Abstract
We examined independent and cumulative effects of two Alzheimer's-related genetic polymorphisms, Apolipoprotein E (APOE) and Clusterin (CLU), in relation to the deleterious effects of poor vascular health (pulse pressure [PP]) on executive function (EF) performance and change in non-demented older adults. Using a sample (n = 593; age range = 53-95 years) from the Victoria Longitudinal Study, we applied latent growth modeling to test the effect of PP, as moderated by APOE and CLU, on an EF latent variable. EF was affected by higher levels of PP but differentially less so for carriers of low-risk alleles (APOE ε2+; CLU TT) than for moderate-or high-risk alleles (APOE ε2−; CLU C+). The cumulative genetic risk of APOE plus CLU provided similar moderation of PP level effects on EF. Future research may focus on how APOE and CLU might provide different but complementary contributions to predicting EF level and change. Vascular health risk in synergistic association with risk-related polymorphisms can elucidate the neurobiological underpinnings of cognitive trajectories in non-demented aging.
Keywords: Apolipoprotein E, Clusterin, Pulse pressure, Executive Function, Victoria Longitudinal Study
1. Introduction
Biomarkers associated with the risk of developing Alzheimer's disease (AD) may affect cognition long before clinical symptoms of AD occur (Anstey et al., 2015). AD-related risk or protection factors derive from genetic, biological, health, lifestyle, and other domains. Such factors may operate independently or interactively to predict level and slope of neurocognitive performance. Among key AD risk genes, Apoliprotein E (APOE) and Clusterin (CLU) have also been prominently implicated in differential cognitive decline in non-demented aging (Small et al., 2004; Thambisetty et al., 2013). Independently, these genes present relatively low penetrance and consequently low effect sizes, but together they may account for substantial cognitive risk (Barral et al., 2012; McFall et al., 2015a) especially within the context of other AD and vascular health risk factors (Josefsson et al., 2012; McFall et al., 2014; McFall et al., 2015b). One important vascular health factor is pulse pressure (PP), a proxy measure of arterial stiffness. Higher levels of PP are associated with decreased vascular health (Steppan et al., 2011), poorer cognitive outcomes (Al Hazzouri and Yaffe, 2014; McFall et al., 2014; Raz et al., 2011), and risk of dementia or AD (Peters et al., 2013; Qiu et al., 2003). This study examines the independent and additive effects of genetic variants within the APOE and CLU genes in interaction with a key vascular risk factor (i.e., PP) on executive function (EF) level and 9-year change in non-demented older adults.
APOE (rs429358 and rs7412) has three isoforms (ε2, ε3, ε4) that exert varying levels of risk on cognitive decline and AD. The isoforms differentially regulate amyloid beta (Aβ) aggregation and clearance, glucose metabolism, neuro-inflammation, lipid transport, mitochondrial function, and neuronal signalling (Bennet et al., 2007; Castellano et al., 2011; Corder et al., 1993; Liu et al., 2013). In general, the ε2 allele has been associated with reduced risk of cognitive decline and AD (Suri et al., 2013). The ε3 allele, the most common, is generally considered neutral (Corbo and Scacchi, 1999). Finally, the ε4 allele is an established risk factor for cognitive decline and AD, alone or in combination with biomarker risk (Bangen et al., 2013; Corder et al., 1993; Schiepers et al., 2012). The CLU (rs11136000) SNP is involved in Aβ clearance, apoptosis, brain atrophy, and disease progression. CLU allelic risk carriers (C+) show decreased white matter integrity and 1.16 greater odds of developing sporadic AD than low-risk homozygotes (TT; Bertram et al., 2007). APOE and CLU both have similar molten globule structures (Morrow et al., 2002) and may influence each other in specific brain regions (Wu et al., 2012). Together, APOE and CLU may co-influence physiologic and pathologic risk by contributing to AD pathology through their similar involvement in the reduced clearance of Aβ peptides which, in turn, lead to neuronal loss and cognitive decline (Lambert and Amouyel, 2011; Wu et al., 2012).
Whereas these AD genetic risk factors are generally not modifiable, vascular health is a prominent, changing, and potentially modifiable influence on brain and cognitive aging. PP, represented by the difference between systolic and diastolic blood pressure, is a measure related to arterial stiffness and is considered a better indicator of declining vascular health than either mean arterial pressure or systolic blood pressure. PP typically shows a steep linear age-related increase (toward worse health) in older adults (Raz et al., 2011). PP is an independent marker of (a) cardiovascular disease and mortality (Bérard et al., 2013; Singer et al., 2014), (b) cognitive decline (Al Hazzouri and Yaffe, 2014; McFall et al., 2014; Singer et al., 2014; Waldstein et al., 2008), (c) mild cognitive impairment (Yaneva-Sirakova et al., 2012), (d) AD biomarkers (Nation et al., 2013), and (e) dementia and AD risk (Peters et al., 2013; Qiu et al., 2003). Increases in systolic blood pressure or PP have been associated with neuropathology such as brain atrophy, lesions, and white matter hyperintensities (Jochemsen et al., 2015; Tsao et al., 2013; van Sloten et al., 2015), especially in prefrontal structures, leading to decreases in EF performance (McFall et al., 2014; Raz et al., 2003).
EFs are a collection of cognitive control processes involved in higher-order thinking such as strategic planning, goal-directed behavior, and problem solving (Luszcz, 2011). Performance scores on cognitive tests representing each component can be combined quantitatively to produce latent EF variable(s). Two characteristics of EF associated with aging should be noted. First, with normal and impaired aging, EF latent structure exhibits dedifferentiation or consolidation into a single factor, although differentiation may continue for exceptional brain aging (de Frias et al., 2006, 2009). Second, EF performance generally declines with non-demented aging, but considerable variability in timing and trajectories across individuals is observed. Notably, below average or steeply declining EF performance in older adults is associated with development of cognitive impairment (de Frias et al., 2009; Nathan et al., 2001) or AD (Bäckman et al., 2005; Grober et al., 2008; Rapp and Reischies, 2005). Neurobiological, health, and lifestyle markers may contribute independently to differential EF performance and decline, but also interactively with genetic or other biomarkers (Lindenberger et al., 2008; McFall et al., 2013; Papenberg et al., 2014). Identifying specific factors, moderating influences and synergistic combinations that contribute to variability in EF trajectories is an important avenue of research in neurocognitive aging.
Single-gene risk associated with typical cognitive decline is often difficult to detect. However, in the cumulative or interactive presence of other genetic or biomedical factors, associations may become evident. Increasingly, researchers are investigating cumulative or interactive effects (from genetic, biological, or health domains) in order to better understand mechanisms associated with variability in trajectories of non-demented and impaired neurocognitive aging (Ferencz et al., 2014; McFall et al., 2015a; Sapkota et al., 2015; Sleegers et al., 2015). We examine EF performance and change in older adults as related to interactive and cumulative risk with selected genetic polymorphisms and vascular health. We address two specific research questions. Research Question (RQ)1: Do APOE or CLU low-risk (protective) alleles reduce the negative effects of poor vascular health (higher PP) on EF performance and change in non-demented older adults (e.g., APOE × PP)? RQ2: Does the combination of APOE and CLU clarify the negative effects of poor vascular health (high PP) on EF performance and change in non-demented older adults beyond that of APOE or CLU alone? We expected genetic low-risk (protective) alleles of APOE and CLU, both independently and in combination, to reduce the deleterious effects of higher PP on EF performance and 9-year change.
2. Material and Methods
2.1. Participants
Participants were community-dwelling adults (initially aged 53-95 years) drawn from the Victoria Longitudinal Study (VLS). The VLS is a longitudinal sequential study designed to examine human aging in relation to biomedical, genetic, health, cognitive, and neuropsychological aspects (Dixon and de Frias, 2004). The VLS and all present data collection procedures were in full and certified compliance with prevailing human research ethics guidelines and boards. Informed written consent was provided by all participants. Using standard procedures (e.g., Dixon et al., 2012; Small et al., 2011), we assembled longitudinal data consisting of three samples and all available waves (up to three) since the early 2000s. The EF tasks required for this study were installed in the VLS neuropsychological battery at this point. Therefore, the first included wave for each sample was the first exposure to the EF tasks. This study assembled (a) Sample 1 (S1) Waves 6, 7, and 8; (b) Sample 2 (S2) Waves 4 and 5; and (c) Sample 3 (S3) Waves 1, 2, and 3. For terminological efficiency, the respective earliest wave of each sample became Wave 1 (W1 or baseline) and the respective second and third wave became Wave 2 (W2) and Wave 3 (W3). The mean intervals between the waves of data collection were approximately 4.5 years (W1-W2; W2-W3). Although we used the three waves to organize the demographic information (Table 1) it is important to note that wave was not used as the metric of longitudinal change in the analyses. Specifically, age was used as the metric of change for this study. Statistically, using age in this manner permits us to account for variability associated with age as well as, or better than, if it were used as a covariate in the statistical models. Moreover, testing genetic-health interactions on EF across multiple linked longitudinal periods of up to 9 years (M = 8.9) allowed us to produce an accelerated longitudinal design covering a 40-year band of aging (i.e., 53-95 years).
Table 1.
Participant Characteristics Categorized by Time Point
| Wave 1 | Wave 2 | Wave 3 | |
|---|---|---|---|
| N | 592a | 495 | 319 |
| Years between waves | - | 4.45 (.56) | 4.42 (.70) |
| Age | 70.3 (8.66) | 74.5 (8.53) | 76.2 (8.22) |
| Range | 53-95 | 57-95 | 62-96 |
| Sex (% Female) | 67.3 | 67.1 | 69.6 |
| Education | 15.3 (2.95) | 15.4 (3.01) | 15.3 (3.20) |
| Health to perfectb | 1.78 (.719) | 1.85 (.714) | 1.84 (.789) |
| Health to peersc | 1.56 (.683) | 1.63 (.652) | 1.65 (.730) |
| Pulse Pressure | 51.9 (10.2) | 55.0 (12.3) | 55.2 (12.2) |
| Range | 32.1-99.2 | 26.0-102.6 | 29.0-95.5 |
| Systolic Blood Pressure | 126.2 (14.3) | 126.8 (15.3) | 126.8 (14.9) |
| Range | 86.2-171.8 | 89.1-164.1 | 94.5-172.8 |
| Hypertension ≥160 n (%) | 7 (1.2) | 7 (1.4) | 8 (2.6) |
| Diastolic Blood Pressure | 74.3 (9.31) | 71.8 (8.87) | 71.7 (8.61) |
| Range | 45.9-105.9 | 50.9-106.4 | 52.1-100.6 |
| Hypotension ≤60 n (%) | 34 (5.7) | 39 (6.6) | 30 (5.1) |
| Hypertension Med (%) | 26 | 38 | 36 |
| Body Mass Index (kg/m2) | 26.8 (4.14) | 26.5 (4.28) | 26.6 (4.38) |
| Range | 15.0-43.3 | 16.2-41.0 | 17.6-40.5 |
| Type 2 diabetes (%) | 7.8 | 7.3 | 6.9 |
| Smoking Status (%) | |||
| Present | 4.1 | 3.3 | 1.3 |
| Previous | 52.7 | 55.0 | 52.5 |
| Never | 43.2 | 41.8 | 45.6 |
| Mini Mental State Exam | 28.7 (1.23) | 28.5 (1.39) | 28.6 (1.50) |
| APOE | ε2+ | ε3ε3 | ε4+ |
| n | 74 | 352 | 140 |
| Lipid Lowering Med. | 4.3 | 14.2 | 16.3 |
| Hypertension Med. | 31 | 28 | 19 |
| CLU | TT | TC | CC |
| n | 88 | 286 | 188 |
| Lipid Lowering Med. | 11.4 | 14.0 | 14.4 |
| Hypertension Med. | 26 | 24 | 30 |
Note. Results presented as Mean (Standard Deviation) unless otherwise stated. Age and education presented in years. Smoking and drinking status are reported in percentages of total sample.
One participant contributed data at Wave 2 but not at Wave 1.
Self-reported health relative to perfect.
Self-reported health relative to peers. Self-report measures are based on 1 “very good” to 5 “very poor”. All vascular health measures are reported in mmHg. Medication use is reported as percentage of self-reported use. These characteristics are represented by wave for convenience only: all longitudinal analyses were conducted with chronological age as the metric of change.
Given the necessity for both genetic and longitudinal data in this study, these factors defined the initial opportunity in sample recruitment. VLS genotyping occurred in the 2009-2011 period and was limited by funding arrangement to about 700 continuing VLS participants. After initial evaluations, the eligible source sample consisted of 695 participants. Several exclusionary criteria were then applied to this source sample: (a) a diagnosis of Alzheimer's disease or any other dementia, (b) a Mini-Mental Status Exam (MMSE; Folstein et al., 1975) score of less than 24, (c) a self-report of “severe” for potential comorbid conditions (e.g., epilepsy, head injury, depression), (d) a self-report of “severe” or “moderate” for potential comorbid diseases such as neurological conditions (e.g., stroke, Parkinson's disease), and (e) insufficient EF data. The final study sample consisted of n = 593 adults. One participant (female) contributed data to W2 only. At W1 there were 592 adults, including 398 females and 194 males (M age = 70.3 years, SD = 8.66, range 53.2 – 95.2). At W2 there were 495 adults, including 332 females and 163 males (M age = 74.5 years, SD = 8.53, range 57.3 – 94.5). At W3 there were 319 adults, including 222 females and 97 males (M age = 76.2 years, SD = 8.22, range 62.4 – 95.6). The design stipulated that whereas S1 and S3 participants could contribute data to all three waves, S2 participants contributed data to W1 and W2 (the required data from the third wave are not yet available). The retention rates for each available and defined two-wave interval are as follows (a) S1 W1-W2 = 88%; (b) S1 W2-W3 = 80%; (c) S2 W1-W2 = 82%; (d) S3 W1-W2 = 84%; (e) S3 W2-W3 = 90%. Structural equation modeling estimates all missing data using maximum likelihood estimations. All missing data in conditional latent growth models were estimated by multiple imputations using Mplus 7 (Muthén and Muthén, 1998 - 2012). Specifically, the VLS practice is to generate 50 imputations of the data set and pool for all growth models (McFall et al., 2015b).
2.2. Executive Function (EF) Measures
We assembled a robust latent EF variable using four manifest indicators of two key EF abilities: shifting (Brixton Spatial Anticipation, Color Trails Test Part 2) and inhibition (Hayling Sentence Completion, Stroop test). For a more detailed description of these EF tasks see McFall and colleagues (2013). All EF tests have been used widely and frequently within the VLS and other projects, with established measurement and structural characteristics (e.g., Bielak et al., 2006; de Frias et al., 2006, 2009) and demonstrated sensitivity to health, genetic, and neurocognitive factors (e.g., McFall et al., 2014; Sapkota et al., 2015) in various older adult populations.
2.3. Pulse Pressure (PP)
PP is calculated as follows: PP = systolic – diastolic blood pressure. For all analyses PP centered at 51.9 mmHg, the population mean at baseline. Although the analyses are conducted with PP as a continuous variable, the results are displayed in terms of three clusters of PP (Figures 1-4). We planned to investigate typically aging older adults; thus, high blood pressure and participants taking antihypertension and lipid lowering medications were included in the study (Table 1).
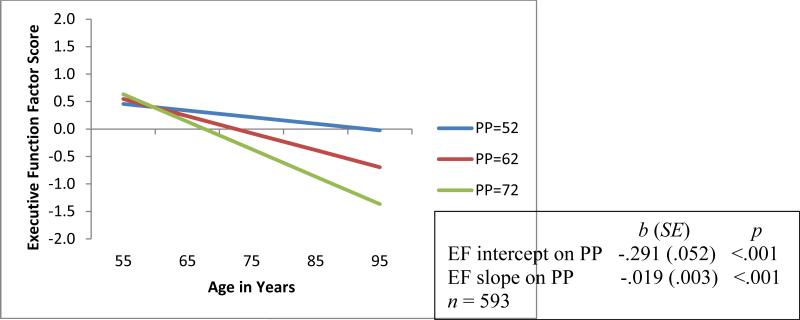
Figure 1.
Predicted growth curve model of executive function (EF) with continuous pulse pressure (PP, mm Hg) as predictor. Three categories of PP are depicted for convenience of display. Age in actual years was the metric of change. The age variable was centered at 75 years.
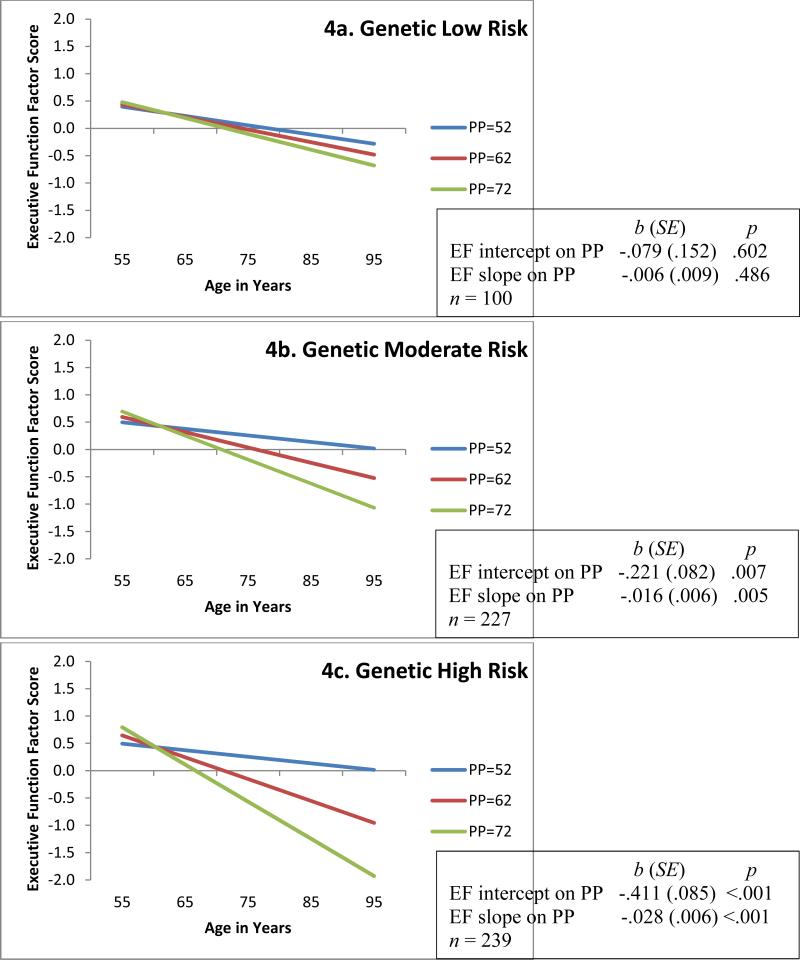
Figure 4.
Predicted growth curve model of executive function (EF) with continuous pulse pressure (PP; mm Hg) as predictor and moderated by APOE plus CLU (Low, Moderate, High) risk groupings. Three categories of PP are depicted for convenience of display. Age in actual years was the metric of change. The age variable was centered at 75 years. Moderation analyses D = 27.7, Δdf = 14, p = .016.
2.4. Saliva Collection, DNA Extraction and Genotyping, and Gene Groupings
Saliva was collected according to standard procedures from Oragene-DNA Genotek and stored at room temperature in the Oragene® disks until DNA extraction. DNA was manually extracted from the saliva sample mix using the manufacturer's protocol. Genotyping was carried out by using a PCR-RFLP strategy as previously described (McFall et al., 2015a).
Genotypic distribution for APOE (clustered according to the presence of the ε4 risk allele; χ2 = 0.58 (1), p >.05) and CLU (χ2 = 0.77 (1), p >.05) are in Hardy-Weinberg equilibrium. As per typical VLS protocol, 27 ε2ε4 participants were excluded from all analyses. Genetic risk analyses were based on low, moderate, and high risk for APOE (ε2+ [ε2ε2, ε2ε3], ε3 [ε3ε3], ε4+ [ε3ε4, ε4ε4]) and CLU (TT, TC, CC). We then tested the additive risk of APOE and CLU. The genetic risk index was created by summing allelic risk for APOE (i.e., low risk (ε2ε2, ε2ε3) = 0, moderate risk (ε3ε3) = 1, high risk (ε3ε4, ε4ε4) = 2) plus CLU (i.e., low risk = 0 (TT), moderate risk = 1 (TC), and high risk = 2 (CC); see Ferencz et al., 2014). Given a maximum genetic risk score of 4, we determined three levels of additive genetic risk as follows: low risk = 0-1, moderate risk = 2, and high risk = 3-4. The low risk group (n = 74) is within the power protocols of multi-group structural equation model analyses (Little, 2013).
2.5. Statistical Analyses
Analyses pertaining to our RQs included confirmatory factor analysis and latent growth modeling. Statistical model fit for all analyses was determined using standard indexes: (a) χ2 for which a good fit would produce a non-significant test (p > .05) indicating that the data are not significantly different from the estimates associated with the model, (b) the comparative fit index (CFI) for which fit is judged by a value of ≥ .95 as good and ≥ .90 as adequate, (c) root mean square error of approximation (RMSEA) for which fit is judged by a value of ≤ .05 as good and ≤ .08 as adequate, and (d) standardized root mean square residual (SRMR) for which fit is judged by a value of ≤ .08 as good (Kline, 2011).
Mplus 7 (Muthén and Muthén, 1998 - 2012) was used to confirm a one-factor EF latent variable reflecting contributions from the four manifest indicators. Using the best fitting EF model we calculated factor scores and these were used for all subsequent models. Invariance testing across the three waves of data resulted in metric and partial scalar invariance (Table 2). Achieving partial scalar invariance indicated that any potential EF practice effects were not significant and accounted for. As described earlier, age was used as the metric of change (i.e., the data were arrayed and analysed by age) a procedure that directly (not indirectly through covariation) includes actual chronological age in the analyses. We centered age at 75 years (the frequently used center point of the 40-year band of VLS W1 data; ranging from 53-95 years). We used multiple imputations to estimate missing values for PP, age, and EF factor scores for all growth models. Best fitting growth model resulted in random intercept, random slope (Table 2). We observed the expected patterns of EF performance and change. Specifically, individuals (a) varied in level of EF performance at age 75 (b = .958, p < .001), (b) exhibited significant 9-year EF decline (M = −.010, p = .021), and (c) showed variable patterns of decline (b = .001, p < .001). Power associated with our measurement model was 0.9. Chi-square difference tests (D)were calculated for all latent growth nested models. D statistics are equivalent to a Scheffe-like procedure accounting for multiple testing (McCoach et al., 2007).
Table 2.
Confirmatory Factor Analyses and Latent Growth Goodness of Fit Indexes for Executive Function Models
| Model | AIC | BIC | χ 2 | df | p | RMSEA | CFI | SRMR |
|---|---|---|---|---|---|---|---|---|
| CFA for One Factor Model | ||||||||
| Configural Invariance | 21288.77 | 21538.73 | 41.33 | 33 | .151 | .021 (.000-.038) | .995 | .029 |
| Metric Invariance | 21285.59 | 21500.46 | 54.14 | 41 | .082 | .023 (.000-.039) | .992 | .051 |
| Scalar Invariance | 21431.61 | 21620.17 | 212.16 | 47 | <.001 | .077 (.067-.088) | .895 | .101 |
| Partial Scalar Invariancea,b | 21316.01 | 21522.11 | 88.56 | 43 | <.001 | .042 (.030-.055) | .971 | .076 |
| Model | AIC | BIC | −2LL | D | Δdf | p | ||
| Latent Growth Model | ||||||||
| Fixed intercept | 4349.89 | 4358.66 | 4345.89 | - | - | |||
| Random intercept | 2897.46 | 2910.46 | 2891.31 | 1454.58 | 1 | <.001 | ||
| Random intercept Fixed slope | 2848.24 | 2865.78 | 2840.24 | 51.07 | 1 | <.001 | ||
| Random intercept Random slopeb | 2055.61 | 2081.92 | 2043.61 | 796.63 | 2 | <.001 | ||
Brixton and Color Trials free to vary.
Best fitting model.
AIC = Akaike Information Criteria. BIC = Bayesian Information Criteria. χ2 = chi-square test of model fit. df = degrees of freedom for model fit. RMSEA = root mean square error of approximation. CFI = comparative fix index. SRMR = standardized root mean square residual. CFA = confirmatory factor analysis. −2LL = −2 log likelihood. D = difference statistic (using −2LL). Δdf = change in degrees of freedom.
For both RQs we tested conditional growth models for EF with PP as a predictor using independent APOE or CLU groupings and cumulative APOE plus CLU groupings as outlined in section 2.4. Moderation effects were calculated using the D statistic between the unconstrained and constrained model of the interaction. In addition, for all analyses we ran a model that included several covariates measured at W1: sex, education level, smoking status, body mass index, type 2 diabetes status, lipid lowering medication use, and antihypertension medication use. Two covariates exhibited a significant effect on EF level or change but these findings did not change the results of the PP and genetic risk models. Specifically, education significantly predicted EF level of performance at age 75 in all PP and gene models and 9-year EF change for all but the APOE × PP model. Antihypertension medication use showed significant covariant effects in the models; consequently, all analyses were repeated after removal of 152 participants using the medications. The analyses of this subgroup did not alter the original results.
3. Results
In preliminary analyses, we tested independent associations of PP, APOE, and CLU with EF level and change. First, as expected, better vascular health (lower levels of PP) was associated with higher EF performance at the centering age 75 (p < .001) and with less 9-year decline (p < .001; see Figure 1). As can be seen in the figure, the group with the lowest (healthiest) level of baseline PP (i.e., PP = 52 mm Hg) exhibited better EF performance (Mi = .216) and less 9-year decline (Ms = −.012) than did the groups with the medium (Mi = −.075; Ms = −.031) and highest levels of PP (Mi = −.366; Ms = −.050). Overall, this result established the benchmark for testing genetic moderation. Second, we observed no significant independent genotype associations with EF performance or decline (APOE bi = −.198, SE = .136, p = .146, bs = .012, SE = .008, p = .186; CLU bi = .008, SE = .074, p = .919, bs = .001, SE = .005, p = .873). Moderation effects are still possible in the absence of direct effects.
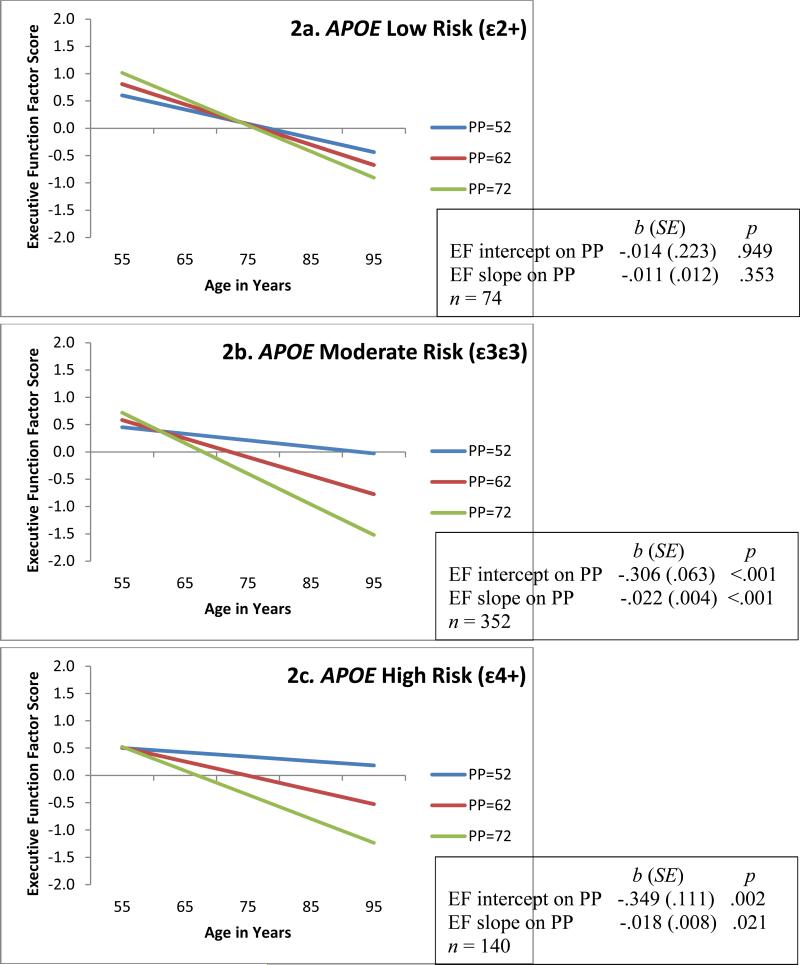
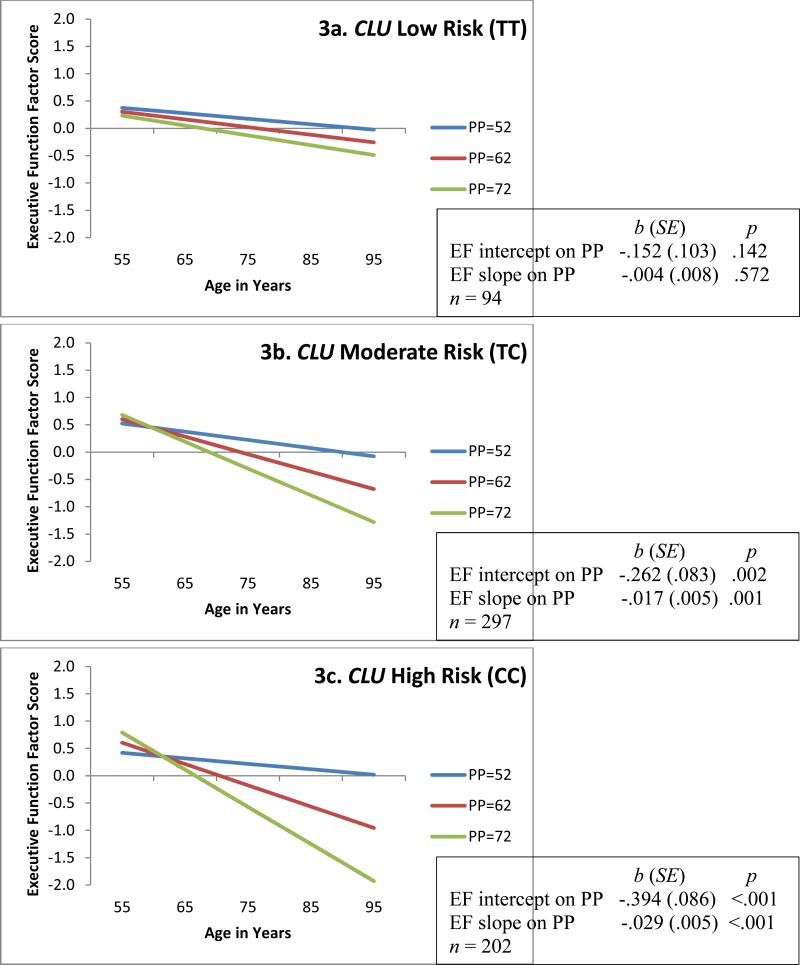
For RQ1, separate analyses showed that both genotypes exhibited significant gene × PP interaction effects on EF performance and change. The contrasting PP-EF associations across levels of genetic risk are displayed in Figure 2 (APOE) and Figure 3 (CLU). For both APOE and CLU the low genetic risk groups exhibited no deleterious effects of PP on EF. This contrasts markedly with the patterns observed for the moderate and high risk groups. Regarding APOE, the ε2+ group (Figure 2a) exhibited non-significant diversity in EF performance (bi = −.014, p = .949) and change (bs = −.011, p = .353) across the levels of PP. In contrast, PP levels within the APOE ε4+ group (Figure 2c) predicted both EF level at age 75 years (bi = −.349, p = .002) and 9-year EF change (bs = −.018, p =.021). We observed a similar pattern in the APOE ε3ε3 group (Figure 2b) for EF performance (b = −.306, p <.001) and change (b = −.022, p <.001). Regarding CLU, the low risk group (TT; Figure 3a) exhibited non-significant diversity in EF performance (bi = −.152, p = .142) and change (bs = −.004, p = .572) due to level of PP. In contrast, PP levels within the CLU high risk group (CC; Figure 3c) predicted both EF level at age 75 years (bi = −.394, p <.001) and 9-year EF change (bs = −.029, p <.001). We observed a similar pattern in the CLU moderate risk group (TC; Figure 3b) for EF performance (bi = −.262, p = .002) and change (bs = −.017, p = .001).
Figure 2.
Predicted growth curve model of executive function (EF) with continuous pulse pressure (PP; mm Hg) as predictor and moderated by APOE (ε2+, ε3ε3, ε4+) groupings. Three categories of PP are depicted for convenience of display. Age in actual years was the metric of change. The age variable was centered at 75 years. Moderation analyses D = 45.4, Δdf = 14, p < .001.
Figure 3.
Predicted growth curve model of executive function (EF) with continuous pulse pressure (PP; mm Hg) as predictor and moderated by CLU (TT, TC, CC) groupings. Three categories of PP are depicted for convenience of display. Age in actual years was the metric of change. The age variable was centered at 75 years. Moderation analyses D = 25.2, Δdf = 14, p = .033.
For RQ2, we examined the combined genetic effects of these two polymorphisms. The analyses showed that the APOE plus CLU combination exhibited a significant interaction effect with PP on EF performance and change (Figure 4). Specifically, the low risk group (Figure 4a) exhibited non-significant diversity in EF performance (bi = −.079, p = .602) and change (bs = −.006, p = .486) across the three levels of PP. In contrast, in the highest genetic risk group (Figure 4c), PP predicted both EF level at age 75 years (bi = −.411, p <.001) and 9-year EF change (bs = −.028, p <.001). We observed a similar pattern in the moderate risk group (Figure 4b) for EF performance (bi = −.221, p = .007) and change (bs = −.016, p = .005). Examining the spreading of slope effect over the PP levels, we noted that APOE ε2+ (Figure 2a) exhibits the least diversity between levels of PP in EF performance at age 75 (intercept) whereas, CLU TT (Figure 3a) exhibits the least diversity in 9-year EF change (slope) compared to their genetic risk counterparts. This may indicate that APOE ε2 carriers influence more risk-reduction in level of EF performance and CLU TT homozygotes are associated with more risk-reduction in EF change. Thus, the combination of APOE and CLU low risk (Figure 4a) may add important information for both EF level and change to that available with APOE or CLU alone.
4. Discussion
The general aim of this research was to examine whether two established AD genetic risk factors moderated the effect of a known vascular health risk factor on cognitive changes in non-demented older adults. Using conditional growth modelling of longitudinal data we found significant interactive effects of PP with APOE or CLU (independently and in combination) on EF performance and nine-year change. In both cases, the effect of poor vascular health was mitigated for carriers of risk-reducing (or protective) alleles of AD-related genotypes.
We began by confirming the expected “fan” (or spreading of slope) effect of PP on EF performance and change, with worsening vascular health predicting steeper cognitive decline (Figure 1; see also McFall et al., 2014). These results, corroborating the systematic stepwise effect of vascular health on cognitive performance and change in non-demented aging, constituted the necessary benchmark for the planned interaction analyses. We next tested the direct independent effects of the two genotypes on cognitive level or change, observing no significant association. Such null results are often observed in independent SNP-phenotype analyses (Harris and Deary, 2011) and positive associations are not required for examining gene × health interactions.
Accordingly, as shown in Figures 2 and 3, we observed two significant interaction effects. In both cases, the results showed that the simple fan effects of PP on EF change in non-demented aging are moderated independently by each of the AD-related genetic polymorphisms. Notably, we observed that the deleterious effects of poor vascular health on EF performance and change were evident in both moderate- and high- risk APOE and CLU genotypes. Essentially, the overall fan effect of PP on cognition was replicated in these genetic risk sub-groups. In contrast, for both polymorphisms, not only was the fan effect not observed in the low-risk genotypes but the level (intercept) differences were not significant and the decline slopes were somewhat reduced, relative to those seen in carriers of the risk-elevating genotypes. These results are consistent with an inference of protection against the powerful negative effects associated with increasingly poorer vascular health.
Putting these concordant interaction results in context, we note first that the APOE ε4 and CLU C carriers showed poorer EF performance at age 75 and 9-year decline with higher PP levels. Although similar results (on other cognitive domains) have been reported for APOE ε4 carriers (McFall et al., 2015b), we are not aware of closely corresponding results for CLU. Both APOE and CLU are commonly studied and replicated SNPs for dementia and occasionally (but not always) show associations with cognitive deficits in non-demented aging. For example, APOE ε4+ allelic risk has been associated with poorer cognitive performance (Bender and Raz, 2012; Fotuhi et al., 2009) and APOE ε2+ with preserved cognitive functioning in non-demented adults (Deary et al., 2004a; Deary et al., 2004b; Lindahl-Jacobsen et al., 2013). Similarly, in a smaller literature, cognitive decline has been observed among CLU C+ risk carriers who eventually reached MCI status (Thambisetty et al., 2013). However, that they both moderate the negative effects of worsening pulse pressure—in homologous patterns—has not been previously reported. The apolipoprotein E (ApoE) protein is involved in brain lipid metabolism and recent studies suggest that this role may be acquired by other lipoproteins in the brain such as clusterin (CLU), which is also known as Apolipoprotein J. CLU is a multifunctional protein that interacts with a variety of molecules including lipids and amyloid proteins. Both ApoE and CLU play a role in amyloid beta clearance in the brain by binding to lipoprotein receptors (Jones et al., 2010). As hypothesized, the two genetic variants, with some similar structure and function, independently moderated the effects of poor vascular health on older adult EF performance and change—and they did so with similar patterns of selective protection for low-risk genotype carriers in the context of substantial vascular health risk for the moderate and high-risk genotype carriers.
Given these favorable results we then moved to our second research question. We tested the cumulative effects of APOE and CLU in interaction with PP on EF performance and change. We again observed results consistent with our risk-reduction hypothesis (Figure 4). Low combined allelic risk from both APOE and CLU showed patterns that were consistent with an interpretation of risk suppression or protection for even those adults with poor vascular health. Poor vascular health leads to microvascular disease that can result in reduced brain tissue volume and widespread changes in brain function, especially in the prefrontal cortex (Chuang et al., 2014; Raz et al., 2003; Raz et al., 2007). Notably, ε2+ carriers exhibit increased levels of ApoE protein when compared to ε3/ε3 or ε4+ carriers. This increase may lead to a preserved ability to repair neuronal damage in ε2 carriers (McFall et al., 2015b). In addition, CLU has been linked with nerve cell survival and post-injury neuroplasticity, and both CLU and ApoE have similar neuronal function. Cumulatively, CLU and APOE may influence the impact of vascular health damage specifically in the frontal lobe (Wu et al., 2012). As expected, we also observed that moderate and high genetic risk of APOE and CLU combined to magnify the negative effects of poor vascular health, as shown by lower EF performance and steeper EF decline. Prior reports indicate that APOE allelic risk may change the amount of CLU present in the frontal lobe in AD (Harr et al., 1996; Nuutinen et al., 2009; Wu et al., 2012), thereby reducing neuronal repair. Our results support this observation and further link APOE and CLU to EF performance and the frontal cortex. However, we observed no evidence to suggest that the combined effect of the two AD-risk genes produced more than marginally different patterns than those observed separately for each polymorphism. Future work may examine other genetic risk scores as constituted with additional complementary biological mechanisms in order to perhaps separate the patterns observed with the moderate and high risk carriers.
There are five main limitations of our study. First, VLS participants may represent a segment of the older adult population with some relevant advantages, including access to universal health care and relatively high levels of education. Although our sample is not representative of all older adults, it does provide a good estimation of genetic and health factors affecting cognitive performance in a growing segment of the population of older adults in western countries. Second, we considered one important aspect of vascular health (PP), but future research could benefit from a broader representation of vascular health or reactivity. However, PP as a proxy for pulse wave velocity has previously demonstrated sensitivity to cognitive change in non-demented older adults (McFall et al., 2014) and was observed to be sensitive, as expected, in this study. Third, although this sample is relatively large and includes three waves (over 9 years) it should be noted, again, that not all participants had an opportunity to contribute to a third wave. However, this design characteristic did not affect the results at least as evidenced by the invariance testing (as reported in Table 2) and change-related analyses. Fourth, we investigated two selected AD-related genes even though many others exist with the potential to influence EF through synergistic association with vascular health. We chose APOE and CLU (APOJ) specifically given their robust association with cognitive change and mechanistic similarities. Future studies could examine other cognition- and AD-related genetic polymorphisms. Fifth, our results are robust and theoretically coherent, but they are not tested beyond the interactive nexus of connections among these AD-related genetic and bio-health risk factors and the EF phenotype. We underscore the extent to which dynamically interactive factors in non-demented aging require careful a priori biological coherence in order to produce viable mechanistic interpretations.
There are also several strengths of this study. First, we used multiple standard and well-established manifest variables which contributed to a validated, longitudinal EF latent variable. Second, we used an accelerated longitudinal design such that the combination of multiple age cohorts allowed for the inclusion of adults spanning a wide range of age (a band of about 40 years). Third, we employed contemporary statistical methods designed specifically to most accurately investigate our research goals. Fourth, our approach and results emphasize neurobiological and neurocognitive coherence in the context of dynamic changes and interactions predicting an important phenotype in non-demented aging.
In sum, we observed that the risk of poor vascular health on EF performance and change in non-demented older adults is evident in the presence of APOE and CLU genetic risk polymorphisms, both independently and cumulatively. Moreover, most interestingly, we found that risk-reducing APOE and CLU genotypes protected against the deleterious effects of moderate to high PP on EF. Notably, our results suggested that the combination of neurodegenerative-related genetic polymorphisms may provide important EF level and slope information beyond that of either individual SNP. Further investigation of the cumulative risk of these (and other) genetic polymorphisms related to neurodegeneration holds promise for understanding the complex genetic and health interactions that produce differential trajectories (from shallow to steep) of cognitive decline with biological aging.
Highlights.
Poor vascular health is associated with poorer older adult executive function (EF)
APOE low risk carriers are protected from negative effects of poor vascular health
CLU low risk carriers are protected from negative effects of poor vascular health
APOE plus CLU risk may contribute different but complementary EF predictions
Acknowledgments
The VLS is supported by a grant from the National Institutes of Health (National Institute on Aging) to R. A. Dixon (R01 AG008235), who is also supported by the Canada Research Chairs program. The VLS genetic initiative also received support by a grant from Alberta Health Services and University Hospital Foundation to Roger A. Dixon, Jack Jhamandas, and David Westaway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication., As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Hazzouri AZ, Yaffe K. Arterial stiffness and cognitive function in the elderly. J Alzheimers Dis. 2014;42(0 4):S503–14. doi: 10.3233/JAD-141563. doi:10.3233/JAD-141563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ, Eramudugolla R, Hosking DE, Lautenschlager NT, Dixon RA. Bridging the translation gap: From dementia risk assessment to advice on risk reduction. J Prev Alzheimers Dis. 2015;2(3):189–98. doi: 10.14283/jpad.2015.75. doi:10.14283/jpad.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger A, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: A meta-analysis. Neuropsychology. 2005;19(4):520–31. doi: 10.1037/0894-4105.19.4.520. doi:org/10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bangen KJ, Beiser A, Delano-Wood L, Nation DA, Lamar M, Libon DJ, Bondi MW, Seshadri S, Wolf PA, Au R. APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis. 2013;22(8):1361–9. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.013. doi:org/10.1016/j.jstrokecerebrovasdis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral S, Bird T, Goate A, Farlow MR, Diaz-Arrastia R, Bennett DA, Graff-Radford N, Boeve BF, Sweet RA, Stern Y, Wilson RS, Foroud T, Ott J, Mayeux R. National Institute on Aging Late-Onset Alzheimer's Disease Genetics, S. Genotype patterns at PICALM, CR1, BIN1, CLU, and APOE genes are associated with episodic memory. Neurology. 2012;78(19):1464–71. doi: 10.1212/WNL.0b013e3182553c48. doi:10.1212/WNL.0b013e3182553c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AR, Raz N. Age-related differences in memory and executive functions in healthy APOE ε4 carriers: The contribution of individual differences in prefrontal volumes and systolic blood pressure. Neuropsychologia. 2012;50(5):704–14. doi: 10.1016/j.neuropsychologia.2011.12.025. doi: 10.1016/j.neuropsychologia.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298(11):1300–11. doi: 10.1001/jama.298.11.1300. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- Bérard E, Bongard V, Ruidavets J, Amar J, Ferrieres J. Pulse wave velocity, pulse pressure and number of carotid or femoral plaques improve prediction of cardiovascular death in a population at low risk. J Hum Hypertens. 2013;27(9):529–34. doi: 10.1038/jhh.2013.8. doi: 10.1038/jhh.2013.8. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934. doi:10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Bielak AA, Mansueti L, Strauss E, Dixon RA. Performance on the Hayling and Brixton tests in older adults: Norms and correlates. Arch Clin Neuropsychol. 2006;21(2):141–9. doi: 10.1016/j.acn.2005.08.006. doi:10.1016/j.acn.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang Y-F, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, Rebok GW, Tanner EK, Carlson MC. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol Aging. 2014;35(6):1396–403. doi: 10.1016/j.neurobiolaging.2013.12.008. doi: 10.1016/j.neurobiolaging.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo R, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE* 4 a ‘thrifty’allele? Ann Hum Genet. 1999;63(4):301–10. doi: 10.1046/j.1469-1809.1999.6340301.x. doi:10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, Roses A, Haines J, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–3. doi: 10.1126/science.8346443. doi:10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Structure of four executive functioning tests in healthy older adults. Neuropsychology. 2006;20(2):206–14. doi: 10.1037/0894-4105.20.2.206. doi:10.1037/0894-4105.20.2.206. [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, Strauss E. Characterizing executive functioning in older special populations: From cognitively elite to cognitively impaired. Neuropsychology. 2009;23(6):778–91. doi: 10.1037/a0016743. doi:10.1037/a0016743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, Starr JM, Hayward C, Wright AF, Visscher PM, Tynan MC, Whalley LJ. Apolipoprotein e gene variability and cognitive functions at age 79: a follow-up of the Scottish mental survey of 1932. Psychol Aging. 2004a;19(2):367–71. doi: 10.1037/0882-7974.19.2.367. doi:10.1037/0882-7974.19.2.367. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Wright AF, Harris SE, Whalley LJ, Starr JM. Searching for genetic influences on normal cognitive ageing. Trends Cogn Sci. 2004b;8(4):178–84. doi: 10.1016/j.tics.2004.02.008. doi:10.1016/j.tics.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Dixon RA, de Frias CM. The Victoria longitudinal study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsych C. 2004;11(2-3):346–76. doi:10.1080/13825580490511161. [Google Scholar]
- Dixon RA, Small BJ, MacDonald SWS, McArdle JJ. Yes, memory declines with aging— but when, how, and why? In: Naveh-Benjamin M, Ohta N, editors. Memory and aging: Current issues and future directions. Psychology Press; New York, NY: 2012. pp. 325–47. [Google Scholar]
- Ferencz B, Laukka EJ, Welmer AK, Kalpouzos G, Angleman S, Keller L, Graff C, Lövdén M, Bäckman L. The benefits of staying active in old age: physical activity counteracts the negative influence of PICALM, BIN1, and CLU risk alleles on episodic memory functioning. Psychol Aging. 2014;29(2):440–9. doi: 10.1037/a0035465. doi:org/10.1037/a0035465. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. doi:10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5(12):649–58. doi: 10.1038/nrneurol.2009.175. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2008;14(02):266–78. doi: 10.1017/S1355617708080302. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harr SD, Uint L, Hollister R, Hyman BT, Mendez AJ. Brain expression of apolipoproteins E, J, and A-I in Alzheimer's disease. J Neurochem. 1996;66(6):2429–35. doi: 10.1046/j.1471-4159.1996.66062429.x. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends Cogn Sci. 2011;15(9):388–94. doi: 10.1016/j.tics.2011.07.004. doi:org/10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Jochemsen HM, Muller M, Bots ML, Scheltens P, Vincken KL, Mali WP, van der Graaf Y, Geerlings MI, Algra A, Doevendans P. Arterial stiffness and progression of structural brain changes The SMART-MR study. Neurology. 2015;84(5):448–55. doi: 10.1212/WNL.0000000000001201. doi: 10.1212/WNL.0000000000001201. [DOI] [PubMed] [Google Scholar]
- Jones L, Harold D, Williams J. Genetic evidence for the involvement of lipid metabolism in Alzheimer's disease. BBA-Mol Cell Biol L. 2010;1801(8):754–61. doi: 10.1016/j.bbalip.2010.04.005. doi: 10.1016/j.bbalip.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Josefsson M, Luna X, Pudas S, Nilsson LG, Nyberg L. Genetic and lifestyle predictors of 15-year longitudinal change in episodic memory. J Am Geriatr Soc. 2012;60(12):2308–12. doi: 10.1111/jgs.12000. doi: 10.1111/jgs.12000. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. Guilford Press; New York, NY: 2011. [Google Scholar]
- Lambert JC, Amouyel P. Genetics of Alzheimer's disease: new evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21(3):295–301. doi: 10.1016/j.gde.2011.02.002. doi:10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Lindahl-Jacobsen R, Tan Q, Mengel-From J, Christensen K, Nebel A, Christiansen L. Effects of the APOE ε2 allele on mortality and cognitive function in the oldest old. J Gerontol A-Biol. 2013;68(4):389–94. doi: 10.1093/gerona/gls192. doi: 10.1093/gerona/gls192. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li S-C, Heekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci. 2008;2(2):234–44. doi: 10.3389/neuro.01.039.2008. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD. Longitudinal structural equation modeling. Guilford Press; New York, NY: 2013. [Google Scholar]
- Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18. doi: 10.1038/nrneurol.2012.263. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszcz M. Executive function and cognitive aging. In: Schaie KW, Willis SL, editors. The handbook of the psychology of aging. Academic Press; San Diego, CA: 2011. pp. 59–72. [Google Scholar]
- McCoach DB, Black AC, O'Connell AA. Errors of inference in structural equation modeling. Psychol Sch. 2007;44(5):461–70. doi: 10.1002/pits.20238. [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Anstey KJ, Dixon RA. Alzheimer's genetic risk intensifies neurocognitive slowing associated with diabetes in nondemented older adults. Alzheimers Dement: Diagnosis, Assessment & Disease Monitoring. 2015a;1(4):395–402. doi: 10.1016/j.dadm.2015.08.002. doi:10.1016/j.dadm.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Jhamandas J, Westaway D, Dixon RA. IDE (rs6583817) polymorphism and pulse pressure are independently and interactively associated with level and change in executive function in older adults. Psychol Aging. 2014;29(2):418–30. doi: 10.1037/a0034656. doi:org/10.1037/a0034656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Westaway D, Jhamandas J, Bäckman L, Dixon RA. ApoE and pulse pressure interactively influence level and change in the aging of episodic memory: Protective effects among ε2 carriers. Neuropsychology. 2015b;29(3):388–401. doi: 10.1037/neu0000150. doi:org/10.1037/neu0000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Westaway D, Jhamandas J, Dixon RA. IDE (rs6583817) polymorphism and type 2 diabetes differentially modify executive function in older adults. Neurobiol Aging. 2013;34(9):2208–16. doi: 10.1016/j.neurobiolaging.2013.03.010. doi:10.1016/j.neurobiolaging.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JA, Hatters DM, Lu B, Höchtl P, Oberg KA, Rupp B, Weisgraber KH. Apolipoprotein E4 forms a molten globule. A potential basis for its association with disease. J Biol Chem. 2002;277(52):50380–5. doi: 10.1074/jbc.M204898200. doi: 10.1074/jbc.M204898200. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Muthén & Muthén; Los Angeles, CA: 1998-2012. [Google Scholar]
- Nathan J, Wilkinson D, Stammers S, Low JL. The role of tests of frontal executive function in the detection of mild dementia. Int J Geriatr Psychiatry. 2001;16(1):18–26. doi: 10.1002/1099-1166(200101)16:1<18::aid-gps265>3.0.co;2-w. doi: 10.1002/1099-1166(200101)16:1<18::AID-GPS265>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81(23):2024–7. doi: 10.1212/01.wnl.0000436935.47657.78. doi: 10.1212/01.wnl.0000436935.47657.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer's disease. Brain Res Rev. 2009;61(2):89–104. doi: 10.1016/j.brainresrev.2009.05.007. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Bäckman L, Nagel IE, Nietfeld W, Schröder J, Bertram L, Heekeren HR, Lindenberger U, Li S-C. COMT polymorphism and memory dedifferentiation in old age. Psychol Aging. 2014;29(2):374–83. doi: 10.1037/a0033225. doi: org/10.1037/a0033225. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Fagard R, Thijs L, Wang J-G, Forette F, Pereira L, Fletcher A, Bulpitt C. Increased pulse pressure linked to dementia: further results from the Hypertension in the Very Elderly Trial–HYVET. J Hypertens. 2013;31(9):1868–75. doi: 10.1097/HJH.0b013e3283622cc6. doi: 10.1097/HJH.0b013e3283622cc6. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Viitanen M, Fratiglioni L. Pulse pressure and risk of Alzheimer disease in persons aged 75 years and older a community-based, longitudinal study. Stroke. 2003;34(3):594–9. doi: 10.1161/01.STR.0000060127.96986.F4. doi: 10.1161/01.STR.0000060127.96986.F4. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). Am J Geriatr Psychiatry. 2005;13(2):134–41. doi: 10.1176/appi.ajgp.13.2.134. doi: 10.1176/appi.ajgp.13.2.134. [DOI] [PubMed] [Google Scholar]
- Raz N, Dahle CL, Rodrigue KM, Kennedy KM, Land S. Effects of age, genes, and pulse pressure on executive functions in healthy adults. Neurobiol Aging. 2011;32(6):1124–37. doi: 10.1016/j.neurobiolaging.2009.05.015. doi: 10.1016/j.neurobiolaging.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117(6):1169–80. doi: 10.1037/0735-7044.117.6.1169. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke E. Brain aging and its modifiers. Ann N Y Acad Sci. 2007;1097(1):84–93. doi: 10.1196/annals.1379.018. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota S, Vergote D, Westaway D, Jhamandas J, Dixon RA. Synergistic associations of catechol-O-methyltransferase and brain-derived neurotrophic factor with executive function in aging are selective and modified by apolipoprotein E. Neurobiol Aging. 2015;36(1):249–56. doi: 10.1016/j.neurobiolaging.2014.06.020. doi: 10.1016/j.neurobiolaging.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers O, Harris S, Gow A, Pattie A, Brett C, Starr J, Deary I. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17(3):315–24. doi: 10.1038/mp.2010.137. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E. Arterial stiffness, the brain and cognition: a systematic review. Ageing Res Rev. 2014;15:16–27. doi: 10.1016/j.arr.2014.02.002. doi: 10.1016/j.arr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Sleegers K, Bettens K, De Roeck A, Van Cauwenberghe C, Cuyvers E, Verheijen J, Struyfs H, Van Dongen J, Vermeulen S, Engelborghs S. A 22-single nucleotide polymorphism Alzheimer risk score correlates with family history, onset age, and cerebrospinal fluid Aβ 42. Alzheimers Dement. 2015;11(22):1452–60. doi: 10.1016/j.jalz.2015.02.013. doi:10.1016/j.jalz.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ. Tracking cognition-health changes from 55 to 95 years of age. J Gerontol B Psychol. 2011;66(Suppl 1):153–61. doi: 10.1093/geronb/gbq093. doi:org/10.1093/geronb/gbq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. doi:10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 20112011 doi: 10.4061/2011/263585. doi:org/10.4061/2011/2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neurosci Biobehav Rev. 2013;37(10):2878–86. doi: 10.1016/j.neubiorev.2013.10.010. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Beason-Held LL, An Y, Kraut M, Nalls M, Hernandez DG, Singleton AB, Zonderman AB, Ferrucci L, Lovestone S, Resnick SM. Alzheimer risk variant CLU and brain function during aging. Biol Psychiatry. 2013;73(5):399–405. doi: 10.1016/j.biopsych.2012.05.026. doi:10.1016/j.biopsych.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81(11):984–91. doi: 10.1212/WNL.0b013e3182a43e1c. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;53:121–30. doi: 10.1016/j.neubiorev.2015.03.011. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wu Z-C, Yu J-T, Li Y, Tan L. Clusterin in Alzheimer's disease. Adv Clin Chem. 2012;56:155. doi: 10.1016/b978-0-12-394317-0.00011-x. doi: 10.1016/B978-0-12-394317-0.00011-X. [DOI] [PubMed] [Google Scholar]
- Yaneva-Sirakova T, Tarnovska-Kadreva R, Traykov L. Pulse pressure and mild cognitive impairment. J Cardiovasc Med. 2012;13(11):735–40. doi: 10.2459/JCM.0b013e328357ba78. doi: 10.2459/JCM.0b013e328357ba78. [DOI] [PubMed] [Google Scholar]