Abstract
Cognitive impairment is common in Parkinson's disease (PD), but often not improved by dopaminergic treatment. New treatment strategies targeting other neurotransmitter deficits are therefore of growing interest. Imaging the brain at rest (‘task-free') provides the opportunity to examine the impact of a candidate drug on many of the brain networks that underpin cognition, while minimizing task-related performance confounds. We test this approach using atomoxetine, a selective noradrenaline reuptake inhibitor that modulates the prefrontal cortical activity and can facilitate some executive functions and response inhibition. Thirty-three patients with idiopathic PD underwent task-free fMRI. Patients were scanned twice in a double-blind, placebo-controlled crossover design, following either placebo or 40-mg oral atomoxetine. Seventy-six controls were scanned once without medication to provide normative data. Seed-based correlation analyses were used to measure changes in functional connectivity, with the right inferior frontal gyrus (IFG) a critical region for executive function. Patients on placebo had reduced connectivity relative to controls from right IFG to dorsal anterior cingulate cortex and to left IFG and dorsolateral prefrontal cortex. Atomoxetine increased connectivity from the right IFG to the dorsal anterior cingulate. In addition, the atomoxetine-induced change in connectivity from right IFG to dorsolateral prefrontal cortex was proportional to the change in verbal fluency, a simple index of executive function. The results support the hypothesis that atomoxetine may restore prefrontal networks related to executive functions. We suggest that task-free imaging can support translational pharmacological studies of new drug therapies and provide evidence for engagement of the relevant neurocognitive systems.
Introduction
Parkinson's disease (PD) impairs cognition, including executive functions, attentional control, decision-making, verbal fluency, and response inhibition. These deficits may be present soon after diagnosis and impair quality of life despite dopaminergic therapy (Watson and Leverenz, 2010; Yarnall et al, 2013). New approaches are required to maintain or restore cognitive function, in addition to the mainstay of dopaminergic therapy.
Dopaminergic therapy does not satisfactorily restore executive functions in PD (Weintraub et al, 2006; Rowe et al, 2008), due in part to deficits in non-dopaminergic systems. For example, noradrenergic projections from the locus coeruleus to the cortex are severely affected by PD pathology (Braak et al, 2003). Loss of noradrenaline has been implicated in diverse deficits in executive functions such as inhibition, attention, working memory, cognitive flexibility, and verbal fluency (Ye et al, 2015; Kehagia et al, 2014; Vazey and Aston-Jones, 2012; Williams-Gray et al, 2007; Robbins and Arnsten, 2009). Noradrenergic drugs therefore provide a potential mechanism to restore cognitive functions in selected patients (Kehagia et al, 2014; Ye et al, 2015).
Noradrenergic projections from the locus coeruleus reach the inferior frontal gyrus (IFG), presupplementary motor area (pre-SMA), dorsolateral prefrontal cortex, and anterior cingulate cortex (Goldstein et al, 2011). These are critical areas for executive function, inhibition, working memory, and fluency (Gauthier et al, 2009). For example, the right IFG and pre-SMA are important for response inhibition and attentional processing (Rae et al, 2015; Sharp et al, 2010) and are modulated by noradrenaline (Ye et al, 2015; Vazey and Aston-Jones, 2012).
The selective noradrenaline reuptake inhibitor atomoxetine increases noradrenergic neurotransmission within the prefrontal cortex (Bymaster et al, 2002). In preclinical studies, atomoxetine improves attentional set-shifting (Newman et al, 2008) and response inhibition (Robinson et al, 2008). Atomoxetine also improves response inhibition and increases right IFG activation during inhibition by healthy humans (Chamberlain et al, 2009).
Preliminary trials of PD have shown that atomoxetine can improve inhibition and attention in some patients (Kehagia et al, 2014; Ye et al, 2015). Using task-based functional magnetic resonance imaging (fMRI), Ye et al (2015, 2016) showed that the behavioral effect of atomoxetine on inhibitory control in Parkinson's patients was related to enhanced right IFG activity and stronger frontostriatal connectivity.
However, the majority of pharmacological imaging studies, including Ye et al (2015, 2016), use task-based paradigms. This approach has several potential disadvantages including performance confounds, practice effects across sessions, and the ambiguity arising from activation differences in the context of very different performance by patients (Price and Friston, 1999). They also require participant training and are limited to the relatively narrow range of neural systems related to the task.
There is an alternative approach, using task-free fMRI (also known as resting-state fMRI). This minimizes training demands, task-related confounds, and practice effects across sessions. It also allows for inclusion of cognitively and physically impaired patients. In the absence of a task, one cannot study task-related activations but one can examine changes in brain network connectivity.
We therefore used task-free fMRI to investigate the effect of atomoxetine on brain function in patients with PD, using a double-blinded, randomized placebo-controlled crossover design. We measured functional connectivity of the right IFG because of this region's central role in executive function, including inhibition and fluency, and in mediating the behavioral benefits of atomoxetine (Levy and Wagner, 2011). We used seed-based correlation methods to compare functional connectivity of the right IFG, first contrasting patients on placebo with controls (between-group design) and then contrasting patients on placebo to patients on atomoxetine (within-group design). Individual differences were accommodated using covariates of age, drug plasma concentration, disease severity, and the change in out-of-scanner cognitive performance under atomoxetine vs placebo.
We tested the specific hypotheses that (i) PD reduces functional connectivity of the right IFG with other regions implicated in executive function including the pre-SMA, dorsolateral prefrontal, and anterior cingulate cortex; and (ii) atomoxetine restores the functional connectivity of the right IFG to these regions.
Materials and methods
Participants
Thirty-three people with idiopathic PD were recruited from the Cambridge University PD Research Clinic using UK PD Society Brain Bank criteria. Subsets of these patients have been included in previous studies reporting data sets from functional tasks (Ye et al, 2015, 2016). Seventy-six healthy age- and sex-matched controls were recruited from the Medical Research Council's Cognition and Brain Sciences Unit volunteer panel and healthy volunteers registered with the Cambridge University PD Research Clinic, at the John van Geest Centre for Brain Repair. Inclusion criteria were (1) age between 45 and 80 years; (2) non-demented, with Mini-Mental State Examination (MMSE) >26/30, noting that this does not exclude mild cognitive impairment; and (3) no significant current depression. None of the patients reported symptoms or behaviors of impulse control disorders at interview.
Participants underwent assessment with the MMSE, digit span forward and backward, category and letter fluency (Rittman et al, 2013) plus the revised Beck Depression Inventory. Patients were assessed with the Unified PD Rating Scale motor subscale III at the start of each session. All participants provided written informed consent. The study was approved by the local research ethics committee and was exempted from clinical trials status by the United Kingdom Medicines for Human use Regulatory Agency.
We anticipated that any potential future use of noradrenergic drugs for cognition would be adjunctive to dopaminergic medication, not a replacement. Therefore, all patients were tested on their regular medication, to assess the effect of atomoxetine in the context of clinically optimized standard dopaminergic and/or cholinergic therapy. Levodopa equivalent dose (LED) was calculated for each patient (Tomlinson et al, 2010). Participant demographics and clinical characteristics are summarized in Table 1.
Table 1. Participant Clinical and Demographic Characteristics.
| PD patients, mean (SD) | Controls, mean (SD) | Difference (p-value) | |
|---|---|---|---|
| Male:female | 19 : 11 | 41 : 34 | NS |
| Age (years) | 67 (7.3) | 67.1 (8.4) | NS |
| Education (years) | 14.2 (3.6) | 14.8 (4.0) | NS |
| MMSE | 28.4 (1.7) | 29.2 (1.1) | 0.009 |
| Category fluency | 18.3 (5.5) | 24.3 (6.2) | 0.0001 |
| Letter fluency | 16.0 (4.4) | 18.3 (5.7) | NS |
| Digit span forward | 7.0 (1.1) | 7.3 (0.8) | NS |
| Digit span backward | 5.5 (1.2) | 6.0 (1.3) | NS |
| Disease duration (years) | 10.5 (4.4) | — | — |
| LED (mg per day) | 870 (469) | — | — |
| UPDRS III ‘on' | 22.6 (6.8) | — | — |
| Atomoxetine plasma concentration (ng/ml) | 372.1 (167.4) | — | — |
Abbreviations: LED, levodopa equivalent dose; MMSE, Mini-Mental State Examination; NS, nonsignificant; PD, Parkinson's disease; UPDRS III, the Unified Parkinson's disease rating scale motor subscale.
Groups are compared by unpaired t-test or χ2 test as appropriate (NS, p>0.05 uncorrected). LED according to the formula of Tomlinson et al (2010).
Experimental Design
The study used a double-blinded placebo-controlled crossover design for patient treatment by atomoxetine and placebo, randomized within successive blocks of six recruits to maintain balanced groups. Each patient underwent two separate sessions of cognitive and neurological assessments and brain imaging, at least 6 days apart but at approximately the same time of day on each session. At the start of each session patients received a 40 mg oral dose of atomoxetine or placebo. They were transferred to the MRI suite 2 h after the drug administration to coincide with the peak plasma concentration of atomoxetine (Sauer et al, 2005: unpublished day-curve data in a separate group of 20 PD patients confirms peak plasma levels between 120 and 180 min). Control participants were scanned once without the drug to provide normative data. The principal analysis is of the main effect of drug treatment within PD, not a drug by group interaction.
fMRI Data Acquisition and Preprocessing
Task-free functional imaging was performed at rest using a TIM-Trio 3T Magnetic Resonance Imaging (MRI) scanner (Siemens Medical Systems, Erlangen, Germany). A minimum of 145 volumes was acquired using an echo-planar imaging (EPI) sequence (repetition time (TR) 2000 ms, echo time (TE) 30 ms, matrix=64 × 64, in-plane resolution of 3 × 3 mm, 32 slices of 3 mm thickness with a 0.75 mm interslice gap, and a flip angle (FA) of 78°). Structural Magnetization-Prepared Rapid Acquisition with Gradient Echo (MPRAGE) scans (TR of 2300 ms, TE of 2.86 ms, matrix=192 × 192, in-plane resolution of 1.25 × 1.25 mm, 144 slices of 1.25 mm thickness, inversion time of 900 ms and FA of 9°) were also acquired during the same session.
We used a preprocessing pipeline optimized for older subjects to take into account atrophy and subject's head movement in the scanner (Patel et al, 2014). The Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm (Ashburner, 2007) created a study-specific template from MPRAGE images. Unified six-tissue class segmentation was applied to structural images and grey and white matter segments from all participants were warped together iteratively over six steps to create a study-specific template, which was then affine transformed to MNI space.
We used a customized version of the brainwavelet toolbox (www.brainwavelet.org) to perform preprocessing of functional images. The first five volumes were removed and the mean EPI image was coregistered to the T1 image and then transformed to MNI space using the flow fields generated during the DARTEL processing. Subsequent processing of the functional time series included slice-timing correction to correct for acquisition delay, combined regression of cerebrospinal fluid (CSF) signal and motion derivatives, high-pass band filter (0.01 Hz), and wavelet despiking (Patel et al, 2014). Spatial smoothing was applied with an 8 mm isotropic Gaussian kernel.
In-scanner head movements can produce spurious correlations in task-free fMRI data (Power et al, 2012). In the current study, we combined several approaches to minimize these motion-related effects on the blood oxygen level-dependent (BOLD) signal. First, the wavelet despiking toolbox was used during preprocessing: movements related to non-stationary events in each voxel were identified and despiked from the time course. Second, six parameters of head motion were used as regressors during seed-correlation analysis (see Functional connectivity analysis section below). Third, four participants were excluded (one control, three patients) based on a high average root mean-squared (RMS) displacement computed from the translation parameters of head motion: average RMS displacement over 2 SDs from the mean and/or 2 SDs from the mean difference between placebo and atomoxetine sessions.
We also assessed motion between imaging sessions within the patient group. The average RMS displacement did not differ significantly between placebo and atomoxetine sessions (p=0.79, t=0.26). It is therefore unlikely that drug effects on measures of functional connectivity were driven by cross-session differences in head movement.
Functional Connectivity Analysis
To investigate the effect of disease and drug on functional connectivity between brain regions, seed-to-voxel connectivity maps were created for each subject per condition. Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm) was used to perform seed-based correlation functional connectivity analysis. A seed was created for the right IFG as a 5 mm radius sphere (MNI coordinates: 56, 16, and 12). The coordinates for this seed region were derived from a separate data set acquired by Ye et al (2015). The time series of the seed region was extracted from the rs-fMRI data for each individual. Signals deriving from the CSF and white matter (WM) were extracted using template masks.
The mean time series of the seed region was then correlated with the time series of each voxel in the whole brain in a multiple regression model. Head motion parameters as well as WM and CSF signals were included as nuisance regressors to minimize the influence of these non-neuronal signals on correlations with the IFG seed region. The voxel-wise parameter estimates of linear regression were used to create correlation maps for each subject and for each session, which were then Fisher z-transformed to correct the variance in the distribution of correlation coefficients. Individual correlation maps were used in a second-level general linear model to compare functional connectivity of the right IFG between controls and patients (on placebo) and to determine if the drug-enhanced connectivity in patients on atomoxetine vs placebo. Two-sample t-test maps were thresholded at p<0.05 voxel-level FWE-whole brain-corrected plus an exploratory analysis at threshold p<0.001 uncorrected.
Age, LED, drug plasma concentration, UPDRS-III, and the change in neuropsychological performance (eg: fluency) between atomoxetine and placebo sessions were included as covariates to investigate the influence of individual differences on treatment response.
Results
Participant Demographics
Data from 75 controls and 30 patients were used in the final analysis. A summary of demographic and clinical measures for controls and patients is reported in Table 1. Patients and healthy controls were matched in terms of sex, age, and education. PD subjects had lower MMSE and category fluency scores compared with controls as expected.
Right IFG Connectivity Between Controls and Patients
Comparing controls and patients on placebo revealed a reduction in functional connectivity between the right IFG and left IFG/dorsolateral prefrontal cortex as well as the left cerebellum (Table 2 and Supplementary Figure S1). Regions with reduced functional connectivity between the right IFG at the exploratory threshold also included the dorsal anterior cingulate and pre-SMA.
Table 2. Regions with Altered Connectivity with the Right Inferior Frontal Gyrus Seed Region (Controls vs Patients and Atomoxetine versus Placebo).
| Coordinates | Z-score | Cluster size (voxels) | |
|---|---|---|---|
| Region with reduced connectivity in Parkinson's disease | |||
| Left IFG/dorsolateral prefrontal cortex | −44 6 24 | 4.73 | 2669 |
| Dorsal anterior cingulate | 10 42 32 | 3.68* | 48 |
| Pre-SMA | −10 2 56 | 3.85* | 217 |
| Cerebellum | −10 −54 −8 | 5.22 | 3376 |
| Region with increased connectivity on atomoxetine | |||
| Dorsal anterior cingulate (main effect of drug) | 12 34 18 | 4.73 | 416 |
| Left dorsolateral prefrontal cortex (drug interaction with fluency) | −50 20 34 | 3.98* | 52 |
Abbreviations: DARTEL, Diffeomorphic Anatomical Registration Through Exponentiated L; IFG, inferior frontal gyrus; MNI, Montreal Neurological Institute; SMA, supplementary motor area.
Results are reported at whole brain family-wise error correction p<0.05, or as indicated by asterisk also at the exploratory threshold uncorrected p<0.001. Coordinates refer to the local peak in MNI space using a normalized study-specific template (DARTEL).
The Effect of Atomoxetine on Connectivity
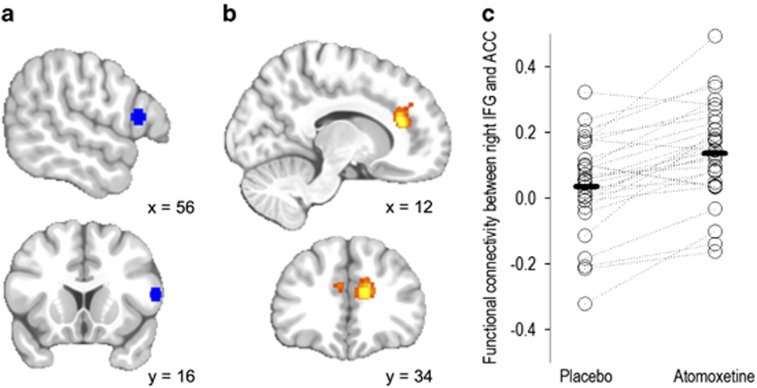
Atomoxetine increased the functional connectivity between the right IFG and dorsal anterior cingulate in PD patients (p<0.05 FWE-corrected, see Figure 1 and Table 2). The dorsal anterior cingulate region with increased connectivity with the right IFG was bilateral but asymmetrical, mainly on the right side. Age, LED, drug plasma concentration, and UPDRS-III did not significantly interact with the drug-induced changes in connectivity.
Figure 1.
Whole-brain seed-correlation comparing right inferior frontal gyrus (IFG) connectivity between placebo and atomoxetine sessions in patients with Parkinson's disease. (a) Right IFG seed region centered on 56, 16, and 12 (in blue). (b) Voxel-wise correlation map showing increased connectivity after atomoxetine in patients, between right IFG and bilateral dorsal anterior cingulate (in orange) (atomoxetine>placebo; peak p<0.05 family-wise error (FWE)-corrected, z=4.73). (c) Scatter plot showing the functional connectivity between right IFG and dorsal anterior cingulate peak during placebo and atomoxetine sessions for each patient. ACC, anterior cingulate cortex.
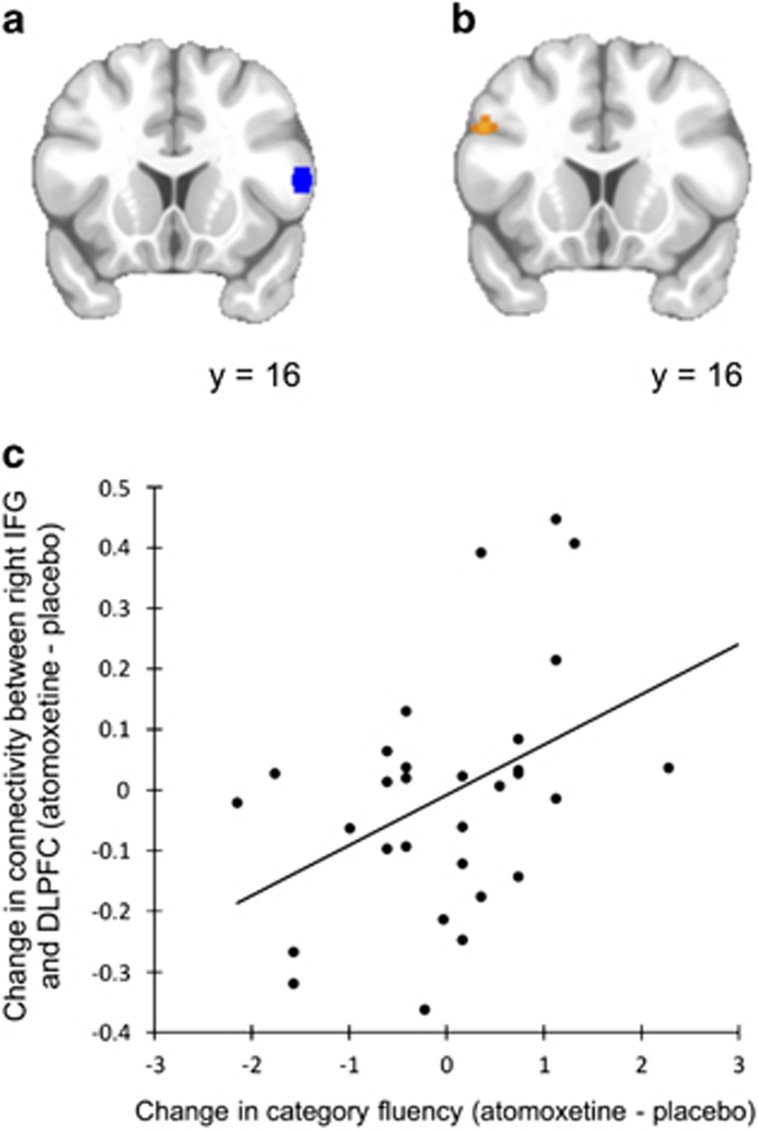
There was an interaction between the effect of atomoxetine on right IFG connectivity and category fluency during each session. Specifically, patients with greater improvement in their out-of-scanner category fluency immediately before imaging (on atomoxetine relative to placebo) also demonstrated greater increases in functional connectivity between the right IFG and the left dorsolateral prefrontal cortex (see Figure 2 and Table 2). There was no interaction between drug plasma concentration, or any other covariate, and the effect of atomoxetine on right IFG functional connectivity.
Figure 2.
Seed-correlation comparing connectivity between placebo and atomoxetine sessions in patients with Parkinson's disease. (a) The seed region in right inferior frontal gyrus (IFG) (in blue). (b and c) Altered connectivity between the right IFG and left dorsolateral prefrontal cortex (DLPFC) on atomoxetine compared with placebo interacted with the change in category fluency between these two sessions (in orange). Patients who improved on the verbal fluency task while on atomoxetine also demonstrated increased connectivity between these regions (p=0.001 uncorrected).
Discussion
We have shown that the selective noradrenergic reuptake inhibitor atomoxetine increases the functional connectivity between the right IFG and dorsal anterior cingulate cortex in PD. These two regions form a critical network for executive function, as evidenced by studies of task-based fMRI and focal brain lesions, but their interaction was revealed in this study by task-free fMRI. Atomoxetine also increased the connectivity between the IFG and dorsolateral prefrontal cortex, in proportion to the change in executive function (indexed by verbal fluency). Previous task-based investigations have indicated noradrenergic enhancement of regional activity and/or functional connectivity in the frontal lobe (Ye et al, 2015; Cubillo et al, 2014; Chamberlain et al, 2009). However, the ability to detect homologous modulations of network connectivity in the resting state, without complex tasks, opens up the use of fMRI to examine candidate therapeutic strategies for cognitive enhancement with reduced demands on timing constraints, training, and performance confounds.
The reduction in task-free connectivity correlates with both cognitive decline (Olde Dubbelink et al, 2014) and neuropsychiatric complications (Yao et al, 2014) and abnormal connectivity in PD has previously been reported (Kwak et al, 2010). In our study, patients showed reduced connectivity compared with healthy controls, between right IFG and the left IFG/dorsolateral prefrontal cortex and dorsal anterior cingulate. This accords with task-based studies of connectivity (Ye et al, 2015) and cognitive performance (Kehagia et al, 2014).
Taken together, these results support the neurobiological model of noradrenergic regulation of executive functions (Robbins and Arnsten, 2009), based on connectivity within the medial and lateral prefrontal cortex. Atomoxetine increased connectivity in this network, on a whole-group level. These patient data are consistent with the preclinical evidence. For example, enhanced noradrenergic neurotransmission in the medial prefrontal cortex facilitates attentional set-shifting performance in rats (Lapiz and Morilak, 2006), whereas noradrenergic depletion impairs attention during distracting conditions (Carli et al, 1983). Human imaging studies also suggest that interactions between dorsal anterior cingulate and right IFG support the executive function of attentional control and inhibition. For example, they are active during low-frequency events and error trials in choice discrimination and response inhibition tasks (Braver et al, 2001); during performance monitoring and attentional control (Botvinick et al, 2004); and in mediating response strategies during inhibition via the pre-SMA and subcortical structures (Sharp et al, 2010; Rae et al, 2015). The right IFG was the only seed region included in the analysis, because of task-based evidence of its involvement in executive dysfunction in PD and the neural mechanisms mediating the effect of atomoxetine on cognition. However, it is possible that atomoxetine also acts on other regions/networks and that these are also relevant to the drugs' cognitive benefits.
Given the integration of these regions within networks regulating executive control, we speculate that atomoxetine increased causal connections between them, known as effective connectivity. However, a seed-based correlational analysis does not provide direct evidence for effective integration. The analysis of effective connectivity is facilitated by strong anatomical priors and a task-based design, even where stochastic dynamic causal models are used to study task-free networks. It complements task-free methods that were the focus of this study in view of their advantages in terms of training, performance, and generalization to multiple networks.
The partial restoration of noradrenergic function in PD patients significantly modulated right IFG-left dorsolateral prefrontal cortex connectivity as a function of atomoxetine-induced changes in verbal fluency (category fluency task). In other words, people showing the highest degree of improvement in verbal fluency were also those who displayed the greatest effect of atomoxetine on functional connectivity within the prefrontal cortex. This result may explain the heterogeneity of drug response in PD and suggests that task-free fMRI might be used to develop biomarkers of individual differences modulating response to pharmacological treatments targeting cognition. Verbal fluency is impaired in PD (Williams-Gray et al, 2007) and we hypothesized that this was related to noradrenergic and structural deficits in prefrontal networks (Goldstein et al, 2011; Rae et al, 2012). The dorsolateral prefrontal cortex has also been implicated in verbal fluency (Frith et al, 1991; Cuenod et al, 1995) and noradrenergic transmission in this region is important for other executive functions (Arnsten, 2011). In PD patients, transcranial direct current stimulation of the dorsolateral prefrontal cortex improved verbal fluency and enhanced functional connectivity in verbal fluency networks (Pereira et al, 2013). The right IFG is active during verbal fluency tasks (Gaillard et al, 2000) and lesions to this region impair category fluency (Biesbroek et al, 2015). Therefore, we suggest that the functional connectivity between the right IFG and left dorsolateral prefrontal cortex represents the integration of executive strategies to support verbal category fluency.
There are several limitations to the current study. First, our patients received a single dose of atomoxetine, in conjunction with the brain imaging. However, it is possible that changes in functional connectivity following chronic drug administration are different (Koda et al, 2010). Homeostatic regulation, including for example, downregulation of noradrenergic receptors or synthesis, may ameliorate the effects of the drug. In practice, this could be offset by dose escalation, and in chronic therapy, patients with PD have tolerated up to 100 mg daily (Marsh et al, 2009). Nonetheless, future trials of clinical efficacy would need to assess longer-term treatment. Noradrenergic neurons in the LC exhibit both tonic and phasic responses, and our data alone do not discriminate the impact of atomoxetine on them. However, preclinical data indicate that atomoxetine increases the phasic-to-tonic firing ratio of the LC, thereby enhancing noradrenaline release in the frontal cortex (Florin-Lechner et al, 1996). We speculate that this effect mediates these changes in functional connectivity. However, the neurochemical mechanisms underlying the effect of atomoxetine may not be exclusively related to its effect on noradrenergic transmission. For example, atomoxetine may act via noradrenergic systems alone or together with dopaminergic transmission (Bymaster et al, 2002). Whereas animal studies suggest that the benefits of atomoxetine on cognitive control are mediated primarily by noradrenergic systems (Bari et al, 2009), interactions with dopamine cannot be excluded. For example, connectivity between the lateral and medial prefrontal cortex is partially dopamine dependent in the context of motor control (Rowe et al, 2010), and cognitive control is enhanced by drugs with joint dopaminergic and noradrenergic effects such as methylphenidate (Moeller et al, 2014). It should also be noted that dopaminergic drugs can modulate BOLD signal fluctuations (Kwak et al, 2012); however, this variable was kept constant in the within-subject analysis comparing PD patients on and off atomoxetine.
This study is not a clinical trial, and ultimately any new therapy would be judged by its clinical impact, on cognition, behavior, or motor control. However, we have demonstrated that the modulation of functional connectivity in prefrontal regions by atomoxetine in the task-free state is concordant with understandings of networks relevant to executive function as well as with changes in performance on neuropsychological assessments performed outside the scanner. This is in line with the correspondence between brain networks identified in task-free and task-based imaging (Smith et al, 2009). This technique also offers insight into how individual differences influence treatment response in terms of one or more brain networks, implicitly demonstrating target engagement in the central nervous system while minimizing the difficulties in learning or executing challenging cognitive tasks. We suggest that this technique is a useful contributor to understanding drug effects on the brain, especially in early stages of translation, but it would be a prelude to rather than a substitute for clinical trials.
In conclusion, this study supports the noradrenergic hypothesis for frontal lobe function and, indirectly, its role in cognition. We demonstrate that task-free fMRI can be used to examine therapies targeting cognitive systems and investigate individual differences associated with treatment response. Further work is needed for optimization of potential noradrenergic therapies for PD, and to establish the limits of this approach more generally in defining the impact of drugs on neurocognitive systems.
Funding and disclosure
This work was funded by the Wellcome trust (103838), Parkinson's UK, National Institute for Health Research's Cambridge Biomedical Research Centre, and the Medical Research Council (MC-A060-5PQ30 and RG62761) and the James F McDonnell Foundation (twenty-first century science initiative on Understanding Human Cognition). The BCNI is supported by a joint award from the Wellcome Trust and Medical Research Council.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Arnsten AFT (2011). Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 69: e89–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. NeuroImage 38(95): 113. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ESJ, Robbins TW (2009). Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 205: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesbroek JM, Zandvoort MJE, van, Kappelle LJ, Velthuis BK, Biessels GJ, Postma A (2015). Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Struct Funct (e-pub ahead of print; doi:10.1007/s00429-015-1033-8). [DOI] [PMC free article] [PubMed]
- Botvinick MM, Cohen JD, Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci (Regul Ed) 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Braak H, Rüb U, Gai WP, Tredici KD (2003). Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110: 517–536. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A (2001). Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex 11: 825–836. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH et al (2002). Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27: 699–711. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ (1983). Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9: 361–380. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Müller U, Rubia K, Del Campo N, Craig K et al (2009). Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry 65: 550–555. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Smith AB, Barrett N, Giampietro V, Brammer M, Simmons A et al (2014). Drug-specific laterality effects on frontal lobe activation of atomoxetine and methylphenidate in attention deficit hyperactivity disorder boys during working memory. Psychol Med 44: 633–646. [DOI] [PubMed] [Google Scholar]
- Cuenod CA, Bookheimer SY, Hertz-Pannier L, Zeffiro TA, Theodore WH, Le Bihan D (1995). Functional MRI during word generation, using conventional equipment: a potential tool for language localization in the clinical environment. Neurology 45: 1821–1827. [DOI] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ (1996). Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res 742: 89–97. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RS (1991). Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci 244: 241–246. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH (2000). Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology 54: 180–185. [DOI] [PubMed] [Google Scholar]
- Gauthier CT, Duyme M, Zanca M, Capron C (2009). Sex and performance level effects on brain activation during a verbal fluency task: a functional magnetic resonance imaging study. Cortex 45: 164–176. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Sullivan P, Holmes C, Kopin IJ, Basile MJ, Mash DC (2011). Catechols in post-mortem brain of patients with Parkinson disease. Eur J Neurol 18: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Housden CR, Regenthal R, Barker RA, Müller U, Rowe J et al (2014). Targeting impulsivity in Parkinson's disease using atomoxetine. Brain 137: 1986–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T (2010). Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem 114: 259–270. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Müller ML, Dayalu P, Seidler RD (2010). Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front Syst Neurosci 4: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Peltier SJ, Bohnen NI, Müller ML, Dayalu P, Seidler RD (2012). L-DOPA changes spontaneous low-frequency BOLD signal oscillations in Parkinson's disease: a resting state fMRI study. Front Syst Neurosci 6: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA (2006). Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137: 1039–1049. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann NY Acad Sci 1224: 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L, Biglan K, Gerstenhaber M, Williams JR (2009). Atomoxetine for the treatment of executive dysfunction in Parkinson's disease: a pilot open-label study. Mov Disord 24: 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Honorio J, Tomasi D, Parvaz MA, Woicik PA, Volkow ND et al (2014). Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cereb Cortex 24: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Darling J, McGaughy J (2008). Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berl) 200: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olde Dubbelink KTE, Schoonheim MM, Deijen JB, Twisk JWR, Barkhof F, Berendse HW (2014). Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology 83: 2046–2053. [DOI] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vértes PE, Ersche KD et al (2014). A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. NeuroImage 95: 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Junqué C, Bartrés-Faz D, Martí MJ, Sala-Llonch R, Compta Y et al (2013). Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson's disease. Brain Stimul 6: 16–24. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59: 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1999). Scanning patients with tasks they can perform. Hum Brain Mapp 8: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Correia MM, Altena E, Hughes LE, Barker RA, Rowe JB (2012). White matter pathology in Parkinson's disease: the effect of imaging protocol differences and relevance to executive function. NeuroImage 62: 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Anderson MC, Rowe JB (2015). The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci 35: 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittman T, Ghosh BC, McColgan P, Breen DP, Evans J, Williams-Gray CH et al (2013). The Addenbrooke's Cognitive Examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J Neurol Neurosurg Psychiatry 84: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT (2009). The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci 32: 267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ESJ, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X et al (2008). Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33: 1028–1037. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes LE, Barker RA, Owen AM (2010). Dynamic causal modelling of effective connectivity from fMRI: Are results reproducible and sensitive to Parkinson's disease and its treatment? NeuroImage 52: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BCP, Eckstein D, Williams-Gray CH, Fallon S et al (2008). Parkinson's disease and dopaminergic therapy— differential effects on movement, reward and cognition. Brain 131: 2094–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J-M, Ring BJ, Witcher JW (2005). Clinical pharmacokinetics of atomoxetine. Clin Pharmacokinet 44: 571–590. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, Boissezon XD, Beckmann CF, James SG, Patel MC et al (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA 107: 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE et al (2009). Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106: 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010). Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25: 2649–2653. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G (2012). The emerging role of norepinephrine in cognitive dysfunctions of Parkinson's disease. Front Behav Neurosci 6: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Leverenz JB (2010). Profile of cognitive impairment in Parkinson's disease. Brain Pathol 20: 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN et al (2006). Association of dopamine agonist use with impulse control disorders in parkinson disease. Arch Neurol 63: 969–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA (2007). Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain 130: 1787–1798. [DOI] [PubMed] [Google Scholar]
- Yao N, Shek-Kwan Chang R, Cheung C, Pang S, Lau KK, Suckling J et al (2014). The default mode network is disrupted in parkinson's disease with visual hallucinations. Hum Brain Mapp 35: 5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ et al (2013). Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 82: 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T et al (2015). Improving response inhibition in Parkinson's disease with atomoxetine. Biol Psychiatry 77: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Rae CL, Nombela C, Ham T, Rittman T, Jones PS et al (2016). Predicting beneficial effects of atomoxetine and citalopram on response inhibition in Parkinson's disease with clinical and neuroimaging measures. Hum Brain Mapp 37: 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.