Abstract
Liver X receptor (LXR) plays an important role in reverse cholesterol transport (RCT), and activation of LXR could reduce atherosclerosis. In the present study we used a cell-based screening method to identify new potential LXRβ agonists. A novel benzofuran-2-carboxylate derivative was identified with LXRβ agonist activity: E17110 showed a significant activation effect on LXRβ with an EC50 value of 0.72 μmol/L. E17110 also increased the expression of ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1) in RAW264.7 macrophages. Moreover, E17110 significantly reduced cellular lipid accumulation and promoted cholesterol efflux in RAW264.7 macrophages. Interestingly, we found that the key amino acids in the LXRβ ligand-binding domain had distinct interactions with E17110 as compared to TO901317. These results suggest that E17110 was identified as a novel compound with LXRβ agonist activity in vitro via screening, and could be developed as a potential anti-atherosclerotic lead compound.
Abbreviations: ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; ApoA-I, apolipoprotein A-I; GAPDH, glyceraldehyde-phosphate dehydrogenase; HDL, high-density lipoprotein; LBD, ligand-binding domain; LXR, liver X receptor; LXRE, LXR response element; NR, nuclear receptor; ox-LDL, oxidized low-density lipoprotein; RCT, reverse cholesterol transport; RXR, retinoid X receptor
Key words: LXRβ, Atherosclerosis, ABCA1, ABCG1, Reverse cholesterol transport, Cholesterol efflux
Graphical abstract
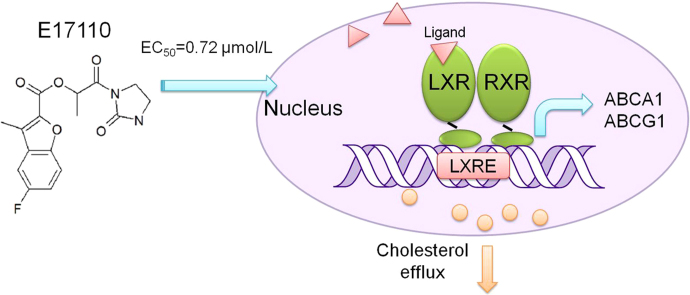
E17110 was identified as a novel LXRβ agonist by using a cell-based screening method. E17110 could increase the expression of ABCA1 and ABCG1 in RAW264.7 macrophages and significantly reduce cellular lipid accumulation and promote cholesterol efflux. Interestingly, we found that LXRβ had distinct interactions with E17110 compared to TO901317.
1. Introduction
The liver X receptors (LXRα and LXRβ) are ligand-activated transcription factors that belong to the nuclear receptor (NR) superfamily1, 2. LXRβ (NR1H2) is ubiquitously expressed at a moderate level in most physiological systems, whereas LXRα (NR1H3) is mainly expressed in the intestine, kidney, spleen and adipose tissue, especially in the liver3. LXRs generally function as permissive heterodimers with retinoid X receptor (RXR) that bind to specific response elements in the regulatory region of their target genes to regulate their expression4. LXRs sense excess cholesterol and trigger various adaptive mechanisms to protect the cells from cholesterol overload. ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1) are regulated by LXRs via functional LXR response elements (LXREs) found in their genes, which play important roles in cholesterol efflux5, 6, 7. ABCA1 can transfer both cholesterol and phospholipids to lipid-free apolipoprotein A-I (apoA-I), and ABCG1 can transfer cholesterol to high-density lipoprotein (HDL)7, 8.
Excessive absorption of lipoproteins in macrophages causes foam cell formation within arterial walls, and these cells subsequently rupture and promote early atherosclerotic plaque formation9, 10. The efflux of excess cellular cholesterol from peripheral tissues and its return to the liver for excretion in the bile occurs by a process referred to as reverse cholesterol transport (RCT)11. Furthermore, RCT is regarded as a major mechanism that removes cholesterol from the cells and transports it to the liver in order to protect against atherosclerotic cardiovascular disease, and this process can be stimulated by LXRs11.
Previous studies showed that treatment of atherosclerotic mice with synthetic LXR ligands effectively inhibited progression and promoted regression of atherosclerotic plaques12, 13. Meanwhile, macrophage-specific deletion of LXR in mice enhances atherogenesis14. Several LXR ligands, such as endogenous ligand 22(R)-hydroxycholesterol and synthetic agonists TO901317 and GW3965, have recently been reported and investigated substantially15, 16, 17. However, these ligands have the undesirable side effect of inducing lipogenesis and hypertriglyceridemia because of their up-regulation of sterol response element binding protein 1c (SREBP-1c) transcription18. Therefore, the identification of novel LXRβ agonists which could achieve beneficial effects from regulating cholesterol metabolism is necessary.
In this study, we discovered E17110 as a novel benzofuran-2-carboxylate derivative with potential LXRβ agonist activity using an LXRβ-GAL4 chimera reporter assay. We then investigated the effect and mechanism of this compound on the target genes of LXRβ and cholesterol efflux in murine macrophages. Furthermore, based on the molecular docking of E17110 and LXRβ ligand-binding domain (LBD) structures, we illustrated the probable interaction mode between LXRβ and E17110.
2. Materials and methods
2.1. Reagents
The compound E17110 was donated by the National Laboratory for Screening New Microbial Drugs, Peking Union Medical College (PUMC, Beijing, China). TO901317 (also referred as T1317 in this paper), oil red O stain and phorbol-12-myristate-13-acetate (PMA) were purchased from Sigma (St. Louis, MO, USA). HEK293T cells and RAW264.7 macrophages were obtained from the Cell Center of PUMC. Fetal bovine serum (FBS) and Opti-MEM® reduced serum medium used for transfection were purchased from Gibco (Invitrogen, Carlsbad, CA, USA). Dulbecco׳s modified Eagle׳s medium (DMEM) was purchased from Hyclone (Thermo Scientific, Rockford, USA). Lipofectamine 2000 and 22-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)−23,24-bisnor-5-cholen-3β-ol (22-NBD-cholesterol) was purchased from Invitrogen. ApoA-I, HDL and oxidized low-density lipoprotein (ox-LDL) was obtained from Union-Biology Company (Beijing, China).
2.2. Plasmids construction and cell culture
The wild-type gene of human LXRβ-LBD was obtained by PCR from HepG2 cells and cloned into the pBIND vector (Promega, Madison, WI, USA), which included the GAL4 DNA-binding domain (GAL4-DBD) in order to construct the pBIND-LXRβ-LBD plasmid. The LXRβ-LBD forward primer was 5′-ATTCGGGATCCCAGCGGCTCAA-3′, and the reverse primer was 5′-TGGGGTACCTCACTCGTGGACGT-3′. GAL4-pGL4-luc plasmid was constructed by inserting the 5×GAL4 response elements into the promoter region of the pGL4.17 reporter vector (Promega) as described previously19.
Mutations in pBIND-LXRβ-LBD were created by site-directed mutagenesis using the Fast Mutagenesis System (TransGen Biotech, Beijing, China). Several key amino acids were changed to alanines in LXRβ-LBD. The mutated plasmids were generated as follows: F271A (Phe271 to Ala) and T316A (Thr316 to Ala).
2.3. LXRβ-GAL4 chimera reporter assay and screening
In brief, HEK293T cells and RAW264.7 macrophages were cultured in different media separately as described previously20.
A synthetic compound library with 20,000 drug-like structures from the National Laboratory for Screening New Microbial Drugs was used for screening in this study. This compound library was purchased from J&K Chemical (synthesized by Enamine, Kyiv, Ukraine), and all of the compounds in this library are modified based on existing drug structures. These compounds were stocked in 96-well plates at 10 mg/mL in 100% DMSO. HEK293T cells were seeded in 96-well plates at 2×104 cells/well in 100 µL DMEM containing 10% FBS. After incubation for 12 h, the cells at 90% confluence were washed once with phosphate-buffered saline (PBS) and transfected with GAL4-pGL4-luc reporter plasmids (180 ng/well) and pBIND-LXRβ-LBD expression plasmids (18 ng/well) using Lipofectamine 2000 (Invitrogen; 0.5 µL/well). After 6 h, the transfected cells were washed twice with PBS and the buffer replaced with 200 µL DMEM, containing the indicated screening compounds (10 µg/mL of various screening samples, 1 µmol/L TO901317 as a positive control, and 0.1% DMSO as a negative control). For initial screening, each compound was assayed only singly. For rescreening, each initially positive compound was tested in triplicate. For E17110 activity assay the cells were treated with various concentrations of E17110 in serum-free DMEM. After 18 h, the cells were washed with PBS once and lysed with 20 µL 1×CCLR (Promega) per well. The luciferase activity was measured as relative luminescence units (RLUs) in a final volume of 60 µL with the Luciferase Assay System (Promega) on a microplate reader (PerkinElmer, Waltham, MA, USA)19.
2.4. Real-time quantitative RT-PCR analysis
RAW264.7 macrophages were seeded in 6-well plates at 6×105 cells/mL in DMEM containing 10% FBS. After cell attachment (24 h), compound E17110 was added at various concentrations. The cells were harvested after 18 h, total RNA was extracted from the cells using TRIzol® reagent (Invitrogen) according to the manufacturer׳s instructions, and the first-strand cDNA was synthesized from the total RNA in a 20-µL system using a reverse transcriptase kit (TransGen Biotech). Real-time quantitative PCR assay with SYBR Green (Roche Diagnostics, Lewes, UK) detection chemistry was performed on a CFX96™ Real-Time PCR Detection System (Bio-Rad, Hercules, USA). The sequences of the primers are listed in Table 1. Melting curves were recorded, and the specificity of the PCR products was checked by agarose gel analysis. The mRNA levels of all genes were normalized for glyceraldehyde-phosphate dehydrogenase (GAPDH) levels, and the quantitative measurements were carried out by the ΔΔCt method.
Table 1.
Primers for real-time quantitative PCR.
| Gene | Accession No. | Forward primer | Reverse primer |
|---|---|---|---|
| mGAPDH | NM_008084.2 | 5′-AACGACCCCTTCATTGAC-3′ | 5′-TCCACGACATACTCAGCAC-3′ |
| mABCA1 | NM_013454.3 | 5′-GTTCCTGCAGAAACAGTAGCA-3′ | 5′-ATGAGGTTGGAGATAGCAGAGA-3′ |
| mABCG1 | NM_009593.2 | 5′-AGGTCTCAGCCTTCTAAAGTTCCTC-3′ | 5′-TCTCTCGAAGTGAATGAAATTTATCG-3′ |
2.5. Western blotting
RAW264.7 macrophages were seeded on 6-well plates at 6×105 cells/mL. The cells were stimulated with different concentrations E17110 for 18 h after which protein extracts were prepared as previously described21. Protein concentrations were determined by a BCA protein assay kit (Thermo Scientific). Equal amounts of protein were analyzed by 10% SDS-PAGE gel electrophoresis and electroblotted onto a 0.45 μm polyvinylidene fluoride membranes (Millipore Corp., Bedford, MA, USA). The membranes were blocked with 5% (w/v) skimmed milk in Tris-buffered saline containing 0.2% Tween-20 (TBST) for 1 h, and then incubated with the following primary antibodies which were diluted in 5% (w/v) skimmed milk in TBST buffer: mouse anti-ABCA1 (1:1000, Novus, Littleton, CO, USA), anti-ABCG1 (1:1000, Abcam) and anti-β-actin (1:2000, Sigma) for 4 °C overnight. The membranes then were washed with TBST three times, followed by incubation with horseradish peroxidase-conjugated secondary antibodies: anti-mouse and anti-rabbit IgG antibodies (1:5000, Novus) for 2 h at temperature. After being washed with TBST three times, the protein bands were detected with an Enhanced Chemiluminescence (ECL) reaction kit (Millipore), and quantified by Quantity One Software (Bio-Rad). All the proteins were normalized to β-actin.
2.6. Oil red O staining
Cellular lipid accumulation was evaluated by means of oil red O staining in RAW264.7 macrophages. The cells were cultured in 96-well plates at 6×104 cells/well, and 60 μg/mL ox-LDL was added after cell attachment. After 12 h, when the cells were grown to 90%–95% confluence, they were stimulated with the compound E17110 for 18 h at various concentrations. Then the samples were treated as described in the previous methods, and observed by light microscopy19, 22. To extract oil red O, isopropanol was added to each well which was then shaken at room temperature for 5 min. Samples were read at 510 nm using a microplate reader23.
2.7. Cholesterol efflux
Cellular cholesterol efflux experiments were performed using 22-NBD-cholesterol in RAW264.7 macrophages20. The cells were seeded in 96-well clear-bottom black plates (Costar) at 4×105 cells/mL. After they attached to the plates the medium was removed and the cells were labeled with 22-NBD-cholesterol (2.0 µmol/L at the final concentration) in serum-free medium containing 0.2% (w/v) bovine serum albumin (BSA) (Sigma Chemical) (medium A) for 24 h in a 37 °C 5% CO2 incubator. After 24 h of labeling, cells were washed twice with PBS and incubated with 100 µL medium A containing E17110 (0, 0.3, 1, 3 and 10 µmol/L) for an additional 18 h. 10 µg/mL ApoA-I or 50 µg/mL HDL was added as the receptor protein to start the efflux experiment at 37 °C for 6 h. Then the amounts of cholesterol in medium and cells were assayed using a microplate reader respectively (PerkinElmer, excitation 485 nm, emission 535 nm). The percentage of 22-NBD-cholesterol efflux (%) was calculated as (medium)/(medium+cell)×100. Each efflux assay was performed in duplicate in three times.
2.8. Molecular docking
To evaluate the activity of E17110, the docking program Discovery Studio 4.1 (Accelrys Inc., CA, USA) was used to dock the structure of LXRβ (PDB code: 1PQC, LXRβ with TO901317). First, all crystal water molecules were removed from the original structure and hydrogen was added in the DS CDOCKER module. To obtain an optimal starting conformation, the compound was minimized to reach the lowest energy state before docking.
2.9. Statistical analysis
Statistics and best-fit curves were calculated with Graphpad Prism 5.0 software (San Diego, CA, USA). The data are expressed as mean±SEM. Results were analyzed by the student׳s t-test and one-way ANOVA analysis by SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). All P values <0.05 were considered statistically significant (*P<0.05, **P<0.01 and ***P<0.001).
3. Results
3.1. Cell-based assay optimization
To assess an assay system, the reproducibility and signal variation at the activity range must be evaluated. In our screening system, TO901317 (1 µmol/L) was the positive control and four assay parameters influencing the signal and noise of the cellular reaction were taken into consideration, including: DMSO concentration: 0.1%; ratio between the reporter plasmid and the expression plasmid: 10:1; cell number: 2×104 cells/well; and incubation time: 18 h (Supplementary Fig. 1). The signal-to-noise ratio (S/N), signal-to-background ratio (S/B), coefficient of variation (CV%) and Z′ factor are classic and scientific indices for evaluation of the quality of assays, and can be utilized in assay validation and optimization24. According to our evaluation, this transient transfection system can be used for screening (Table 2).
Table 2.
The parameters of the LXRβ screening model.
| Parameter | LXRβ screening model | High-throughput screening |
|---|---|---|
| S/B | 43 | >3 |
| S/N | 12.13 | >10 |
| CV (%) | 4.98 | <10 |
| Z′ factor | 0.78 | >0.5 |
3.2. E17110 has LXRβ agonist activity
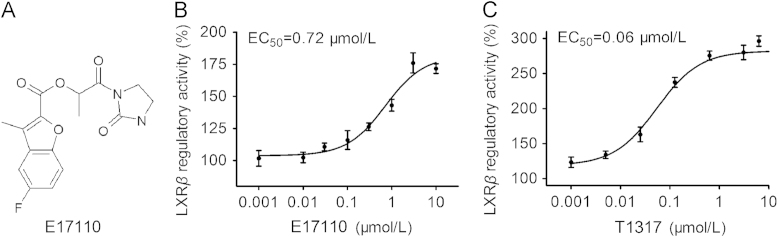
In this study we identified E17110, a structural analog of benzofuran-2-carboxylate (Fig. 1A), with LXRβ agonist activity by the LXRβ-GAL4 luciferase reporter screening as described in Materials and Methods. The chemical name of E17110 is [1-oxo-1-(2-oxoimidazolidin-1-yl)propan-2-yl]5-fluoro-3-methylbenzofuran-2-carboxylate, which has not been reported previously to possess pharmacological activity. E17110 significantly and dose-dependently induced the activation of LXRβ from 0.001 μmol/L to 10 μmol/L with an EC50 of 0.72 μmol/L, and showed a maximal activity of approximately 1.76-fold (Fig. 1B). In contrast, TO901317 exhibited approximately 3-fold LXRβ activation, with an EC50 of 0.06 μmol/L (Fig. 1C). TO901317 is regarded as a positive control, therefore this result was consistent with other prior studies, and our cell-based screening model was stable and credible22.
Figure 1.
E17110 regulates LXRβ. (A) Structure of E17110. (B) LXRβ regulatory activity of E17110. HEK293T cells were transfected with GAL4-pGL4-luc reporter plasmid and pBIND-LXRβ expression plasmid. E17110 showed significant LXRβ agonistic activity in the luciferase activity assay described in the methods section. (C) LXRβ regulatory activity of TO901317. Similar results were obtained in three independent experiments. Data are means±SEM (n=3).
3.3. E17110 induces ABCA1 and ABCG1 expression in vitro
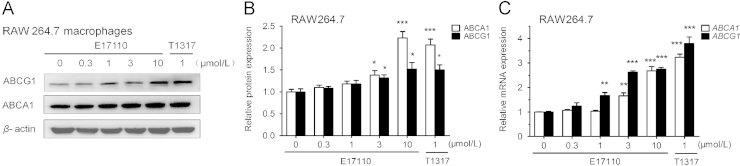
ABCA1 and ABCG1 are crucial target genes of LXR involved in the RCT process in macrophages17. The effects of E17110 on the expression of ABCA1 and ABCG1 in murine macrophages were first detected by western blotting. E17110 significantly increased the protein expression of ABCA1 and ABCG1 in RAW264.7 macrophages (Fig. 2A and B). Furthermore, it up-regulated the ABCA1 and ABCG1 mRNA levels at the same time (Fig. 2C). However, a greater effect was observed when the cells were stimulated with TO901317.
Figure 2.
Effect of E17110 on ABCA1 and ABCG1 expression. (A and B) RAW264.7 macrophages were incubated with E17110 at various concentrations for 18 h, and the levels of ABCA1 and ABCG1 proteins were determined by western blotting. Induction factors were normalized to β-actin, and the control groups were treated with DMSO (0.1%). (C) RAW264.7 macrophages were treated with E17110 at various concentrations for 18 h. Then mRNAs levels of ABCA1 and ABCG1 were measured by real-time quantitative PCR. Induction factors were normalized to GAPDH. Similar results were obtained in four independent experiments. Data are means±SEM (n=4, *P<0.05, **P<0.01 and ***P<0.001 vs. control).
3.4. E17110 promotes cholesterol efflux from macrophages
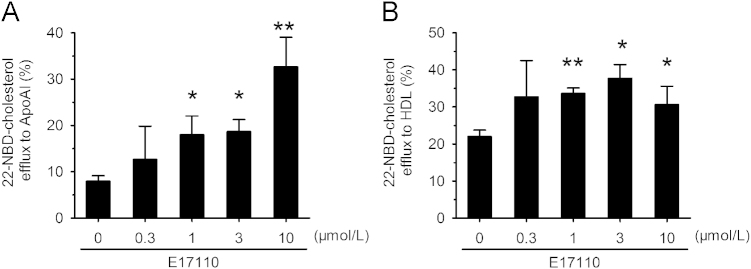
LXR activation in macrophages induced the expression of genes encoding ABCA1 and ABCG1, which facilitate cholesterol efflux from macrophages to plasma HDL and ApoA-I25. We next determined the effect on the cholesterol efflux in RAW264.7 macrophages. ApoA-I (10 µg/mL) or HDL (50 µg/mL) was added to the medium to promote cholesterol efflux. Obviously, E17110 dose-dependently increased cholesterol efflux to ApoA-I or HDL, and reduced the cellular cholesterol concentration in this cell line (Fig. 3).
Figure 3.
E17110 induced cholesterol efflux in RAW264.7 macrophages. RAW264.7 macrophages were preincubated with 22-NBD-cholesterol for 24 h, after which the cells were washed with PBS and incubated with E17110 (0, 0.3, 1, 3 and 10 μmol/L). After 18 h, (A) 10 mg/mL ApoA-I or (B) 50 mg/mL HDL was added and the incubation continued for 6 h at 37 °C. The amounts of cholesterol in medium and cell were separately measured. Relative 22-NBD-cholesterol efflux to ApoA-I or HDL induced by E17110 was calculated as described in the Methods section. Similar results were obtained in three independent experiments. Data are means±SEM (n=3, *P<0.05 and **P<0.01 vs. control).
3.5. E17110 reduces cellular lipid accumulation
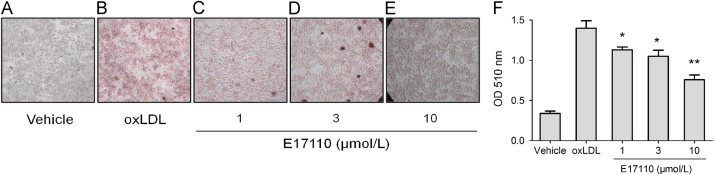
Here, we investigated the potential role of E17110 to inhibit lipid accumulation and foam cell formation in RAW264.7 cells, to evaluate whether it could promote lipid fluxing from mouse peritoneal macrophages. As shown in Fig. 4C–E, treatment of RAW264.7 cells with E17110 effectively reduced lipid accumulation compared with ox-LDL alone (Fig. 4B). Furthermore, foam cells were obviously inhibited when stimulated with 10 μmol/L E17110 (Fig. 4E), with levels similar to the vehicle group (Fig. 4A). At the same time, we used a fast and simple method to quantitate the content of lipid in cells. The result showed that E17110 could significantly reduce lipid accumulation from 1 to 10 μmol/L (Fig. 4F).
Figure 4.
E17110 reduces ox-LDL-induced lipid accumulation in RAW264.7 macrophages. RAW264.7 macrophages were preincubated with (A) PBS for vehicle and (B)–(E) ox-LDL (60 μg/mL) for samples. After 24 h, these cells were separately treated with (B) DMSO, (C)–(E) E17110 (1, 3 and 10 μmol/L) for 18 h. The cells were fixed with 4% paraformaldehyde and stained with 0.5% oil red O to detect lipid accumulation. Representative images of the five study group samples are shown (×400 magnification). Similar results were obtained in three independent experiments. (F) PBS, ox-LDL (60 μg/mL) and E17110 (1, 3 and 10 μmol/L) were added to the cultures throughout the experiment. After oil red O staining, bound dye was solubilized and quantified spectrophotometrically at 510 nm.
3.6. E17110 docks to the LXRβ-LBD
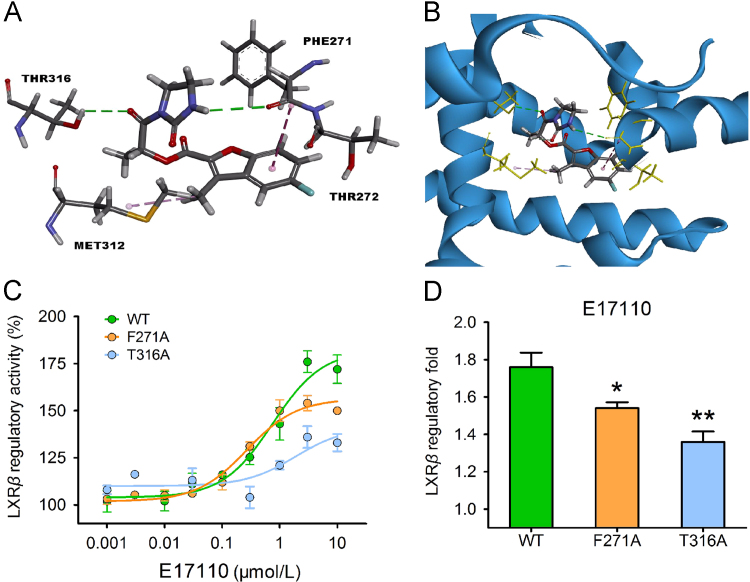
To determine the putative binding mode and potential ligand-pocket interactions of E17110, the structure of E17110 was docked into the ligand-binding domain of LXRβ (PDB code: 1PQC) using the docking program DS CDOCKER. The predicted binding mode suggested that E17110 can fit nicely into the LXRβ ligand-binding domain (Fig. 5A and B), and included two hydrogen bonds and two π–π stacking interactions with the surrounding amino acids. Specifically, one hydrogen bond formed between the oxygen atom of E17110 and the hydrogen atom of Thr316, and other one formed between the hydrogen atom of E17110 and the oxygen atom of Phe271. Meanwhile, two atypical π–π stacking interactions formed between E17110 and Thr272 and Met312.
Figure 5.
(A and B) The result of E17110 docking into the active site of the ligand-binding domain of LXRβ based on the X-ray co-crystal structure of T1317. (C) Activation of various LXRβ mutants by E17110, using the LXRβ-GAL4 chimera reporter assay. (D) E17110 (3 μmol/L) showed different LXRβ agonist activity on the wild-type group and different mutants in the LXRβ-GAL4 chimera reporter assays. Similar results were obtained in three independent experiments. Data are mean±SEM (n=3, *P<0.05 and **P<0.01 vs. control).
3.7. E17110 has interaction sites distinct from those of TO901317
Two different amino acids of LXRβ that were shown as crucial residues for binding of E17110 were individually replaced with alanine residues, and the resulting LXRβ mutants were assayed for activation by E17110 (Fig. 5C and D). The F271A and T316A mutants exhibited a significant decrease of agonistic activation by E17110, indicating a crucial role for these amino acid residues in transcriptional activation. Consistent with this finding, compared with the wild-type group, the different mutants showed distinct agonist activity when treated with 3 μmol/L of E17110.
4. Discussion
In our study, we identified E17110, a benzofuran-2-carboxylate derivative with LXRβ agonistic activity with an EC50 of 0.72 μmol/L. ABCA1 and ABCG1 are major transporters involved in cholesterol efflux from macrophages and play a vital role in maintaining cellular cholesterol homeostasis. Here we demonstrated that in RAW264.7 macrophages, E17110 dose-dependently induced the expression of ABCA1 and ABCG1 proteins and mRNAs. At the same time, we found that E17110 could reduce cellular lipid accumulation in RAW264.7 macrophages. ABCA1 can transfer both cholesterol and phospholipids from plasma membranes to HDL or to lipid-free ApoA-I26, while ABCG1 only transfers cholesterol to HDL but not to lipid-free ApoA-I27. We also performed cholesterol efflux experiments in RAW264.7 macrophages. We found that E17110 significantly increased cholesterol efflux to ApoA-I or HDL, and reduced the cellular cholesterol concentration in a dose-dependent manner. Therefore, we speculated that the cholesterol efflux induced by E17110 was related to the upregulation of ABCA1 and ABCG1 expression via activation of LXRβ in macrophages. This could be of benefit in the prevention of atherosclerosis.
Molecular docking was carried out to analyze ligand characteristics of E17110. Several potentially crucial amino acid residues were identified from the docking results, and they were replaced with alanine residues by site-directed mutagenesis. Interestingly, we found that the amino acids in LXRβ-LBD proposed to interact with E17110 differed from those identified for TO901317. Two amino acids (Phe271 and Thr316) formed the most important interaction forces with E17110. In contrast, H435 and W457, which are very important for binding TO901317, did not show significant impact on E17110 binding (data not shown). Thus, we suggest that E17110 has a distinct mechanism for promoting LXRβ agonist activity in vitro.
LXRs are members of the nuclear receptor superfamily and are present in two isoforms, LXRα and LXRβ2, 28. LXRs act as cholesterol sensors that control the expression of target genes when activated by ligands. LXR activation promotes cholesterol efflux and reduces cellular lipid accumulation, to prevent macrophage foam cell formation. Recently, LXRs have been regarded as potential targets for treating atherosclerosis, and synthetic agonists have been the key subject of many studies29, 30. However, full LXR agonists commonly lead to lipid accumulation in the liver because they activate the LXRα subtype and increase the expression of SREBP-1c regulated genes in the lipogenesis pathway25, 31. Therefore, in this study, our goal was to find a novel compound targeted to LXRβ with potential anti-atherosclerotic activity by screening. LXRα and LXRβ have a similar structure in both DBD and LBD domains, so the effect of this new compound on the LXRα subtype still needs to be tested and the possible effects on triglyceride metabolism evaluated.
5. Conclusions
Overall, through screening we identified E17110, a derivative of benzofuran-2-carboxylate as an anti-atherosclerotic lead compound with potential LXRβ agonist activity in vitro. E17110 increased the expression of ABCA1 and ABCG1 dependently on LXRβ activation, and promoted cholesterol efflux in macrophages. Meanwhile, E17110 could reduce lipid accumulation and inhibit the foam cell formation. In summary, our study suggests that E17110 may be useful for the development of pharmaceutical agents for treating atherosclerosis.
Acknowledgments
This work was kindly supported by the National Natural Science Foundation of China (Nos. 81273515, 81321004 and 81503065), the Key New Drug Creation and Manufacturing Program (Nos. 2012ZX09301002-003 and 2012ZX09301002-001), and the Basic Scientific Research Program of Materia Medica, CAMS (2014ZD03).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.apsb.2016.03.005.
Contributor Information
Yanni Xu, Email: xuyanniwendeng@hotmail.com.
Shuyi Si, Email: sisyimb@hotmail.com.
Appendix A. Supplementary material
Supplementary Fig. 1 Optimization of the cell-based screening model. (A) The sensitivity to DMSO concentration. Cells were seeded at 2×104 cells/well and treated with blank control (DMEM only), or serial dilution of DMSO (0.01%, 0.05%, 0.1%, 0.5%, 1%, 5% and 10%). (B) The ratio of reporter plasmid GAL4-pGL4-luc to expression plasmid pBIND-LXRβ-LBD. The cells were transfected with reporter plasmid to expression plasmid at the ratio of 20:1. 10:1, 5:1, 1:1, 1:5, 1:10 and 1:20 respectively, followed by 18 h treatment of positive control (1 µmol/L TO901317) and negative control (0.1% DMSO). (C) Cell number. HEK293T cells were seeded at 0.5×104, 1×104, 2×104, 5×104 and 10×104 cells/well respectively, followed by 18 h treatment of positive control (1 µmol/L TO901317) and negative control (0.1% DMSO). (D) Incubation time. The cells were incubated for 6, 12, 18, 24 and 36 h respectively, with positive control (1 µmol/L TO901317) and negative control (0.1% DMSO). Data are means±SEM (*P<0.05, **P<0.01, ***P<0.001 vs. control).
References
- 1.Wojcicka G., Jamroz-Wiśniewska A., Horoszewicz K., Beltowski J. Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw. 2007;61:736–759. [PubMed] [Google Scholar]
- 2.Willy P.J., Umesono K., Ong E.S., Evans R.M., Heyman R.A., Mangelsdorf D.J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 3.Auboeuf D., Rieusset J., Fajas L., Vallier P., Frering V., Riou J.P. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-α in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 4.Tamura K., Chen Y.E., Horiuchi M., Chen Q., Daviet L., Yang Z. LXRα functions as a cAMP-responsive transcriptional regulator of gene expression. Proc Natl Acad Sci U S A. 2000;97:8513–8518. doi: 10.1073/pnas.100519097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costet P., Luo Y., Wang N., Tall A.R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 6.Sabol S.L., Brewer H.B., Jr., Santamarina-Fojo S. The human ABCG1 gene: identification of LXR response elements that modulate expression in macrophages and liver. J Lipid Res. 2005;46:2151–2167. doi: 10.1194/jlr.M500080-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Baranowski M. Biological role of liver X receptors. J Physiol Pharmacol. 2008;59(Suppl. 7):31–55. [PubMed] [Google Scholar]
- 8.Cavelier C., Lorenzi I., Rohrer L., von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim Biophys Acta. 2006;1761:655–666. doi: 10.1016/j.bbalip.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Naik S.U., Wang X., Da Silva J.S., Jaye M., Macphee C.H., Reilly M.P. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–97. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 10.Shibata N., Glass C.K. Macrophages, oxysterols and atherosclerosis. Circ J. 2010;74:2045–2051. doi: 10.1253/circj.cj-10-0860. [DOI] [PubMed] [Google Scholar]
- 11.Rader D.J., Alexander E.T., Weibel G.L., Billheimer J., Rothblat G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph S.B., McKilligin E., Pei L., Watson M.A., Collins A.R., Laffitte B.A. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin N., Bischoff E.D., Daige C.L., Thomas D., Vu C.T., Heyman R.A. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 14.Tangirala R.K., Bischoff E.D., Joseph S.B., Wagner B.L., Walczak R., Laffitte B.A. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janowski B.A., Willy P.J., Devi T.R., Falck J.R., Mangelsdorf D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 16.Houck K.A., Borchert K.M., Hepler C.D., Thomas J.S., Bramlett K.S., Michael L.F. T0901317 is a dual LXR/FXR agonist. Mol Genet Metab. 2004;83:184–187. doi: 10.1016/j.ymgme.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Geyeregger R., Zeyda M., Stulnig T.M. Liver X receptors in cardiovascular and metabolic disease. Cell Mol Life Sci. 2006;63:524–539. doi: 10.1007/s00018-005-5398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peet D.J., Turley S.D., Ma W.Z., Janowski B.A., Lobaccaro J.M., Hammer R.E. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 19.Li N., Xu Y., Feng T., Liu C., Li Y., Wang X. Identification of a selective agonist for liver X receptor α (LXRα) via screening of a synthetic compound library. J Biomol Screen. 2014;19:566–574. doi: 10.1177/1087057113516004. [DOI] [PubMed] [Google Scholar]
- 20.Li N., Wang X., Zhang J., Liu C., Li Y.Z., Feng T.T. Identification of a novel partial agonist of liver X receptor α (LXRα) via screening. Biochem Pharmacol. 2014;92:438–447. doi: 10.1016/j.bcp.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Amoruso A., Bardelli C., Gunella G., Ribichini F., Brunelleschi S. A novel activity for substance P: stimulation of peroxisome proliferator-activated receptor-γ protein expression in human monocytes and macrophages. Br J Pharmacol. 2008;154:144–152. doi: 10.1038/bjp.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoang M.H., Jia Y.Y., Jun H.J., Lee J.H., Lee B.Y., Lee S.J. Fucosterol is a selective liver X receptor modulator that regulates the expression of key genes in cholesterol homeostasis in macrophages, hepatocytes, and intestinal cells. J Agric Food Chem. 2012;60:11567–11575. doi: 10.1021/jf3019084. [DOI] [PubMed] [Google Scholar]
- 23.Zou C.H., Shen Z.F. One-step intracellular triglycerides extraction and quantitative measurement in vitro. J Pharmacol Toxicol Methods. 2007;56:63–66. doi: 10.1016/j.vascn.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J.H., Chung T.D., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 25.Repa J.J., Mangelsdorf D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 26.Dean M., Hamon Y., Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- 27.Kennedy M.A., Barrera G.C., Nakamura K., Baldan A., Tarr P., Fishbein M.C. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Miyata K.S., McCaw S.E., Patel H.V., Rachubinski R.A., Capone J.P. The orphan nuclear hormone receptor LXRα interacts with the peroxisome proliferator-activated receptor and inhibits peroxisome proliferator signaling. J Biol Chem. 1996;271:9189–9192. doi: 10.1074/jbc.271.16.9189. [DOI] [PubMed] [Google Scholar]
- 29.Huang C. Natural modulators of liver X receptors. J Integr Med. 2014;12:76–85. doi: 10.1016/S2095-4964(14)60013-3. [DOI] [PubMed] [Google Scholar]
- 30.Hong C., Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- 31.Quinet E.M., Savio D.A., Halpern A.R., Chen L., Schuster G.U., Gustafsson J.A. Liver X receptor (LXR)-β regulation in LXRα-deficient mice: implications for therapeutic targeting. Mol Pharmacol. 2006;70:1340–1349. doi: 10.1124/mol.106.022608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Optimization of the cell-based screening model. (A) The sensitivity to DMSO concentration. Cells were seeded at 2×104 cells/well and treated with blank control (DMEM only), or serial dilution of DMSO (0.01%, 0.05%, 0.1%, 0.5%, 1%, 5% and 10%). (B) The ratio of reporter plasmid GAL4-pGL4-luc to expression plasmid pBIND-LXRβ-LBD. The cells were transfected with reporter plasmid to expression plasmid at the ratio of 20:1. 10:1, 5:1, 1:1, 1:5, 1:10 and 1:20 respectively, followed by 18 h treatment of positive control (1 µmol/L TO901317) and negative control (0.1% DMSO). (C) Cell number. HEK293T cells were seeded at 0.5×104, 1×104, 2×104, 5×104 and 10×104 cells/well respectively, followed by 18 h treatment of positive control (1 µmol/L TO901317) and negative control (0.1% DMSO). (D) Incubation time. The cells were incubated for 6, 12, 18, 24 and 36 h respectively, with positive control (1 µmol/L TO901317) and negative control (0.1% DMSO). Data are means±SEM (*P<0.05, **P<0.01, ***P<0.001 vs. control).