Abstract
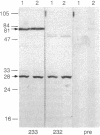
The presence of a human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, in the human genome has been documented. The HRES-1 genomic locus is transcriptionally active and contains open reading frames. Antibodies 232 and 233, specific for synthetic peptides pep14-24 and pep117-127, corresponding to two nonoverlapping HTLV-related regions in the longer open reading frame of HRES-1, recognize an identical 28-kDa protein in H9 human T cells. Thus, HRES-1 is a human endogenous retroviral sequence capable of protein expression. HRES-1/p28 is localized to the cytoplasm and nuclear bodies. While HTLV-I-specific antibodies react with HRES-1 peptides, antibody 233 cross-reacts with HTLV-I gag p24 protein. Three consecutive highly charged amino acid residues, Arg-Arg-Glu, present in both HRES-1 pep117-127 and HTLV-I gag p24 are likely to be the core of cross-reactive epitopes. The prevalence of antibodies to HRES-1 peptides pep14-24 and pep117-127 was determined in 65 normal blood donors and 146 patients with immunological disorders. Sera of patients with multiple sclerosis (19 out of 65, 29%), progressive systemic sclerosis (4 out of 17, 23%), systemic lupus erythematosus (4 out of 19, 21%), and Sjogren syndrome (2 out of 19, 10%) contained significantly higher HRES-1 peptide binding activity than sera of normal donors. Sera of patients with AIDS showed no specific binding to HRES-1 peptides. Nine of 30 HRES-1-seropositive patients showed immunoreactivity to HTLV-I gag p24. The data indicate that HRES-1/p28 may serve as an autoantigen eliciting autoantibodies cross-reactive with HTLV-I gag antigens.
Full text
PDF

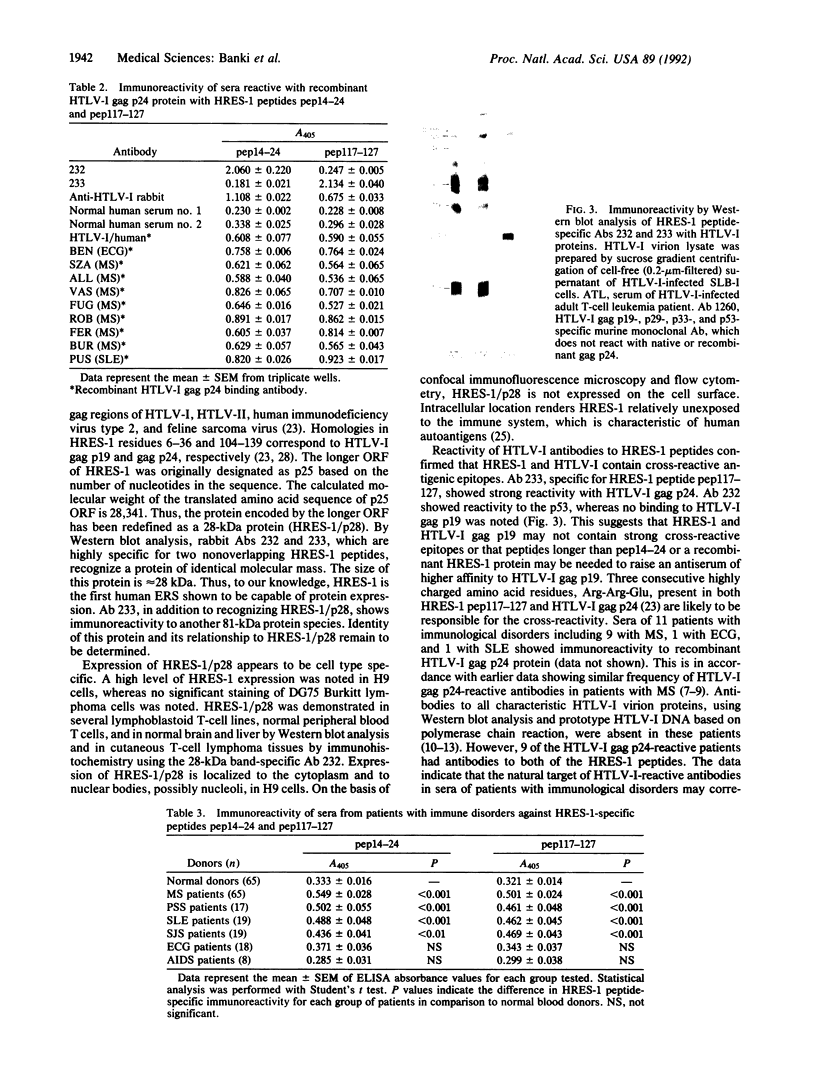

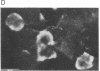
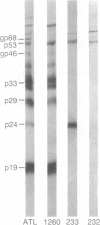
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. N., Khan A. S. Human endogenous retroviruses and immune disease. Clin Immunol Immunopathol. 1990 Jul;56(1):1–8. doi: 10.1016/0090-1229(90)90163-k. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Retroviruses and retrotransposons: the role of reverse transcription in shaping the eukaryotic genome. Cell. 1985 Mar;40(3):481–482. doi: 10.1016/0092-8674(85)90190-4. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., O'Connell C., Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Tronick S., Schlom J. Detection and cloning of human DNA sequences related to the mouse mammary tumor virus genome. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5503–5507. doi: 10.1073/pnas.79.18.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Haislip A. M., Myers L. W., Ellison G. W., Merrill J. E. Failure to detect human T-cell leukemia virus-related sequences in multiple sclerosis blood. Arch Neurol. 1990 Oct;47(10):1064–1065. doi: 10.1001/archneur.1990.00530100026008. [DOI] [PubMed] [Google Scholar]
- Emerit I. Chromosomal instability in collagen disease. Z Rheumatol. 1980 Mar-Apr;39(3-4):84–90. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985 Nov 29;230(4729):1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B., Wroblewska Z., Frankel M. E., Koprowski H. Molecular mimicry in virus infection: crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2346–2350. doi: 10.1073/pnas.80.8.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. M., Eichwald E. J., Martin J. H., Smith C. B., DeWitt C. W. Immunogenetic control of experimental type II collagen-induced arthritis. I. Susceptibility and resistance among inbred strains of rats. Arthritis Rheum. 1981 Jun;24(6):781–789. doi: 10.1002/art.1780240605. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Harada F., Tsukada N., Kato N. Isolation of three kinds of human endogenous retrovirus-like sequences using tRNA(Pro) as a probe. Nucleic Acids Res. 1987 Nov 25;15(22):9153–9162. doi: 10.1093/nar/15.22.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka H., Yamamoto K., Fujii H., Miura H., Miyasaka N., Nishioka K., Miyamoto T. Fine epitope mapping of the human SS-B/La protein. Identification of a distinct autoepitope homologous to a viral gag polyprotein. J Clin Invest. 1990 May;85(5):1566–1574. doi: 10.1172/JCI114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., DeFreitas E. C., Harper M. E., Sandberg-Wollheim M., Sheremata W. A., Robert-Guroff M., Saxinger C. W., Feinberg M. B., Wong-Staal F., Gallo R. C. Multiple sclerosis and human T-cell lymphotropic retroviruses. Nature. 1985 Nov 14;318(6042):154–160. doi: 10.1038/318154a0. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Steinberg A. D. Retroviruses and autoimmunity. J Autoimmun. 1990 Apr;3(2):137–166. doi: 10.1016/0896-8411(90)90137-h. [DOI] [PubMed] [Google Scholar]
- Kröger B., Horak I. Isolation of novel human retrovirus-related sequences by hybridization to synthetic oligonucleotides complementary to the tRNA(Pro) primer-binding site. J Virol. 1987 Jul;61(7):2071–2075. doi: 10.1128/jvi.61.7.2071-2075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985 Jun 10;260(11):6698–6709. [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Jimenez S. A., Riggs E., Ziemnicka-Kotula D. Determination of an epitope of the diffuse systemic sclerosis marker antigen DNA topoisomerase I: sequence similarity with retroviral p30gag protein suggests a possible cause for autoimmunity in systemic sclerosis. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8492–8496. doi: 10.1073/pnas.86.21.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Kurihara M., Takano T. Retrovirus-related sequences in human DNA: detection and cloning of sequences which hybridize with the long terminal repeat of baboon endogenous virus. Nucleic Acids Res. 1982 May 11;10(9):2865–2878. doi: 10.1093/nar/10.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Ohta K., Mori F., Nishitani H., Saida T. Sera from patients with multiple sclerosis react with human cell T lymphotropic virus-I gag proteins but not env proteins--Western blotting analysis. J Immunol. 1986 Dec 1;137(11):3440–3443. [PubMed] [Google Scholar]
- PCR analysis of DNA from multiple sclerosis patients for the presence of HTLV-I. Science. 1989 Nov 10;246(4931):821–824. [PubMed] [Google Scholar]
- PCR analysis of DNA from multiple sclerosis patients for the presence of HTLV-I. Science. 1989 Nov 10;246(4931):821–824. [PubMed] [Google Scholar]
- Perl A., Isaacs C. M., Eddy R. L., Byers M. G., Sait S. N., Shows T. B. The human T-cell leukemia virus-related endogenous sequence (HRES1) is located on chromosome 1 at q42. Genomics. 1991 Dec;11(4):1172–1173. doi: 10.1016/0888-7543(91)90052-g. [DOI] [PubMed] [Google Scholar]
- Perl A., Nagy K., Pazmany T., Isaacs C., Baraczka K., Szabo T., Feher J. No evidence for human T-cell leukemia virus type I or human T-cell leukemia virus type II infection in patients with multiple sclerosis. Arch Neurol. 1990 Oct;47(10):1061–1063. doi: 10.1001/archneur.1990.00530100023007. [DOI] [PubMed] [Google Scholar]
- Perl A., Rosenblatt J. D., Chen I. S., DiVincenzo J. P., Bever R., Poiesz B. J., Abraham G. N. Detection and cloning of new HTLV-related endogenous sequences in man. Nucleic Acids Res. 1989 Sep 12;17(17):6841–6854. doi: 10.1093/nar/17.17.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. E., Johnston S. L., Runge L. A., Moore J. L., Poiesz B. J. High IgM antibody to human T-lymphotropic virus type I in systemic lupus erythematosus. J Clin Immunol. 1986 May;6(3):234–241. doi: 10.1007/BF00918703. [DOI] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Rabson A. B., Hamagishi Y., Steele P. E., Tykocinski M., Martin M. A. Characterization of human endogenous retroviral envelope RNA transcripts. J Virol. 1985 Oct;56(1):176–182. doi: 10.1128/jvi.56.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki A., Johansson E., Krohn K. Interpretation of antibodies reacting solely with human retroviral core proteins. N Engl J Med. 1988 Feb 18;318(7):448–449. doi: 10.1056/NEJM198802183180712. [DOI] [PubMed] [Google Scholar]
- Reuter R., Lührmann R. Immunization of mice with purified U1 small nuclear ribonucleoprotein (RNP) induces a pattern of antibody specificities characteristic of the anti-Sm and anti-RNP autoimmune response of patients with lupus erythematosus, as measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8689–8693. doi: 10.1073/pnas.83.22.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittner G., Schwanitz G., Baur M. P., Black C. M., Welsh K. I., Kühnl P., Rittner C. Family studies in scleroderma (systemic sclerosis) demonstrating an HLA-linked increased chromosomal breakage rate in cultured lymphocytes. Hum Genet. 1988 Dec;81(1):64–70. doi: 10.1007/BF00283732. [DOI] [PubMed] [Google Scholar]
- Schmid M., Ott G., Haaf T., Scheres J. M. Evolutionary conservation of fragile sites induced by 5-azacytidine and 5-azadeoxycytidine in man, gorilla, and chimpanzee. Hum Genet. 1985;71(4):342–350. doi: 10.1007/BF00388461. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland G. R., Parslow M. I., Baker E. New classes of common fragile sites induced by 5-azacytidine and bromodeoxyuridine. Hum Genet. 1985;69(3):233–237. doi: 10.1007/BF00293031. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]