compare the continuous variables. Univariate and multivariate logistic regression analysis were done to evaluate the association of the miRNA signature and clinicopathological data to MVI, respectively. The P values were calculated using the Wald test.
Logistic regression analysis was performed to analyze various combinations of clinical parameters and miR-125b. Using the independent predictors of MVI according to multivariate analysis, the receiver operating characteristic (ROC) curves were created and the area under the curve (AUC) was used to determine the feasibility. The Youden’s Index was used to identify the optimal cut-off point. As defined, the corresponding sensitivity and specificity was showed.
Results
The Level of Serum miR-125b was Correlates With MVI and Envelop Invasion in 108 HCC Patients
We divided the 108 patients into two groups based on the median value of the expression level of miR-125b. As shown in Table 1, low expression of miR-125b was significantly correlated with MVI and envelope invasion (P = .003 and P = .011, respectively). No significant association was found between miR-125b and other clinic-pathological features, such as the patient’s age, gender, tumor size, tumor multiplicity, histological grade and BCLC stage, viral hepatitis, cirrhosis, AFP and GGT (P > .05).
Table 1.
Correlation Between the Level of Serum miR-125b and Clinicopathologic Features in 108 HCC Patients Underwent Hepatectomy
| All Cases | miR-125b Expression Level |
P Value | ||
|---|---|---|---|---|
| Low Expression (n = 54) | High Expression (n = 54) | |||
| Age | ||||
| ≥ 60 | 38 | 21 | 17 | .420 |
| < 60 | 70 | 33 | 37 | |
| Gender | ||||
| Male | 89 | 45 | 44 | .800 |
| Female | 19 | 9 | 10 | |
| Tumor size | ||||
| ≥ 5cm | 43 | 24 | 18 | .236 |
| < 5cm | 66 | 30 | 36 | |
| Tumor multiplicity | ||||
| Single | 97 | 49 | 48 | .750 |
| Multiple | 11 | 5 | 6 | |
| MVI | ||||
| Present | 32 | 23 | 9 | .003 |
| Absent | 76 | 31 | 45 | |
| Envelope invasion | ||||
| Present | 63 | 38 | 25 | .011 |
| Absent | 45 | 16 | 29 | |
| Histological grade | ||||
| Well | 14 | 5 | 9 | .396 |
| Moderate | 78 | 42 | 36 | |
| Poorly | 16 | 7 | 9 | |
| BCLC | ||||
| A | 72 | 33 | 39 | .221 |
| B | 36 | 21 | 15 | |
| Viral hepatitis | ||||
| Negative | 4 | 1 | 3 | .435 |
| HBV | 98 | 49 | 49 | |
| HCV | 6 | 4 | 2 | |
| Cirrhosis | ||||
| Present | 91 | 48 | 43 | .186 |
| Absent | 17 | 6 | 11 | |
| AFP (ng/ml) | ||||
| ≥ 400 | 25 | 12 | 13 | .820 |
| < 400 | 83 | 42 | 41 | |
| GGT (U/ml) | ||||
| ≥ 55 | 49 | 21 | 28 | .176 |
| < 55 | 59 | 33 | 26 | |
MVI was Associated With Relapse Free Survival of PostOperative HCC Patients
As shown in Table 2, association of MVI and the other clinical parameters were analyzed with univariate analysis. Tumor size, BCLC stage, AFP and envelop invasion were significantly associated with MVI, whereas other features, including age at diagnosis, gender, differentiation, tumor multiplicity, cirrhosis, viral hepatitis and GGT were not.
Table 2.
Correlation Between MVI and Clinicopathologic Features in 108 HCC Patients Underwent Hepatectomy
| MVI Present (n=32) | MVI Absent (n=76) | P Value | |
|---|---|---|---|
| Age (years) | 53.88±11.69 | 53.64±11.26 | .924 |
| Gender (Male/Female) | 26/6 | 63/13 | .838 |
| Tumor size (cm) | 6.53±3.92 | 3.93±2.10 | .001 |
| Tumor multiplicity (Single/Multiple) | 29/3 | 68/8 | 1.000 |
| Envelope invasion (Present/Absent) | 26/6 | 37/39 | .002 |
| Differentiation (Well/Moderate/Poorly) | 1/26/5 | 13/52/11 | .140 |
| BCLC (A/B) | 14/18 | 58/18 | .001 |
| Viral hepatitis (Negative/HBV/HCV) | 1/29/2 | 3/69/4 | .960 |
| Cirrhosis (Present/Absent) | 27/5 | 64/12 | .983 |
| Ln (AFP)⁎ | 5.56±3.37 | 3.29±2.48 | .001 |
| Ln (GGT)⁎ | 3.95±0.79 | 3.87±0.71 | .569 |
Ln (natural logarithms): the primitive value of AFP and GGT was skew distribution in the groups, the logarithmic transformation was performed to make the value fitted Gaussian distribution.
According to the presence or absence of MVI, 108 HCC patients were divided into two groups. The Kaplan-Meier curves of relapse free survival (RFS) according MVI was plotted in Figure 1, the group of patients without MVI had a longer RFS than the group with MVI, the median RFS was 9.07 month and 30.73 month, respectively (P = .000).
Figure 1.
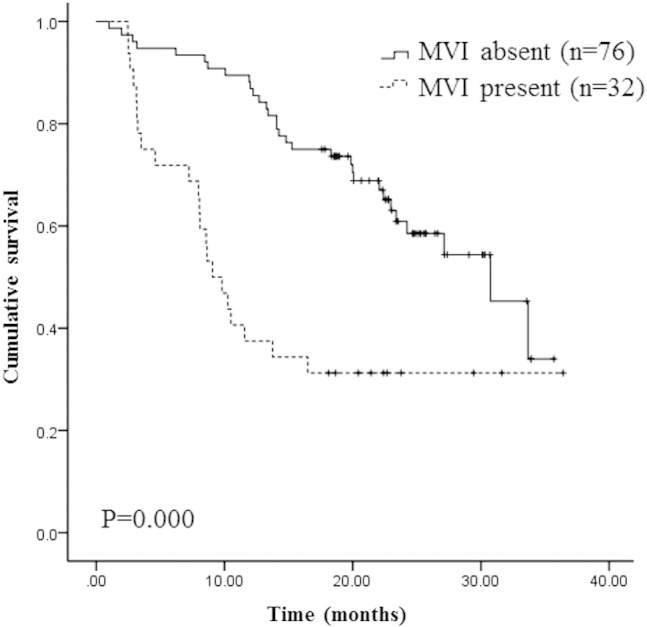
Microvascular invasion (MVI) was associated with relapse free survival.
The Prognostic Value of the Width of the Surgical Margin in HCC Patients
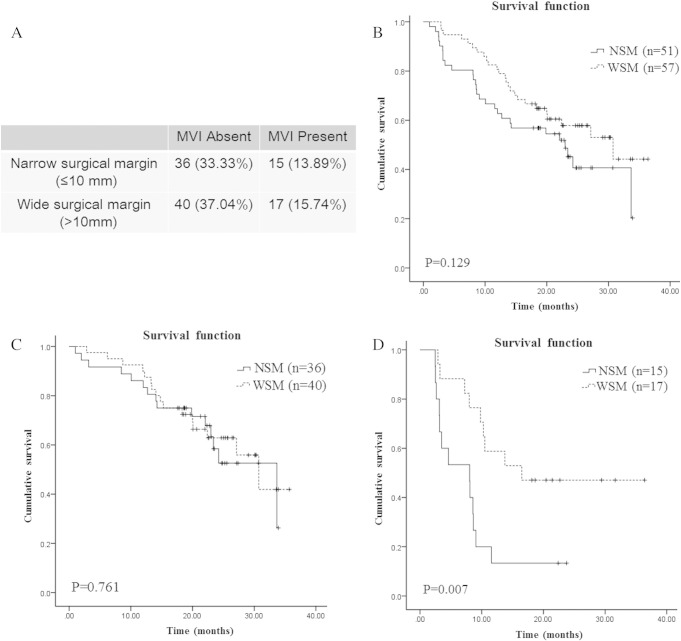
As shown in Figure 2A, 57 patients had a wide surgical margin (> 10 mm), 51 patients had a narrow surgical margin (≤ 10 mm) based on a microscopic examination. The surgical margin width was significantly different between the groups (15.61 ± 3.5 mm vs. 2.61 ± 2.15 mm). The median relapse-free survival time in patients with a wide surgical margin (> 10 mm) and a narrow surgical margin (≤ 10 mm) were 30.73 months and 22.97 months, respectively. There was no significant difference among the groups (P = .129, Figure 2B).
Figure 2.
Association of the width of the surgical margin and relapse free survival by Kaplan-Meier curves and the log-rank test.
A, According to the surgical margin width, the case number in each subgroup was showed in the table. B, 108 patients with narrow surgical margin (NSM) or wide surgical margin (WSM). C, In the subgroup of HCC patients without MVI, patients with narrow surgical margin or wide surgical margin. D, In the subgroup of HCC patients with MVI, patients with narrow surgical margin or wide surgical margin. MVI: microvascular invasion. NSM: narrow surgical margin. WSM: wide surgical margin.
The Kaplan-Meier curves were also used to analyze the association of the surgical margin width and RFS in the subgroup of patients with or without MVI, respectively. As shown in Figure 2C and D, a wide surgical margin status provided a favorable RFS time in the patients with MVI: the median relapse-free survival time for the narrow (n = 15) and the wide (n = 17) margin groups were 8.03 months and 16.50 months, respectively. The difference was significant (P = .007, Figure 2C). On the other hand, the surgical margin status did not influence the RFS in patients without MVI: the median relapse-free survival time for the narrow (n = 36) and the wide (n = 40) margin groups were 33.67 months and 30.73 months, respectively. The difference was not significant (P = .761, Figure 2D). Our results revealed that surgical margin width was associated with postoperative relapse free survival in MVI present HCC patients, but not associated with postoperative relapse free survival in MVI absent HCC patients.
The Classifier of miR-125b for Predicting the Presence of MVI of Preoperative HCC Patients
As positive postoperative MVI is a strong predictor of recurrence for HCC patients after resection, we wanted to identify pre-operative factors that are independently associated with MVI. Multivariate analysis revealed that miR-125b, AFP and tumor size were the independent predictive factors associated with MVI (Table 3). Then, the discriminative power of these factors in predicting MVI of HCC patients before operation was verified. To evaluate the predictive value, the ROC curve was used to analyze the sensitivity and specificity. As shown in Figure 3, the ROC curve of miR-125b showed an AUC of 76.97% (87.50% sensitivity and 51.32% specificity), which was almost equal to the classifier established by combination of AFP and tumor size (78.82% probability, 84.21% sensitivity and 65.63% specificity). Furthermore, the combination of AFP, MVI and miR-125b yielded a ROC curve area of 86.68% (84.38% sensitivity and 72.37% specificity). The results demonstrated that the combination of AFP, MVI and miR-125b was a much more powerful discrimination tool in predicting MVI of HCC patients before they received operation.
Table 3.
Univariate and Multivariate Cox Proportional Hazards Regression Analysis of MVI in Relation to miR-125b and Clinical Parameters of 108 HCC Patients
| Variable | Logistic |
|||
|---|---|---|---|---|
| Univariate Analysis |
Multivariate Analysis |
|||
| HR (95%CI) | P Value | HR (95%CI) | P Value | |
| miR-125b | 0.409 (0.263-0.635) | .000 | 0.371 (0.211-0.654) | .001 |
| Tumor size | 1.449 (1.193-1.761) | .000 | 1.522 (1.181-1.960) | .001 |
| AFP | 1.301 (1.121-1.510) | .001 | 1.348 (1.104-1.645) | .003 |
| Envelope invasion | 4.568 (1.688-12.356) | .003 | ||
| BCLC | 4.143 (1.725-9.949) | .001 | ||
| Differentiation | 1.743 (0.778-3.902) | .177 | ||
| GGT | 1.181 (0.670-2.081) | .566 | ||
| Hepatitis | 1.216 (0.313-4.717) | .778 | ||
| Cirrhosis | 1.012 (0.325-3.154) | .983 | ||
| Tumor multiplicity | 0.879 (0.218-3.553) | .857 | ||
| Gender | 0.894 (0.307-2.606) | .838 | ||
| Age | 1.002 (0.966-1.039) | .923 | ||
Figure 3.
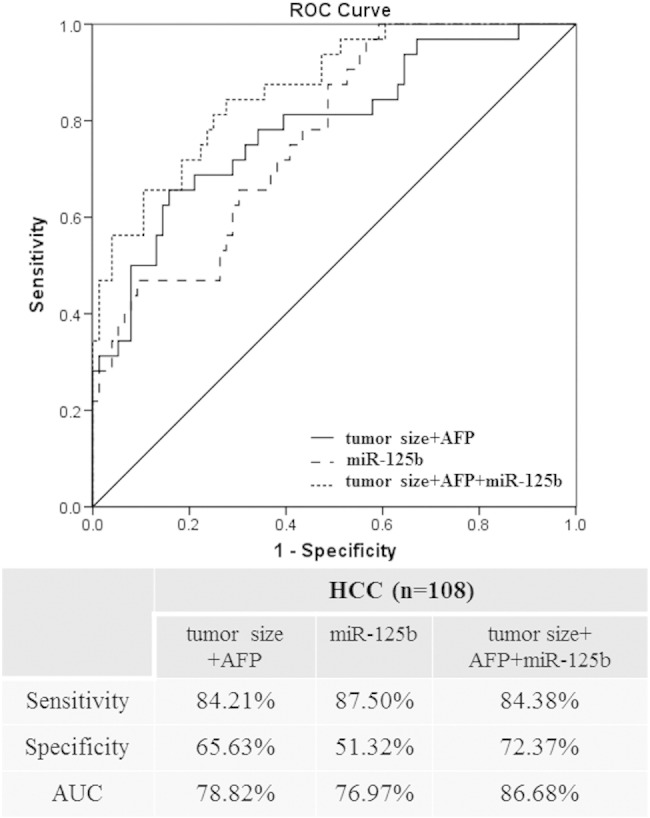
Receiver operating characteristic curve analysis for predicting the presence of microvascular invasion of HCC patients preoperatively.
ROC curves for the combination of tumor size and AFP, miR-125b and the combination of tumor size, AFP and miR-125b in total 108 patients, respectively. The sensitivity, specificity and AUC were indicated below the ROC graph.
Discussion
Many risk factors may contribute to the postoperative recurrence, including AFP, satellite nodal, tumor size, MVI and TNM stage [19]. Among them, the presence of MVI has been reported to be the most significant independent risk factor affecting relapse-free survival following curative resection and/or liver transplantation [20], [21], [22], [23], the odds ratio (OR) reach the level as high as 28.40 [8]. In the present study, the RFS rates at 1 year for HCC patients with or without MVI were 62.5% (20/32) and 13.2% (10/76), respectively, which are consistent with the previous study [24]. Our data combined with the results of other studies indicated that MVI in HCC patients could provide a more consistent and reliable prediction of poor prognosis.
In addition, the invasion of the microvasculature is regarded as the main way to spread cancer cells both in the hepatic circulation and the systemic circulation before liver resection and/or liver transplantation in patients with MVI [25]. And, the incidence of MVI was closely related to the distance from the tumor capsule of the primary HCC [26]. A previous study concluded that MVI was found beyond 1 cm from the tumor capsule of the primary tumor in only a few HCC patients [5]. Accordingly, a more than 1-cm resection margin might be adequate for the majority of patients to reduce tumor recurrence. However, preserving non-tumorous liver parenchyma is also an important consideration, especially in cirrhotic liver, for decreasing the incidence of postoperative liver failure and improving the chance of performing multidiscipline-team treatment in case of tumor recurrence. A recent study demonstrated anatomic resection could achieve better survival than non-anatomic resection in HCC patients with MVI [23]. On the contrary, anatomic resection and non-anatomic resection brought similar survival in patients without MVI, whereas non-anatomic resection could preserve more remnants liver [24]. In this cohort, the RFS of the wide margin group was better than the narrow margin group, but the differences did not reached statistical significance (P = .129). However, the RFS for patients with MVI was significantly better in the wide margin group than the narrow margin group, despite the small sample size (wide/narrow, 17/15). The result implied that a wide surgical margin is more advantageous for HCC patients with MVI. On the other hand, the RFS for patients without MVI was no significant difference between the wide and narrow margin group, implying that a narrow resection margin could be benefit for HCC patients without MVI and could preserve as much non-tumorous liver parenchyma as possible. All of these indicated that the subgroup of HCC patients with or without MVI can benefit from different surgical approach.
Since MVI is a histopathologic diagnosis, surgical specimens examination is still the only accurate method for assessing MVI until now [27]. It cannot be confirmed prior to the surgery. This naturally leads to questions about how to identify the existence of MVI preoperatively. Although previous studies showed that diffusion-weighted imaging (DWI) of MR was useful in predicting MVI for HCCs [28] and infiltrative tumor margin in CT scan had a significant correlation with MVI [29], detection of MVI using preoperative radiological imaging still remains a difficulty even using the latest imaging procedures, such as ultrasonography, CT and MRI. Some other studies demonstrated that tumor size and high AFP level [8], [30], and histological grade [31] were associated with higher rates of MVI, but using clinical data to identify MVI accurately is also far from satisfaction. In our study, tumor size and preoperative AFP level were independent risk factor associated with MVI, which are consistent with previous reports. But the predictive accuracy and efficiency of these two factors was dissatisfaction. In recent years, miRNAs have been proposed as novel diagnostic tools for classification and prognostic stratification of HCC. Here, when a third parameter, serum miR-125b, was added to the classifier that established by combined tumor size and AFP to predict MVI preoperatively, the probability was further improved from 78.82% to 86.68%. The results demonstrated that the combination of tumor size, AFP and miR-125b was a much more powerful discrimination tool in predicting MVI preoperatively and may provide clinicians with an opportunity to make a more accurate predictive diagnosis of MVI.
In summary, given the fact that MVI has a pivotal impact on recurrence and surgery plan selection of HCC patients, determining an accurate preoperative prediction of MVI is essential needed. Our study found that the combined classifier of tumor size, serum miR-125b and preoperative AFP level is a reliable tool with sufficient accuracy in predicting MVI in HCC patients before they received hepatic resection. But the results of our study have some limitations due to its retrospective, single-center design and limited sample size. More randomized controlled trails with a large sample size are needed to further confirm the results.
Disclosure of Potential Conflicts of Interest
There are no conflicts to disclose.
Acknowledgement
This work was supported by National Natural Science Foundation (81302279, 81321091), Beijing Hope Run Special Fund (LC2015A12), PR China.
Contributor Information
Ningzhi Xu, Email: xuningzhi@cicams.ac.cn.
Jianxiong Wu, Email: dr.wujx@hotmail.com.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Fan ST, Poon RT, Yeung C, Lam CM, Lo CM, Yuen WK, Ng KK, Liu CL, Chan SC. Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg. 2011;98(9):1292–1300. doi: 10.1002/bjs.7583. [DOI] [PubMed] [Google Scholar]
- 3.Colecchia A, Schiumerini R, Cucchetti A, Cescon M, Taddia M, Marasco G, Festi D. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol. 2014;20(20):5935–5950. doi: 10.3748/wjg.v20.i20.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373(9664):614–616. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 5.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137(3):850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi KK, Kim SH, Choi SB, Lim JH, Choi GH, Choi JS, Kim KS. Portal venous invasion: the single most independent risk factor for immediate postoperative recurrence of hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26(11):1646–1651. doi: 10.1111/j.1440-1746.2011.06780.x. [DOI] [PubMed] [Google Scholar]
- 7.Shirabe K, Kajiyama K, Harimoto N, Masumoto H, Fukuya T, Ooya M, Maehara Y. Prognosis of hepatocellular carcinoma accompanied by microscopic portal vein invasion. World J Gastroenterol. 2009;15(21):2632–2637. doi: 10.3748/wjg.15.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford) 2010;12(1):56–61. doi: 10.1111/j.1477-2574.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Liu S, Ding G, Liu W, Zhou J, Kuang M, Ji Y, Kondo T, Fan J. Quantitative proteomic analysis identified paraoxonase 1 as a novel serum biomarker for microvascular invasion in hepatocellular carcinoma. J Proteome Res. 2013;12(4):1838–1846. doi: 10.1021/pr3011815. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104(7):528–540. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronte F, Bronte G, Fanale D, Caruso S, Bronte E, Bavetta MG, Fiorentino E, Rolfo C, Bazan V, Marco VD. HepatomiRNoma: The proposal of a new network of targets for diagnosis, prognosis and therapy in hepatocellular carcinoma. Crit Rev Oncol Hematol. 2016;97:312–321. doi: 10.1016/j.critrevonc.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Liang L, Wong CM, Ying Q, Fan DN, Huang S, Ding J, Yao J, Yan M, Li J, Yao M. MicroRNA-125b suppressesed human liver cancer cell proliferation and metastasis by directly targeting oncogene LIN28B2. Hepatology. 2010;52(5):1731–1740. doi: 10.1002/hep.23904. [DOI] [PubMed] [Google Scholar]
- 14.Zhou JN, Zeng Q, Wang HY, Zhang B, Li ST, Nan X, Cao N, Fu CJ, Yan XL, Jia YL. MicroRNA-125b attenuates epithelial-mesenchymal transitions and targets stem-like liver cancer cells through small mothers against decapentaplegic 2 and 4. Hepatology. 2015;62(3):801–815. doi: 10.1002/hep.27887. [DOI] [PubMed] [Google Scholar]
- 15.Giray BG, Emekdas G, Tezcan S, Ulger M, Serin MS, Sezgin O, Altintas E, Tiftik EN. Profiles of serum microRNAs; miR-125b-5p and miR223-3p serve as novel biomarkers for HBV-positive hepatocellular carcinoma. Mol Biol Rep. 2014;41(7):4513–4519. doi: 10.1007/s11033-014-3322-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Liu M, Zhu H, Rong W, Wu F, An S, Liu F, Feng L, Wu J, Xu N. Identification of recurrence-related serum microRNAs in hepatocellular carcinoma following hepatectomy. Cancer Biol Ther. 2015;16(10):1445–1452. doi: 10.1080/15384047.2015.1071730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi R, Weiland M, Gao XH, Zhou L, Mi QS. Identification of endogenous normalizers for serum microRNAs by microarray profiling: U6 small nuclear RNA is not a reliable normalizer. Hepatology. 2012;55(5):1640–1642. doi: 10.1002/hep.25558. [author reply 1642–1643] [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X, Du L, Wang L, Li J, Liu Y, Zheng G, Qu A, Zhang X, Pan H, Yang Y. Serum microRNA expression signatures identified from genome-wide microRNA profiling serve as novel noninvasive biomarkers for diagnosis and recurrence of bladder cancer. Int J Cancer. 2015;136(4):854–862. doi: 10.1002/ijc.29041. [DOI] [PubMed] [Google Scholar]
- 20.Andreou A, Vauthey JN, Cherqui D, Zimmitti G, Ribero D, Truty MJ, Wei SH, Curley SA, Laurent A, Poon RT. Improved long-term survival after major resection for hepatocellular carcinoma: a multicenter analysis based on a new definition of major hepatectomy. J Gastrointest Surg. 2013;17(1):66–77. doi: 10.1007/s11605-012-2005-4. [discussion p 77] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barreto SG, Brooke-Smith M, Dolan P, Wilson TG, Padbury RT, Chen JW. Cirrhosis and microvascular invasion predict outcomes in hepatocellular carcinoma. ANZ J Surg. 2013;83(5):331–335. doi: 10.1111/j.1445-2197.2012.06196.x. [DOI] [PubMed] [Google Scholar]
- 22.Moon JI, Kwon CH, Joh JW, Choi GS, Jung GO, Kim JM, Shin M, Choi SJ, Kim SJ, Lee SK. Primary versus salvage living donor liver transplantation for patients with hepatocellular carcinoma: impact of microvascular invasion on survival. Transplant Proc. 2012;44(2):487–493. doi: 10.1016/j.transproceed.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Fan LF, Zhao WC, Yang N, Yang GS. Alpha-fetoprotein: the predictor of microvascular invasion in solitary small hepatocellular carcinoma and criterion for anatomic or non-anatomic hepatic resection. Hepatogastroenterology. 2013;60(124):825–836. doi: 10.5754/hge121039. [DOI] [PubMed] [Google Scholar]
- 24.Zhao WC, Fan LF, Yang N, Zhang HB, Chen BD, Yang GS. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol. 2013;39(8):858–864. doi: 10.1016/j.ejso.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, Maehara Y. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol. 2007;95(3):235–240. doi: 10.1002/jso.20655. [DOI] [PubMed] [Google Scholar]
- 26.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28(4):376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 27.Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, Chung AY, Ooi LL, Tan SB. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108–113. doi: 10.1097/SLA.0b013e31821ad884. [DOI] [PubMed] [Google Scholar]
- 28.Suh YJ, Kim MJ, Choi JY, Park MS, Kim KW. Preoperative prediction of the microvascular invasion of hepatocellular carcinoma with diffusion-weighted imaging. Liver Transpl. 2012;18(10):1171–1178. doi: 10.1002/lt.23502. [DOI] [PubMed] [Google Scholar]
- 29.Chou CT, Chen RC, Lee CW, Ko CJ, Wu HK, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br J Radiol. 2012;85(1014):778–783. doi: 10.1259/bjr/65897774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, Kanematsu T. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34(5):1034–1038. doi: 10.1007/s00268-010-0424-5. [DOI] [PubMed] [Google Scholar]
- 31.Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, Sun H, Zhou J, Ji Y. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. doi: 10.1186/1471-2407-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]