Abstract
In this study, we examined the effect of progesterone on histopathologic and functional outcomes of intracerebral hemorrhage (ICH) in 10–12-month-old mice. Progesterone or vehicle was administered by intraperitoneal injection 1 hour after collagenase-induced ICH and then by subcutaneous injections at 6, 24, and 48 hours. Oxidative and nitrosative stress were assayed at 12 hours post-ICH. Injury markers were examined on day 1, and lesion was examined on day 3. Neurologic deficits were examined for 28 days. Progesterone posttreatment reduced lesion volume, brain swelling, edema, and cell degeneration and improved long-term neurologic function. These protective effects were associated with reductions in protein carbonyl formation, protein nitrosylation, and MMP-9 activity and attenuated cellular and molecular inflammatory responses. Progesterone also reduced VEGF expression, increased neuronal-specific Na+/K+ ATPase α3 subunit expression, and reduced PKC-dependent Na+/K+ ATPase phosphorylation. Furthermore, progesterone reduced glial scar thickness, myelin loss, brain atrophy, and residual injury volume on day 28 after ICH. With multiple brain targets, progesterone warrants further investigation for its potential use in ICH therapy.
Keywords: Progesterone, neuroprotective effects, inflammatory response, neurologic function, intracerebral hemorrhage
1. Introduction
Intracerebral hemorrhage (ICH), a common and devastating subtype of stroke, accounts for 15–20% of all strokes and affects more than 2 million people worldwide each year (Adeoye and Broderick, 2010, Poon, et al., 2015, Wang, 2010). With limited therapeutic options, ICH is associated with high morbidity and poor prognosis. It causes perihematomal edema, elevations in intracranial pressure, and neurologic deficits (Keep, et al., 2014). Hematoma formation, expansion, and mass effect cause the primary damage, whereas inflammatory response and oxidative stress contribute to the progression of secondary injury (Wang, 2010, Wu, et al., 2010). Because outcomes are poor, it is worth exploring approaches that can ameliorate the detrimental effects of neuroinflammation and improve functional recovery after ICH.
We and others have revealed that inflammatory mediators cyclooxygenase (COX)-1, COX-2, prostaglandin receptors, high-mobility group box 1 (HMGB1), and interleukin 1β (IL-1β), may play major roles in ICH-induced secondary injury (Han, et al., 2015, Mracsko and Veltkamp, 2014, Wang, 2010, Wu, et al., 2016, Wu, et al., 2015, T. Wu, et al., 2011). A recent clinical trial reported that patients treated in the acute stage of ICH with celecoxib, a selective COX-2 inhibitor, had a smaller expansion of perihematomal edema than controls (Lee, et al., 2013). Increased release of HMGB1 and IL-1β after ICH may boost inflammatory reaction in the early stage by promoting secretion of other chemotactic factors and adhesion molecules from the vascular endothelium. These factors can lead to the activation of glial cells and early infiltration of neutrophils and macrophages to the injury lesion (Li, et al., 2015, Wu, et al., 2015). Infiltrating polymorphonuclear neutrophils that accumulate within and around the lesion release toxic substances such as matrix metalloproteinase (MMP)-2 and MMP-9, and promote further inflammatory damage (Wang, 2010, Wang and Dore, 2007b, Xue and Yong, 2008). Inflammatory response also enhances the release of reactive oxygen species (ROS) that lead to cell death and brain tissue damage after ICH (Wang, 2010, Wang and Dore, 2007b). In addition to activation of MMP-2 and MMP-9 released from inflammatory cells, increased expression of vascular endothelial growth factor (VEGF) and decreased activity of Na+/K+ ATPase (NKA) also play a role in brain edema formation after stroke (Keep, et al., 2014, Won, et al., 2014, Zan, et al., 2014). Therefore, modulation of inflammatory response after ICH could offer a promising therapeutic approach to ICH.
Progesterone, primarily a sex hormone, has been shown to exert neuroprotective effects by alleviating inflammatory response and improving neurologic function after ischemic stroke (Jiang, et al., 2011, Jiang, et al., 2009, Jiang, et al., 2016, Won, et al., 2015). To our knowledge, no study has reported a therapeutic effect of progesterone after ICH in middle-aged mice. Therefore, we investigated whether progesterone administered after ICH provides neuroprotection and improves long-term neurologic outcome for ICH. Because a 12-month-old mouse is equivalent to a person of 58 years (www.age-converter.com/mouse-age-calculator.html), middle age in mice equates to 10–15 months (Dutta and Sengupta, 2015). Therefore, to enhance the clinical relevance, we used 10–12-month-old mice, as ICH occurs more often in middle-aged and elderly individuals. For early outcomes of ICH, we evaluated inflammatory response, oxidative stress, neuronal death, brain injury volume, brain swelling, and brain edema in the acute phase after ICH; for long-term outcomes of ICH, we evaluated astrogliosis (glial scar thickness), myelin loss, brain atrophy, and residual lesion volume on day 28. Neurologic deficits were assessed on days 1, 3, 7, 14, 21, and 28 post-ICH.
2. Materials and Methods
2.1 Animals, ICH model, and treatment regimen
All animal procedures were performed in accordance with the National Institutes of Health and institutional guidelines for animal research under a protocol approved by the Animal Care and Use Committee of Johns Hopkins University. One hundred and thirty middle-aged male C57BL/6 mice weighing 25 to 35 g (10–12 months old) were used and were given free access to food and water throughout the study. Animal experiments were reported in accordance with the ARRIVE guidelines.
The procedure for inducing ICH by collagenase injection in mice has been established in our laboratory (Chang, et al., 2014, Zhao, et al., 2015). Briefly, mice were anesthetized by isoflurane (3.0% for induction, 1.0% for maintenance) and ventilated with oxygen-enriched air (20%:80%) via a nose cone. To induce hemorrhage, we injected the left caudate putamen of mice with collagenase VII-S (0.075 U in 0.5 μL saline, Sigma-Aldrich, St Louis, MO) at the following stereotactic coordinates: 0.8 mm anterior and 2.0 mm lateral of the bregma, 2.9 mm in depth. Collagenase was delivered over 5 minutes, and the needle was left in place for an additional 10 minutes to prevent any reflux. Rectal temperature was maintained at 37.0 ± 0.5°C throughout the experimental and recove ry periods. The rectal temperature and percent change in body weight of each mouse was recorded as previously described (Wu, et al., 2015).
Computer-generated random numbers were used for randomization of mice into three study groups: sham-operated group, vehicle-treated ICH group, and progesterone-treated ICH group. Progesterone (8 mg/kg, Sigma-Aldrich) or vehicle [22.5% 2-hydroxypropylcyclodextrin (Sigma-Aldrich)] was injected intraperitoneally at 1 hour after surgery and subcutaneously at 6, 24, and 48 hours (Wali, et al., 2014). Investigators blinded to the treatment groups evaluated outcomes in all mice and performed data analysis.
2.2 Brain lesion volume, swelling, and atrophy
Mice (n=10–11 per group) were euthanized after neurologic examination on day 3 or 28 post-ICH. The entire brain of each mouse was cut with a cryostat into 50-μm sections at 10 rostral-caudal levels that were spaced 360 μm apart. Sections were stained with Luxol fast blue (LFB, for myelin) and Cresyl Violet (for neurons) before being quantified for grey- and white-matter injury with SigmaScan Pro software (version 5.0.0 for Windows; Systat, San Jose, CA). The lesion volume in cubic millimeters was calculated by multiplying the section thickness by the damaged area, as determined by the lack of specific staining (Wang, et al., 2003).
Brain swelling (n=10–11 mice/group) was quantified by calculating the percentage of hemispheric enlargement on day 3 after ICH (H. Wu, et al., 2011). Hemisphere enlargement (%) was expressed as: [(ipsilateral hemisphere volume − contralateral hemisphere volume) / contralateral hemisphere volume] × 100%.
Brain atrophy (tissue loss, n=12 mice/group) was quantified on day 28 after ICH according to the formula: (contralateral hemisphere volume ipsilateral hemisphere volume) / contralateral hemisphere volume ×100% (Wu, et al., 2015).
2.3 Brain water content
Mice (n=6 per group) were anesthetized and decapitated on day 3 after ICH as previously described (Jiang, et al., 2009, Zhu, et al., 2014) for determination of brain water content. Briefly, cerebral tissue was divided into two hemispheres. We immediately dissected and weighed the ipsilateral and contralateral striatum and cerebellum (which served as an internal control) with an electronic analytical balance to obtain the wet weight. Then, after brain samples were dried at 100°C in an electric blast drying oven for 24 hours, we obtained the dry weight. The percentage of brain water content was calculated as: (wet weight − dry weight) / wet weight × 100%.
2.4 Neurologic deficit assessment
The modified neurologic severity score (mNSS) was used to assess the neurologic deficits of mice on days 1, 3, 7, 14, and 28 after ICH or sham surgery (n=12 mice/group) (Jiang, et al., 2013, Wang and Dore, 2007a, Zhao, et al., 2015). The mNSS comprises body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling. Neurologic deficit was graded on a scale of 0–4 for each test, establishing a maximum deficit score of 24.
2.5 Gelatin gel zymography
The MMP-2 (gelatinase A, 72 kD) and MMP-9 (gelatinase B, 98 kD) proteolytic activities in hemorrhagic brain were measured with gelatin gel zymography on day 1 post-ICH as previously described (Wang and Tsirka, 2005). Briefly, the brains were collected, and the hemorrhagic hemispheres were sonicated in ice-cold homogenization buffer (20 mM Tris, 1 mM EGTA, 1 mM EDTA, 10% sucrose, pH 7.4) containing protease inhibitors. Protein samples were obtained by centrifugation at 1,000 rpm for 10 minutes at 4°C. Samples were loaded onto 10% Tris-tricine gels with 0.1% gelatin as a substrate, and separated by electrophoresis. Next, the gel was renatured and incubated with development buffer at 37°C for 48 hours. After development, the gel was stained with 0.5% wt/vol Coomassie blue R-250 for 2 hours and then destained appropriately to be photographed. MMP-2/9 activity was measured by optical density and quantified as fold increase compared with sham controls using NIH Image J software (n=5 mice for each treatment group, and n=3 mice for sham group).
2.6 Immunofluorescence
Anesthetized mice underwent transcardial perfusion with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M PBS on day 3 or 28 after ICH (n=6 mice/group). The brains were removed and postfixed in 4% paraformaldehyde at 4°C overnight and then placed in 15%, 20%, and 30% sucrose consecutively until they sank. Coronal brain sections of 10 μm thickness were obtained with a freezing microtome (Leica, Germany) and were kept at −20°C. Immunofluorescence was carried out as described previously (Wang and Dore, 2008, T. Wu, et al., 2011). Sections were blocked in 5% bovine serum albumin for 60 minutes at room temperature and then were incubated with rabbit anti-ionized calcium-binding adapter molecule 1 (Iba-1, microglial marker; 1:200; Wako Chemicals, Richmond, VA), rabbit anti-myeloperoxidase (MPO, neutrophil marker; 1:300; Dako, Carpinteria, CA), or rabbit anti-glial fibrillary acidic protein (GFAP, astrocyte marker; 1:500; Dako) at 4°C overnight. After being washed three times with PBS, the sections were incubated with AlexaFluor 488-conjugated goat anti-rabbit IgG (1:1500; Molecular Probes, Eugene, OR) or AlexaFluor 594-conjugated goat anti-rabbit IgG (1:1500; Molecular Probes) for 60 minutes at room temperature. The sections were rinsed three times for 5 minutes each with PBS. Stained sections were examined with a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). In selected sections with similar lesion areas, the numbers of cells immunoreactive for Iba1, MPO, or GFAP at 12 locations per mouse were quantified and averaged as positive cells per square millimeter (4 comparable fields per section and 3 sections per mouse) in the perihematomal brain region (Zhao, et al., 2015). We defined microglia and/or macrophages as activated if the cells were spherical, amoeboid, or rod-like in appearance; had a diameter of more than 7.5 μm in at least one direction, with short, thick processes; and exhibited intense Iba1 immunoreactivity. Resting microglia/macrophages were characterized by small cell bodies (<7.5 μm in diameter) with long processes and weak Iba1 immunoreactivity (Wang, et al., 2008). We express the results as positive cells per square millimeter.
2.7 Fluoro-Jade B Staining
Fluoro-Jade B (FJB) histochemical staining was performed on day 3 after ICH as we described previously to quantify degenerating cells (n=6 mice per group) (Wu, et al., 2012, H. Wu, et al., 2011). Stained sections were examined with a fluorescence microscope, and images were captured and analyzed by SPOT image software (Diagnostic Instruments Inc., Sterling Heights, MI). Areas with large blood vessels were avoided. The FJB+ cells were quantified with the same strategy as described above for immunoreactive cells.
2.8 Western blotting
Five brains from each treatment group and three brains from the sham group were used for measurement of protein expression at 12 hours and 1 day post-ICH. The brains were carefully removed and placed in chilled saline. Total protein was isolated from the hemorrhagic hemisphere by lysing the tissue with Laemmli buffer containing 2% SDS, 10% glycerol, 2% 2-mercaptoethanol, and 0.002% bromphenol blue in 75 mM Tris-HCl. The samples were heated to 95°C for 10 minutes before being separated on 10% Tris/glycine/SDS acrylamide gels (Bio-Rad, Hercules, CA). The proteins were subsequently transblotted onto polyvinylidene difluoride membranes (Millipore, Bedford, MA) and blocked for 2 hours at room temperature in 5% dry milk. The immunoblots were incubated for 2 hours at 37°C with rabbit anti-COX-1 (1:500; Cayman Chemical, Ann Arbor, MI), rabbit anti-COX-2 (1:5000; Cayman Chemical), rabbit anti-HMGB1 (1:1000, Abcam, Cambridge, MA), rabbit anti-IL-1β (1:1000, Millipore, Temecula, CA, USA), mouse anti-VEGF (1:500, Santa Cruz Biotechnology, Dallas, TX), mouse anti-NKA α1 (1:10,000; Sigma-Aldrich) or NKA α3 (1:10,000; Affinity Bioreagents, Golden, CO), rabbit anti-phospho NKA pSer23 or NKA pSer943 (1:1,000; Santa Cruz Biotechnology), or mouse anti-nitrotyrosine (1:40,000; Millipore). β-Actin protein served as a loading control. After three washes with Tris-buffered saline/0.05% Tween-20, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibody (Santa Cruz Biotechnology) for 1 hour at 37°C. Protein signal was visualized with the SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and detected with an imaging system. Sigma scan software (Scion Corp., Frederick, MD) was used for densitometric analysis of bands. Data were expressed as fold change over the loading control. Background values were subtracted, and multiple blots were combined for statistical analysis.
2.9 Protein Oxidation Assay
An OxyBlot protein oxidation detection kit (Millipore) for protein carbonyl groups was used to determine the oxidative modification of proteins in striatum at 12 hours after ICH, as described previously (n=5 mice for each treatment group, and n=3 for sham group) (Wu, et al., 2015, Zhao, et al., 2015).
2.10 White Matter Damage and Myelin Loss
We stained brain sections used for immunofluorescence with LFB to evaluate white matter damage on day 28 by measuring the amount of myelin in LFB-stained intact myelin tracts, as previously demonstrated (n=12 mice/group) (Chen, et al., 2011, Wu, et al., 2012). Light microscopy was used at the same exposure level to analyze three different sections from each mouse. At least three comparable white-matter fields from the middle of the corpus striatum were selected from each section. The areas covered by the LFB stain were quantified with ImageJ software, averaged, and expressed as a percentage of the total area of the white matter examined.
2.11 Statistical Analysis
Sample sizes were determined with a power analysis based on the results of one of our previous studies (Wu, et al., 2015). We used a power of 0.9 and a significance level of 0.05. A chi-square test was used for analyzing mortality. The power analysis showed that 8 mice per group would be sufficient to detect a significant difference in corrected lesion volume on day 3 after collagenase-induced ICH. For behavior studies, we calculated the sample size based on difference in means and standard deviation of the means of the neurologic deficit score on day 28 after ICH reported in our previous study. The results revealed that 10 completed mice in each group would enable us to detect a significant difference in neurologic deficit on day 28 after collagenase-induced ICH.
Parametric data are expressed as mean ± SD. Student’s t-test was applied for comparisons of the difference between two groups. The statistical comparisons among multiple groups were made by using 1-way analysis of variance (ANOVA) followed by Bonferroni correction. Repeated ANOVA followed by Bonferroni correction was used for detection of changes in rectal body temperature, body weight and neurologic deficit score between treatment groups over time. Differences were considered statistically significant at p < 0.05.
3. Results
3.1 Effect of progesterone on mortality, rectal temperature, and body weight
In the collagenase-induced ICH model, the mortality of progesterone-treated mice (13.33%, 6 of 45) was not different from that of vehicle-treated mice (9.30%, 4 of 45, p>0.05). Progesterone treatment did not affect rectal body temperature at any time during the research period (Supplementary Fig. 1A). Mouse body weight was decreased compared with baseline during the first week after ICH. Percent change in body weight from baseline did not differ between progesterone-treated mice and vehicle-treated mice on days 1, 3, 7, 14, and 21 after ICH (Supplementary Fig. 1B, n=12 mice/group, all p>0.05), but on day 28, weight gain in progesterone-treated mice was significantly greater than that of vehicle-treated mice (p<0.05).
3.2 Effect of progesterone on brain lesion volume, brain swelling, and edema
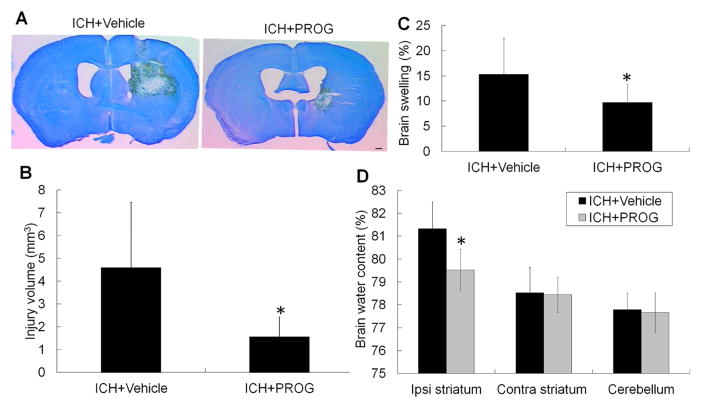
Brain lesions were identified by lack of color on sections stained with LFB/Cresyl Violet (Fig. 1A). The brain lesion volume comprised the hematoma, perihematomal edema, and surrounding damaged grey matter and white matter tracts. Progesterone treatment reduced brain lesion volume compared with that in the vehicle-treated group on day 3 after ICH (p<0.05, n=10–11 mice/group; Fig. 1B).
Fig. 1.
Progesterone (PROG) decreases brain lesion volume and edema on day 3 after collagenase-induced ICH. (A) Representative images of Luxol fast blue/Cresyl Violet-stained brain sections on day 3 after collagenase-induced ICH. The area of the lesion is indicated by a lack of staining; scale bar = 2 mm. (B) Brain lesion volume was measured on Luxol fast blue/Cresyl Violet-stained brain sections. Quantification analysis revealed that brain lesion volume was smaller in the progesterone-treated group than in the vehicle-treated group 3 days after ICH (n=10–11 mice/group, *p<0.05). (C) Progesterone post-treatment reduced brain swelling after collagenase-induced ICH compared with that of the vehicle-treated group (n=10–11 mice/group, *p<0.05). (D) Progesterone post-treatment reduced brain water content in the ipsilateral striatum after collagenase-induced ICH compared with that of the vehicle-treated group (n=6 mice/group, *p<0.05). All values are means ± SD. Ipsi, ipsilateral; Contra, contralateral.
Because swelling contributes to brain damage and death after severe stroke, we also measured the percentage of hemispheric enlargement to evaluate brain swelling. Progesterone treatment significantly reduced brain swelling on day 3 after ICH compared with that in the vehicle-treated group (n=10–11 mice/group, p<0.05; Fig. 1C). We also measured the brain water content in the ipsilateral and contralateral striatum and cerebellum. As with brain swelling, brain water content in the ipsilateral striatum was significantly less in the progesterone-treated group than in the vehicle-treated group (p<0.05; n=6 mice/group, Fig. 1D).
3.3 Effect of progesterone on neurobehavioral deficits
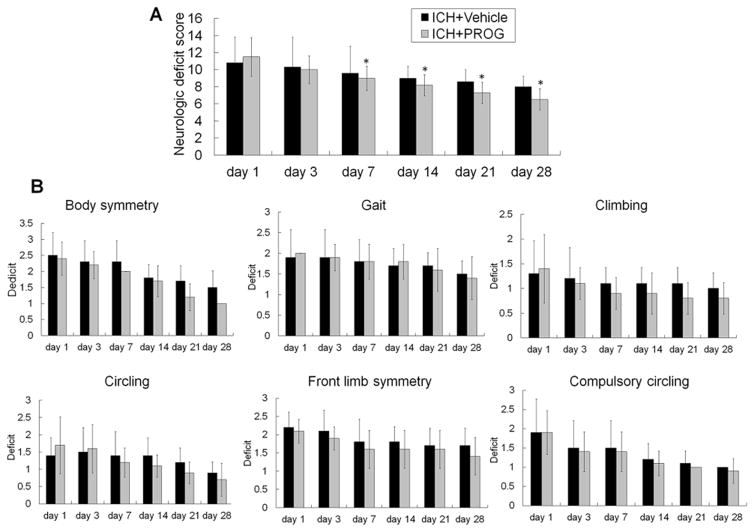
To examine whether reduced brain injury results in improved neurologic function, we assessed neurologic deficit score at baseline and on days 1, 3, 7, 14, 21, and 28 after ICH. Mice treated with progesterone after ICH had lower neurologic deficit scores than did vehicle-treated mice from days 7 to 28 after ICH (n=12 mice/group, all p<0.05; Fig. 2). However, although an obvious trend was apparent, neurologic deficit scores for the individual tests were not significantly different between the treatment and vehicle groups (all p>0.05).
Fig. 2.
Progesterone (PROG) post-treatment ameliorates neurologic deficits after collagenase-induced ICH. (A) Progesterone post-treatment improved the neurologic function of mice on days 7, 14, 21, and 28 after ICH compared with that of the vehicle-treated group (n=12 mice/group, *p<0.05). (B) Neurologic deficit scores for each of the individual tests on days 1, 3, 7, 14, 21, and 28 after ICH (n=12 mice/group, all p>0.05). Values are means ± SD.
3.4 Effect of progesterone on oxidative and nitrosative stress
Protein carbonyl formation and 3-nitrotyrosine immunoreactivity were used as markers of oxidative and nitrosative stress (Zhao, et al., 2015). The levels of carbonylated and nitrosylated proteins were significantly increased in the ICH brain at 12 hours after ICH, but were significantly lower in the progesterone-treated group than in the vehicle-treated group (p<0.05, n=5 mice for each treatment group, and n=3 for sham group; Fig. 3A and B).
Fig. 3.
Progesterone (PROG) decreases oxidative stress and MMP-9 activity after collagenase-induced ICH. Immunoblotting analysis showed that progesterone decreased protein carbonyl (A) and 3-nitrotyrosine (B) immunoreactivity on multiple protein bands at 12 hours after ICH. (C) Representative gel showing relative activity of MMP-2 and MMP-9 on day 1 after ICH in brain lysates from sham and ICH mice post-treated with vehicle or progesterone. Bar graphs show the quantitative analysis of nitrotyrosine (A, 14 to 191 kDa), carbonyls (B, 29 to 98 kDa), and MMP-2 and MMP-9 activity (C) from each group (n=5 mice for each treatment group, and n=3 mice for sham group). All data are shown as mean ± SD. *p<0.05 vs. ICH+vehicle.
3.5 Effect of progesterone on MMP-9 activity
Gelatin zymograms revealed that the activity of pro-MMP-9 and pro-MMP-2 increased significantly in vehicle-treated mice on day 1 after ICH and that progesterone treatment significantly reduced the activity of pro-MMP-9, but not that of pro-MMP-2, when compared with that of vehicle-treated mice (p<0.05, n=5 mice for each treatment group, and n=3 mice for sham group; Fig. 3C).
3.6 Effect of progesterone on microglial/macrophage activation, astrocytic activation, neutrophil infiltration, and neuronal death
Iba-1 immunofluorescence labeling was used to detect the effect of progesterone treatment on microglial/macrophage activation after ICH (Wang and Dore, 2007a). Microglia/macrophages were classified as either resting or activated by using a combination of morphological criteria and a cell body diameter cutoff of 7.5 μm (H. Wu, et al., 2011). The number of activated microglia/macrophages in the perihematomal region on day 3 after ICH was significantly reduced in mice treated with progesterone (p<0.01; n=6 mice/group, Fig. 4A and E).
Fig. 4.
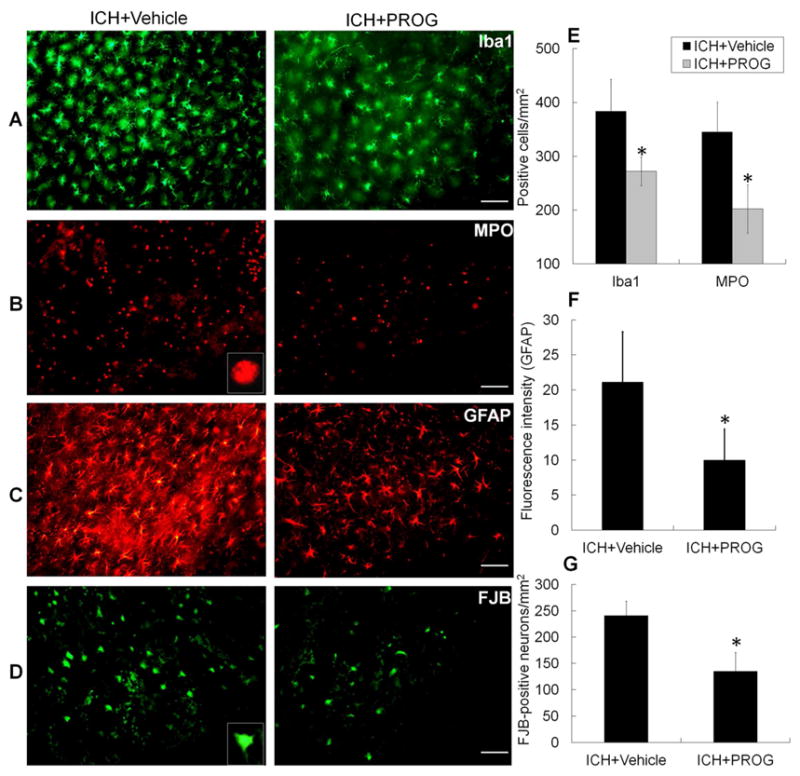
Progesterone (PROG) reduces microglial/macrophage and astrocytic activation, neutrophil infiltration, and Fluoro-Jade B (FJB)+ cell number after collagenase-induced ICH. (A–C) Immunostaining for Iba1 (A), myeloperoxidase (MPO; B), and glial fibrillary acidic protein (GFAP; C) in the perihematomal region on day 3 after collagenase-induced ICH. Inset represents higher magnification of MPO-positive neutrophil. Scale bar = 50 μm. (D) FJB histological staining of degenerating cells in sections collected 3 days after collagenase injection. Inset represents higher magnification of FJB-positive cell. Scale bar = 25 μm. (E–G) Bar graphs show quantification analysis of activated microglia/macrophages and astrocytes, infiltrating neutrophils, and FJB-positive cells (n=6 mice/group, *p<0.01 or 0.05 versus vehicle-treated group). Values are means ± SD.
Neutrophil infiltration occurs after activation of microglia/macrophages and contributes to early hemorrhagic brain injury after ICH (Moxon-Emre and Schlichter, 2011, Wang, 2010, Wang and Tsirka, 2005). MPO-immunoreactive neutrophils were evident in the hemorrhagic striatum on day 3 after ICH. An inset in Fig. 4B shows higher magnification of an MPO-positive neutrophil. Progesterone treatment reduced their number when compared with that in vehicle-treated mice (p<0.05; n=6 mice/group, Fig. 4B and E).
GFAP immunofluorescence labeling was used to examine the effect of progesterone treatment on astrocyte reactivity. Reactive astrocytes in the peri-ICH area had more GFAP-immunoreactive processes than did resting astrocytes on the contralateral side. Additionally, the immunoreactivity was more intense and the processes were longer and thicker (Wu, et al., 2012). Progesterone treatment reduced the fluorescence intensity of GFAP in the perihematomal area on day 3 after ICH (p<0.05; n=6 mice/group, Fig. 4C and F).
Cell degeneration was assessed with FJB staining. We found that progesterone treatment significantly reduced the number of FJB+ cells in the perihematomal region on day 3 after ICH (p<0.01; n=6 mice/group, Fig. 4D and G).
3.7 Effect of progesterone on the expression of inflammatory factors
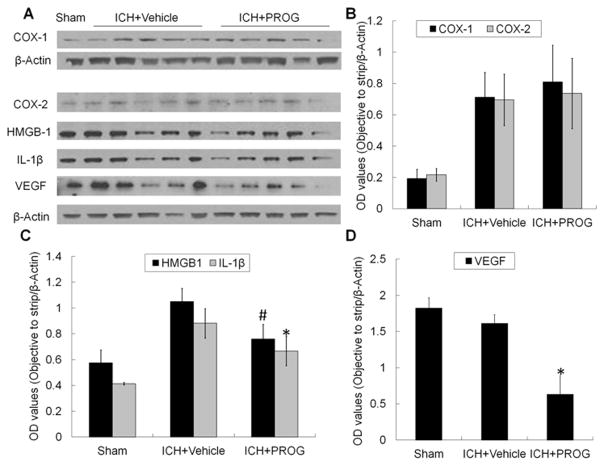
Using Western blot analysis, we examined the effect of progesterone on COX-1, COX-2, HMGB1, and IL-1β expression in the acute stage of ICH. Progesterone administration did not change COX-1 or COX-2 expression on day 1 compared with that in vehicle-treated ICH mice. However, it significantly reduced expression of HMGB1 (p<0.05) and IL-1β (p<0.01) in the ipsilateral striatum (n=5 mice per treatment group, and n=3 mice for sham group; Fig. 5A–C).
Fig. 5.
Progesterone (PROG) decreases HMGB-1, IL-1β, and VEGF expression on day 1 after collagenase-induced ICH. (A) Representative Western blot showing relative protein expression of COX-1, COX-2, HMGB-1, IL-1β, and VEGF in brain lysates from sham and ICH rats post-treated with vehicle or progesterone. β-Actin was used as a loading control (n=5 mice for each treatment group, and n=3 for sham group). (B–D) Bar graphs show the quantitative analysis of COX-1, COX-2, HMGB-1, IL-1β, and VEGF expression from each group (n=5 mice for each treatment group, and n=3 mice for sham group, #p<0.05 or *p<0.01 versus vehicle-treated group). Values are means ± SD.
3.8 Effect of progesterone on VEGF and NKA activity
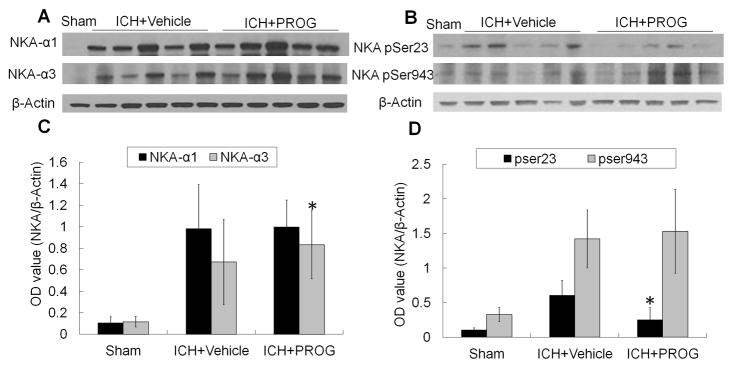
Studies have shown that an increase in VEGF expression and downregulation of NKA activity are associated with the aggravation of brain injury (Jiang, et al., 2014, Yang, et al., 2012). The activity of NKA in brain can be downregulated by phosphorylation of the α subunit at Ser23 by PKC or Ser943 by PKA (Yang, et al., 2012). Changes in NKA expression in the ICH brain have not been reported. Immunoblotting results showed that the expression of NKA α1 subunit and PKA-dependent phosphorylation at Ser943 did not change significantly in progesterone-treated ICH mice compared with that in vehicle-treated ICH mice on day 1 (p>0.05, Fig. 6). However, progesterone significantly reduced the expression of VEGF and PKC-dependent phosphorylation of NKA at Ser23 and increased the expression of NKA α3 subunit on day 1 after ICH when compared with that of vehicle-treated ICH mice (p<0.01; n=5 mice for each treatment group, and n=3 mice for sham group; Fig. 5A and D and Fig. 6A–D).
Fig. 6.
Progesterone (PROG) increases protein expression of Na+/K+ ATPase (NKA) α3, but decreases protein expression of NKA pSer-23 on day 1 after collagenase-induced ICH. (A and B) Representative Western blots showing relative protein expression of NKA α1 and NKA α3 (A), and NKA pSer23 and NKA pSer943 (B) in brain lysates from sham-operated and ICH rats post-treated with vehicle or progesterone. β-Actin was used as a loading control. (C and D) Bar graphs show the quantitative analysis of NKA α1, NKA α3, NKA pSer23, and NKA pSer943 expression from each group (n=5 mice for each treatment group, and n=3 mice for sham group, *p<0.01 versus vehicle-treated group). Values are means ± SD.
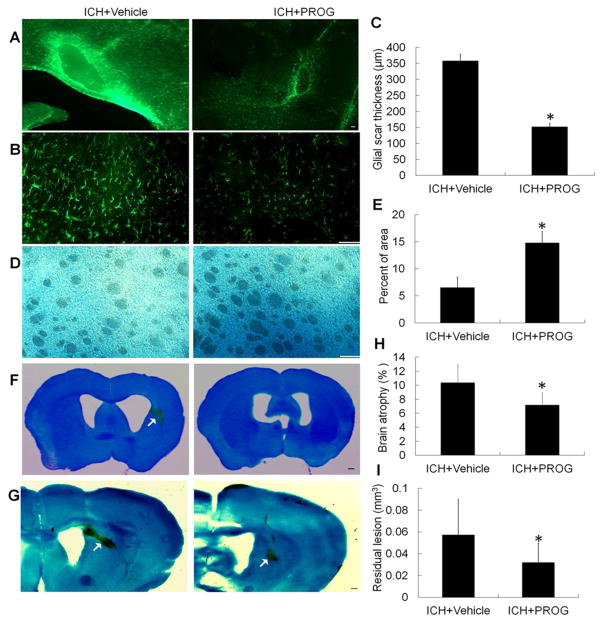
3.9 Effect of progesterone on astrogliosis, myelin loss, brain atrophy, and residual lesion volume
After ICH, astrocytes undergo morphologic changes known as astrogliosis (Qin, et al., 2015). We examined the astrocytic proliferation around the lesion site with immunostaining of GFAP on day 28 after ICH (Fig. 7A and B). Progesterone significantly decreased astrogliosis (glial scar thickness) in ICH mice when compared with that in vehicle-treated ICH mice (p<0.01, Fig. 7C). We also used LFB to label normal myelin (Chen, et al., 2011, Zhao, et al., 2015). Progesterone treatment reduced the loss of LFB-stained myelin in the perihematomal region on day 28 after ICH (n=12 mice/group, p<0.01, Fig. 7D and E). It also significantly reduced brain atrophy and residual lesion volume on day 28 after ICH (both p<0.01, n=12 mice/group, Fig. 7F–I).
Fig. 7.
Progesterone (PROG) post-treatment decreases glial scar thickness (astrogliosis), myelin loss, brain atrophy, and lesion volume on day 28 after collagenase-induced ICH. (A and B) Immunostaining for glial fibrillary acidic protein in the perihematomal region on day 28 after collagenase-induced ICH; scale bar = 50 μm. (C) Quantitative analysis of glial scar thickness (n=12 mice/group, *p<0.01 vs. ICH + vehicle). (D) Immunostaining with Luxol fast blue in the perihematomal region on day 28 after collagenase-induced ICH; scale bar = 50 μm. (E) Quantitative analysis of white matter damage (n=12 mice/group, *p<0.01 vs. ICH + vehicle). (F and G) Representative images of Luxol fast blue/Cresyl Violet-stained brain sections on day 28 after collagenase-induced ICH; scale bar = 2 mm. (H and I) Bar graphs show the quantitative analysis of brain atrophy (H) and lesion volume (I) from each group (n=12 mice/group). Quantitative data are shown as mean ± SD. *p<0.01 vs. ICH + vehicle.
4. Discussion
We and others have shown that progesterone is neuroprotective and might be a useful therapeutic agent for traumatic brain injury (TBI) (Howard, et al., 2015), ischemic stroke injury (Jiang, et al., 2016, Won, et al., 2015), and spinal cord injury (Garcia-Ovejero, et al., 2014). To our knowledge, this is the first study to test the efficacy of progesterone in a clinically relevant ICH model, in which the hematoma develops over several hours. We found that progesterone treatment beginning 1 hour after ICH reduced brain injury volume, brain swelling, brain edema, and the number of the FJB+ degenerating cells and improved long-term neurologic recovery. These changes were associated with decreases in protein oxidation, protein nitrosylation, and MMP-9 activity; alleviation of cellular and molecular inflammatory responses (microglial/macrophage and astrocyte activation, neutrophil infiltration, HMGB1 and IL-1β expression); and decreases in VEGF and NKA pSer23 expression, but increases in NKA α3 expression. In addition, we showed that progesterone treatment reduced astrogliosis/glial scar formation, myelin loss, brain atrophy, and residual lesion. Furthermore, progesterone increased body weight on day 28 but did not increase mortality rate or core body temperature. Together, these results suggest that progesterone has anti-inflammatory and anti-oxidant effects, reduces MMP-9 activity, and inhibits NKA pSer23 expression, which may lead to reductions in secondary brain injury and improvements in functional recovery after ICH.
Accumulating evidence suggests that inflammation is an important contributor to secondary brain injury after ICH (Keep, et al., 2012, Zhou, et al., 2014). ICH results in a rapid and robust cellular inflammatory response characterized in part by activation of resident microglia and astrocytes and infiltration of neutrophils and macrophages that release proinflammatory cytokines, chemokines, ROS, prostaglandins, proteases, ferrous iron, and other immunoreactive molecules (Hwang, et al., 2011, Wang, 2010). Therefore, inflammation may contribute to blood-brain barrier (BBB) breakdown and brain edema (Wang, 2010). Conversely, BBB disruption also contributes to inflammation by promoting leukocyte and macrophage infiltration, which exacerbates vasogenic edema after ICH (Hwang, et al., 2011, Wang, 2010). It is known that perihematomal edema increases by approximately 75% in the first 24 hours after ICH, peaks around 5–6 days, lasts for up to 14 days, and is associated with adverse outcomes (Gebel, et al., 2002, Hwang, et al., 2011). In this study, we showed that progesterone treatment reduces brain lesion volume and brain swelling/edema early after ICH, likely by reducing cellular inflammatory responses from microglia and astrocytes and infiltrating neutrophils and macrophages. To minimize concern that decreases in cellular inflammatory responses and neuronal death by progesterone are due to differences in lesion volume, we performed profile-based cell counting in vehicle and treatment groups using brain sections with similar lesion areas.
Cellular inflammatory response triggers release of various immunoreactive molecules that regulate brain cell function directly (Wang, 2010). HMGB1, defined as a cytokine, has shown a toxic role early after ICH in preclinical and clinical studies (Zhou, et al., 2010). Proinflammatory cytokine IL-1β, released primarily from stimulated macrophages and monocytes, can increase endothelial permeability and lead to vasogenic edema (Holmin and Mathiesen, 2000). We and others have shown that inhibition of HMGB1 and IL-1β expression alleviates brain injury and improves neurologic function (Ohnishi, et al., 2011, Wu, et al., 2015). Here, we showed that progesterone treatment markedly decreases protein expression of HMGB1 and IL-1β at day 1 after ICH. We and others also have shown that COX-2 is upregulated mainly in astrocytes in the perihematomal region after ICH (Gao, et al., 2008, T. Wu, et al., 2011) and that the selective COX-2 inhibitor celecoxib reduces expansion of perihematomal edema in ICH patients compared with that in controls (Lee, et al., 2013). We also have shown that COX-1 is constitutively expressed in neurons and microglia in the striatum after ICH (T. Wu, et al., 2011). Interestingly, we demonstrated here that progesterone treatment does not affect COX-1 and COX-2 expression. These data suggest that progesterone can modulate molecular inflammatory responses after ICH that are independent of COX1 and COX-2.
Inflammatory cells, mainly microglia/macrophages and leukocytes, are major sources of ROS, which can activate MMP-2 and MMP-9 and damage the neurovascular unit and BBB (Wang, 2010, Xue and Yong, 2008, Zhou, et al., 2014). The efficacy of free radical scavengers has provided direct evidence for a causal relationship between ROS and ICH injury. Specifically, overexpression of copper/zinc-superoxide dismutase (Wakai, et al., 2014) or treatment with deferoxamine (Cui, et al., 2015, Ni, et al., 2015, H. Wu, et al., 2011), a chelator of pro-oxidative iron, significantly reduced brain injury in animal models of ICH. In agreement with these experiments, mice with a genetic deletion of NADPH-oxidase, a key enzyme involved in ROS generation, exhibited reduced damage after ICH (Tang, et al., 2005, Wakisaka, et al., 2010). Recently, it was demonstrated that estrogen reduces ferrous iron toxicity both in vivo and in vitro, indicating that sex differences in susceptibility to ICH may be associated with differences in how the body handles iron toxicity (Umeano, et al., 2013). Our study revealed that progesterone, another female sex hormone, also reduces oxidative stress early after ICH by reducing protein carbonyl formation and protein nitrosylation, which is associated with reduction of MMP-9 activity. This result is consistent with our previous research in the ischemic stroke model (Jiang, et al., 2009). A recent study showed that progesterone attenuates BBB breakdown and hemorrhagic transformation after delayed treatment with tissue plasminogen activator in a middle cerebral artery occlusion model of stroke. Additionally, its neurovascular protective effects were associated with a reduction of VEGF-MMP-9 expression (Won, et al., 2014). Consistent with this observation, we and others have shown that exogenous application of VEGF can increase BBB permeability (Jiang, et al., 2014, J. Wang, et al., 2015). Here, we showed that progesterone treatment decreases VEGF expression and MMP-9 activity. Together, these data indicate that progesterone has antioxidant effects and anti-MMP-9 activity that may reduce BBB permeability.
NKA is important for cell physiology. Reduced brain NKA activity has been reported in a variety of pathologic brain conditions but has not been shown in ICH (de Lores Arnaiz and Ordieres, 2014, Yang, et al., 2012). In the brain, the NKA α subunit has three isoforms—α1, α2, and α3—and multiple isoforms are expressed by both neurons and glia (de Lores Arnaiz and Ordieres, 2014). Phosphorylation of the α subunit at Ser23 by PKC and at Ser943 by PKA reduces the NKA activity (Yang, et al., 2012). We demonstrated here that progesterone treatment does not affect the expression of the widespread NKA α1 subunit and does not inhibit PKA-dependent phosphorylation at Ser943. In contrast, it increased the expression of the neuronal-specific NKA α3 subunit and inhibited PKC-dependent phosphorylation at Ser23 on day 1 after ICH. Thus, progesterone treatment after ICH may offer neurovascular protection by inhibiting PKC-dependent phosphorylation of Ser23 and thereby partially restoring NKA activity.
As discussed above, progesterone most likely exerts neuroprotective effects through anti-inflammatory, anti-oxidant, and anti-MMP-9 activity, and by inhibiting PKC-dependent phosphorylation of NKA, which may lead to reduction in early ICH injury and improvements in long-term outcomes, including histopathology and neurologic recovery after ICH. However, long-term histopathology in mouse ICH models has not been well studied. Our results revealed that the residual lesion on day 28 after ICH was surrounded by an astroglial scar and that neuronal and axonal/ myelin loss are likely the major causes of brain atrophy. Importantly, progesterone treatment not only decreased neurobehavioral deficit scores gradually from days 7 to 28, but also mitigated astrogliosis/glial scar formation, myelin loss, and brain atrophy on day 28 after ICH. The alleviation of secondary brain injury in the acute phase and the attenuation of long-term histopathology by progesterone led to improved functional recovery up to day 28 after ICH when compared with that of vehicle-treated animals.
Although the Women’s Health Initiative-Memory Study (WHIMS) failed to find beneficial effects of progestin in age-associated brain dysfunction, some explained that the differences in the neurobiology of progesterone and the clinically used synthetic progestin, medroxyprogesterone acetate, may account for the negative results. Indeed, the type of progestin used may dictate the outcome of preclinical and clinical studies that address brain function (Singh and Su, 2013a, Singh and Su, 2013b). Another study showed that hormone replacement therapy that combines progesterone with estrogen may increase the risk of ischemic stroke with no effect on hemorrhagic stroke (Davis, 2008, Speroff, 2010). Based on these previous reports and the limited therapeutic options for ICH, we tested the therapeutic effect of progesterone for ICH in a rodent model. As in the animal models of ischemic stroke and TBI (Barha, et al., 2011, Jiang, et al., 2016), we found that progesterone is neuroprotective in the mouse collagenase-induced ICH model. However, recently completed clinical trials with progesterone failed to demonstrate any neuroprotective effect in patients with TBI (Jickling and Sharp, 2015, Schwamm, 2014). The authors of these studies commented that the failure of these trials may relate to the limited/insensitive outcome measures, unidimensional TBI characterization approaches (e.g., Glascow coma scale), and the heterogeneity of severe TBI (Stein, 2015, Webster, et al., 2015). To increase the clinical relevance of our study, we used middle-aged mice because ICH occurs more often in middle-aged and elderly individuals. Moreover, we chose the collagenase-induced ICH model because the hematoma develops gradually over 4–6 hours (Wang, 2010, Wang and Dore, 2007b, M. Wang, et al., 2015). This time course mimics that of the clinical condition in which a small penetrating artery ruptures and continues to bleed for an extended period. Indeed 17% of patients exhibit bleeding that lasts for more than 6 hours (Kazui, et al., 1996, Wang, 2010). In translational medicine, testing in multiple related preclinical models and in different laboratories is strongly encouraged before advancing any novel medicine or therapy to a clinical trial (Wang, 2010). Therefore, data from the blood injection-induced ICH model and from different laboratories are needed to provide additional evidence to support the therapeutic efficacy of progesterone for ICH. The results could lay the foundation for future clinical trials.
Sex differences in patient outcomes after ICH have been reported (Gokhale, et al., 2015). Our study is limited because it included only middle-aged male mice. Validation in middle-aged females and in aged males and females is needed to confirm the efficacy of progesterone for ICH. Progesterone has been shown to have multiple neuroprotective effects. Previous studies also have shown that it has neurotrophic effects and anti-excitotoxic effects (Jiang, et al., 2016, Singh and Su, 2013a). Moreover, progesterone-regulated neural responses may be mediated by membrane progesterone receptors (α-, β-, and γ-isoforms), putative γ-aminobutyric acid type A receptors, or progesterone membrane receptor component 1 (PGRMC1) (Liu, et al., 2012). It will be important to assess the effects of progesterone on excitotoxicity and neurogenesis and to determine which receptor is involved in the neuroprotective effects of progesterone after ICH.
In conclusion, our findings indicate that progesterone protects brain against ICH injury in middle-aged male mice. With multiple cellular and molecular targets, progesterone warrants further preclinical investigation and holds therapeutic promise for patients with ICH.
Supplementary Material
Progesterone (PROG) post-treatment does not affect rectal temperature but increases body weight on day 28 after collagenase-induced ICH. (A) Progesterone post-treatment had no influence on rectal temperature throughout the 28-day research period when compared with that of the vehicle-treated ICH group. (B) Changes in body weight did not differ significantly between vehicle-treated and progesterone-treated ICH mice at any time point except for day 28 after ICH, at which point the progesterone-treated mice exhibited greater weight gain (n=12 mice/group, *p<0.05). Values are means ± SD.
Highlights.
We examined the efficacy of progesterone in a clinically relevant ICH model.
Progesterone decreased inflammatory response and oxidative stress, restored Na+/K+ ATPase activity after ICH.
Progesterone reduced brain lesion volume and edema, attenuated long-term histopathology in ICH brain.
Progesterone provides neuroprotection and improves long-term neurologic outcome after ICH.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81301006), American Heart Association grant 13GRNT15730001, and the National Institutes of Health (R01NS078026, R01AT007317). We thank Claire Levine, MS, ELS, for assistance with this manuscript.
Abbreviations
- ICH
intracerebral hemorrhage
- COX
cyclooxygenase
- HMGB1
high-mobility group box 1
- IL-1β
interleukin 1β
- MMP
matrix metalloproteinase
- NKA
Na+/K+ ATPase
- VEGF
vascular endothelial growth factor
- ROS
reactive oxygen species
- LFB
luxol fast blue
- mNSS
modified neurologic severity score
- FJB
Fluoro-Jade B
- Iba-1
ionized calcium-binding adapter molecule 1
- MPO
myeloperoxidase
- GFAP
glial fibrillary acidic protein
Footnotes
Disclosures/Conflict of Interest
The authors report no disclosures relevant to the manuscript.
Authors’ contributions
Chao Jiang and Jian Wang were responsible for the study design and for drafting and revising the manuscript. Chao Jiang, Fangfang Zuo, Yuejuan Wang, Jieru Wan, and Zengjin Yang were responsible for the biochemical and molecular study. Chao Jiang, Wenwu Chen, Weidong Zang, Hong Lu, and Qingwu Yang were responsible for the assessment of histopathologic and functional outcomes of intracerebral hemorrhage. The data were analyzed by Chao Jiang and Jian Wang.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeoye O, Broderick JP. Advances in the management of intracerebral hemorrhage. Nature reviews Neurology. 2010;6(11):593–601. doi: 10.1038/nrneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Experimental neurology. 2011;231(1):72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CF, Cho S, Wang J. (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Annals of clinical and translational neurology. 2014;1(4):258–71. doi: 10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke; a journal of cerebral circulation. 2011;42(2):445–52. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui HJ, He HY, Yang AL, Zhou HJ, Wang C, Luo JK, Lin Y, Tang T. Efficacy of deferoxamine in animal models of intracerebral hemorrhage: a systematic review and stratified meta-analysis. PloS one. 2015;10(5):e0127256. doi: 10.1371/journal.pone.0127256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. Use of oral contraceptives and postmenopausal hormone replacement: Evidence on risk of stroke. Curr Treat Options Neurol. 2008;10(6):468–74. doi: 10.1007/s11940-008-0049-2. [DOI] [PubMed] [Google Scholar]
- de Lores Arnaiz GR, Ordieres MG. Brain Na(+), K(+)-ATPase Activity In Aging and Disease. International journal of biomedical science : IJBS. 2014;10(2):85–102. [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Sengupta P. Men and mice: Relating their ages. Life sciences. 2015 doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Gao Z, Wang J, Thiex R, Rogove AD, Heppner FL, Tsirka SE. Microglial activation and intracerebral hemorrhage. Acta neurochirurgica Supplement. 2008;105:51–3. doi: 10.1007/978-3-211-09469-3_11. [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Gonzalez S, Paniagua-Torija B, Lima A, Molina-Holgado E, De Nicola AF, Labombarda F. Progesterone reduces secondary damage, preserves white matter, and improves locomotor outcome after spinal cord contusion. Journal of neurotrauma. 2014;31(9):857–71. doi: 10.1089/neu.2013.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebel JM, Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2002;33(11):2631–5. doi: 10.1161/01.str.0000035284.12699.84. [DOI] [PubMed] [Google Scholar]
- Gokhale S, Caplan LR, James ML. Sex differences in incidence, pathophysiology, and outcome of primary intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2015;46(3):886–92. doi: 10.1161/STROKEAHA.114.007682. [DOI] [PubMed] [Google Scholar]
- Han X, Lan X, Li Q, Gao Y, Zhu W, Cheng T, Maruyama T, Wang J. Inhibition of prostaglandin E2 receptor EP3 mitigates thrombin-induced brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015 doi: 10.1177/0271678X15606462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T. Intracerebral administration of interleukin-1beta and induction of inflammation, apoptosis, and vasogenic edema. Journal of neurosurgery. 2000;92(1):108–20. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- Howard RB, Sayeed I, Stein D. Suboptimal dosing parameters as possible factors in the negative Phase III clinical trials of progesterone in TBI. Journal of neurotrauma. 2015 doi: 10.1089/neu.2015.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BY, Appelboom G, Ayer A, Kellner CP, Kotchetkov IS, Gigante PR, Haque R, Kellner M, Connolly ES. Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovasc Dis. 2011;31(3):211–22. doi: 10.1159/000321870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Cui K, Wang J, He Y. Microglia and cyclooxygenase-2: possible therapeutic targets of progesterone for stroke. International immunopharmacology. 2011;11(11):1925–31. doi: 10.1016/j.intimp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wang J, Li X, Liu C, Chen N, Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflammation research : official journal of the European Histamine Research Society [et al] 2009;58(9):619–24. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- Jiang C, Wang J, Yu L, Ou C, Liu X, Zhao X, Wang J. Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia. Behavioural brain research. 2013;250:222–9. doi: 10.1016/j.bbr.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Zuo F, Wang Y, Lu H, Yang Q, Wang J. Progesterone Changes VEGF and BDNF Expression and Promotes Neurogenesis After Ischemic Stroke. Molecular neurobiology. 2016 doi: 10.1007/s12035-015-9651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Xia R, Jiang Y, Wang L, Gao F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PloS one. 2014;9(2):e86407. doi: 10.1371/journal.pone.0086407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling GC, Sharp FR. Improving the translation of animal ischemic stroke studies to humans. Metabolic brain disease. 2015;30(2):461–7. doi: 10.1007/s11011-014-9499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke; a journal of cerebral circulation. 1996;27(10):1783–7. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. The Lancet Neurology. 2012;11(8):720–31. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids and barriers of the CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Park HK, Ryu WS, Lee JS, Bae HJ, Han MK, Lee YS, Kwon HM, Kim CK, Park ES, Chung JW, Jung KH, Roh JK. Effects of celecoxib on hematoma and edema volumes in primary intracerebral hemorrhage: a multicenter randomized controlled trial. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2013;20(8):1161–9. doi: 10.1111/ene.12140. [DOI] [PubMed] [Google Scholar]
- Li D, Lei C, Zhang S, Liu M, Wu B. Blockade of high mobility group box-1 signaling via the receptor for advanced glycation end-products ameliorates inflammatory damage after acute intracerebral hemorrhage. Neuroscience letters. 2015;609:109–19. doi: 10.1016/j.neulet.2015.10.035. [DOI] [PubMed] [Google Scholar]
- Liu A, Margaill I, Zhang S, Labombarda F, Coqueran B, Delespierre B, Liere P, Marchand-Leroux C, O’Malley BW, Lydon JP, De Nicola AF, Sitruk-Ware R, Mattern C, Plotkine M, Schumacher M, Guennoun R. Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology. 2012;153(8):3747–57. doi: 10.1210/en.2012-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011;70(3):218–35. doi: 10.1097/NEN.0b013e31820d94a5. [DOI] [PubMed] [Google Scholar]
- Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Frontiers in cellular neuroscience. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Okauchi M, Hatakeyama T, Gu Y, Keep RF, Xi G, Hua Y. Deferoxamine reduces intracerebral hemorrhage-induced white matter damage in aged rats. Experimental neurology. 2015;272:128–34. doi: 10.1016/j.expneurol.2015.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi M, Katsuki H, Fukutomi C, Takahashi M, Motomura M, Fukunaga M, Matsuoka Y, Isohama Y, Izumi Y, Kume T, Inoue A, Akaike A. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology. 2011;61(5–6):975–80. doi: 10.1016/j.neuropharm.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Poon MT, Bell SM, Al-Shahi Salman R. Epidemiology of Intracerebral Haemorrhage. Frontiers of neurology and neuroscience. 2015;37:1–12. doi: 10.1159/000437109. [DOI] [PubMed] [Google Scholar]
- Qin J, Ma X, Qi H, Song B, Wang Y, Wen X, Wang QM, Sun S, Li Y, Zhang R, Liu X, Hou H, Gong G, Xu Y. Transplantation of Induced Pluripotent Stem Cells Alleviates Cerebral Inflammation and Neural Damage in Hemorrhagic Stroke. PloS one. 2015;10(6):e0129881. doi: 10.1371/journal.pone.0129881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamm LH. Progesterone for traumatic brain injury--resisting the sirens’ song. The New England journal of medicine. 2014;371(26):2522–3. doi: 10.1056/NEJMe1412951. [DOI] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone and neuroprotection. Hormones and behavior. 2013a;63(2):284–90. doi: 10.1016/j.yhbeh.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience. 2013b;239:84–91. doi: 10.1016/j.neuroscience.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L. Transdermal hormone therapy and the risk of stroke and venous thrombosis. Climacteric : the journal of the International Menopause Society. 2010;13(5):429–32. doi: 10.3109/13697137.2010.507111. [DOI] [PubMed] [Google Scholar]
- Stein DG. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain injury. 2015;29(11):1259–72. doi: 10.3109/02699052.2015.1065344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil Granger D, Zhang JH. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. Journal of neurochemistry. 2005;94(5):1342–50. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- Umeano O, Phillips-Bute B, Hailey CE, Sun W, Gray MC, Roulhac-Wilson B, McDonagh DL, Kranz PG, Laskowitz DT, James ML. Gender and age interact to affect early outcome after intracerebral hemorrhage. PloS one. 2013;8(11):e81664. doi: 10.1371/journal.pone.0081664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakai T, Sakata H, Narasimhan P, Yoshioka H, Kinouchi H, Chan PH. Transplantation of neural stem cells that overexpress SOD1 enhances amelioration of intracerebral hemorrhage in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(3):441–9. doi: 10.1038/jcbfm.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Critical role for copper/zinc-superoxide dismutase in preventing spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. Stroke; a journal of cerebral circulation. 2010;41(4):790–7. doi: 10.1161/STROKEAHA.109.569616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali B, Ishrat T, Won S, Stein DG, Sayeed I. Progesterone in experimental permanent stroke: a dose-response and therapeutic time-window study. Brain : a journal of neurology. 2014;137(Pt 2):486–502. doi: 10.1093/brain/awt319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in neurobiology. 2010;92(4):463–77. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain : a journal of neurology. 2007a;130(Pt 6):1643–52. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dore S. Inflammation after intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007b;27(5):894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- Wang J, Dore S. Heme oxygenase 2 deficiency increases brain swelling and inflammation after intracerebral hemorrhage. Neuroscience. 2008;155(4):1133–41. doi: 10.1016/j.neuroscience.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fields J, Dore S. The development of an improved preclinical mouse model of intracerebral hemorrhage using double infusion of autologous whole blood. Brain research. 2008;1222:214–21. doi: 10.1016/j.brainres.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fu X, Yu L, Li N, Wang M, Liu X, Zhang D, Han W, Zhou C. Preconditioning with VEGF Enhances Angiogenic and Neuroprotective Effects of Bone Marrow Mononuclear Cell Transplantation in a Rat Model of Chronic Cerebral Hypoperfusion. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rogove AD, Tsirka AE, Tsirka SE. Protective role of tuftsin fragment 1–3 in an animal model of intracerebral hemorrhage. Annals of neurology. 2003;54(5):655–64. doi: 10.1002/ana.10750. [DOI] [PubMed] [Google Scholar]
- Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain : a journal of neurology. 2005;128(Pt 7):1622–33. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- Wang M, Hong X, Chang CF, Li Q, Ma B, Zhang H, Xiang S, Heo HY, Zhang Y, Lee DH, Jiang S, Leigh R, Koehler RC, van Zijl PC, Wang J, Zhou J. Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI. Magnetic resonance in medicine. 2015 doi: 10.1002/mrm.25690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KM, Wright DK, Sun M, Semple BD, Ozturk E, Stein DG, O’Brien TJ, Shultz SR. Progesterone treatment reduces neuroinflammation, oxidative stress and brain damage and improves long-term outcomes in a rat model of repeated mild traumatic brain injury. Journal of neuroinflammation. 2015;12(1):238. doi: 10.1186/s12974-015-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won S, Lee JH, Wali B, Stein DG, Sayeed I. Progesterone attenuates hemorrhagic transformation after delayed tPA treatment in an experimental model of stroke in rats: involvement of the VEGF-MMP pathway. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(1):72–80. doi: 10.1038/jcbfm.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won S, Lee JK, Stein DG. Recombinant tissue plasminogen activator promotes, and progesterone attenuates, microglia/macrophage M1 polarization and recruitment of microglia after MCAO stroke in rats. Brain, behavior, and immunity. 2015;49:267–79. doi: 10.1016/j.bbi.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Wu H, Wu T, Han X, Wan J, Jiang C, Chen W, Lu H, Yang Q, Wang J. Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2016 doi: 10.1177/0271678X15625351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wu T, Hua W, Dong X, Gao Y, Zhao X, Chen W, Cao W, Yang Q, Qi J, Zhou J, Wang J. PGE2 receptor agonist misoprostol protects brain against intracerebral hemorrhage in mice. Neurobiology of aging. 2015;36(3):1439–50. doi: 10.1016/j.neurobiolaging.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wu T, Li M, Wang J. Efficacy of the lipid-soluble iron chelator 2,2′-dipyridyl against hemorrhagic brain injury. Neurobiology of disease. 2012;45(1):388–94. doi: 10.1016/j.nbd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wu T, Xu X, Wang J, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011;31(5):1243–50. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Z, Hu X, Zhao R, Song Y, Ban X, Qi J, Wang J. Dynamic changes of inflammatory markers in brain after hemorrhagic stroke in humans: a postmortem study. Brain research. 2010;1342:111–7. doi: 10.1016/j.brainres.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wu H, Wang J, Wang J. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation. 2011;8:22. doi: 10.1186/1742-2094-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Yong VW. Matrix metalloproteinases in intracerebral hemorrhage. Neurological research. 2008;30(8):775–82. doi: 10.1179/174313208X341102. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Carter EL, Kibler KK, Kwansa H, Crafa DA, Martin LJ, Roman RJ, Harder DR, Koehler RC. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. Journal of neurochemistry. 2012;121(1):168–79. doi: 10.1111/j.1471-4159.2012.07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zan L, Zhang X, Xi Y, Wu H, Song Y, Teng G, Li H, Qi J, Wang J. Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion. Neuroscience. 2014;262:118–28. doi: 10.1016/j.neuroscience.2013.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wu T, Chang CF, Wu H, Han X, Li Q, Gao Y, Hou Z, Maruyama T, Zhang J, Wang J. Toxic role of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain, behavior, and immunity. 2015;46:293–310. doi: 10.1016/j.bbi.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Progress in neurobiology. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xiong KL, Lin S, Zhong Q, Lu FL, Liang H, Li JC, Wang JZ, Yang QW. Elevation of high-mobility group protein box-1 in serum correlates with severity of acute intracerebral hemorrhage. Mediators of inflammation 2010. 2010 doi: 10.1155/2010/142458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS, Wang J. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PloS one. 2014;9(5):e97423. doi: 10.1371/journal.pone.0097423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Progesterone (PROG) post-treatment does not affect rectal temperature but increases body weight on day 28 after collagenase-induced ICH. (A) Progesterone post-treatment had no influence on rectal temperature throughout the 28-day research period when compared with that of the vehicle-treated ICH group. (B) Changes in body weight did not differ significantly between vehicle-treated and progesterone-treated ICH mice at any time point except for day 28 after ICH, at which point the progesterone-treated mice exhibited greater weight gain (n=12 mice/group, *p<0.05). Values are means ± SD.