Abstract
High mobility group box 1 (HMGB1) is a nuclear protein that can bind to DNA and act as a co-factor for gene transcription. When released into extracellular fluid, it plays a proinflammatory role by acting as a damage-associated molecular pattern molecule (DAMP) (also known as an alarmin) to initiate innate immune responses by activating multiple cell surface receptors such as the receptor for advanced glycation end-products (RAGE) and toll-like receptors (TLRs), TLR2, TLR4 or TLR9. This proinflammatory role is now considered to be important in the pathogenesis of a wide range of kidney diseases whether they result from hemodynamic changes, renal tubular epithelial cell apoptosis, kidney tissue fibrosis or inflammation. This review summarizes our current understanding of the role of HMGB1 in kidney diseases and how the HMGB1-mediated signaling pathway may constitute a new strategy for the treatment of kidney diseases.
KEY WORDS: High mobility group box 1, Inflammation, Acute kidney injury, Chronic kidney disease, Diabetic nephropathy, Antineutrophil cytoplasmic autoantibody-associated vasculitis, Clear cell renal cell carcinoma, Nephritis
Graphical abstract
HMGB1 plays an important pathological role in different kidney diseases by activating receptors on cell membranes, particularly TLR2/4 and RAGE, and inducing inflammation. This review summarizes our current understanding of the role of HMGB1 in kidney diseases and further discusses its potential to provide protection against them.
1. Introduction
The kidney is made up of a heterogeneous population of cells which function together to perform a number of tightly controlled, complex and interdependent processes. It is frequently targeted by pathogenic immune responses against renal autoantigens or by local manifestation of systemic autoimmunity. In fact, many studies have revealed that kidney disease is associated with inflammation1.
High mobility group box 1 (HMGB1) is a nuclear DNA-binding protein discovered over 30 years ago2. Under normal circumstances, it participates in a variety of biological processes including transcription, DNA repair, differentiation and development3. However, when released from necrotic or activated cells it functions as a potent proinflammatory cytokine which exerts its actions through multiple cell-surface receptors including the receptor for advanced glycation end products (RAGE) and toll-like receptors (TLRs), TLR2, TLR4 or TLR9. Recently, studies have shown that HMGB1 plays an important role in the pathogenesis of a variety of kidney diseases.
This review focuses on the role of HMGB1 in the pathogenesis of different kidney diseases including acute kidney injury (AKI), chronic kidney disease (CKD), diabetic nephropathy (DN), antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV), clear cell renal cell carcinoma (ccRCC) and both granulomatous and lupus nephritis (GN and LN respectively). It also attempts to evaluate the potential for modulating the HMGB1-mediated signaling pathway in the treatment of kidney diseases.
2. Characteristics of HMGB1
HMGB1 is a member of the high mobility group nuclear protein family and one of the most evolutionarily conserved proteins4. The human HMGB1 gene is located on chromosome 13q12 and six polymorphic loci throughout the gene locus have recently been identified. HMGB1 is almost always present in the nuclei of mammalian cells and is released into the extracellular medium in response to appropriate stimuli, a process in which the inflammasome plays an important role. The release occurs both by active secretion and a passive process subsequent to inflammation factor stimulation of inflammatory cells such as dendritic cells and monocyte/macrophages. After transport from the nucleus to the cytoplasm, HMGB1 moves into the secretory lysosome and is secreted from the cell through exocytosis5. When released from necrotic or burst cells, the damage signal is passed to adjacent cells6.
In the cytoplasm, HMGB1 regulates cellular processes such as autophagy and apoptosis7, 8, 9, 10. Autophagy is a process associated with the degradation of intracellular organelles following sequestration within double-membrane delimited vacuoles. HMGB1 is important for oxidative stress–mediated autophagy and serves as a new target for the treatment of stress-associated disorders8. Through autophagy, HMGB1 contributes to cell proliferation11 but it also activates endonuclease G and DNA fragmenting factor (DFF) to promote apoptosis12. In fact, recent research suggests that heat shock protein β1 (HSPB1) is a response to cardiomyocyte apoptosis to which HMGB1 contributes13.
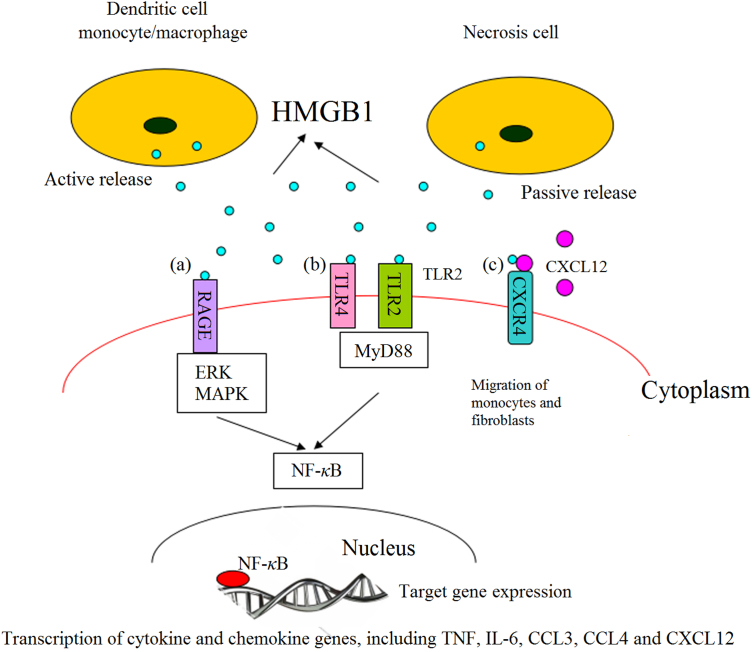
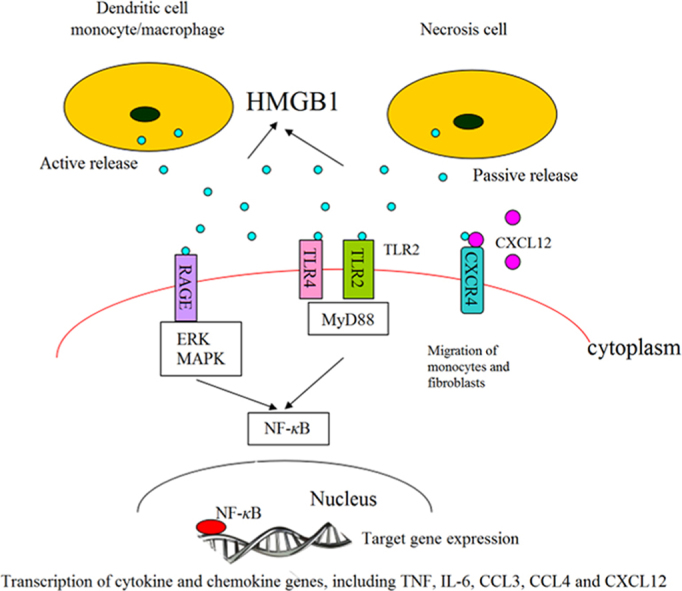
HMGB1 released into the extracellular fluid serves as a damage-associated molecular pattern molecule (DAMP) to mediate the noninfectious inflammatory response14. As shown in Fig. 1, extracellular HMGB1 can interact with other soluble molecules, cellular receptors and surface molecules such as RAGE, TLR2, TLR4, TLR9, syndecan-3, CD24-Siglec-10, macrophage antigen-1, chemokine (C-X-C motif) receptor 4 (CXCR4), certain integrins, and the T cell Ig domain and mucin domain proteins. It interacts with RAGE at its COOH-terminal motif to inhibit invasive migration and metastasis15. It interacts with TLR2/4 receptors16 to promote the translocation of cytoplasmic NF-κB into the nucleus and induce an inflammatory response. It binds to chemokine (C-X-C motif) ligand 12 (CXCL12) to form a HMGB1–CXCL12 heterocomplex which interacts with CXCR4 to promote the migration of monocytes and fibroblasts17. Finally, HMGB1 stimulation contributes to tumor progression by causing overexpression of miR-21 depending on the interleukin-6 (IL-6)/Stat3 signaling axis18.
Figure 1.
Interaction of extracellular HMGB1 released from inflammatory and necrotic cells with cell surface receptors. (a) HMGB1 binds to RAGE which induces nuclear transcription of NF-κB, leading to transcription and expression of target genes of cytokines and chemokines; (b) HMGB1 causes inflammation by interacting with TLR2/TLR4 through MyD88 dependent and independent pathways; (c) the heterocomplex formed by binding of HMGB1 and CXCL12 promotes the migration of monocytes and fibroblasts.
3. Expression of HMGB1 in renal diseases
As a potential inflammatory cytokine, HMGB1 plays multiple roles in the pathogenesis of renal disease. In recent years, numerous studies have reported the association between HMGB1 expression and renal disease19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34. Evidence from these studies reveals that in renal disease HMGB1 levels in blood and urine, the expression of HMGB1 in renal tissue, and the levels of HMGB1 in the cytoplasm and extracellular medium are all elevated. Table 1 lists studies implicating the expression of HMGB1 in various renal diseases.
Table 1.
Sites of expression of HMGB1 in various renal diseases.
| Renal disease | Expression of HMGB1 | Reference |
|---|---|---|
| Acute kidney injury | Serum | 23 |
| Chronic kidney disease | Serum; urine; | 24, 25, 26 |
| Renal fibrosis | Renal proximal tubule epithelial cells (PTECs); M1 macrophage | 32, 33 |
| Diabetic nephropathy | Renal glomerular cells; tubular epithelial cells | 34 |
| Renal involvement of ANCA-AAV | Serum; renal tissue | 19, 20 |
| Clear cell renal cell carcinoma | Cytosol | 22 |
| Granulomatous nephritis | Renal tissue | 31 |
| Lupus nephritis | Macrophages; renal tissue; serum; urine | 27, 28, 29, 30 |
4. HMGB1 and renal disease
4.1. HMGB1 and AKI
AKI is defined as an abrupt and sustained decrease in kidney function35. It occurs frequently in patients with end-stage liver disease and cirrhosis and portends a poor prognosis. AKI can result from a number of causes including ischemia/reperfusion injury (IRI), sepsis, hemodynamic changes, inflammation and nephrotoxity36.
A cross-sectional study23 in patients with AKI showed an elevated serum HMGB1 level related to inflammatory parameters such as ferritin and orosomucoid. A study in TLR4 deficient (TLR4−/−) mice37 with IRI of the kidney not only indicated an increased expression of HMGB1 in the kidney but also found serum creatinine and tubular injury were reduced after treatment with anti-HMGB1 antibodies. The results show that HMGB1 promotes kidney injury through TLR4 suggesting inhibiting the release of HMGB1 could protect against AKI. In fact, Wu et al.38 showed that preconditioning wild-type mice with recombinant HMGB1 (rHMGB1) before ischemia protects the kidney from TLR4-dependent IRI and that Siglec-G upregulation is involved.
A study exploring the effect of mycophenolate mofetil (MMF) on IRI39 demonstrated that plasma creatinine levels and renal damage were reduced by MMF treatment. TLR4 expression and the plasma concentration of cytokines were also reduced. However, there was no change in the concentration of HMGB1 suggesting MMF reduces TLR4 expression directly to improve renal function. A recent study40 reported CO-releasing molecule-2 decreased the activity of nuclear histone acetyltransferase and consequently inhibited the acetylation and release of HMGB1 to exert a protective effect against potentially lethal IRI of the kidney. HMGB1 released due to IRI reduces the survival of tubular epithelial cells (TECs) and augments inflammation. Glycyrrhizic acid inhibits the interaction of HMGB1 with TECs and attenuates renal injury following IRI and transplantation41. These findings demonstrate that HMGB1 plays an important role in IRI through the TLR4 pathway.
4.2. HMGB1 and CKD
It has been shown that CKD is associated with chronic inflammation42. A clinical trial involving 110 patients with CKD revealed that serum HMGB1 was significantly elevated and correlated with glomerular filtration rate (GFR) as well as with markers of inflammation and malnutrition. These included high-sensitivity C-reactive-protein (hs-CRP), IL-6, tumor necrosis factor (TNF), serum-albumin, hemoglobin A (1c) (HbA (1c)) and hemoglobin25. The correlation was particularly strong in patients with vasculitis including Henoch–Schönlein purpura nephritis, and IgA nephropathy (IgAN) with glomerular crescents43. In another study in rats with adenine-induced CKD26, the HMGB1 concentration was significantly increased suggesting it may be a potentially useful biomarker for this condition. Leelahavanichkul et al.24, using a recently characterized 5/6 nephrectomy (5/6Nx) mouse model of progressive CKD, found that HMGB1 was released from apoptotic cells and that CKD-sepsis could be attenuated by anti-HMGB1. Nakamura et al.44 studied the relationship between the RAGE ligand and asymmetric dimethylarginine (ADMA) in 20 nondiabetic normotensive CKD patients and found that HMGB1 elevation raised the level of ADMA. This suggests the active involvement of the AGE/HMGB-1–RAGE–ADMA axis in CKD.
Renal fibrosis resulting from CKD is an important public health concern. Tubulointerstitial fibrosis is characterized by the loss of renal tubules, an increased myofibroblast population, and accumulation of extracellular matrix proteins (ECM)45. In a model of immune-mediated epithelial–mesenchymal transition (EMT) established in human proximal TECs (PTECs), human rHMGB1 treatment induced alterations in epithelial morphology consistent with EMT32. The effect of HMGB1 was mediated at least in part by RAGE products and through induction of transforming growth factor-β1 secretion from PTECs. TLR2 is upregulated in kidneys of patients with tubulointerstitial damage46 and levels of TLR2, its danger ligands Gp96 and biglycan, and HMGB1 are increased in mice with obstructive nephropathy. The results demonstrate that TLR2 can initiate renal inflammation during progressive renal injury and that the absence of TLR2 does not affect the development of tubulointerstitial fibrosis. It is possible that the inhibition of HMGB1 alone or of HMGB1 and TLR2 together is more effective than inhibition of TLR2 alone in renal fibrosis. Recently, Tian et al.33 found HMGB1 facilitates M1 macrophage polarization in the early stage of unilateral ureter obstruction. Thus inhibition of HMGB1 release may alter macrophage phenotype and protect kidney tissue from injury and fibrosis. In summary, HMGB1 plays an important role in the pathogenesis of renal fibrosis by activating RAGE/TLR2/TLR4.
4.3. HMGB1 and DN
DN is mainly the result of inflammatory processes and metabolic alterations caused by hyperglycemia47. Kim et al.34 first reported the association between HMGB1 and DN, showing that in both cytoplasmic and nuclear patterns of diabetic renal glomerular cells and TECs, HMGB1 is highly expressed in contrast to cells in normal rats where HMGB1 is expressed in the nuclei only. RAGE expression and NF-κB activity were also elevated in diabetic rats where the binding of NF-κB to the RAGE promoter was increased. These findings suggest that HMGB1 released during hyperglycemia may induce renal injury in diabetic rats through the participation of RAGE and activation of NF-κB.
TLR4/TLR2 may also play important roles in DN. Lin et al.48 first studied the effect of TLR4 on DN in human renal biopsies, human PTECs and an animal model and found that tubulointerstitial inflammation is promoted through the TLR4-mediated pathway. Furthermore, TLR2 contributes to inflammation in DN through NF-κB activation49. Recently, Mudaliar et al.50 showed that high glucose levels upregulate HMGB1 in cell supernatants and TLR4/TLR2 expression in human microvascular endothelial cells (HMEC-1). In addition, rHMGB1 was found to induce NF-κB activation and synthesis of proinflammatory cytokines and chemokines, which were attenuated by inhibition of TLR2 or TLR4 signaling. Therefore it appears likely that hyperglycemia increases HMGB1 release and that it then binds to RAGE and TLR2/TLR4 to transduce inflammation through NF-κB activation and contribute to DN.
4.4. HMGB1 and AAV
AAV is characterized by pauci-immune necrotizing inflammation of the small blood vessels. In a study of 30 AAV patients19, HMGB1 was found to be significantly higher in AAV with renal involvement and to remain higher in inactive cases than in historic healthy controls. Similarly, a study20 comparing plasma levels of HMGB1 in patients with active AAV with those in remission and normal controls found levels were higher in active AAV patients suggesting HMGB1 may be a marker of disease activity and a predictor of outcome in AAV.
4.5. HMGB1 and ccRCC
ccRCC is the most common form of cancer of the kidney. In a study of 39 patients with pathologically confirmed ccRCC by Takeuchi et al.22, the immunohistological expression of HMGB1 in the cytoplasm was found to correlate with PT1b classification and tumor grade as previously found by Wu et al.51. Takeuchi et al. also found evidence that the methylation of HMGB1 at lysine 112 in ccRCC affected its ability to bind to DNA and mediate its translocation. In a study52 examining the expression of HMGB1, RAGE and ERK1/2 phosphorylation in patients with ccRCC, HMGB1 released to cytoplasm was found to promote the development and progression of the disease via ERK1/2 activation, a process partially mediated by RAGE.
4.6. HMGB1 and nephritis
4.6.1. HMGB1 and GN
Granulomas are distinctive chronic inflammatory lesions characterized by aggregations of activated macrophages and marked fibrosis. Infections, particulates and unidentified factors are thought to initiate their formation. However, the pathogenetic mechanisms remain obscure. Oyama et al.31 examined the expression of HMGB1 and MCP-1 in rats with crystal-induced GN and found the HMGB1 concentration was higher in renal granulomas, urine and serum, and that injection of HMGB1 worsened renal function and upregulated MCP-1. They concluded that HMGB1 was involved in GN and could be a novel target for inhibiting chronic granulomatous disease.
4.6.2. HMGB1 and LN
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease characterized by multiple organ involvement, production of autoantibodies to nuclear components, and immune complex deposition53. LN is common in SLE and evidence suggests HMGB1 may play an important role. Thus a comparison of HMGB1 levels in 70 SLE patients and 35 healthy controls showed that serum levels in SLE patients were higher particularly in those with active renal disease54. Furthermore, a study of 35 patients with active LN55 evaluated renal biopsies and serum levels of HMGB1 and found that renal tissue expression and serum levels were elevated in LN. Abdulahad et al.56 repeated this finding confirming that HMGB1 is associated with LN but providing no clarification as to its actual role. However, a recent study by Li et al.27 revealed macrophage activation induced by activated lymphocyte-derived DNA (ALD-DNA) contributes to the pathogenesis of murine LN and that only extracellular but not intracellular HMGB1 can significantly facilitate this activation and lead to LN. This suggests that reducing the release of HMGB1 from intracellular stores could ameliorate inflammation in LN.
5. Conclusions
HMGB1 induces inflammation by binding to receptors on cell membranes (especially TLR2/4 and RAGE) and plays an important pathological role in many kidney diseases (Table 2). Although the mechanisms by which HMGB1 is released and the signaling pathways it activates require further elucidation, evidence suggests that modulating HMGB1-mediated signaling may constitute a new strategy for the treatment of kidney diseases. Further animal and cell studies are required to evaluate how extracellular and intracellular HMGB1 are implicated in the pathogenesis of different kidney diseases.
Table 2.
Roles of HMGB1 in the pathogenesis of renal diseases.
| Renal disease | Receptor | Role of HMGB1 | Protection |
|---|---|---|---|
| Acute kidney disease | TLR4 | Increase systemic circulating cyto/chemokines; mobilize bone marrow CD34-Flk1-cells to the circulation | Preconditioning with rHMGB1; MMF; CO-releasing molecule-2; glycyrrhizic acid |
| Chronic kidney injury | RAGE | Enhance asymmetric dimethylarginine (ADMA) | Inhibition of HMGB1-RAGE-ADMA axis |
| Renal fibrosis | RAGE TLR2 | Induce transforming growth factor-β1 secretion from PTECs; facilitate M1 polarization | Inhibit HMGB1 release |
| Diabetic nephropathy | RAGE TLR2/TLR4 | Activate NF-κB | Inhibit HMGB1 release |
| Clear cell renal cell carcinoma | RAGE | Promote the development and progression of ccRCC via ERK1/2 activation | – |
| Lupus nephritis | – | Facilitate the ALD-DNA induced macrophage activation | – |
Note: “–” indicates no literature.
Acknowledgments
This study was funded by the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (No. 20150218), Program for New Century Excellent Talents in University (NCET-13-0605), the National Natural Science Foundation of China (No. 81102512), and Hunan Provincial Natural Science Foundation of China (No. 14JJ7001).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Kurts C., Panzer U., Anders H.J., Rees A.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin G.H., Sanders C., Johns E.W. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem. 1973;38:14–19. doi: 10.1111/j.1432-1033.1973.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 3.Tang D.L., Kang R., Livesey K.M., Kroemer G., Billiar T.R., van Houten B. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab. 2011;13:701–711. doi: 10.1016/j.cmet.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsung A., Tohme S., Billiar T.R. High-mobility group box-1 in sterile inflammation. J Intern Med. 2014;276:425–443. doi: 10.1111/joim.12276. [DOI] [PubMed] [Google Scholar]
- 5.Bonaldi T., Talamo F., Scaffidi P., Ferrera D., Porto A., Bachi A. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 7.Celona B., Weiner A., di Felice F., Mancuso F.M., Cesarini E., Rossi R.L. Substantial histone reduction modulates genomewide nucleosomal occupancy and global transcriptional output. PLoS Biol. 2011;9:e1001086. doi: 10.1371/journal.pbio.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang D.L., Kang R., Livesey K.M., Zeh H.J., Lotze M.T. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid Redox Signal. 2011;15:2185–2195. doi: 10.1089/ars.2010.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang D.L., Kang R., Cheh C.W., Livesey K.M., Liang X., Schapiro N.E. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 2010;29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang D.L., Kang R., Livesey K.M., Cheh C.W., Farkas A., Loughran P. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y.S., Song L.J. HMGB1-induced autophagy in Schwann cells promotes neuroblastoma proliferation. Int J Clin Exp Pathol. 2015;8:504–510. [PMC free article] [PubMed] [Google Scholar]
- 12.Widlak P., Garrard W.T. Discovery regulation, and action of the major apoptotic nucleases DFF40/CAD and endonuclease G. J Cell Biochem. 2005;94:1078–1087. doi: 10.1002/jcb.20409. [DOI] [PubMed] [Google Scholar]
- 13.Narumi T., Shishido T., Otaki Y., Kadowaki S., Honda Y., Funayama A. High-mobility group box 1-mediated heat shock protein beta 1 expression attenuates mitochondrial dysfunction and apoptosis. J Mol Cell Cardiol. 2015;82:1–12. doi: 10.1016/j.yjmcc.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Andersson U., Antoine D.J., Tracey K.J. The functions of HMGB1 depend on molecular localization and post-translational modifications. J Intern Med. 2014;276:420–424. doi: 10.1111/joim.12309. [DOI] [PubMed] [Google Scholar]
- 15.Huttunen H.J., Fages C., Kuja-Panula J., Ridley A.J., Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Rets. 2002;62:4805–4811. [PubMed] [Google Scholar]
- 16.Park J.S., Gamboni-Robertson F., He Q., Svetkauskaite D., Kim J.Y., Strassheim D. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;29:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 17.Campana L., Bosurgi L., Bianchi M.E., Manfredi A.A., Rovere-Querini P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol. 2009;86:609–615. doi: 10.1189/jlb.0908576. [DOI] [PubMed] [Google Scholar]
- 18.Chen M., Liu Y., Varley P., Chang Y., He X.X., Huang H. High mobility group box-1 promotes hepatocellular carcinoma progression through miR-21-mediated matrix metalloproteinase activity. Cancer Res. 2015;75:1645. doi: 10.1158/0008-5472.CAN-14-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruchfeld A., Wendt M., Bratt J., Qureshi A.R., Chavan S., Tracey K.J. High-mobility group box-1 protein (HMGB1) is increased in antineutrophilic cytoplasmatic antibody (ANCA)-associated vasculitis with renal manifestations. Mol Med. 2011;17:29–35. doi: 10.2119/molmed.2010.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Gou S.J., Chang D.Y., Yu F., Zhao M.H., Chen M. Association of circulating level of high mobility group box 1 with disease activity in antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Care Res. 2013;65:1828–1834. doi: 10.1002/acr.22187. [DOI] [PubMed] [Google Scholar]
- 21.Rabadi M.M., Ghaly T., Goligorksy M.S., Ratliff B.B. HMGB1 in renal ischemic injury. Am J Physiol Ren Physiol. 2012;303:F873–F885. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi T., Sakazume K., Tonooka A., Zaitsu M., Takeshima Y., Mikami K. Cytosolic HMGB1 expression in human renal clear cell cancer indicates higher pathological T classifications and tumor grades. Urol J. 2013;10:960–965. [PubMed] [Google Scholar]
- 23.Zakiyanov O., Kriha V., Vachek J., Zima T., Tesar V., Kalousova M. Placental growth factor, pregnancy-associated plasma protein-a, soluble receptor for advanced glycation end products, extracellular newly identified receptor for receptor for advanced glycation end products binding protein and high mobility group box 1 levels in patients with acute kidney injury: a cross sectional study. BMC Nephrol. 2013;14:245. doi: 10.1186/1471-2369-14-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leelahavanichkul A., Huang Y.N., Hu X.Z., Zhou H., Tsuji T., Chen R. Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing high mobility group box protein-1. Kidney Int. 2011;80:1198–1211. doi: 10.1038/ki.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruchfeld A., Qureshi A.R., Lindholm B., Barany P., Yang L.H., Stenvinkel P. High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD) Mol Med. 2008;14:109–115. doi: 10.2119/2007-00107.Bruchfeld. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali B.H., Al Z.M., Al S.A., Nemmar A. High-mobility group box-1 protein in adenine-induced chronic renal failure and the influence of gum arabic thereon. Physiol Res. 2015;64:147–151. doi: 10.33549/physiolres.932759. [DOI] [PubMed] [Google Scholar]
- 27.Li X.Y., Yue Y., Zhu Y.Y., Xiong S.D. Extracellular, but not intracellular HMGB1, facilitates self-DNA induced macrophage activation via promoting DNA accumulation in endosomes and contributes to the pathogenesis of lupus nephritis. Mol Immunol. 2015;65:177–188. doi: 10.1016/j.molimm.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Abdulahad D.A., Westra J., Bijzet J., Limburg P.C., Kallenberg C.G.M., Bijl M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zickert A., Palmblad K., Sundelin B., Chavan S., Tracey K.J., Bruchfeld A. Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res Ther. 2012;14:R36. doi: 10.1186/ar3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdulahad D.A., Westra J., Bijzet J., Dolff S., van Dijk M.C., Limburg P.C. Urine levels of HMGB1 in systemic lupus erythematosus patients with and without renal manifestations. Arthritis Res Ther. 2012;14:R184. doi: 10.1186/ar4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyama Y., Hashiguchi T., Taniguchi N., Tancharoen S., Uchimura T., Biswas K.K. High-mobility group box-1 protein promotes granulomatous nephritis in adenine-induced nephropathy. Lab Invest. 2010;90:853–866. doi: 10.1038/labinvest.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch J., Nolan S., Slattery C., Feighery R., Ryan M.P., McMorrow T. High-mobility group box protein 1: a novel mediator of inflammatory-induced renal epithelial-mesenchymal transition. Am J Nephrol. 2010;32:590–602. doi: 10.1159/000320485. [DOI] [PubMed] [Google Scholar]
- 33.Tian S.J., Zhang L., Tang J.M., Guo X., Dong K., Chen S.Y. HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Ren Physiol. 2015;308:F69–75. doi: 10.1152/ajprenal.00484.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J., Sohn E., Kim C.S., Jo K., Kim J.S. The role of high-mobility group box-1 protein in the development of diabetic nephropathy. Am J Nephrol. 2011;33:524–529. doi: 10.1159/000327992. [DOI] [PubMed] [Google Scholar]
- 35.Hoste E.A., Clermont G., Kersten A., Venkataraman R., Angus D.C., De Bacquer D. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glodowski S.D., Wagener G. New insights into the mechanisms of acute kidney injury in the intensive care unit. J Clin Anesth. 2015;27:175–180. doi: 10.1016/j.jclinane.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Wu H.L., Ma J., Wang P., Corpuz T.M., Panchapakesan U., Wyburn K.R. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H.L., Steenstra R., de Boer E.C.S., Zhao C.Y., Ma J., van der Stelt J.M. Preconditioning with recombinant high-mobility group box 1 protein protects the kidney against ischemia-reperfusion injury in mice. Kidney Int. 2014;85:824–832. doi: 10.1038/ki.2013.475. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y.X., Zhang J.R., Wang Z.G. Mycophenolate mofetil affects monocyte Toll-like receptor 4 signaling during mouse renal ischemia/reperfusion injury. Chin Med J (Engl) 2013;126:1224–1229. [PubMed] [Google Scholar]
- 40.Ruan Y.L., Wang L., Zhao Y., Yao Y., Chen S., Li J.H. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int. 2014;86:525–537. doi: 10.1038/ki.2014.80. [DOI] [PubMed] [Google Scholar]
- 41.Lau A., Wang S., Liu W., Haig A., Zhang Z.X., Jevnikar A.M. Glycyrrhizic acid ameliorates HMGB1-mediated cell death and inflammation after renal ischemia reperfusion injury. Am J Nephrol. 2014;40:84–95. doi: 10.1159/000364908. [DOI] [PubMed] [Google Scholar]
- 42.Bao Y.S., Na S.P., Zhang P., Jia X.B., Liu R.C., Yu C.Y. Characterization of interleukin-33 and soluble ST2 in serum and their association with disease severity in patients with chronic kidney disease. J Clin Immunol. 2012;32:587–594. doi: 10.1007/s10875-011-9622-7. [DOI] [PubMed] [Google Scholar]
- 43.Sato F., Maruyama S., Hayashi H., Sakamoto I., Yamada S., Uchimura T. High mobility group box chromosomal protein 1 in patients with renal diseases. Nephron Clin Pract. 2008;108:C194–C201. doi: 10.1159/000118942. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura T., Sato E., Fujiwara N., Kawagoe Y., Ueda Y., Suzuki T. Positive association of serum levels of advanced glycation end products and high mobility group box-1 with asymmetric dimethylarginine in nondiabetic chronic kidney disease patients. Metabolism. 2009;58:1624–1628. doi: 10.1016/j.metabol.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Lan H.Y. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Leemans J.C., Butter L.M., Pulskens W.P.C., Teske G.J.D., Claessen N., van der Poll T. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS One. 2009;4:e5704. doi: 10.1371/journal.pone.0005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King G.L. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79:1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 48.Lin M., Yiu W.H., Wu H.J., Chan L.Y.Y., Leung J.C.K., Au W.S. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol. 2012;23:86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mudaliar H., Pollock C., Komala M.G., Chadban S., Wu H.L., Panchapakesan U. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Ren Physiol. 2013;305:F143–F154. doi: 10.1152/ajprenal.00398.2012. [DOI] [PubMed] [Google Scholar]
- 50.Mudaliar H., Pollock C., Ma J., Wu H.L., Chadban S., Panchapakesan U. The role of TLR2 and 4-mediated inflammatory pathways in endothelial cells exposed to high glucose. PLoS One. 2014;9:e108844. doi: 10.1371/journal.pone.0108844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu F., Zhao Z.H., Ding S.T., Wu H.H., Lu J.J. High mobility group box 1 protein is methylated and transported to cytoplasm in clear cell renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:5789–5795. doi: 10.7314/apjcp.2013.14.10.5789. [DOI] [PubMed] [Google Scholar]
- 52.Lin L.G., Zhong K.H., Sun Z.K., Wu G.Z., Ding G.D. Receptor for advanced glycation end products (RAGE) partially mediates HMGB1-ERKs activation in clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2012;138:11–22. doi: 10.1007/s00432-011-1067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zickert A., Palmblad K., Sundelin B., Chavan S., Tracey K.J., Bruchfeld A. Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res Ther. 2012;14:R36. doi: 10.1186/ar3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdulahad D.A., Westra J., Bijzet J., Limburg P.C., Kallenberg C.G., Bijl M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R71. doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zickert A., Palmblad K., Sundelin B., Chavan S., Tracey K.J., Bruchfeld A. Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res Ther. 2012;14:R36. doi: 10.1186/ar3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdulahad D.A., Westra J., Bijzet J., Dolff S., van Dijk M.C., Limburg P.C. Urine levels of HMGB1 in systemic lupus erythematosus patients with and without renal manifestations. Arthritis Res Ther. 2012;14:R184. doi: 10.1186/ar4015. [DOI] [PMC free article] [PubMed] [Google Scholar]