Abstract
Background
Cryptococcal disease remains an important cause of morbidity and mortality in HIV infected individuals in sub-Saharan Africa, despite the introduction of antiretroviral therapy (ART). Fluconazole has not been previously studied as primary prophylaxis against cryptococcal disease in patients awaiting or starting ART.
Methods
This prospective, double blind randomized controlled trial compared 200mg fluconazole three times per week to identical placebo in Uganda. 1519 ART naïve adults with CD4 counts <200 cells/μL were enrolled. Patients were excluded if baseline cryptococcal antigen (CrAg) was positive. Patients were randomly allocated to placebo or control (1:1) in blocks of 40 without stratification. Participants were seen after 4 weeks and referred for ART, then seen every 8 weeks. Participants discontinued trial treatment when CD4 counts reached 200 (median 197 days). Analyses were carried out on an intention to treat basis and included all participants enrolled in the study. Primary end points were invasive cryptococcal disease and all cause mortality. Secondary endpoints were first episode and incidence of oesophageal candidiasis, time to first episode and incidence of oro-pharyngeal or vaginal candidiasis, time to first hospital admission or death. The primary safety end point was stopping trial drug due to elevated transaminases above 5x upper limit of normal or other major adverse events. The trial is registered on controlled-trials.com; ISRCTN 76481529
Results
760 participants received fluconazole and 759 received placebo. 19 developed cryptococcal disease, one on fluconazole and 18 on placebo (P<0.001); adjusted Hazard Ratio (aHR) 18.7 (95%CI 2.5-140.7). One case of cryptococcal disease could be prevented by treating 44.6 patients with baseline CD4 counts <200 cells/μL. Fluconazole was effective both pre-ART (aHR=11.0; 95%CI 1.4-85.3) and after starting ART (no cases on fluconazole vs. 7 cases on placebo after starting ART). Seven participants died from cryptococcal disease, none on fluconazole. All cause mortality (N=189) did not differ between the two arms (P=0.46). The frequency of elevated transaminases (>5x upper limit) was similar in both arms (aHR=0.94, 95% CI 0.65-1.35).
Conclusions
Fluconazole was safe and effective as primary prophylaxis against cryptococcal disease, both before starting and during early ART. Cryptococcal infection was less common than anticipated because of the rapid commencement of ART and exclusion of those with positive CrAg. In patients with negative CrAg on screening, fluconazole prophylaxis can prevent cryptococcal disease while waiting for ART and in the early weeks of ART, particularly in those with CD4 counts <100 cells/μL.
Funding
Medical Research Council, UK and Rockefeller Foundation
Keywords: Cryptococcus, primary prophylaxis, Fluconazole
Background
Cryptococcal disease is one of the commonest central nervous system infections in HIV infected individuals. Infection is acquired by the inhalation of environmental spores or desiccated yeast cells: clinical disease may not occur for months to years after exposure and may be preceded by asymptomatic cryptococcal antigenaemia1. The disease is particularly problematic in sub-Saharan Africa, where the incidence in severe immunosuppression may reach 10% and it may cause up to 17% of deaths in HIV infected individuals1, 2. Untreated, the mortality is 100%, and even with optimal treatment, around 30% of individuals die3, 4. Survivors often have severe disabilities5. The gold standard treatment of amphotericin and flucytosine is costly and difficult to administer in resource poor settings4, 6, fluconazole is therefore often used with poorer outcomes7, 8.
Prior to the availability of ART in the USA, a randomised controlled trial (RCT) of primary prophylaxis using fluconazole showed a reduction in the incidence of cryptococcal disease but no effect on mortality9. Other Western studies suggested benefit from routine prophylaxis with itraconazole or fluconazole, but most were small and retrospective10–18. A Cochrane review of cryptococcal primary prophylaxis concluded that azoles reduced incidence of cryptococcal disease but that the effect on mortality was unclear, and that studies were needed in the developing world19. Only two studies have been done in developing countries, both in Thailand and with less than 10% of participants receiving ART. One suggested that fluconazole prophylaxis reduced invasive fungal infections and mortality but had no effect on cryptococcal events20; the other demonstrated a reduction in systemic fungal diseases (including cryptococcosis) with itraconazole but no survival advantage21.
The incidence of cryptococcal disease has recently declined in industrialised countries, predominantly due to ART22–24. International initiatives led to progress in the provision of ART in resource poor countries but only 30% of those who need ART in sub-Saharan Africa are receiving it25. Cryptococcal disease remains common in sub-Saharan Africa, both in those who are yet to start ART or who are in the first months of ART26, 27.
Primary prophylaxis against cryptococcal disease has never been formally tested in Africa or any setting where effective ART is available. We performed a double-blind, randomized, placebo controlled trial to examine the efficacy and safety of fluconazole as primary prophylaxis against cryptococcal disease prior to ART initiation and in the first few months of ART.
Methods
Ethics statement
Information on the trial was provided during group and individual meetings and through leaflets in the local language. Participants gave written or (if illiterate) witnessed (by a person independent of the trial team) verbal consent to screening and enrolment. Ethical approval was gained from the Uganda National Council for Science and Technology and the Ethics Committees of UVRI and the Liverpool School of Tropical Medicine. An independent data monitoring committee monitored accumulating data regularly. At completion of the trial, all participants were offered fluconazole if their CD4 count was still below 200 cells/µL.
Study design and population
Participants were recruited from five local HIV/AIDS organisations in Masaka district, Uganda, between November 2004 and January 2008: the AIDS Support Organisation (TASO), Masaka Regional Referral Hospital, Uganda Cares Masaka, Kalangala District Health Services and Kitovu Mobile AIDS Organisation. Participants were from predominantly rural communities, including the Ssese Islands in Lake Victoria.
Potential participants were screened for eligibility at a dedicated study clinic sited at TASO; from April 2006 participants were also screened and enrolled at Ministry of Health clinics on the Ssese islands. ART naive adults with laboratory confirmation of HIV infection (Murex HIV-1.2.0, Murex Biotech; HIV Uni-form II plus O, Biomerieux; Cambridge Biotech HIV-1 Western blot) and a CD4 count less than 200 cellsµ/L (FACSCount Becton Dickinson, USA) were eligible for the study. Cryptococcal antigen (CrAg) (Remel, Lexana, USA) testing was done (dilution first performed to exclude pronase effect) and participants with a serum CrAg titre >1:8 on 2 occasions were excluded; it was considered unethical to randomise these patients. Other exclusion criteria were pregnancy or lactation, liver transaminases (LFT) > 3 x upper limit of normal (ULN), and moribund patients. Participants with oral and vaginal candidiasis at screening were treated with topical clotrimazole or nystatin, or if refractory, ketoconazole (200-400mg daily for 5 days); symptomatic oesophageal candidiasis was treated with fluconazole (minimum 14 days) and enrolment delayed for 4 weeks.
An independent statistician prepared a list for 1:1 randomisation to fluconazole or matching placebo in random permuted blocks of size 40. Randomization was not stratified by site. Trial drug was packaged and labelled by an independent clinician and pharmacist. Participants were allocated to sequential trial numbers on enrolment and received the corresponding sealed trial drug pack. Blinded samples of each batch of trial drug were assessed at the University of Liverpool, UK for consistency with trial drug labelling.
Trial procedures
Eligible and consenting participants were enrolled and received either 200 mg of fluconazole or matching placebo (manufactured by Cipla, India) three times a week. Participants were seen 4 weeks after enrolment and then 8-weekly for follow up. New clinical symptoms or signs were assessed and intercurrent illnesses treated. Pill counts and adherence to trial medication were assessed at routine visits to encourage adherence. LFTs were performed 8- weekly and CD4 counts measured every 16 weeks. A serum sample was stored at each follow-up for subsequent CrAg testing. Women underwent a pregnancy test, were counselled about avoiding pregnancy and offered contraception at every appointment. Patients were offered co-trimoxazole prophylaxis (480mg daily) according to national guidelines. Participants were encouraged to attend the clinic or contact the trial team if they felt unwell between routine visits and if necessary were hospitalised under the care of the trial team. Field workers attempted to contact non-attendees at home if a routine appointment was missed.
Suspected cryptococcal cases were investigated with a serum CrAg test, chest X-ray, blood cultures (BACTEC 9120 blood culture system, Becton Dickinson) and lumbar puncture (routine CSF microscopy, India ink microscopy, glucose analysis, and CSF culture at 37°C for 14 days on Sabouraud dextrose agar). Invasive cryptococcal disease was defined as symptoms of cryptococcal disease with a serum CrAg titre>1:8 on two occasions, or a positive CSF CrAg or Cryptococcus neoformans grown from blood or CSF culture.
Participants who developed cryptococcal disease were treated with amphotericin (0.8- 1mg/kg/day iv) for 14 days followed by fluconazole 400mg daily for 8 weeks, and secondary fluconazole prophylaxis (200mg daily). Oral and vaginal candidiasis was diagnosed by culture and treated with topical nystatin or clotrimazole; refractory cases received oral ketoconazole (200mg-400mg daily for 5 days). Oesophageal candidiasis was diagnosed by dysphagia with a positive oro-pharyngeal culture, and treated with fluconazole 200mg daily for 2 weeks; trial drug was suspended during this period.
The trial was designed prior to the availability of ART in Uganda. In the first year of the trial free of charge ART became widely available and trial protocols were modified. At the initial four week follow up, LFTs were performed to exclude side effects of trial medication and participants given a referral letter to their preferred ART care provider documenting CD4 count, full blood count, LFTs and medical problems. ART providers had a minimum six week client preparation time for ART initiation. Participants entered this pathway at the four week point so as not to delay ART initiation. ART regimens (of two nucleoside reverse transcriptase inhibitors and one non-nucleoside reverse transcriptase inhibitor) were chosen and monitored by providers independent of the trial team. .
Participants continued trial medication until the end of the trial (minimum 12 weeks) or until their CD4 count reached 200 cells/µL when they were no longer considered to be at risk of cryptococcal disease. Trial drug was also stopped if transaminases exceeded 5x ULN, if there was an adverse event considered to be related to trial drug, if women became pregnant, if participants wanted to leave the trial or if they moved away from study area. Once trial drug was stopped, participants were reviewed at the clinic every six months.
Deaths and potential episodes of cryptococcal disease were retrospectively reviewed by an independent end point review committee (EPRC). A verbal autopsy was performed on those who died outside hospital and the most recently stored serum was tested for CrAg. The (EPRC) had access to participants’ files, hospital notes, verbal autopsy data and retrospective CrAg results, but were blind to treatment group.
Endpoints
The two co-primary endpoints were time to first episode of invasive cryptococcal disease and all cause mortality. All cause mortality was redefined as a primary endpoint (previously a secondary endpoint) in place of mortality from cryptococcal disease in 2006, when it became clear that both the number of cryptococcal events and the case fatality rate was lower than anticipated (after enrolment of 796). Secondary efficacy endpoints were: time to first episode of oesophageal candidiasis, time to first episode of oro-pharyngeal or vaginal candidiasis, time to first hospital admission or death, incidence of candidiasis (allowing for multiple episodes) and incidence of hospital admission (allowing for multiple admissions). The primary safety end point was stopping trial drug due to elevated transaminases above 5x ULN or other major adverse event.
Statistical Analysis
The original sample size of 590 participants was based on an annual rate of invasive cryptococcal disease of 10.3% and had 80% power to detect a 75% reduction in the incidence of cryptococcal disease at the 5% significance level. As ART became available in Uganda, the sample size was re-estimated to account for the reduction in cryptococcal incidence in participants who started ART. Recruitment of 770 participants in each arm (to contribute 530 person years of observation (PYO)) was estimated to give 80% power to detect a reduction of 75% in cryptococcal disease at the 5% level (22 cryptococcal events).
Analyses were carried out on an intention to treat basis and included all enrolled participants. Subjects were considered to be at risk of an event until they experienced the event, stopped taking trial drug due to their CD4 count reaching 200, died or the trial ended. Subjects who stopped trial drug due to an adverse event or pregnancy were considered at risk until trial end. Participants lost to follow-up or who withdrew were considered at risk until the last time seen. Primary outcomes were analysed using survival analysis. Kaplan-Meier survival curves were used to illustrate the time to event in the two treatment arms; a log rank test was used to determine whether distributions differed between arms. A further log-rank test stratified the exposure time by ART status. Cox regression models were fitted with terms for baseline CD4 (categorized as <50, 50-99, 100-149 or 150-199 cells/μL for mortality and as <50 or 50-199 cells/μL for cryptococcal events (due to small numbers) and for ART status as a time-varying covariate. A formal test examining a potential ART by treatment arm interaction was carried out for all cause mortality; there were too few events to do this for the cryptococcal primary endpoint. A Bonferroni correction was applied to adjust for multiple significance testing and a 2.5% significance level used for the two primary endpoints. Similar methods were used for analysis of secondary outcomes. To assess the incidence of any episode of candidiasis or hospital admissions, survival analysis methods were adapted to allow for multiple events within participants as described by Cleves28
Role of the funding source
This research was supported by the Medical Research Council, UK and the Rockefeller Foundation. Neither had a role in design, analysis or writing of this paper. The corresponding author had full access to all study data and responsibility for the decision to submit for publication.
Results
Study Population
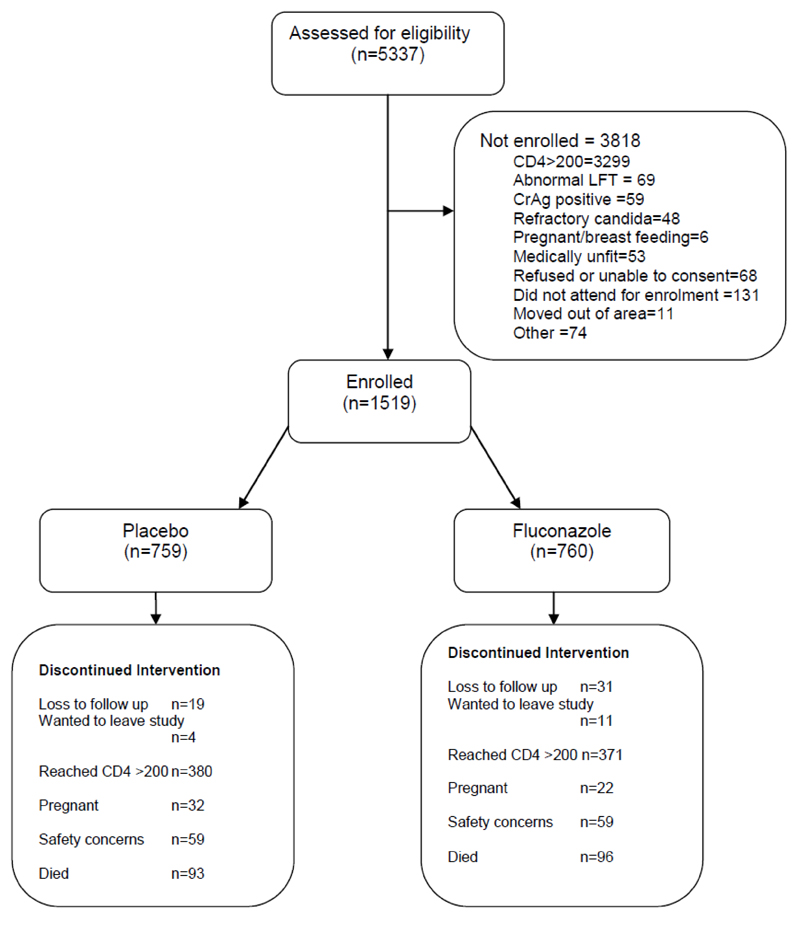
5337 participants were screened and 1519 enrolled. 759 participants received placebo and 760 received fluconazole (Figure 1). There were no baseline differences between the groups (Table 1). More women were enrolled than men, consistent with the usual pattern of HIV care seeking in Uganda29.
Figure 1. Trial Profile.
Table 1. Baseline Characteristics.
| Variable | Placebo (n=759) | Fluconazole (n=760) | Overall (n=1519) |
|---|---|---|---|
| Sex Male n (%) | 251 (33.1%) | 286 (37.6%) | 537 (35.4%) |
| Female n (%) | 508 (66.9%) | 474 (62.4%) | 982 (64.6%) |
| Age (years Mean (s.d.)) | 35.8 (8.8) | 35.9 (9.1) | 35.8 (9.0) |
| Age (grouped) <25 | 47 (6.2%) | 58 (7.6%) | 105 (6.9%) |
| 25-34 | 323 (42.6%) | 306 (40.3%) | 629 (41.4%) |
| 35-44 | 270 (35.6%) | 269 (35.4%) | 539 (35.5%) |
| 45 + | 119 (15.7%) | 127 (16.7%) | 246 (16.2%) |
| CD4 count (median (IQR)) | 112 (48 – 157) | 110 ( 45 – 160) | 111 (46 – 159) |
| CD4 count (grouped) | |||
| 150 – 199 | 231 (30.4%) | 237 (31.2%) | 468 (30.8%) |
| 100 – 149 | 185 (24.4%) | 168 (22.1%) | 353 (23.2%) |
| 50 – 99 | 150 (19.8%) | 152 (20.0%) | 302 (19.9%) |
| 1 - 49 | 193 (25.4%) | 203 (26.7%) | 396 (26.1%) |
| WHO stage 1 | 20 (2.6%) | 18 (2.4%) | 38 (2.5%) |
| 2 | 164 (21.6%) | 175 (23.0%) | 339 (22.3%) |
| 3 | 524 (69.0%) | 506 (66.6%) | 1030 (67.8%) |
| 4 | 51 (6.7%) | 61 (8.0%) | 112 (7.4%) |
Participants were at risk in the primary analysis for a median of 30 weeks (IQR 25-53) on placebo and 30 weeks (IQR 25-54) on fluconazole. The median total follow up was 60 weeks (IQR 28-123) on placebo and 59 weeks (IQR 27-124) on fluconazole. 751 (49.4%) participants stopped trial drug because their CD4 count reached 200 cells/μL; 407 (26.8%) stopped at the end of the trial; 54 (3.5%) stopped due to pregnancy; 50 (3.3%) were lost to follow-up and 15 (1.0%) withdrew consent. All other participants stopped because of a cryptococcal or safety endpoint. 1298 participants (85.4%) started ART (641 on fluconazole and 657 on placebo) at a median time of 11 weeks (IQR 7-17 weeks) after enrolment, of whom 1063 (81.9%) received a regimen containing nevirapine. The median time to ART was 82 and 87 days for the fluconazole and placebo arms respectively.
Cryptococcal disease
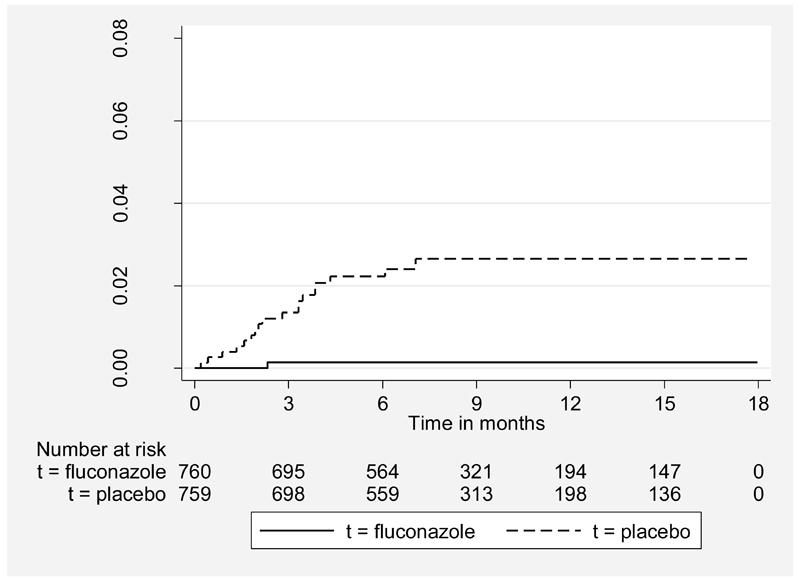
Eighteen participants taking placebo and one taking fluconazole developed cryptococcal disease (table 2). The risk was significantly higher in the placebo than in the fluconazole arm (log-rank X2 = 15.27, P<0.001) (figure 2). The hazard ratio for developing cryptococcal disease on placebo compared to fluconazole was 18.74 (95% CI 2.5-140.7), adjusting for baseline CD4 count and whether or not the participant was on ART. Fluconazole reduced cryptococcal events both before and after starting ART (table 3). Before ART, there were 11 cryptococcal events on placebo and one event on fluconazole. After starting ART, seven participants on placebo but none on fluconazole developed cryptococcal disease. No cryptococcal events occurred in participants who stopped taking trial drug when their CD4 count reached 200. The overall rate of cryptococcal events was 2.80 per 100 PYO in the placebo arm and 0.15 per 100 PYO in the fluconazole arm (Table 3). On average 44.6 patients would require fluconazole prophylaxis to prevent one case of cryptococcal disease. 13 (68.4%) of those developing cryptococcal disease had a CD4 count <50 cells/μL at the time of diagnosis, 4 (21.0%) had a CD4 count between 50-99 cells/μL, and the remaining 2 (10.5%) had CD4 counts of 124 and 139 cells/μL. Cryptococcal infection occurred predominantly in patients with WHO stage 3 (12 [63.2%]) or 4 (4 [21.0%]) at baseline. The NNT was 22.8 for those with a baseline CD4 count< 100 cells/μL (two events missed) and 44.1for baseline WHO stage 3 and 4 together. Positive cryptococcal cultures, including the one in the fluconazole arm, were all sensitive to fluconazole.
Table 2. Diagnosis of Cryptococcal Events.
| Age | Sex | Time to Event§ (days) | Time on ART (days) | CD4 count¥ (cells /µl) | Serum CrAg titre (at diagnosis) | Blood Culture | CSF Crag | CSF culture | Died within four weeks | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 32 | M | 71 | -- | 14 | 1:512 | C. neoformans | pos | C. neoformans | N |
| 2 | 31 | M | 215 | 78 | 18 | neg | C. neoformans | neg | neg | Y |
| 3 | 36 | F | 117 | 56 | 139 | 1:32^ | neg | neg | neg | N |
| 4 | 35 | M | 132 | 39 | 30 | 1:128 | neg | pos | neg | N |
| 5 | 38 | M | 105 | 7 | 124 | 1:1024 | C. neoformans | pos | C. neoformans | N |
| 6 | 46 | M | 101 | 3 | 31 | 1:1024 | C. neoformans | pos | C. neoformans | N |
| 7 | 33 | F | 117 | -- | 14 | 1:128 | C. neoformans | neg | neg | N |
| 8 | 30 | F | 27 | -- | 3 | 1:1024 | C. neoformans | pos | C. neoformans | N |
| 9 | 35 | F | 62 | -- | 2 | 1:1024 | N/A | N/A | C. neoformans | Y |
| 10 | 33 | M | 59 | -- | 78 | 1:512 | neg | pos | C. neoformans | N |
| 11 | 58 | F | 85 | 13 | 66 | 1:16^ | neg | neg | neg | N |
| 12 | 52 | M | 66 | -- | 27 | 1:512 | C. neoformans | pos | C. neoformans | N |
| 13 | 34 | M | 185 | -- | 76 | 1:2048 | C. neoformans | pos | C. neoformans | N |
| 14 | 31 | F | 6 | -- | 70 | 1:256 | C. neoformans | pos | C. neoformans | N |
| 15 | 39 | M | 101 | 38 | 7 | 1:128 | neg | pos | neg | N |
| 16 | 37 | M | 55 | -- | 26 | 1:512 | C. neoformans | pos | C. neoformans | N |
| 17 | 32 | M | 48 | -- | 8 | 1:512^ | neg | N/A | N/A | Y |
| 18 | 38 | F | 41 | -- | 8 | 1:512 | C. neoformans | pos | C. neoformans | N |
| 19 | 28 | F | 13 | -- | 15 | 1:8 | C. neoformans | pos | neg | N |
Time from study enrolment to cryptococcal event (second CrAg for those diagnosed on CrAg alone)
Diagnosed on serum CrAg alone
CD4 count at study enrolment
On fluconazole
N/A- not available
neg=negative
pos=positive
Figure 2. Incidence of cryptococcal disease by treatment arm.
Table 3. Efficacy Outcomes.
| Overall | Before ART | On ART | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Fluconazole | Placebo | Fluconazole | Placebo | Fluconazole | ||||||||||||
| Events | Rate / 100 PYO | Events | Rate /100 PYO | Unadjusted log rank X2 (P-value) | aHR1 (95% C.I.) | Heterogeneity LR X2 (P-value) | Events | Rate / 100 PYO | Events | Rate / 100 PYO | aHR2 (95% C.I.) | Events | Rate / 100 PYO | Events | Rate / 100 PYO | aHR2 (95% C.I.) | |
| Primary outcomes | |||||||||||||||||
| Cryptococcal disease | 18 | 2.8 | 1 | 0.15 | 15.27 (P=0.001) | 18.74 (2.5 – 140.7) | Non-estimable | 11 | 5.14 | 1 | 0.48 | 11.0 (1.4- 85.3) | 7 | 1.63 | 0 | 0 | ∞* (1.45-∞) |
| Deaths on trial drug | 93 | 14.1 | 96 | 14.5 | 0.05 (P=0.82) | 0.96 (0.72 – 1.27) | 0.55 (P=0.46) | ||||||||||
| Secondary outcomes | |||||||||||||||||
| First episode of oesophageal candidiasis | 55 | 8.7 | 6 | 0.91 | 40.76 (P<0.001) | 9.4 (4.0–21.8) | 5.44 (P=0.02) | 45 | 21.3 | 2 | 0.96 | 22.2 (5.4- 91.7) | 10 | 2.39 | 4 | 0.89 | 2.9 (0.91-9.3) |
| First episode of oropharyngeal or vaginal candidiasis | 159 | 29.5 | 24 | 3.7 | 116.64 (P<0.001) | 7.4 (4.8 - 11.4) | 5.40 (P=0.02) | 119 | 61.4 | 11 | 5.3 | 11.4 (6.1- 21.1) | 40 | 11.6 | 13 | 3 | 4.0 (2.1-7.4) |
| Hospital admission or death | 235 | 44.3 | 229 | 42.5 | 0.18 (P=0.67) | 1.05(0.87 - 1.26) | 1.04 (P=0.31) | ||||||||||
| Incidence of hospital admissions | 284 | 43.7 | 278 | 42.6 | 0.12 (P=0.73) | 1.04 (0.88-1.22) | 0.34 (P=0.56) | ||||||||||
| Incidence of all candidiasis | 272 | 41.2 | 29 | 4.4 | 198.6 (P<0.001) | 9.6 (6.5-14.1) | 7.10 (P=0.008) | 187 | 85.7 | 12 | 5.7 | 14.8 (8.2- 26.5) | 85 | 19 | 17 | 3.8 | 5.3 (3.1-8.9) |
Hazard ratio adjusted for baseline CD4 group and for before or after starting ART as time varying covariate.
Hazard ratio adjusted for baseline CD4 group
Exact CI for rate ratio has been used as HR cannot be estimated since there were no cryptococcal events on the fluconazole arm after starting ART.
Survival
Fluconazole had no effect on survival; 93 subjects on placebo and 96 on fluconazole died (P=0.82 on log rank test). The hazard ratio for death in placebo vs. fluconazole groups was 0.96, adjusting for baseline CD4 count and ART status as a time dependent covariate. The end point review committee judged cryptococcal disease to be the definite cause of death in seven participants and a possible cause of death in one; all were on placebo. 9 deaths (5 placebo, 4 fluconazole) occurred after participants had stopped trial drug. Including these deaths in the survival analysis changed the adjusted hazard ratio from 0.96 to 0.97.
Secondary endpoints
Fluconazole significantly reduced the incidence rate for the first episode of oesophageal candidiasis, oral and vaginal candidiasis (P<0.001) (table 3). The effect of fluconazole was greater before ART (Table 3). Overall, 63 participants developed 74 episodes of oesophageal candidiasis; 66 episodes in 55 participants on placebo and 8 episodes in 8 participants on fluconazole. The incidence rate for first occurrence of oesophageal candidiasis in the placebo arm dropped from 21.3 per 100 PYO before ART to 2.39 per 100 PYO after ART, but remained relatively constant in the fluconazole arm (table 3). Fluconazole also reduced oral and vaginal candidiasis (P<0.001) with a stronger effect before ART (table 3). The effect of fluconazole on oral candidiasis alone was greater before ART than after ART, although still statistically significant after initiating ART. The effect of fluconazole on vaginal candidiasis alone was similar before and after ART. The incidence of hospital admission or death did not differ between the two arms.
Safety
59 participants on placebo and 59 on fluconazole stopped trial drug because of safety concerns (Table 4). 115 participants had transaminases above 5x ULN (57 placebo, 58 fluconazole) and three had Stevens-Johnson syndrome (2 placebo, 1 fluconazole). Taking nevirapine as ART did not increase the risk of hepatotoxicity. In those on nevirapine, 27/522 (5.2%) and 34/541 (6.3%) taking fluconazole and placebo respectively stopped trial drug due to elevated transaminases. 54 women became pregnant; they were reviewed by an independent physician during pregnancy and infants by a paediatrician. There was no evidence of excess miscarriage (7/32 on placebo, 6/22 on fluconazole, P=0.65), stillbirth (0/13 vs. 1/8 live births, Fishers Exact P=0.38) or of fluconazole related abnormalities in live born babies. Severe (grade 4) anaemia did not differ between arms. Mild side effects attributed by the study physicians to the trial drug (including headache, nausea, and abdominal pain) were experienced by 259 participants (136 placebo, 123 on fluconazole). The loss to follow up (LTFUP) and withdrawal rates were higher in the fluconazole arm than in the placebo arm (table 4). Withdrawal occurred at a median of 83 days (IQR 26-174) and LTFUP at a median of 138 days (IQR 84-195); there was no difference between the arms in the timing of ART or the proportion who commenced ART. 26/50 LTFUP were subsequently located and known to be alive: a chance imbalance in movement from the trial area (10/31 LTFUP on fluconazole, 3/19 LTFUP on placebo) was a major contributor to the difference.
Table 4. Safety, toxicity and loss to follow up.
| Outcome | Placebo n=759 | Fluconazole n=760 | Unadjusted log rank P-value | aHR1 (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Event | rate | Events | rate | ||||
| Withdrawal of trial drug | Due to an adverse event | 59 | 9.9 | 59 | 9.6 | 0.00 (P=0.99) | 1.04 (0.72 –1.49) |
| Due to LFT<5ULN | 57 | 9.6 | 58 | 9.5 | 0.01 (P=0.93) | 1.02 (0.70-1.47) | |
| Due to other AE | 2 | 0.33 | 1 | 0.16 | 0.32 (P=0.57) | 1.99 (0.18-22.2) | |
| Pregnancy | 32 | 7.8 | 22 | 5.4 | 1.83 (P=0.18) | 1.44 (0.84-2.49) | |
| Serious adverse events | Life threatening | 53(49) | 8.29 | 49(42) | 7.62 | 0.16 (P=0.69) | 1.02 (0.69-1.51) |
| Anaemia (Grade 4) | 49 | 10.0 | 62 | 12.8 | 1.38 (P=0.24) | 0.79 (0.54 -1.15) | |
| Events resulting in disability | 9 | 1.38 | 5 | 0.76 | 1.17 (P=0.28) | 1.88 (0.63-5.65) | |
| Reported side effects | Nausea | 38 | 6.11 | 35 | 5.57 | 0.11 (P=0.74) | 1.09 (0.69 – 1.72) |
| Headache | 9 | 1.39 | 10 | 1.53 | 0.06 (P=0.81) | 0.89 (0.36 – 2.19) | |
| Abdominal pain | 25 | 3.91 | 22 | 3.42 | 0.21 (P=0.65) | 1.13 (0.64 – 2.01) | |
| Rash | 27 | 4.27 | 17 | 2.64 | 2.36 (P=0.12) | 1.59 (0.86 – 2.91) | |
| Other | 49 | 8.01 | 46 | 7.41 | 0.10 (P=0.75) | 1.07 (0.72 – 1.61) | |
| Participants reporting ≥1 SEs | 136 | 25.35 | 123 | 22.12 | 0.83 (P=0.36) | 1.12 (0.88 – 1.43) | |
| Loss to follow-up | 19 | 3.30 | 31 | 5.15 | 2.43 (P=0.12) | 0.60 (0.34 – 1.07) | |
| Withdrawal | 4 | 0.69 | 11 | 1.83 | 3.15 (P=0.076) | 0.36 (0.12 – 1.14) | |
Hazard ratio adjusted for baseline CD4 group and for before or after starting ART as time varying covariate.
Discussion
This trial showed fluconazole to be highly effective and safe in preventing invasive cryptococcal disease with a protective effect that occurred both before starting ART and in the first months of ART. The overall degree of protection (aHR 18.7) was much greater than that seen in the only other large RCT of azole prophylaxis9, but despite this, fluconazole prophylaxis had no effect on survival. The incidence of cryptococcal disease and number of cryptococcal events was lower than anticipated when the trial was designed; the rapid roll out of ART in Uganda was unexpected. Patients were randomised with CD4 counts below 200 cells/µL in the expectation that CD4 counts would drop during the trial. However, most patients started ART within three months of enrolment, reducing the time at risk of cryptococcal disease considerably; 774 (51.0%) patients never had a CD4 count below 100 cells/µL. The Trial Steering Group considered this issue during the trial but felt that reducing the CD4 entry criteria in the middle of the trial was not appropriate. In addition, randomising patients with a positive baseline CrAg was considered unethical; the study therefore excluded those with incipient cryptococcal disease or those at highest risk of developing cryptococcal disease.
The low incidence of cryptococcal disease and relatively low case fatality rate (7/19, 37%) due to intensive surveillance and rapid initiation of treatment meant that although there was a strong effect on cryptococcal specific mortality (0 vs. seven deaths), there was no impact on all cause mortality. In fact, only one Thai study (90 patients) has demonstrated a survival advantage in HIV infected patients from azole prophylaxis (HR 4.3. 95% confidence limits (0.9, 19.8) p =0.065); although there was a trend towards a reduction in cryptococcal disease with fluconazole, only two of the nine deaths on placebo were attributed to cryptococcal disease 20.
Fluconazole was safe in routine use: the incidence of grade 3 or 4 hepatic enzyme elevation was similar in the two arms. We found no evidence of hepatoxicity when fluconazole was coadministered with nevirapine, in keeping with other studies31. The safety of fluconazole at this prophylactic dose means that it could be used in clinics without laboratory support.
The trial population was representative of a rural sub-Saharan African setting and the findings were robust with similar baseline findings and ART treatment in each arm. Higher rates of cryptococcal disease have been described previously in Africa and case fatality rates reach 60% even when ART is available1–3. Both the proportion of patients accessing ART and the speed of access to ART were unusual in our study. In routine practice in Sub-Saharan Africa where ART access is often more limited or other continents with limited access, the benefits of fluconazole prophylaxis may be even greater. Cryptococcal events occurred no later than three months after ART initiation suggesting the need for a limited duration of prophylaxis once on ART. The reduction of oesophageal, oral and vaginal candidiasis is an additional benefit.
The results of this trial have considerable policy implications. Less than half those needing ART in sub-Saharan Africa currently access ART and low CD4 counts at presentation are common32, 33. Delays of several weeks before ART initiation often occur and are compounded by stock-outs34, 35. Up to 20% of early mortality on ART is due to cryptococcal disease in sub-Saharan Africa1. In this context, fluconazole prophylaxis “buys time” for the patient to start ART and then protect against cryptococcal disease until immune reconstitution occurs. Fluconazole prophylaxis is therefore of enormous potential benefit for individuals who are a) unable to access ART, b) waiting for ART or c) CrAg negative patients with low CD4 counts (<100 cells/µL) in the early stages of ART.
Studies have shown that a positive screening CrAg predicts a high risk of cryptococcal disease and mortality when starting ART 36 37. In South Africa, no-one with a negative CrAg measured shortly before ART initiation developed cryptococcal disease37; CrAg screening at ART initiation may be cost effective.38 Fluconazole primary prophylaxis is a complementary strategy. CrAg positivity identifies those at highest risk, but we have shown that CrAg negative patients may develop cryptococcal disease when there is a delay between CrAg screening and ART initiation. Cambodian data modelling suggested that screening was more cost-effective than prophylaxis if the CD4 count was higher than 50 cells/µL39 We believe that the relative benefit of screening or prophylaxis for those with CD4 counts <100 predominantly depends upon the delay before ART initiation.
Overall, our results provide substantial evidence to support current WHO recommendations that “where cryptococcal disease is common, antifungal prophylaxis with azoles should be considered for severely immuno-compromised people with HIV (WHO clinical stage 4 or CD4 < 100 cells/µL), whether on antiretroviral therapy or not.” 40, although our data suggest that WHO clinical stage 3 should be included. Fluconazole is a safe, well tolerated intervention that could be given in the community, improving quality of life by reducing candida infections and preventing cryptococcal disease in those waiting to access ART or in the early phase of ART.
Panel: Research in context
Systematic review. A Cochrane Review has examined azole primary prophylaxis against cryptococcal disease. The five studies comprised 1500 patients in total and included two small studies (219 patients) from Thailand, but none from Africa. Azole prophylaxis reduced the incidence of cryptococcal disease (RR 0.21) but did not affect mortality.
Interpretation. This study is the first to investigate the role of primary prophylaxis in Africa where the burden of cryptococcal disease is greatest and the first to include a large proportion of patients commencing antiretroviral therapy. Unlike previous studies, only those who were CrAg negative were included in the study. Fluconazole was highly effective in reducing the risk of cryptococcal disease both before and after initiation of ART. Recent data suggest that there is benefit in CrAg screening and treatment of CrAg positivity prior to ART. This study shows that fluconazole primary prophylaxis is a complementary strategy that can prevent the development of cryptococcal disease in those waiting for ART or those with low CD4 counts in the early stages of ART.
Acknowledgements
The Cryptococcal Trial Team comprised Freddie Kibengo, David Katende, Ivan Namakoola, Abu-BakerGgayi, Jane Margaret Amony, Christine Nagawa, Christine Musoke, Victor Nanono, Doreen Bamukama, Victoria Nabbona, Jane Muwanga, Stella Nakate, Grace Katooko, Rose Tindebywa, Stephen Nkayivu, Eunice Kajura, and Baker Kiyemba. Sebastian Kazibwe, Chadress Kabagenyi, Lubega Dennis, Anita Namagende, Jane Taban and Mike Mukasa.
Footnotes
Conflict of interest
None of the authors declare a conflict of interest
This research was supported by the Medical Research Council, UK and by a small grant from the Rockefeller Foundation. We would like to thank the trial participants, staff of TASO Masaka, Kitovu Mobile AIDS Organisation (especially Carla Symmons), Uganda Cares Masaka and Ministry of Health teams; Neil French and Charles Gilks for initial discussions; John Kissa for data management; Lieve Van der Paal and Henry Barigye for reviewing the pregnant women and infants; Peter Hughes for laboratory support and Christine Watera, Sebastian Owilla, Jessica Nakiyingi-Miiro and Jim Todd for help with randomisation.
Data and Safety Monitoring Committee: Andrew Nunn (chair), Silver Bahendeka, Rod Hay.
Trial Steering Committee Tim Peto (chair), David Denning, Elly Katabira, Jonathan Mermin.
End Point Review Committee Alison M. Elliott, Martin Nsubuga.
References
- 1.French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002 May 3;16(7):1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 2.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009 Feb 20;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 3.Kambugu A, Meya DB, Rhein J, O'Brien M, Janoff EN, Ronald AR, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008 Jun 1;46(11):1694–701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer A, Loyse A, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis. 2008 Jul 1;47(1):123–30. doi: 10.1086/588792. [DOI] [PubMed] [Google Scholar]
- 5.Rex JH, Larsen RA, Dismukes WE, Cloud GA, Bennett JE. Catastrophic visual loss due to Cryptococcus neoformans meningitis. Medicine (Baltimore) 1993 Jul;72(4):207–24. doi: 10.1097/00005792-199307000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Sloan D, Dlamini S, Paul N, Dedicoat M. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings. Cochrane Database Syst Rev. 2008;(4):CD005647. doi: 10.1002/14651858.CD005647.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Schaars CF, Meintjes GA, Morroni C, Post FA, Maartens G. Outcome of AIDS-associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazole. BMC Infect Dis. 2006;6:118. doi: 10.1186/1471-2334-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007 Jul 1;45(1):76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 9.Powderly WG, Finkelstein D, Feinberg J, Frame P, He W, van der Horst C, et al. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. N Engl J Med. 1995 Mar 16;332(11):700–5. doi: 10.1056/NEJM199503163321102. [DOI] [PubMed] [Google Scholar]
- 10.Smith DE, Bell J, Johnson M, Youle M, Gazzard B, Tchamouroff S, et al. A randomized, double-blind, placebo-controlled study of itraconazole capsules for the prevention of deep fungal infections in immunodeficient patients with HIV infection. HIV Med. 2001 Apr;2(2):78–83. doi: 10.1046/j.1468-1293.2001.00060.x. [DOI] [PubMed] [Google Scholar]
- 11.Nightingale SD, Cal SX, Peterson DM, Loss SD, Gamble BA, Watson DA, et al. Primary prophylaxis with fluconazole against systemic fungal infections in HIV-positive patients. Aids. 1992 Feb;6(2):191–4. doi: 10.1097/00002030-199202000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Ammassari A, Linzalone A, Murri R, Marasca G, Morace G, Antinori A. Fluconazole for primary prophylaxis of AIDS-associated cryptococcosis: a case-control study. Scand J Infect Dis. 1995;27(3):235–7. doi: 10.3109/00365549509019015. [DOI] [PubMed] [Google Scholar]
- 13.Cantey PT, Stephens DS, Rimland D. Prevention of cryptococcosis in HIV-infected patients with limited access to highly active antiretroviral therapy: evidence for primary azole prophylaxis. HIV Med. 2005 Jul;6(4):253–9. doi: 10.1111/j.1468-1293.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 14.Newton JA, Jr, Tasker SA, Bone WD, Oldfield EC, 3rd, Olson PE, Nguyen MT, et al. Weekly fluconazole for the suppression of recurrent thrush in HIV-seropositive patients: impact on the incidence of disseminated cryptococcal infection. Aids. 1995 Nov;9(11):1286–7. doi: 10.1097/00002030-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 15.McKinsey DS, Wheat LJ, Cloud GA, Pierce M, Black JR, Bamberger DM, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo-controlled, double-blind study. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin Infect Dis. 1999 May;28(5):1049–56. doi: 10.1086/514744. [DOI] [PubMed] [Google Scholar]
- 16.Havlir DV, Dube MP, McCutchan JA, Forthal DN, Kemper CA, Dunne MW, et al. Prophylaxis with weekly versus daily fluconazole for fungal infections in patients with AIDS. Clin Infect Dis. 1998 Dec;27(6):1369–75. doi: 10.1086/515018. [DOI] [PubMed] [Google Scholar]
- 17.Quagliarello VJ, Viscoli C, Horwitz RI. Primary prevention of cryptococcal meningitis by fluconazole in HIV-infected patients. Lancet. 1995 Mar 4;345(8949):548–52. doi: 10.1016/s0140-6736(95)90465-4. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MR, Fisher M, Cartledge J, Rogers T, Gazzard BG. The role of azoles in the treatment and prophylaxis of cryptococcal disease in HIV infection. Aids. 1994 May;8(5):651–4. doi: 10.1097/00002030-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Chang LW, Phipps WT, Kennedy GE, Rutherford GW. Antifungal interventions for the primary prevention of cryptococcal disease in adults with HIV. Cochrane Database Syst Rev. 2005;(3):CD004773. doi: 10.1002/14651858.CD004773.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P. A multicentre, randomized, double-blind, placebo-controlled trial of primary cryptococcal meningitis prophylaxis in HIV-infected patients with severe immune deficiency. HIV Med. 2004 May;5(3):140–3. doi: 10.1111/j.1468-1293.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 21.Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin Infect Dis. 2002 Jan 15;34(2):277–84. doi: 10.1086/338154. [DOI] [PubMed] [Google Scholar]
- 22.Ives NJ, Gazzard BG, Easterbrook PJ. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART)in a London clinic. J Infect. 2001 Feb;42(2):134–9. doi: 10.1053/jinf.2001.0810. [DOI] [PubMed] [Google Scholar]
- 23.Dromer F, Mathoulin-Pelissier S, Fontanet A, Ronin O, Dupont B, Lortholary O. Epidemiology of HIV-associated cryptococcosis in France (1985-2001): comparison of the pre- and post-HAART eras. Aids. 2004 Feb 20;18(3):555–62. doi: 10.1097/00002030-200402200-00024. [DOI] [PubMed] [Google Scholar]
- 24.Mirza SA, Phelan M, Rimland D, Graviss E, Hamill R, Brandt ME, et al. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992-2000. Clin Infect Dis. 2003 Mar 15;36(6):789–94. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 25.WHO, UNICEF, UNAIDS. Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. Progress Report. WHO; Geneva: 2008. pp. 1–140. [Google Scholar]
- 26.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. Aids. 2005 Nov 18;19(17):2050–2. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy KM, Morgan J, Wannemuehler KA, Mirza SA, Gould SM, Mhlongo N, et al. Population-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalence. Aids. 2006 Nov 14;20(17):2199–206. doi: 10.1097/QAD.0b013e3280106d6a. [DOI] [PubMed] [Google Scholar]
- 28.Cleves MA. ssa13: Analysis of multiple failure-time data with Stata. Stata Technical Bulletin. 1999;49:30–9. [Google Scholar]
- 29.Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt) 2008 Jan-Feb;17(1):47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 30.Pienaar ED, Young T, Holmes H. Interventions for the prevention and management of oropharyngeal candidiasis associated with HIV infection in adults and children. Cochrane Database Syst Rev. 2006;3:CD003940. doi: 10.1002/14651858.CD003940.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Manosuthi W, Athichathanabadi C, Uttayamakul S, Phoorisri T, Sungkanuparph S. Plasma nevirapine levels, adverse events and efficacy of antiretroviral therapy among HIV-infected patients concurrently receiving nevirapine-based antiretroviral therapy and fluconazole. BMC Infect Dis. 2007;7:14. doi: 10.1186/1471-2334-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 33.UNAIDS/WHO. AIDS epidemic update. 2009 http://dataunaidsorg/pub/Report/2009/JC1700_Epi_Update_2009_enpdf
- 34.Parkes-Ratanshi R, Bufumbo L, Nyanzi-Wakholi B, Levin J, Grosskurth H, Lalloo DG, et al. Barriers to starting ART and how they can be overcome: individual and operational factors associated with early and late start of treatment. Trop Med Int Health. 2010 Nov;15(11):1347–56. doi: 10.1111/j.1365-3156.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 35.Lawn SD, Campbell L, Kaplan R, Boulle A, Cornell M, Kerschberger B, et al. Time to Initiation of Antiretroviral Therapy Among Patients With HIV-Associated Tuberculosis in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2011 Mar 23; doi: 10.1097/QAI.0b013e3182199ee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007 Aug;12(8):929–35. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009 Apr 1;48(7):856–62. doi: 10.1086/597262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meya D, Manabe Y, Castelnuovo B, Cook B, Elbireer A, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;15(51(4)):448–55. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micol R, Tajahmady A, Lortholary O, Balkan S, Quillet C, Dousset JP, et al. Cost-effectiveness of primary prophylaxis of AIDS associated cryptococcosis in Cambodia. PLoS One. 2010;5(11):e13856. doi: 10.1371/journal.pone.0013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World, Health, Organisation. Essential prevention and care interventions for adults and adolescents living with HIV in resource limited settings. Geneva: WHO; 2008. pp. 20–3. [Google Scholar]