Abstract
The rhizome of Gastrodia elata (GE), a herb medicine, has been used for treatment of neuronal disorders in Eastern Asia for hundreds of years. Parishin C is a major ingredient of GE. In this study, the i.c.v. injection of soluble Aβ1–42 oligomers model of LTP injury was used. We investigated the effects of parishin C on the improvement of LTP in soluble Aβ1–42 oligomer–injected rats and the underlying electrophysiological mechanisms. Parishin C (i.p. or i.c.v.) significantly ameliorated LTP impairment induced by i.c.v. injection of soluble Aβ1–42 oligomers. In cultured hippocampal neurons, soluble Aβ1–42 oligomers significantly inhibited NMDAR currents while not affecting AMPAR currents and voltage-dependent currents. Pretreatment with parishin C protected NMDA receptor currents from the damage induced by Aβ. In summary, parishin C improved LTP deficits induced by soluble Aβ1–42 oligomers. The protection by parishin C against Aβ-induced LTP damage might be related to NMDA receptors.
KEY WORDS: Parishin C, Long-term potentiation, Neuroprotection, NMDA receptors, Ion channels
Graphical abstract
Parishin C given i.p. or i.c.v. prevented inhibitory effects of soluble Aβ1–42 oligomers on LTP induction. Soluble Aβ1–42 oligomers inhibited NMDAR currents without affecting AMPAR currents or voltage dependent currents. Parishin C pre-perfusion protected NMDAR currents from soluble Aβ1–42 oligomers.

1. Introduction
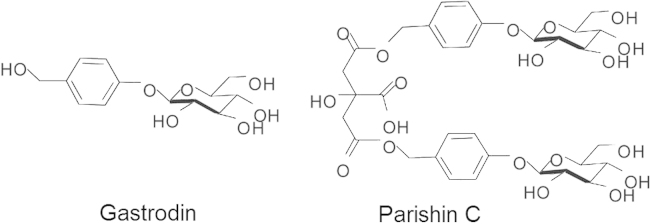
The rhizome of Gastrodia elata Blume (GE) is a traditional herb medicine which has been commonly used in Eastern Asia for centuries. It is widely used especially for treatment of headache, dizziness, epilepsy, stroke and dementia. GE has been reported to have therapeutic effects on animal models of Alzheimer׳s disease (AD), including improvements in spatial memory deficits and Aβ deposit in the hippocampus1. In clinical studies, the GE extract has been used to treat vascular dementia and showed improvement on cognition in the patients2. Parishin C is known as major component of GE3. As shown in Fig. 1, parishin C is bis-gastrodin citrates constituted of two gastrodin molecules esterified with two terminal carboxyl groups of citric acid. Previous studies demonstrated that parishins improved animal performances in a variety of cognitive-behavioral tests, such as step-down test, passive avoidance task and the Morris water maze task3, 4, 5. We found that parishin C was more potent than other parishins or gastrodin.
Figure 1.
Chemical structures of gastrodin and parishin C.
AD is the most common type of dementia in aging adults. Learning and memory declines progressively and can linger for many years. Beta-amyloid (Aβ) is one of the most important pathological features in AD patients and animal models, including Aβ plaques and soluble Aβ oligomers6. Recent studies focused on soluble Aβ oligomers, which were thought to correlate with disease progression better than insoluble fibrillary plaques7, 8, 9. To mimic symptoms of AD and investigate the effects of the parishin C, the soluble Aβ oligomers–induced cognitive deficits animal model was employed in our study.
Long-term potentiation (LTP) recording is a well-known and widely documented model for investigating the synaptic basis of learning and memory10, 11, 12. The induction of LTP is affected by changing activity of ion channels, including voltage-dependent ion channels such as Na+, Ca2+ and K+ channels as well as ionotropic glutamate receptors. Functional N-methy-d-aspartate receptors (NMDARs) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) are essential for LTP induction13, 14.
Therefore, in the present study we focused on the electrophysiological mechanisms of parishin C in protection of LTP after Aβ1–42 injection into the cerebral ventricle of rats. We presently performed LTP in vivo recordings and whole cell patch clamp recordings for voltage-dependent ion channels and NMDA receptors in cultured neurons.
2. Materials and methods
2.1. Animals
Male Wistar rats (240–260 g) were used in the LTP recordings. Rats were housed in a temperature- and light-controlled environment (23 ± 1 °C and 12 h light cycle), with free access to food and water. They arrived at least 3 days before the experiments and were handled carefully. All the experiment procedures were performed in accordance with the guidelines for the care and use of laboratory animals and were approved by the Animal Care Committee of Peking Union Medical College and Chinese Academy of Medical Sciences.
2.2. Drugs and materials
Parishin C was provided by Professor Jiangong Shi (HPLC purity >98%, Institute Meteria Medica). NMDA, MK-801, memantine and Aβ1–42 were purchased from Sigma–Aldrich Chemicals Inc. (St Louis, MO, USA). All drugs were dissolved in saline and freshly prepared before use.
2.3. Soluble Aβ1–42 oligomers preparation
Soluble Aβ1–42 oligomers were prepared as reported previously15, 16. Aβ1–42 was dissolved to 1 mmol/L with hexafluoroisopropanol (HFIP, Sigma), separated into aliquots, and was stored at –80 °C after evaporation of HFIP. Soluble Aβ1–42 oligomers were prepared freshly before use. The peptide was resuspended in DMSO to 5 mmol/L, and diluted by cold F12 medium to yield a 100 μmol/L stock solution, then incubated at 4 °C for 24 h. The preparation was then centrifuged at 14,000×g for 10 min at 4 °C. The supernatants were kept at 4 °C and further used for electrophysiological experiments. Solvents processed meanwhile were used as control.
2.4. Hippocampal neuron culture
Rat hippocampal neurons were isolated and cultured from Wistar rats on postnatal day 0–1 according to protocols modified from Kaech and Banker17. Hippocampi were isolated and incubated in trypsin (0.1%) for 30 min. Neurons were initially cultured in DMEM with 10% fetal bovine serum and 10% horse serum on poly-d-lysine coated glass coverslips. After 4 h the medium was removed and changed to Neurobasal-A with B27 medium (2%). Then culture medium was half-changed every 3 days. 10 μmol/L cytosine arabinoside (AraC) was added on day in vitro (DIV) 2, and removed on DIV 3. Whole cell recordings were performed between DIV 9–14.
2.5. LTP recordings in vivo
The rats were anesthetized with urethane (20%, 1.3 g/kg, i.p.) and then positioned in a stereotaxic frame. The skull was exposed and two holes were drilled for the placement of electrodes. A stainless bipolar stimulating electrode was placed in the perforant path (7.5 mm posterior to bregma, 4.2 mm lateral to the midline, 2.8–3.5 mm ventral). A recording electrode was placed in the dentate gyrus of the same side (3.8 mm posterior to bregma, 2.0 mm lateral to the midline, 3.0–3.5 mm ventral). A separate hole (1 mm posterior to bregma, 1.2 mm lateral to the midline, 3.5 mm ventral) was drilled to allow a guide cannula for intracerebroventricular (i.c.v.) injection of drugs or soluble Aβ1–42 oligomers. Silver wire was fixed to the bone or skin and used as reference and ground. The population spike (PS) was obtained from the dentate gyrus in response to stimulation in the perforant path at a frequency of 0.033 Hz with single constant current pulse (100 μs in duration). The PS was collected and amplified by TDT RA16PA amplifier and digitized by TDT RX7-5 processor (Tucker-Davis Technologies, USA). The depth of both electrodes was adjusted until the maximal response was observed. At that point, the intensity of the test stimuli was adjusted until it evoked about 40% of the maximum response of PS amplitude. LTP was induced by high frequency stimuli (HFS) delivered at 100 Hz, 10 stimuli, repeated 10 times at an interval of 300 ms. Pulse width stayed the same with the test stimuli, and intensity doubled.
Stable baseline PS responses were recorded for at least 15–20 min prior to drug applications. Soluble Aβ1–42 oligomers (1 nmol/L in 5 μL) or vehicle or drugs were injected i.c.v. at 1 μL/min. After injection, the cannula remained in place for 5 min before starting injections. Thus it took 10 min for injection of one drug. Volume of drugs or soluble Aβ1–42 oligomers was calculated based on the volume of rat cerebrospinal fluid, which was estimated about 500 μL according to previous reports18, 19.
2.6. Whole cell patch clamp recordings
Whole cell patch clamp recordings were performed using EPC-10 amplifier (HEKA Elektronik, German) at room temperature.
For sodium currents: the internal solutions contained (in mmol/L): CsF 140, NaCl 10, EGTA 1, HEPES 10 (300–310 mOsm with sucrose, pH 7.25). The external solution contained (in mmol/L): NaCl 140, MgCl2 1, CaCl2 1, CdCl2 0.2, HEPES 10, Glucose 10, 4-aminopyridine (4-AP) 5 (310–330 mOsm with sucrose, pH 7.4). After whole cell recordings were assessed, membrane potential was clamped at –80 mV. Sodium currents were activated by 50 ms pulse from –80 to +40 mV in 10 mV steps.
For potassium currents: the internal solutions contained (in mmol/L): KCl 140, EGTA 10, CaCl2 1, HEPES 10 (300–310 mOsm with sucrose, pH 7.25). The external solution contained (in mmol/L): Choline Cl 140, KCl 5, MgCl2 1, CaCl2 1, HEPES 10, Glucose 10 (310–330 mOsm with sucrose, pH 7.4). After whole cell recordings were assessed, membrane potential was clamped at –70 mV. For potassium currents activation, firstly holding potential was depolarized to –110 mV for 250 ms, then was applied 250 ms pulse from –40 to +60 mV in 10 mV steps.
For calcium currents: the internal solutions contained (in mmol/L): CsCl 130, EGTA 10, HEPES 10, ATP-Mg 5 (300–310 mOsm with sucrose, pH 7.25). The external solution contained (in mmol/L): Choline Cl 130, MgCl2 1, BaCl2 10, HEPES 10, Glucose 10, TTX 1, 4-AP 5 (310–330 mOsm with sucrose, pH 7.4). After whole cell recordings were assessed, membrane potential was clamped at –90 mV. Calcium currents were activated by 200 ms pulse from –50 to +50 mV in 10 mV steps.
For NMDAR and AMPAR currents: the internal solution contained (in mmol/L): K-gluconate 140, NaCl 10, CaCl2 1, EGTA 10, HEPES 10, ATP-Mg 5, GTP-Na 0.2 (310–320 mOsm with sucrose, pH 7.25 with KOH). The external solution contained (in mmol/L): NaCl 150, KCl 5, CaCl2 2, MgCl2, 1, Glucose 10, HEPES 10 (320–330 mOsm with sucrose, pH 7.4 with NaOH). 0.1 μmol/L TTX was added when used. Patch pipettes were pulled from glass capillaries with resistance 3–4 MΩ. The junction potential was close to 14 mV and corrected. After whole cell recordings were accessed the membrane potential was clamped at –70 mV. After membrane rupture, 5 min were allowed for equilibration between neuron and the internal solution. Currents were sampled at 10 kHz by Pulse v6.74 (HEKA). Series resistance and capacitance were monitored throughout the experiments. Data were discarded if series resistance and capacitance were changed by >20%. NMDAR and AMPAR currents were evoked by pressure application system. A glass capillary (tip diameter 2–5 μm) containing 100 μmol/L NMDA and 10 μmol/L glycine or 3 μmol/L AMPA was positioned at a distance of 10–20 μm to the somata of the neuron. A picospritzer (PV830, WPI, USA) was used to apply a pressure of 2–5 psi for 2 s duration to elicit currents at an interval of 2 min. Tetrodotoxin (TTX), soluble Aβ1–42 oligomers and other drugs were bath perfused.
2.7. Data analysis
For LTP recordings, PS amplitudes were analyzed using Matlab v7.1 (Mathworks) as previously reported18. Briefly, every data point was calculated as average of 10 PS amplitude values in 5 min. The baseline was calculated as average of PS amplitude 30 min before HFS, and each time point was calculated as percentage of the baseline values. LTP was induced successfully if PS amplitudes after HFS increased 30% than baseline. LTP values expressed here are those at 55–60 min after HFS, unless stated otherwise. NMDAR currents and AMPR currents were all normalized to the first current evoked and all results were shown in a relative way. All data were expressed as mean±SEM. Difference between groups was detected by two-way ANOVA with repeated measures in LTP recordings. Subsequently, the student׳s t test or one-way ANOVA was performed in other experiments. The results are statistically significant when P value was less than 0.05.
3. Results
3.1. Soluble Aβ1–42 oligomers inhibited NMDAR-dependent LTP induction
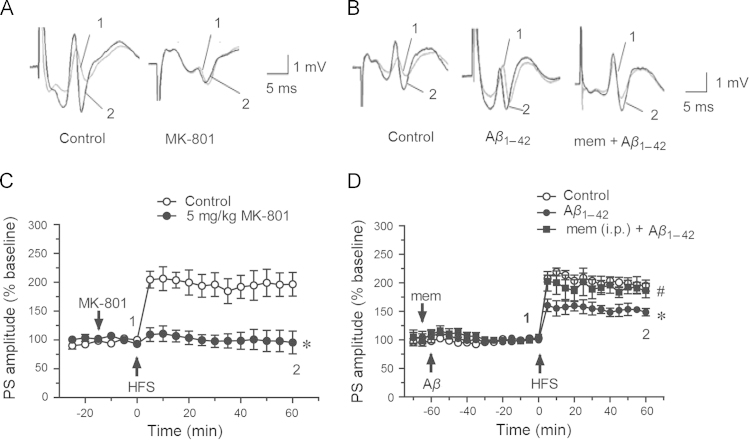
The rats were anesthetized and then positioned in a stereotaxic frame. The population spike (PS) was obtained from the dentate gyrus in response to stimulation in the perforant path. LTP was induced by high frequency stimuli (HFS, described before) successfully and it could be abolished by 5 mg/kg MK-801 (i.p., 95.7±19.7% vs. control 196.7±20.5%, P<0.05, n=4–5, Fig. 2A and 2C). MK-801 is a noncompetitive blocker of NMDA receptors20. This indicated that the LTP from the perforant pathway to the dentate gyrus (PP-DG) was NMDAR-dependent. Soluble Aβ1–42 oligomers were given i.c.v. in rats 60 min before HFS as shown in Fig. 2D, which significantly inhibited LTP induction (148.7±6.5% vs. control group 195.1±9.6%, P<0.05, n=5, Fig. 2B and 2D). Memantine (10 mg/kg, i.p.), an open channel uncompetitive inhibitor of NMDA receptors21, (clinically used for treatment of AD) given before Aβ injection significantly ameliorated Aβ-induced inhibition of LTP (186.6±12.6% vs. Aβ1–42 group 148.7±6.5%, P<0.05, n=5, Fig. 2B and 2D). These results suggested that Aβ-induced inhibition of LTP was related to NMDA receptors.
Figure 2.
Memantine rescued inhibition of LTP induced by soluble Aβ1–42 oligomers in rats. (A) and (B) showed original traces of PS before (1) and after (2) HFS. (C) 5 mg/kg MK-801 abolished HFS induced LTP (control group n=4; MK-801 group n=5). (D) 2 μmol/L soluble Aβ1–42 oligomers significantly inhibited LTP (vs. control group, P< 0.05), and 10 mg/kg memantine administrated i.p. in advance rescued this inhibition (control group n=7, Aβ1–42 group n=5, memantine group n=5). Data were shown as mean ± SEM, ⁎P<0.05 vs. control group, #P< 0.05 vs. Aβ1–42 group.
3.2. Parishin C ameliorated the suppression of LTP induced by Aβ1–42
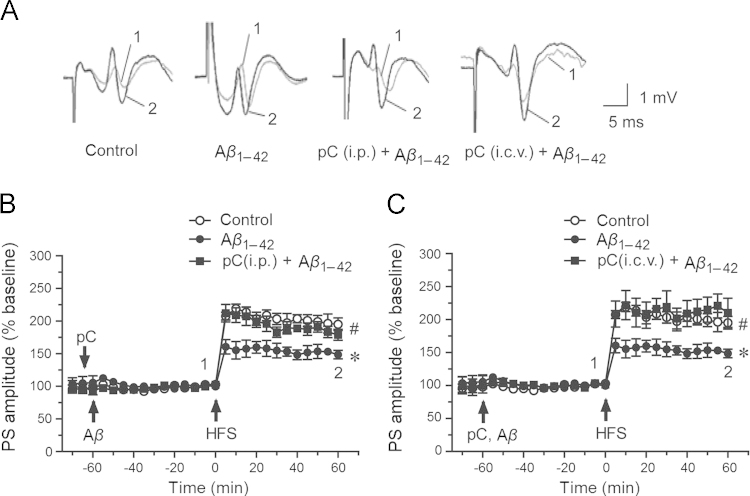
Parishin C 20 mg/kg was given i.p. two days consecutively and then given a third time before the LTP recordings as shown in Fig. 3A and 3B. Attenuation of LTP mediated by soluble Aβ1–42 oligomers was significantly ameliorated by parishin C (179.0±8.4% vs. Aβ1–42 group 148.7±6.5%, P<0.05, n=5). Furthermore, parishin C 10 μmol/L (given i.c.v. followed by Aβ1–42 injection via i.c.v.) also effectively protected Aβ1–42 induced reduction of LTP (210.2±22.1% vs. Aβ1–42 group 148.7±6.5%, P<0.05, n=5, Fig. 3C). The results indicated parishin C had protective effects against Aβ-induced damage of LTP.
Figure 3.
Effects of parishin C on inhibition of LTP induced by soluble Aβ1–42 oligomers in rats. (A) showed original traces of PS before (1) and after (2) HFS in four groups. (B) Parishin C 20 mg/kg was given i.p. for two days before LTP recordings and was given i.p. 10 min before i.c.v. injection of 2 μmol/L soluble Aβ1–42 oligomers. (C) Parishin C 10 μmol/L was given i.c.v. before 2 μmol/L soluble Aβ1–42 oligomers injection. HFS was performed 60 min after injection of soluble Aβ1–42 oligomers in all experiments. Parishin C improved LTP induction significantly after Aβ treatment (P < 0.05). Data were shown as mean ± SEM, control group n=7, Aβ1–42 group n=5, pC (i.p.) group n=5, pC (i.c.v.) group n=5, ⁎P < 0.05 vs. control group, #P<0.05 vs. Aβ1–42 group.
3.3. Effects of parishin C on ion channels on normal cultured rat hippocampal neurons
The above results demonstrated that parishin C is a potent compound for improving LTP deficits caused by Aβ. Further mechanisms of parishin C on LTP and relevant receptors were studied. In LTP recordings, PS amplitudes prior to HFS represented the basic synaptic transmission. The data showed that 20 mg/kg parishin C given i.p. did not influence either basic synaptic transmission or LTP induction in normal rats (Fig. 4A).
Figure 4.

Parishin C had no effects on normal LTP and ion channels. (A) Upper traces of PS before (1) and after (2) HFS in two groups. Parishin C 20 mg/kg given i.p. 30 min before HFS did not influence the baseline and LTP induction in normal rats (control group n=8, pC group n=7). (B) Perfusion of 10 μmol/L parishin C for 10 min had no effects on AMPAR currents in primary cultured hippocampal pyramidal neurons. The bars above current recordings show pressure injection of 1 s, 3 μmol/L AMPA (n=3). (C) Perfusion of 10 μmol/L parishin C for 10 min had no effects on NMDAR currents in primary cultured hippocampal pyramidal neurons. The bars above current recordings show pressure injection of 1 s, 100 μmol/L NMDA and 10 μmol/L glycine (n=5). Data were shown in mean±SEM. The amplitudes of all currents were normalized to the first evoked at –70 mV.
NMDA receptors and AMPA receptors are the main glutamate receptors responsible for excitatory postsynaptic ion channel currents, and they are essential for basic synaptic transmission and LTP formation14, 22. Our results showed that parishin C at 10 μmol/L had no influence on either of these ion channel currents in hippocampal pyramidal neurons (Fig. 4B and C).
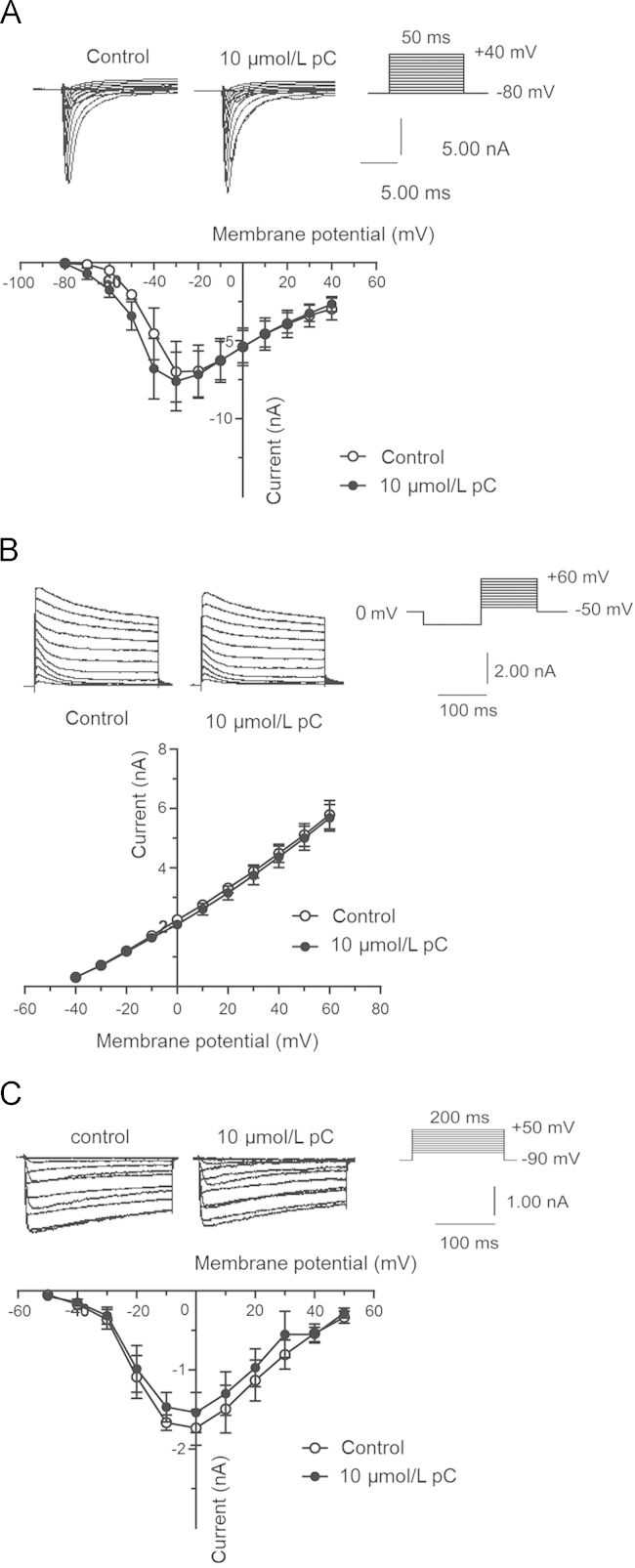
The effects of parishin C on voltage-dependent channels were also studied. At 10 μmol/L, parishin C had no effect on the currents of sodium channels, potassium channels or calcium channels of neuronal cells (Fig. 5A–C). The I–V (current and voltage relationship) curves obtained before and after parishin C treatment did not differ for each channel. These results demonstrated that parishin C did not affect the major voltage-dependent ion channels under normal conditions.
Figure 5.
10 μmol/L parishin C had no effects on voltage-dependent currents in cultured hippocampal neurons of rats. (A) parishin C (10 μmol/L) had no effects on voltage-dependent total sodium currents (n = 5), holding potential =–80 mV. (B) parishin C (10 μmol/L) had no effects on voltage-dependent total outward potassium currents (n = 3), holding potential = –70 mV. (C) parishin C (10 μmol/L) had no effects on voltage-dependent total calcium currents (n = 3), holding potential =–90 mV. Data are shown in mean ± SEM.
3.4. Parishin C rescued attenuation of Aβ1–42 on NMDAR currents
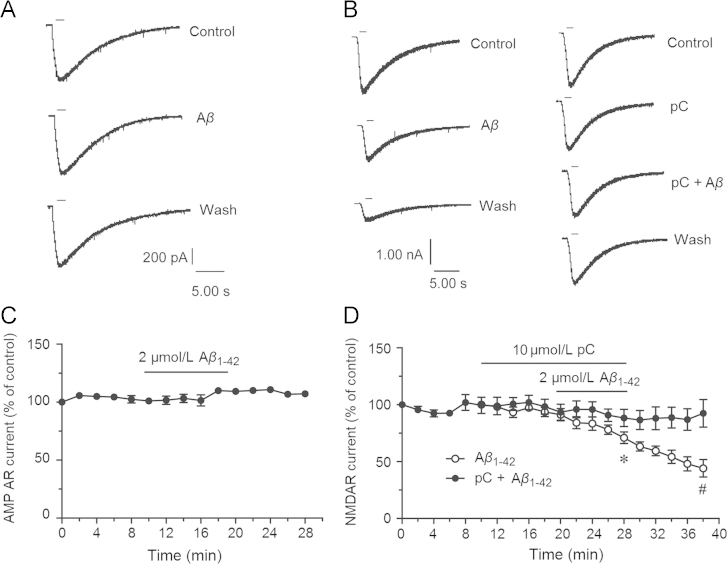
The above results showed that Aβ reduced the activity of NMDAR-dependent LTP but had no effects on PS before HFS. Parishin C also had no effects on AMPAR currents and NMDAR currents, while parishin C protected against the effects of Aβ. The results demonstrated that the mechanism of parishin C might be related to interactions between Aβ and NMDA receptors. Then we studied the effects of Aβ on AMPAR and NMDAR currents in primary cultured hippocampal pyramidal neurons as well as the role of parishin C in this system. As shown in Fig. 6A and C, perfusion of 2 μmol/L Aβ1–42 oligomers did not influence AMPAR currents. However 2 μmol/L soluble Aβ1–42 oligomers reduced NMDAR currents to 71.0±5.0% of control (vs. before perfusion 93.6±4.0%, n=5, P<0.05, Fig. 6B and D). The currents did not recover during washout, and continued to decline to 44.1±7.1% (vs. Aβ1–42 group, P<0.05, Fig. 6D). In the following experiments, parishin C was perfused 10 min in advance and continued during Aβ1–42 perfusion (Fig. 6B and D). The NMDAR currents inhibited by Aβ1–42 were attenuated by parishin C. Since parishin C had no effects on NMDAR currents (Fig. 4C and 6D), these results suggested that parishin C protected NMDA receptors from the damage of Aβ, and subsequently protected against LTP suppression.
Figure 6.
Parishin C protected against inhibition of NMDAR currents by 2 μmol/L soluble Aβ1–42 oligomers but not AMPAR currents in primary cultured hippocampal neurons. Holding potential was –70 mV and external solution was with 0.1 μmol/L TTX, and without Mg2+. (A) Bath perfusion of 2 μmol/L soluble Aβ1–42 oligomers for 10 min had no effects on AMPAR currents. Short bar means pressure injection of 3 μmol/L AMPA. (B) Bath perfusion of 2 μmol/L soluble Aβ1–42 oligomers for 10 min inhibited NMDAR currents, and the inhibition continued during wash. Parishin C (10 μmol/L) perfusion 10 min beforehand prevented Aβ’s inhibitory effects. Short bar meant pressure injection of 100 μmol/L NMDA and 10 μmol/L glycine. (C) The AMPAR currents were not changed during soluble Aβ1–42 oligomers perfusion (n=3). (D) Soluble Aβ1–42 oligomers significantly inhibited NMDAR currents but it was rescued by perfusion of parishin C (Aβ1–42 group n=5, pC+ Aβ1–42 group n=6). ⁎P<0.05 vs. currents before perfusion, #P<0.05 vs. Aβ1–42 group. Data are mean±SEM. The amplitudes of all currents were normalized to the first evoked at –70 mV.
4. Discussion
GE is widely used in China and many Asian countries as a traditional drug and food supplement for improving learning and memory as well as protection of brain function. Recently, it was found that an active fraction isolated from the crude extract of GE contained parishin, parishin B and parishin C, improved the performance of rodents in scopolamine-induced cognitive deficits3, 4, 5. In the present study we aimed at the pharmacological effects of parishin C on cognitive disorders and LTP impairment induced by Aβ, as well as the electrophysiological mechanisms related to NMDA receptors.
In accordance with behavioral tests3, 4, 5, our results indicated that parishin C is a potent agent for improving learning and memory. Parishin C given i.p. had no effects on normal rats, but it protected LTP deficits induced by soluble Aβ1–42 oligomers. Parishin C (10 μmol/L) delivered directly into the brain via i.c.v. also restored the LTP inhibition by soluble Aβ1–42 oligomers. This indicated that parishin C might cross brain blood barrier to protect the central nervous system. In our previous studies, we investigated dose-dependent effects of parishin C on scopolamine mediated inhibition of LTP in vivo. In this study, the high dose (20 mg/kg) of parishin C was selected to observe the electrophysiological mechanisms. The patch clamp recordings indicated that the nootropic effects of parishin C might be involved in protection of NMDA receptors from the injury of Aβ.
Although the mechanisms of AD are not well understood, Aβ is believed to be centrally involved. Compared with Aβ plaques, soluble Aβ oligomers correlate more strongly with AD-type dementia7. Previous reports demonstrated that soluble Aβ oligomers impaired functions of synapse23, 24. AMPA and NMDA receptors are both important glutamate receptors in CNS synapses, and both are required in learning and memory procession and LTP formation25, 26, 27. NMDA receptor activation needs both glutamate and membrane depolarization, in order to reverse Mg2+ block25. AMPAR currents are the main form of spontaneous and fast excitatory synaptic transmission, while NMDA receptors are essential for induction of LTP14. Thus in our research, firstly, we demonstrated that LTP in our system was NMDA-receptor-dependent, and that the inhibitory effects of Aβ might be related to NMDA receptors. Secondly, Aβ had no effects on baseline PS, but significantly inhibited LTP induction. Thirdly, acute Aβ perfusion had no effects on AMPAR currents, but it irreversibly inhibited NMDAR currents. Results in vivo and in vitro are identical, which indicate that acute Aβ administration modulates NMDA receptors and inhibits LTP induction.
In the following, whether soluble Aβ1–42 oligomers–induced impairment of LTP could be prevented by parishin C was investigated. Parishin C neither had effects on basic synaptic transmission, nor on LTP levels in normal rats. It also had no effects on NMDAR currents or AMPAR currents, or voltage-dependent channels in normal cultured hippocampal neurons. However, parishin C attenuated soluble Aβ1–42 oligomers–induced inhibition of LTP given via i.p. and i.c.v. Furthermore, it was interesting that the Aβ1–42-induced reduction of NMDAR currents in primary cultured hippocampal neurons, but did not affect the AMPAR currents at the same concentration. Parishin C obviously prevented Aβ-induced attenuation of NMDAR currents. Therefore we suggested that actions of parishin C might be related to NMDA receptors. It may decrease the toxicity of soluble Aβ1–42 oligomers on the receptors. Soluble Aβ1–42 oligomers–induced dysfunction of NMDA receptors might involve various pathways. For example, Aβ disrupts calcium homeostasis28, and influences phosphorylation of NMDA receptors, especially on NR2A and NR2B subunits18, 29, 30. In addition, Aβ might down-regulate surface expression of NMDA receptors in the synapse31. NMDA receptors have been suggested as an important target of Aβ32, 33, and they are closely related to the inhibition of LTP34, 35, 36, 37, 38. In our study we used NMDAR antagonist d-APV, NMDA receptor subtype NR2A antagonist NVP-AAM077 and NR2B antagonist Ro 25-6981. Parishin C could not reverse inhibitory effects of APV, NVP-AAM077 or Ro 25-6981 (data not shown). Aβ and parishin C might bind to specific sites on the receptors other than the binding sites of APV, NVP-AAM077 and Ro 25-6981. It is possible that parishin C binds to NMDA receptors and prevents Aβ binding to the receptors.
The reports of AMPA receptors in AD were conflict. Tu et al.33 showed that the expression of AMPA receptors was down-regulated in AD. Tozzi et al.39 found that AMPA receptors were not changed while the NMDAR/AMPAR ratio decreased in hippocampus in AD animals. In our study, soluble Aβ1–42 oligomers were perfused to the cells during the experiments and it did not change the amplitude of AMPAR currents. It has been known that basic synaptic transmissions are mainly mediated by AMPA receptors. In our studies MK-801 did not influence baseline, but blocked the potentiation induced by HFS. A previous report showed that AMPAR currents decreased after incubation of neurons with Aβ for three days40. Another study showed the effects of Aβ25--35 on AMPA receptors in an experiment with brain slices 41. Therefore, different experimental conditions and Aβ forms might affect the expression and function of AMPA receptors.
The effects of parishin C might be attributed to its molecular structure, which is a long chain. This might allow parishin C to be associated with targeted protein(s) more efficiently than gastrodin. This structural advantage could explain its potent pharmacological effects. Taking together, parishin C might bind to NMDA receptors and inhibit the binding and injury of Aβ to the receptors. Further research about the mechanisms of parishin C are needed.
5. Conclusions
We studied the effects of parishin C on LTP deficits induced by soluble Aβ1–42 oligomers. Inhibition of ion channel currents by Aβ which were mediated by NMDA receptors could be prevented by parishin C. Therefore, parishin C could be a potent compound for treating neuronal degenerative diseases.
Acknowledgments
This study has been granted by the National Nature Science Foundation of China (No. 81373387), National Major Special Project on New Drug Innovation of China (No. 2012ZX09301002-004).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Huang G.B., Zhao T., Muna S.S., Jin H.M., Park J.I., Jo K.S. Therapeutic potential of Gastrodia elata Blume for the treatment of Alzheimer׳s disease. Neural Regen Res. 2013;8:1061–1070. doi: 10.3969/j.issn.1673-5374.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu A.H., Tian J.Z., Zhong J., Yang C.Z., Shi J., Yin J.X. A clinical study on a randomized, double-blind control of Chinese medicine granules in treatment of vascular dementi. China J Chin Mater Med. 2006;31:1722–1725. [PubMed] [Google Scholar]
- 3.Xu WJ, Tan XC, Zhang M, Zhao LM, Liu Y, Shi JG, et al., inventor; Beijing Kelaibo Pharmaceutical Development, assignee. Gastrodia elata plant extract for preventing senile dementia and its preparing method. China Patent CN200510128785. 2007 June 13
- 4.Wang K, Shi JG, Zhao LM, Zhao DL, Zou DC, Zhang M, et al., inventor; Beijing Collab Pharma Co. Ltd., assignee. Application of Gastrodiaelata blume parishin extractive in preparation of medicament for protecting brain. China Patent CN102727506A. 2012 October 17
- 5.Wang K, Shi JG, Liu Y, Zhao LM, Zhang M, Chen MX, inventor; Beijing Collab Pharma Co. Ltd., assignee. Gastrodiaelata blume plant extract for preventing and treating vascular dementia and preparation method thereof. China Patent CN101628086A 2010 January 20
- 6.Selkoe D.J. Normal and abnormal biology of the β-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- 7.McDonald J.M., Savva G.M., Brayne C., Welzel A.T., Forster G., Shankar G.M. The presence of sodium dodecyl sulphate−stable Aβ dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira S.T., Klein W.L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer׳s disease. Neurobiol Learn Mem. 2011;96:529–543. doi: 10.1016/j.nlm.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean C.A., Cherny R.A., Fraser F.W., Fuller S.J., Smith M.J., Beyreuther K. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer׳s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Teyler T.J., DiScenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- 11.Bliss T.V.P., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 12.Lynch M.A. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 13.Rison R.A., Stanton P.K. Long-term potentiation and N-methyl-d-aspartate receptors: foundations of memory and neurologic disease? Neurosci Biobehav Rev. 1995;19:533–552. doi: 10.1016/0149-7634(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 14.Song I., Huganir R.L. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 15.Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knobloch M., Farinelli M., Konietzko U., Nitsch R.M., Mansuy I.M. Aβ oligomer–mediated long-term potentiation impairment involves protein phosphatase 1–dependent mechanisms. J Neurosci. 2007;27:7648–7653. doi: 10.1523/JNEUROSCI.0395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaech S., Banker G. Culturing hippocampal neurons. Nat Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- 18.Li P.P., Wang W.P., Liu Z.H., Xu S.F., Lu W.W., Wang L. Potassium 2-(1-hydroxypentyl)-benzoate promotes long-term potentiation in Aβ1–42-injected rats and APP/PS1 transgenic mice. Acta Pharmacol Sin. 2014;35:869–878. doi: 10.1038/aps.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai Y.L., Smith P.M., Lamm W.J., Hildebrandt J. Sampling and analysis of cerebrospinal fluid for chronic studies in awake rats. J Appl Physiol Respir Environ Exerc Physiol. 1983;54:1754–1757. doi: 10.1152/jappl.1983.54.6.1754. [DOI] [PubMed] [Google Scholar]
- 20.Kovacic P., Somanathan R. Clinical physiology and mechanism of dizocilpine (MK-801): electron transfer, radicals, redox metabolites and bioactivity. Oxid Med Cell Longev. 2010;3:13–22. doi: 10.4161/oxim.3.1.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H.S., Pellegrini J.W., Aggarwal S.K., Lei S.Z., Warach S., Jensen F.E. Open-channel block of N-methyl-d-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bashir Z.I., Alford S., Davies S.N., Randall A.D., Collingridge G.L. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- 23.Lacor P.N., Buniel M.C., Chang L., Fernandez S.J., Gong Y., Viola K.L. Synaptic targeting by Alzheimer׳s-related amyloid β oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein W.L. Synaptic targeting by Aβ oligomers (ADDLS) as a basis for memory loss in early Alzheimer׳s disease. Alzheimers Dement. 2006;2:43–55. doi: 10.1016/j.jalz.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Rao V.R., Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Bassani S., Folci A., Zapata J., Passafaro M. AMPAR trafficking in synapse maturation and plasticity. Cell Mol Life Sci. 2013;70:4411–4430. doi: 10.1007/s00018-013-1309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cain D.P. LTP, NMDA, genes and learning. Curr Opin Neurobiol. 1997;7:235–242. doi: 10.1016/s0959-4388(97)80012-8. [DOI] [PubMed] [Google Scholar]
- 28.Alberdi E., Sánchez-Gómez M.V., Cavaliere F., Pérez-Samartín A., Zugaza J.L., Trullas R. Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Wu G.M., Hou X.Y. Oligomerized Aβ25--35 induces increased tyrosine phosphorylation of NMDA receptor subunit 2A in rat hippocampal CA1 subfield. Brain Res. 2010;1343:186–193. doi: 10.1016/j.brainres.2010.04.055. [DOI] [PubMed] [Google Scholar]
- 30.Rönicke R., Mikhaylova M., Rönicke S., Meinhardt J., Schröder U.H., Fändrich M. Early neuronal dysfunction by amyloid β oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol Aging. 2011;32:2219–2228. doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Snyder E.M., Nong Y., Almeida C.G., Paul S., Moran T., Choi E.Y. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 32.Danysz W., Parsons C.G. Alzheimer׳s disease, β-amyloid, glutamate, NMDA receptors and memantine–searching for the connections. Br J Pharmacol. 2012;167:324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu S., Okamoto S., Lipton S.A., Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer׳s disease. Mol Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S.M., Jin M., Koeglsperger T., Shepardson N.E., Shankar G.M., Selkoe D.J. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q.W., Rowan M.J., Anwyl R. β-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci. 2004;24:6049–6056. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roselli F., Tirard M., Lu J., Hutzler P., Lamberti P., Livrea P. Soluble β-amyloid1–40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J Neurosci. 2005;25:11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsen K.M., Sheng M. NMDA receptors and BAX are essential for Aβ impairment of LTP. Sci Rep. 2012;2:225. doi: 10.1038/srep00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rammes G., Hasenjager A., Sroka-Saidi K., Deussing J.M., Parsons C.G. Therapeutic significance of NR2B-containing NMDA receptors and mGluR5 metabotropic glutamate receptors in mediating the synaptotoxic effects of β-amyloid oligomers on long-term potentiation (LTP) in murine hippocampal slices. Neuropharmacology. 2011;60:982–990. doi: 10.1016/j.neuropharm.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 39.Tozzi A., Sclip A., Tantucci M., de Iure A., Ghiglieri V., Costa C. Region- and age-dependent reductions of hippocampal long-term potentiation and NMDA to AMPA ratio in a genetic model of Alzheimer׳s disease. Neurobiol Aging. 2015;36:123–133. doi: 10.1016/j.neurobiolaging.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Gu Z.L., Liu W.H., Yan Z. β-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shemer I., Holmgren C., Min R., Fülöp L., Zilberter M., Sousa K.M. Non-fibrillar β-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur J Neurosci. 2006;23:2035–2047. doi: 10.1111/j.1460-9568.2006.04733.x. [DOI] [PubMed] [Google Scholar]