Abstract
The neuroendocrine hormone amylin, also known as islet amyloid polypeptide, is co-localized, co-packaged and co-secreted with insulin from adult pancreatic islet β cells to maintain glucose homeostasis. Specifically, amylin reduces secretion of nutrient-stimulated glucagon, regulates blood pressure with an effect on renin-angiotensin system, and delays gastric emptying. The physiological actions of human amylin attribute to the conformational α-helix monomers whereas the misfolding instable oligomers may be detrimental to the islet β cells and further transform to β-sheet fibrils as amyloid deposits. No direct evidence proves that the amylin fibrils in amyloid deposits cause diabetes. Here we also have performed a systematic review of human amylin gene changes and reported the S20G mutation is minor in the development of diabetes. In addition to the metabolic effects, human amylin may modulate autoimmunity and innate inflammation through regulatory T cells to impact on both human type 1 and type 2 diabetes.
Keywords: Amylin, Neuroendocrine hormone, Diabetes
Core tip: This is a systematic review to describe amylin as a neuroendocrine hormone. Besides the glucose homeostasis and cytotoxicity of amylin, we tried to perform that the S20G mutation of human amylin is also minor in the pathogenesis of diabetes. In addition to the metabolic effects, human amylin may have impact on autoimmunity, implicating a potential as the immunosuppressor to improve autoimmunity conditions in the future therapy of diabetes, allergic diseases and immune rejection.
INTRODUCTION
Amylin, or islet amyloid polypeptide (IAPP), is a neuroendocrine hormone co-localized, co-secreted and co-packaged with insulin from pancreatic β cells[1,2]. Abnormalities in human amylin folding, secretion and action have detrimental effects on islet function and glucose regulation by islet amyloidosis and β cell dysfunction in type 2 diabetes (T2D)[3-5]. The molecules of amylin polypeptide fold to form the α-helix monomers and oligomers and the β-sheet fibrils. The amylin-aggregated amyloid fibrils are thought to form through smaller cell toxic intermediates and deposited amyloid disrupts normal islet architecture[6]. However, amylin plays a critical role in metabolism homeostasis[7] as a neuroendocrine hormone that carries a targeted signal to the brain. Several actions of amylin that impact glucose regulation have been identified, including the effects on nutrient-stimulated glucagon secretion[8], on nutrient delivery from the stomach to the small intestine for absorption[9], on renin-angiotensin system (RAS)[10] and on food intake by delaying gastric emptying[11].
FUNCTIONAL AMYLIN
Amylin functions as part of the neuroendocrine pancreas and contributes to glucose homeostasis with other two pancreatic islet hormones insulin and glucagon. The amylin and insulin is a pair of synergistic partner genes co-expressed by a common promoter[12], and regulates the levels of glucose by complex endocrine and neuronal pathways. In physiological state, the simultaneous release of amylin and insulin from the secretory granules results in a parallel pattern in the islet β-cells in response to glucose stimulation[13]. However, concentration of plasma amylin and insulin decreased in advanced T2D[7]. Glucagon commonly increases blood glucose when nutrients are not available; while insulin and amylin primarily decrease the post-meal glucose by stimulating the uptake of glucose from circulation into muscle and fat cells for storage and by inhibiting the endogeneous glucose output from liver. Complimentary to insulin, amylin regulates postprandial glycaemia by suppressing postmeal glucagon secretion from islet α-cells[8], which is possibly mediated by signals from the vagus nerve at the pancreatic islets. Amylin and insulin also coordinate storage of carbohydrate to transfer triglyceride into muscle glycogen in skeletal muscles[14] probably by phosphorylase activation[15] (Table 1 and Figure 1).
Table 1.
Physiological actions of amylin
| Neuroendocrine effects |
| Inhibiting insulin secretion in a high concentration |
| Inhibiting glucagon secretion at mealtime |
| Delaying nutrient delivery from stomach to the small intestine |
| Reducing food intake by a signal from the central nervous system |
| Metabolic effects |
| Co-regulating glucose with insulin and glucagon |
| Inhibiting muscle glycogen synthesis[15] |
| Stimulating oxidative responses and low density lipoprotein uptake in insulin-producing cells |
| Inhibiting bone resorption |
| Lipolytic-like effects |
| Renal effects |
| Renin ↑ |
| Angiotensin II ↑ |
| Regulating renal growth |
| Regulating water-sodium homeostasis |
| Haemodynamic effect |
| Aldosterone ↑ |
| Hypocalcaemia |
| Vasodilation |
Figure 1.
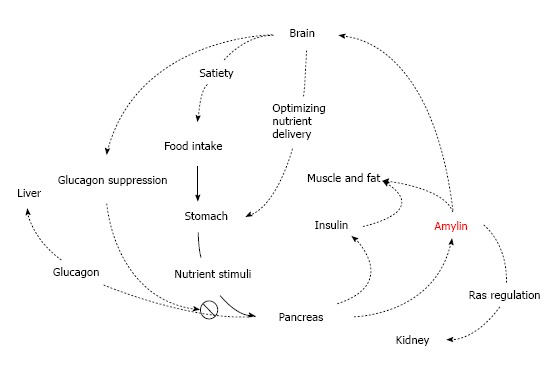
Overview of physiological actions of amylin. (1) Amylin suppresses glucagon secretion from islet alpha cells at mealtime and thus, inhibits glucagons-induced glucose release from the liver; (2) Amylin delays nutrient delivery from the stomach to the small intestine for absorption; (3) Amylin reduces food intake by a signal mediated through the central nervous system; (4) Renal amylin may stimulate Renin-Angiotensin System; and (5) Amylin and insulin coordinate storage of carbohydrate.
As a neuroendocrine hormone, amylin also acts in the central nervous system to produce satiety through brainstem-localized receptors, which have been found at several locations in the brain, including the nucleus accumbens, the dorsal raphe and the area postrema in rat brain[16]. The area postrema may be an important site for amylin action. This area does not have a blood brain barrier and allows access to circulatory peptides. Lesioning studies have indicated that some of amylin’s actions are mediated at this site. The suppression of neuronal amylin on food intake and gastrointestinal motility[17] to slow down the absorption and to limit the rate at which glucose enters the circulation[18] has been found in human. Gastric emptying is considered to be a typical pathological phenomenon and a crucial reason for the postprandial hyperglycemia in T1D. It is believed that most of amylin deficiency in T1D may be pathogenically significant in the gastric behavior[19]. Thus, high plasma amylin concentration in young with newly-diagnosed T1D[20], which may result in a delay in gastric emptying that markedly improved postprandial glucose excursions in new T1D patients[19]. Amylin deficiency significantly affects the lack of delay in gastric emptying in response to hyperglycemia in T1D[19], and is further supported by the highly potent protective effects of amylin on glucose homeostasis[21].
Furthermore, a physiological effect of amylin on the RAS has been implicated in the hemodynamic regulation of blood pressure[22] and kidney function[10]. Inhibition of angiotensin-converting enzyme (ACE) is associated with the reducing density of amylin binding in the renal cortex[10]. Pharmacokinetics pattern of amylin closely resembles that of C-peptide[23-25]. Excreted in the urine, when glomerular filtration decreases, amylin cumulates in the blood stream. Therefore, patients with renal failure may have high levels of circulating amylin[26]. These patients also have a higher than normal prevalence of islet amyloid in the absence of diabetic symptoms[27] (Table 1 and Figure 1).
KINETICS OF AMYLIN
Human amylin is derived from a larger precursor proamylin, coding sequence with 89 amino acids residue. The flanking peptides at N-terminal and C-terminal of mature amylin are removed by a proteolytic enzyme, which is also responsible for proinsulin to insulin conversion in the β cells[28]. The prohormone convertases PC2 and PC3 involved in processing proinsulin are likely responsible for amylin processing as well[5].
Measuring the concentration of circulating amylin is challenging. The minimum detectable concentration of amylin in 50 mL plasma is 0.5 to 2 pmol/L, and the dynamic range is 2 to 100 pmol/L[29]. The basal plasma concentrations of amylin in human in the 2-15 pmol/L range, with an insulin/amylin ratio of 10-100:1[30,31]. In healthy subjects, circulating amylin rises in response to the glucose challenge[32]. The amylin to insulin molar ratio is similar at all time points despite of high-frequency oscillations and inter-race differences in circulating amylin concentrations[33,34].
Circulating amylin levels are increased in individuals with obesity, hypertension, positive family history of insulin resistance in line with hyperinsulinaemia[35-37]. An exaggerated amylin response has been documented in subjects with obesity and impaired glucose tolerance[32,36]. Moreover, the amylin to insulin ratio is consistent in insulin-resistant persons at all time points, although large interindividual variations (0.2% to 1.6%) in amylin/insulin secretory ratios have been documented[35]. Thus, hyperamylinaemia due to insulin resistance precedes the occurrence of T2D[36]. However, in later-stage T2D, the secretion of both amylin and insulin becomes deficient[32]. In diabetic patients on insulin treatment, amylin/insulin is detectable but the response to the glucose challenge is negligible, reflecting functional failure of the islet β cells[38]. Interestingly, diabetes is also characterised by an excess of glucagon, in particularly after mealtimes. The net effect of insulin and amylin deficiency and glucagon excess is an increased postmeal glucose level. Furthermore, prolonged exposure of pancreatic islets to hyperglycaemia favours selective amylin secretion, increasing the risk of islet amyloid formation and β cell apoptosis[39,40].
AMYLIN-DERIVED AMYLOIDOSIS
Amyloidosis is a generic term for aggregation state of amyloid polypeptide with β-sheet conformation that bounds to each other by certain chemical bonds[41]. Amylin is encoded by calcitonin mRNA from a gene made up of three introns on the 12th chromosome[42]. Besides amylin, more than 25 proteins in human are known by their fibrillate aggregation[43,44]. Many of them have similar protein structures with amyloid-like properties and characteristic occurrences in metabolic disturbances, such as amylin, amyloid light-chain and β-amyloid[45,46]. In the islet of T2D patients, amylin fibrils commonly contribute to the form of islet amyloid. In addition, amylin is also found to deposit in brain[16], plays the potential role in the development of Alzheimer’s disease (AD) and cerebrovascular disease (CVD) pathology with β-amyloid, or might impair brain function independently of β-amyloid pathology[47].
Certain gene mutations, amino acid sites in amylin protein and minor components are more or less associated with amyloid deposits. It has been reported some mutations in the human amylin gene leading to amino acid substitutions, such as S20G. S20G is an important amylin gene mutation resulting in a glycine for serine substitution at position 20 of the mature IAPP molecule (Figure 2A). In vitro studies indicate that the S20G mutation amylin is more cytotoxic in forming amyloid and inducing apoptosis in COS-1 cells[48]. A low prevalence (< 5%) of the S20G has been reported in T2D Japanese[49-51], Hong Kong Chinese[52,53], Taiwanese[54], and Mainland Chinese. All the cases with the mutation S20G are heterozygous. Although the heterozygous mutation S20G is more common in diabetic patients than in normal control (2.6% vs 0.9%, P < 0.0001), linkage analysis reveals that mutation in or near amylin gene is unlikely a major course of T2D[55] (Table 2).
Figure 2.
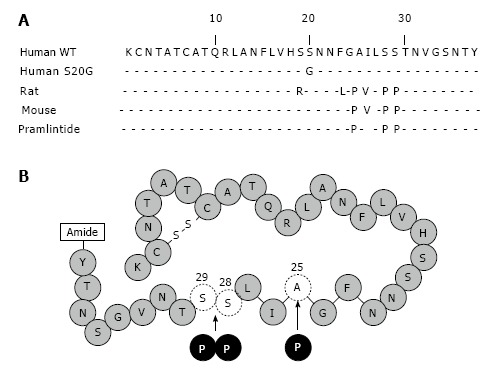
Amino acid sequence and diagrammatic representations of human amylin and pramlintide. A: Amino acid sequence alignment of human (WT, S20G), rat, mouse amylin and pramlintide. Only the amino acids that differ are shown. The sequence between amino acids 20 to 29 represents the amyloidogenic domain; B: The synthetic amylin analog pramlintide differs from human amylin at three amino acid sites (proline at 25, 28, and 29) and this molecule overcomes these disadvantages of human amylin.
Table 2.
Prevalence of the amylin gene mutation S20G in Asian populations (%) n
| Ref. | Type 2 diabetes | Impaired glucose tolerance | Type 1 diabetes | Control | |
| Japanese | Seino et al[50] | 2.6 (1538) | - | - | 0.8 (1108) |
| Japanese | Yamada et al[51] | 4.7 (86) | - | - | 1.6 (182) |
| Japanese | Sakagashira et al[49] | 4.1 (294) | - | 0 (59) | 0 (187) |
| Hong Kong Chinese | Ng et al[53] | 1.5 (462) | - | - | 0 (126) |
| Taiwanese | Chuang et al[54] | 1.6 (182) | 4.2 (24) | 1.6 (122) | 4.4 (99) |
| Mainland Chinese | Lee et al[52] | 2.1 (94) | - | - | 0 (106) |
Many molecular chaperones, like apolipoprotein E (apoE) and heat shock protein (HSP) family, may relates to amylin deposits[56-58], and is generally considered as a major genetic modulator of β-amyloid deposition and risk of AD[59,60]. The apoE ε4 allele particularly affects the increased risk for atherosclerosis[61], brain plaque[61] and islet amyloidosis. In T2D, apoE plays a critical role in lipid metabolism, amylin fibril formation[62] and is a probable link to atherosclerosis[57]. HSP is identified within highly purified β cell granules derived from INS-1E islet β cells such as insulin and amylin[63]. Vita demonstrates the existence of direct functional interactions involved HSP70, can suppress the misfolding of human amylin[58], which is also proved to limit the toxicity of β-amyloid[64]. These chaperones may contribute the AD-diabetes link at the pathophysiological level, including the interactive amyloid of β-amyloid and human amylin[65,66].
CONFORMATIONS OF AMYLIN
The conformation of amylin is considered a significant factor in that abnormal accumulation of amylin fibrils in organs may lead to amyloidosis in T2D. Human amylin is subtyped into three different conformations: Monomers, oligomers and fibrils (Figure 3). Monomers are unfolded random-coiled peptides physiologically. These molecules can be misfolding with α-helix structures, and aggregated into the pathologic oligomers, the soluble amyloid intermediates, which include spherical particles of 2.7 to 4.2 nm in diameter[67]. Amylin fibrils formation is a self-driven process accumulating the misfolded oligomeric proteins with a β-sheet fibrillar structure into insoluble islet amyloid deposits. Islet amylin-amyloid is the pathologic hallmark of most individuals with T2D[22,68].
Figure 3.
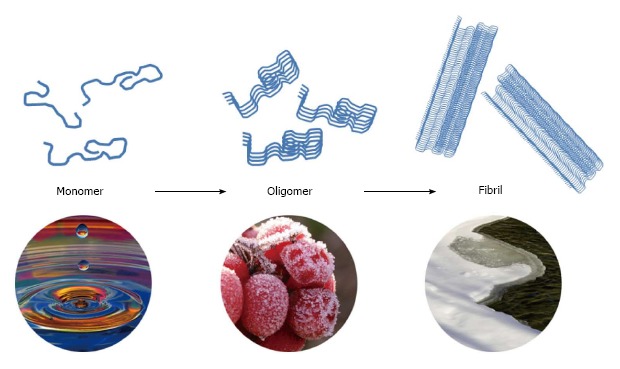
Three conformations of human amylin. Monomers of amylin with physiological functions mainly contribute to glucose and lipid homeostasis and tend to misfold into the cytotoxic oligomers. A self-driven process is accumulating the misfolded oligomers into insoluble nontoxic amylin fibrils with a β-sheet structure.
In physiological state, the toxic oligomers can be rescued into amyloid fibrils by chaperones or eliminated by the ubiquitin-proteasome system[69]. Concentration of amylin fibrils in the intracytoplasmic organelles of human beta cells far exceed the in vitro concentration required for amyloid formation, so there must be an underlying mechanism in normal beta cells to induce the aggregation of amylin monomers into fibrils. Mechanisms that may associate with amylin-amyloid include an acid pH[70], the presence of chaperon proteins (HSP), or the presence of other proteins[58]. Then these possible factors for amylin aggregation may derive from hyperglycaemia, high-fat diet, or low-grade chronic inflammation[3], which are considered as the cardinal symptoms of T2D. The amylin monomer has its special function in endocrine system, or further polymerise to amyloid fibrils, which may play an important role in cell informational transfer, memory and survival prolonging[71].
Mature fibrillar aggregate of amylin has been considered to be nontoxic, and even these small amyloid deposits seen widely located in islets or other organs in T2D, may not have significant contribution to organs damage. Therefore, the formation of fibrils from cytotoxic oligomers can be considered as a protective mechanism of transforming a dynamic protein into inert amyloid. Here we have to be curious whether this process of fibrillar formation initially acts as a rescuer in the pathway of cell failure[71].
The amino acid sequence of amylin derived from islet amyloid in T2D is identical to that present in healthy humans, and amylin from human insulinoma tissue. Moreover, amylin structure exhibits close sequence homology among all species in both the amino terminal (residues 1 to 19) and the carboxy terminal (residues 20 to 29) regions. In contrast, residues 20 to 29 show considerable divergence among species and have been implicated in the conversion of the peptide’s secondary structure from a predominantly α helical one to a β sheet structure[72]. This assignment is based on the comparison of the sequences of human amylin, which is highly amyloidogenic, with those of cat amylin, which is moderately amyloidogenic, and with rat and mouse amylin, which does not aggregate to form amyloid[72,73].
AMYLIN TOXICITY
Mechanisms of islet β cell depletion by amyloid include mechanical replacement, apoptosis, necrosis and β cell membrane damage. Human amylin has been clearly shown toxic to insulin-producing β cells of the adult pancreas of rats and humans[74]. The cytotoxic action of amylin in insulin-producing cells is paralleled by increased oxidative responses and low density lipoprotein (LDL) uptake, suggesting that cytotoxic mechanisms of amylin in insulin-producing cells involve changes in pathways of cellular oxidative stress systems and lipid homeostasis[75].
Soluble oligomer of amylin is recently reported to contribute the primary toxicity in T2D but not unsoluble fibril in the amyloid diseases[76-78]. Different misfolded oligomers of amylin with a conformation-dependent structure suggest that they share a common mechanism of pathogenesis[79]. Like oligomer of β amyloid protein playing an important role in the pathogenesis of AD and CVD[80], oligomic amylin is also a central subject in the risk of the islet β-cell lesion in T2D through formation of toroidal (ion-leaking) pores inserted into membranes[81,82].
THERAPEUTIC APPLICATION AND PROSPECTIVE
Human amylin has a tendency to aggregate, form insoluble particles and stick to surfaces. Learnt from non-amyloidogenic rat amylin, the peptide structure is broken by substituting the positions 25 alanine, 28 and 29 serines into proline residues (Figure 2B). This analog of human amylin, named “pramlintide”, is used for the potential prevention of complications of T1D as an adjunct with insulin and a single agent for T2D[2]. This soluble, stable synthetic analog amylin avoids aggregation of amyloid relating the development of β-cell dysfunction[83]. Like wild-type human amylin, pramlintide can adjust postprandial glucagon release and gastric emptying rate in individuals with T1D and T2D[84-87]. In clinical therapy of diabetes, pramlintide as an assistant treatment of insulin usually decreases postprandial glucose without rising insulin level[88,89]. In T1D, pramlintide therapy significantly reduced 4.4-6.6 mmol/mol haemoglobin a1c at 26 wk vs placebo[90,91]. And mean body weight was significantly reduced (-0.8 to -1.3 kg at week 26 or 29) vs placebo[90-92]. Then in T2D, pramlintide therapy resulted in significant reductions in haemoglobin a1c (-7.7 to -8.7 mmol/mol after 16 or 26 wk) and mean body weight (-1.4 to -1.6 kg after 16 or 26 wk) vs placebo[93-95]. According to these functions, the pramlintide is manufactured and designed as injection in pen injector. Since the pH value of pramlintide buffer is incompatible with most insulin products, pramlintide is recommended not to mix with insulin in the same syringe (shown in Symlin® Package Insert). This analog has the same functions of blood glucose regulation and gastric emptying delay as wild-type human amylin.
Human amylin no doubt plays a significant role in neuroendocrine contribution to glucose homeostasis. Treatment with non-fibrillar pramlintide improves glycaemic control and weight management without adverse events of severe hypoglycaemia in T1D and T2D[96]. However, whether the toxicity of fibrillar amylin contributes significantly to pathogenesis of diabetes is yet unconvincing. Studies data indicate that microscopically evident fibrillar amylin is neither necessary nor sufficient to cause diabetes, but rather that it is positively correlated with protection[97,98].
It is worth noting that amylin may regulate the inflammatory response and immune factor secretion[99,100]. Mouse amylin was reported that can trigger a broad autoimmune response by CD4+ effector T cells in NOD mice[101]. Recent study shows that human amylin can induce CD4+CD25+FoxP3+ Regulatory T cells and reduce risk of autoimmune diabetes[102]. It firstly demonstrates autoimmune inhibition by human amylin. All these findings suggest a novel approach to restore glucose homeostasis and improve autoimmunity conditions such as autoimmune diseases, allergic diseases, immune rejection of organ transplantation and graft vs host reaction (GVHR).
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 9, 2016
First decision: March 1, 2016
Article in press: April 6, 2016
P- Reviewer: Mansour AA, Nishio K, Tarantino G S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Bioph Res Co. 1986;140:827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark A, Chargé SB, Badman MK, de Koning EJ. Islet amyloid in type 2 (non-insulin-dependent) diabetes. APMIS. 1996;104:12–18. doi: 10.1111/j.1699-0463.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark A, Charge SB, Badman MK, MacArthur DA, de Koning EJ. Islet amyloid polypeptide: actions and role in the pathogenesis of diabetes. Biochem Soc Trans. 1996;24:594–599. doi: 10.1042/bst0240594. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 6.Anguiano M, Nowak RJ, Lansbury PT. Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE, Verchere CB, Andrikopoulos S, Asberry PJ, Leonetti DL, Wahl PW, Boyko EJ, Schwartz RS, Newell-Morris L, Fujimoto WY. Reduced amylin release is a characteristic of impaired glucose tolerance and type 2 diabetes in Japanese Americans. Diabetes. 1998;47:640–645. doi: 10.2337/diabetes.47.4.640. [DOI] [PubMed] [Google Scholar]
- 8.Gedulin BR, Rink TJ, Young AA. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46:67–70. doi: 10.1016/s0026-0495(97)90170-0. [DOI] [PubMed] [Google Scholar]
- 9.Edelman SV, Weyer C. Unresolved challenges with insulin therapy in type 1 and type 2 diabetes: potential benefit of replacing amylin, a second beta-cell hormone. Diabetes Technol Ther. 2002;4:175–189. doi: 10.1089/15209150260007390. [DOI] [PubMed] [Google Scholar]
- 10.Wookey PJ, Cao Z, Cooper ME. Interaction of the renal amylin and renin-angiotensin systems in animal models of diabetes and hypertension. Miner Electrolyte Metab. 1998;24:389–399. doi: 10.1159/000057400. [DOI] [PubMed] [Google Scholar]
- 11.Cooper GJ, Day AJ, Willis AC, Roberts AN, Reid KB, Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989;1014:247–258. doi: 10.1016/0167-4889(89)90220-6. [DOI] [PubMed] [Google Scholar]
- 12.German MS, Moss LG, Wang J, Rutter WJ. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical beta-cell nuclear complexes. Mol Cell Biol. 1992;12:1777–1788. doi: 10.1128/mcb.12.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulder H, Ahrén B, Sundler F. Islet amyloid polypeptide and insulin gene expression are regulated in parallel by glucose in vivo in rats. Am J Physiol. 1996;271:E1008–E1014. doi: 10.1152/ajpendo.1996.271.6.E1008. [DOI] [PubMed] [Google Scholar]
- 14.James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, Friend LA, Shelly DA, Paul RJ, Fischer JE. Stimulation of both aerobic glycolysis and Na(+)-K(+)-ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol. 1999;277:E176–E186. doi: 10.1152/ajpendo.1999.277.1.E176. [DOI] [PubMed] [Google Scholar]
- 15.Young AA, Mott DM, Stone K, Cooper GJ. Amylin activates glycogen phosphorylase in the isolated soleus muscle of the rat. FEBS Lett. 1991;281:149–151. doi: 10.1016/0014-5793(91)80380-l. [DOI] [PubMed] [Google Scholar]
- 16.Beaumont K, Kenney MA, Young AA, Rink TJ. High affinity amylin binding sites in rat brain. Mol Pharmacol. 1993;44:493–497. [PubMed] [Google Scholar]
- 17.Grabauskas G, Zhou SY, Das S, Lu Y, Owyang C, Moises HC. Prolactin-releasing peptide affects gastric motor function in rat by modulating synaptic transmission in the dorsal vagal complex. J Physiol. 2004;561:821–839. doi: 10.1113/jphysiol.2004.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samsom M, Szarka LA, Camilleri M, Vella A, Zinsmeister AR, Rizza RA. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol Gastrointest Liver Physiol. 2000;278:G946–G951. doi: 10.1152/ajpgi.2000.278.6.G946. [DOI] [PubMed] [Google Scholar]
- 19.Woerle HJ, Albrecht M, Linke R, Zschau S, Neumann C, Nicolaus M, Gerich JE, Göke B, Schirra J. Impaired hyperglycemia-induced delay in gastric emptying in patients with type 1 diabetes deficient for islet amyloid polypeptide. Diabetes Care. 2008;31:2325–2331. doi: 10.2337/dc07-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsson JF, Ludvigsson J, Carlsson A, Casas R, Forsander G, Ivarsson SA, Kockum I, Lernmark Å, Marcus C, Lindblad B, et al. High plasma levels of islet amyloid polypeptide in young with new-onset of type 1 diabetes mellitus. PLoS One. 2014;9:e93053. doi: 10.1371/journal.pone.0093053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young A. Inhibition of gastric emptying. Adv Pharmacol. 2005;52:99–121. doi: 10.1016/S1054-3589(05)52006-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhao HL, Lai FM, Tong PC, Zhong DR, Yang D, Tomlinson B, Chan JC. Prevalence and clinicopathological characteristics of islet amyloid in chinese patients with type 2 diabetes. Diabetes. 2003;52:2759–2766. doi: 10.2337/diabetes.52.11.2759. [DOI] [PubMed] [Google Scholar]
- 23.Kautzky-Willer A, Thomaseth K, Ludvik B, Nowotny P, Rabensteiner D, Waldhäusl W, Pacini G, Prager R. Elevated islet amyloid pancreatic polypeptide and proinsulin in lean gestational diabetes. Diabetes. 1997;46:607–614. doi: 10.2337/diab.46.4.607. [DOI] [PubMed] [Google Scholar]
- 24.Thomaseth K, Pacini G, Clodi M, Kautzky-Willer A, Nolan JJ, Prager R, Olefsky JM, Ludvik B. Amylin release during oral glucose tolerance test. Diabet Med. 1997;14 Suppl 2:S29–S34. doi: 10.1002/(sici)1096-9136(199706)14:2+<s29::aid-dia401>3.3.co;2-s. [DOI] [PubMed] [Google Scholar]
- 25.Thomaseth K, Kautzky-Willer A, Ludvik B, Prager R, Pacini G. Integrated mathematical model to assess beta-cell activity during the oral glucose test. Am J Physiol. 1996;270:E522–E531. doi: 10.1152/ajpendo.1996.270.3.E522. [DOI] [PubMed] [Google Scholar]
- 26.Ludvik B, Clodi M, Kautzky-Willer A, Schuller M, Graf H, Hartter E, Pacini G, Prager R. Increased levels of circulating islet amyloid polypeptide in patients with chronic renal failure have no effect on insulin secretion. J Clin Invest. 1994;94:2045–2050. doi: 10.1172/JCI117558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Koning EJ, van den Brand JJ, Mott VL, Chargé SB, Hansen BC, Bodkin NL, Morris JF, Clark A. Macrophages and pancreatic islet amyloidosis. Amyloid. 1998;5:247–254. doi: 10.3109/13506129809007297. [DOI] [PubMed] [Google Scholar]
- 28.Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988;263:17243–17246. [PubMed] [Google Scholar]
- 29.Percy AJ, Trainor DA, Rittenhouse J, Phelps J, Koda JE. Development of sensitive immunoassays to detect amylin and amylin-like peptides in unextracted plasma. Clin Chem. 1996;42:576–585. [PubMed] [Google Scholar]
- 30.Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, Chang JK, Rizza RA. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–756. doi: 10.2337/diab.39.6.752. [DOI] [PubMed] [Google Scholar]
- 31.Sanke T, Hanabusa T, Nakano Y, Oki C, Okai K, Nishimura S, Kondo M, Nanjo K. Plasma islet amyloid polypeptide (Amylin) levels and their responses to oral glucose in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1991;34:129–132. doi: 10.1007/BF00500385. [DOI] [PubMed] [Google Scholar]
- 32.Koda JE, Fineman MS, Kolterman OG, Caro JF. 24 hour plasma amylin profiles are elevated in IGT subjects vs. normal controls. Diabetes. 1995;44(suppl 1):238A. [Google Scholar]
- 33.Dimsdale JE, Kolterman O, Koda J, Nelesen R. Effect of race and hypertension on plasma amylin concentrations. Hypertension. 1996;27:1273–1276. doi: 10.1161/01.hyp.27.6.1273. [DOI] [PubMed] [Google Scholar]
- 34.Juhl CB, Pørksen N, Sturis J, Hansen AP, Veldhuis JD, Pincus S, Fineman M, Schmitz O. High-frequency oscillations in circulating amylin concentrations in healthy humans. Am J Physiol Endocrinol Metab. 2000;278:E484–E490. doi: 10.1152/ajpendo.2000.278.3.E484. [DOI] [PubMed] [Google Scholar]
- 35.Blackard WG, Clore JN, Kellum JM. Amylin/insulin secretory ratios in morbidly obese man: inverse relationship with glucose disappearance rate. J Clin Endocrinol Metab. 1994;78:1257–1260. doi: 10.1210/jcem.78.5.8175987. [DOI] [PubMed] [Google Scholar]
- 36.Gulli G, Rossetti L, DeFronzo RA. Hyperamylinemia is associated with hyperinsulinemia in the glucose-tolerant, insulin-resistant offspring of two Mexican-American non-insulin-dependent diabetic parents. Metabolism. 1997;46:1157–1161. doi: 10.1016/s0026-0495(97)90209-2. [DOI] [PubMed] [Google Scholar]
- 37.Kailasam MT, Parmer RJ, Tyrell EA, Henry RR, O’Connor DT. Circulating amylin in human essential hypertension: heritability and early increase in individuals at genetic risk. J Hypertens. 2000;18:1611–1620. doi: 10.1097/00004872-200018110-00012. [DOI] [PubMed] [Google Scholar]
- 38.Koda JE, Fineman M, Rink TJ, Dailey GE, Muchmore DB, Linarelli LG. Amylin concentrations and glucose control. Lancet. 1992;339:1179–1180. doi: 10.1016/0140-6736(92)90785-2. [DOI] [PubMed] [Google Scholar]
- 39.Gasa R, Gomis R, Casamitjana R, Novials A. High glucose concentration favors the selective secretion of islet amyloid polypeptide through a constitutive secretory pathway in human pancreatic islets. Pancreas. 2001;22:307–310. doi: 10.1097/00006676-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Hou X, Ling Z, Quartier E, Foriers A, Schuit F, Pipeleers D, Van Schravendijk C. Prolonged exposure of pancreatic beta cells to raised glucose concentrations results in increased cellular content of islet amyloid polypeptide precursors. Diabetologia. 1999;42:188–194. doi: 10.1007/s001250051138. [DOI] [PubMed] [Google Scholar]
- 41.Rochet JC, Lansbury PT. Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol. 2000;10:60–68. doi: 10.1016/s0959-440x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 42.Höppener JW, Oosterwijk C, van Hulst KL, Verbeek JS, Capel PJ, de Koning EJ, Clark A, Jansz HS, Lips CJ. Molecular physiology of the islet amyloid polypeptide (IAPP)/amylin gene in man, rat, and transgenic mice. J Cell Biochem. 1994;55 Suppl:39–53. doi: 10.1002/jcb.240550006. [DOI] [PubMed] [Google Scholar]
- 43.Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, Ikeda S, Masters CL, Merlini G, Saraiva MJ, Sipe JD. A primer of amyloid nomenclature. Amyloid. 2007;14:179–183. doi: 10.1080/13506120701460923. [DOI] [PubMed] [Google Scholar]
- 44.Westermark P. Aspects on human amyloid forms and their fibril polypeptides. FEBS J. 2005;272:5942–5949. doi: 10.1111/j.1742-4658.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 45.Yao Y, Wang SX, Zhang YK, Qu Z, Liu G, Zou WZ. A clinicopathological analysis in a large cohort of Chinese patients with renal amyloid light-chain amyloidosis. Nephrol Dial Transplant. 2013;28:689–697. doi: 10.1093/ndt/gfs501. [DOI] [PubMed] [Google Scholar]
- 46.Kojro E, Postina R. Regulated proteolysis of RAGE and AbetaPP as possible link between type 2 diabetes mellitus and Alzheimer’s disease. J Alzheimers Dis. 2009;16:865–878. doi: 10.3233/JAD-2009-0998. [DOI] [PubMed] [Google Scholar]
- 47.Srodulski S, Sharma S, Bachstetter AB, Brelsfoard JM, Pascual C, Xie XS, Saatman KE, Van Eldik LJ, Despa F. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol Neurodegener. 2014;9:30. doi: 10.1186/1750-1326-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakagashira S, Hiddinga HJ, Tateishi K, Sanke T, Hanabusa T, Nanjo K, Eberhardt NL. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am J Pathol. 2000;157:2101–2109. doi: 10.1016/S0002-9440(10)64848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakagashira S, Sanke T, Hanabusa T, Shimomura H, Ohagi S, Kumagaye KY, Nakajima K, Nanjo K. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes. 1996;45:1279–1281. doi: 10.2337/diab.45.9.1279. [DOI] [PubMed] [Google Scholar]
- 50.Seino S. S20G mutation of the amylin gene is associated with Type II diabetes in Japanese. Study Group of Comprehensive Analysis of Genetic Factors in Diabetes Mellitus. Diabetologia. 2001;44:906–909. doi: 10.1007/s001250100531. [DOI] [PubMed] [Google Scholar]
- 51.Yamada K, Yuan X, Ishiyama S, Nonaka K. Glucose tolerance in Japanese subjects with S20G mutation of the amylin gene. Diabetologia. 1998;41:125. doi: 10.1007/s001250050878. [DOI] [PubMed] [Google Scholar]
- 52.Lee SC, Hashim Y, Li JK, Ko GT, Critchley JA, Cockram CS, Chan JC. The islet amyloid polypeptide (amylin) gene S20G mutation in Chinese subjects: evidence for associations with type 2 diabetes and cholesterol levels. Clin Endocrinol (Oxf) 2001;54:541–546. doi: 10.1046/j.1365-2265.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 53.Ng MC, Lee SC, Ko GT, Li JK, So WY, Hashim Y, Barnett AH, Mackay IR, Critchley JA, Cockram CS, et al. Familial early-onset type 2 diabetes in Chinese patients: obesity and genetics have more significant roles than autoimmunity. Diabetes Care. 2001;24:663–671. doi: 10.2337/diacare.24.4.663. [DOI] [PubMed] [Google Scholar]
- 54.Chuang LM, Lee KC, Huang CN, Wu HP, Tai TY, Lin BJ. Role of S20G mutation of amylin gene in insulin secretion, insulin sensitivity, and type II diabetes mellitus in Taiwanese patients. Diabetologia. 1998;41:1250–1251. doi: 10.1007/s001250051060. [DOI] [PubMed] [Google Scholar]
- 55.Cook JT, Patel PP, Clark A, Höppener JW, Lips CJ, Mosselman S, O’Rahilly S, Page RC, Wainscoat JS, Turner RC. Non-linkage of the islet amyloid polypeptide gene with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:103–108. doi: 10.1007/BF00500380. [DOI] [PubMed] [Google Scholar]
- 56.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 57.Guan J, Zhao HL, Sui Y, He L, Lee HM, Lai FM, Tong PC, Chan JC. Histopathological correlations of islet amyloidosis with apolipoprotein E polymorphisms in type 2 diabetic Chinese patients. Pancreas. 2013;42:1129–1137. doi: 10.1097/MPA.0b013e3182965e6e. [DOI] [PubMed] [Google Scholar]
- 58.Chien V, Aitken JF, Zhang S, Buchanan CM, Hickey A, Brittain T, Cooper GJ, Loomes KM. The chaperone proteins HSP70, HSP40/DnaJ and GRP78/BiP suppress misfolding and formation of β-sheet-containing aggregates by human amylin: a potential role for defective chaperone biology in Type 2 diabetes. Biochem J. 2010;432:113–121. doi: 10.1042/BJ20100434. [DOI] [PubMed] [Google Scholar]
- 59.Peuralinna T, Tanskanen M, Mäkelä M, Polvikoski T, Paetau A, Kalimo H, Sulkava R, Hardy J, Lai SL, Arepalli S, et al. APOE and AβPP gene variation in cortical and cerebrovascular amyloid-β pathology and Alzheimer’s disease: a population-based analysis. J Alzheimers Dis. 2011;26:377–385. doi: 10.3233/JAD-2011-102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW. Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J Biol Chem. 2006;281:29468–29478. doi: 10.1074/jbc.M602533200. [DOI] [PubMed] [Google Scholar]
- 61.Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- 62.Mahley RW, Rall SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 63.Hickey AJ, Bradley JW, Skea GL, Middleditch MJ, Buchanan CM, Phillips AR, Cooper GJ. Proteins associated with immunopurified granules from a model pancreatic islet beta-cell system: proteomic snapshot of an endocrine secretory granule. J Proteome Res. 2009;8:178–186. doi: 10.1021/pr800675k. [DOI] [PubMed] [Google Scholar]
- 64.Magrané J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang M, Hu R, Chen H, Chang Y, Ma J, Liang G, Mi J, Wang Y, Zheng J. Polymorphic cross-seeding amyloid assemblies of amyloid-β and human islet amyloid polypeptide. Phys Chem Chem Phys. 2015;17:23245–23256. doi: 10.1039/c5cp03329b. [DOI] [PubMed] [Google Scholar]
- 66.O’Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J Biol Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 67.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maloy AL, Longnecker DS, Greenberg ER. The relation of islet amyloid to the clinical type of diabetes. Hum Pathol. 1981;12:917–922. doi: 10.1016/s0046-8177(81)80197-9. [DOI] [PubMed] [Google Scholar]
- 69.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Chargé SB, de Koning EJ, Clark A. Effect of pH and insulin on fibrillogenesis of islet amyloid polypeptide in vitro. Biochemistry. 1995;34:14588–14593. doi: 10.1021/bi00044a038. [DOI] [PubMed] [Google Scholar]
- 71.Dobson CM. Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol. 2004;15:3–16. doi: 10.1016/j.semcdb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 72.Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Betsholtz C, Christmanson L, Engström U, Rorsman F, Jordan K, O’Brien TD, Murtaugh M, Johnson KH, Westermark P. Structure of cat islet amyloid polypeptide and identification of amino acid residues of potential significance for islet amyloid formation. Diabetes. 1990;39:118–122. doi: 10.2337/diacare.39.1.118. [DOI] [PubMed] [Google Scholar]
- 74.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 75.Janciauskiene S, Ahrén B. Fibrillar islet amyloid polypeptide differentially affects oxidative mechanisms and lipoprotein uptake in correlation with cytotoxicity in two insulin-producing cell lines. Biochem Biophys Res Commun. 2000;267:619–625. doi: 10.1006/bbrc.1999.1989. [DOI] [PubMed] [Google Scholar]
- 76.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 77.Zhao HL, Sui Y, Guan J, He L, Gu XM, Wong HK, Baum L, Lai FM, Tong PC, Chan JC. Amyloid oligomers in diabetic and nondiabetic human pancreas. Transl Res. 2009;153:24–32. doi: 10.1016/j.trsl.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Li XL, Chen T, Wong YS, Xu G, Fan RR, Zhao HL, Chan JC. Involvement of mitochondrial dysfunction in human islet amyloid polypeptide-induced apoptosis in INS-1E pancreatic beta cells: An effect attenuated by phycocyanin. Int J Biochem Cell Biol. 2011;43:525–534. doi: 10.1016/j.biocel.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 79.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 80.Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–358. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zraika S, Hull RL, Verchere CB, Clark A, Potter KJ, Fraser PE, Raleigh DP, Kahn SE. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia. 2010;53:1046–1056. doi: 10.1007/s00125-010-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gurlo T, Ryazantsev S, Huang CJ, Yeh MW, Reber HA, Hines OJ, O’Brien TD, Glabe CG, Butler PC. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176:861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pittner RA, Albrandt K, Beaumont K, Gaeta LS, Koda JE, Moore CX, Rittenhouse J, Rink TJ. Molecular physiology of amylin. J Cell Biochem. 1994;55 Suppl:19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
- 84.Fineman MS, Koda JE, Shen LZ, Strobel SA, Maggs DG, Weyer C, Kolterman OG. The human amylin analog, pramlintide, corrects postprandial hyperglucagonemia in patients with type 1 diabetes. Metabolism. 2002;51:636–641. doi: 10.1053/meta.2002.32022. [DOI] [PubMed] [Google Scholar]
- 85.Fineman M, Weyer C, Maggs DG, Strobel S, Kolterman OG. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–508. doi: 10.1055/s-2002-34790. [DOI] [PubMed] [Google Scholar]
- 86.Herrmann K, Frias JP, Edelman SV, Lutz K, Shan K, Chen S, Maggs D, Kolterman OG. Pramlintide improved measures of glycemic control and body weight in patients with type 1 diabetes mellitus undergoing continuous subcutaneous insulin infusion therapy. Postgrad Med. 2013;125:136–144. doi: 10.3810/pgm.2013.05.2635. [DOI] [PubMed] [Google Scholar]
- 87.Weinzimer SA, Sherr JL, Cengiz E, Kim G, Ruiz JL, Carria L, Voskanyan G, Roy A, Tamborlane WV. Effect of pramlintide on prandial glycemic excursions during closed-loop control in adolescents and young adults with type 1 diabetes. Diabetes Care. 2012;35:1994–1999. doi: 10.2337/dc12-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maggs DG, Fineman M, Kornstein J, Burrell T, Schwartz S, Wang Y, Ruggles JA, Kolterman OG, Weyer C. Pramlintide reduces postprandial glucose excursions when added to insulin lispro in subjects with type 2 diabetes: a dose-timing study. Diabetes Metab Res Rev. 2004;20:55–60. doi: 10.1002/dmrr.419. [DOI] [PubMed] [Google Scholar]
- 89.Weyer C, Gottlieb A, Kim DD, Lutz K, Schwartz S, Gutierrez M, Wang Y, Ruggles JA, Kolterman OG, Maggs DG. Pramlintide reduces postprandial glucose excursions when added to regular insulin or insulin lispro in subjects with type 1 diabetes: a dose-timing study. Diabetes Care. 2003;26:3074–3079. doi: 10.2337/diacare.26.11.3074. [DOI] [PubMed] [Google Scholar]
- 90.Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, Weyer C, Kolterman OG. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004;21:1204–1212. doi: 10.1111/j.1464-5491.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 91.Whitehouse F, Kruger DF, Fineman M, Shen L, Ruggles JA, Maggs DG, Weyer C, Kolterman OG. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25:724–730. doi: 10.2337/diacare.25.4.724. [DOI] [PubMed] [Google Scholar]
- 92.Edelman S, Garg S, Frias J, Maggs D, Wang Y, Zhang B, Strobel S, Lutz K, Kolterman O. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006;29:2189–2195. doi: 10.2337/dc06-0042. [DOI] [PubMed] [Google Scholar]
- 93.Ratner RE, Want LL, Fineman MS, Velte MJ, Ruggles JA, Gottlieb A, Weyer C, Kolterman OG. Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes. Diabetes Technol Ther. 2002;4:51–61. doi: 10.1089/15209150252924094. [DOI] [PubMed] [Google Scholar]
- 94.Hollander PA, Levy P, Fineman MS, Maggs DG, Shen LZ, Strobel SA, Weyer C, Kolterman OG. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784–790. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]
- 95.Riddle M, Frias J, Zhang B, Maier H, Brown C, Lutz K, Kolterman O. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care. 2007;30:2794–2799. doi: 10.2337/dc07-0589. [DOI] [PubMed] [Google Scholar]
- 96.Thompson RG, Gottlieb A, Organ K, Koda J, Kisicki J, Kolterman OG. Pramlintide: a human amylin analogue reduced postprandial plasma glucose, insulin, and C-peptide concentrations in patients with type 2 diabetes. Diabet Med. 1997;14:547–555. doi: 10.1002/(SICI)1096-9136(199707)14:7<547::AID-DIA390>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 97.Treusch S, Cyr DM, Lindquist S. Amyloid deposits: protection against toxic protein species? Cell Cycle. 2009;8:1668–1674. doi: 10.4161/cc.8.11.8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aitken JF, Loomes KM, Konarkowska B, Cooper GJ. Suppression by polycyclic compounds of the conversion of human amylin into insoluble amyloid. Biochem J. 2003;374:779–784. doi: 10.1042/BJ20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olcott AP, Tian J, Walker V, Dang H, Middleton B, Adorini L, Washburn L, Kaufman DL. Antigen-based therapies using ignored determinants of beta cell antigens can more effectively inhibit late-stage autoimmune disease in diabetes-prone mice. J Immunol. 2005;175:1991–1999. doi: 10.4049/jimmunol.175.3.1991. [DOI] [PubMed] [Google Scholar]
- 100.Westwell-Roper C, Dunne A, Kim ML, Verchere CB, Masters SL. Activating the NLRP3 inflammasome using the amyloidogenic peptide IAPP. Methods Mol Biol. 2013;1040:9–18. doi: 10.1007/978-1-62703-523-1_2. [DOI] [PubMed] [Google Scholar]
- 101.Baker RL, Delong T, Barbour G, Bradley B, Nakayama M, Haskins K. Cutting edge: CD4 T cells reactive to an islet amyloid polypeptide peptide accumulate in the pancreas and contribute to disease pathogenesis in nonobese diabetic mice. J Immunol. 2013;191:3990–3994. doi: 10.4049/jimmunol.1301480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang XX, Shen J, Liao QY, Li R, Zhang QJ, He L, Zhao HL. Human Amylin Induces CD4 CD25 Foxp3 Regulatory T Cells in the Protection from Autoimmune Diabetes [abstract] Diabetes. 2015;64(suppl 1):1804–P. doi: 10.1007/s12026-017-8956-5. [DOI] [PubMed] [Google Scholar]


