Abstract
Background & aims
It is unknown whether the addition of locoregional therapies (LRTx) to sorafenib improves prognosis over sorafenib alone in patients with advanced hepatocellular carcinoma (HCC). The aim of this study was to assess the effect of LRTx in this population.
Methods
A retrospective analysis was performed of patients with advanced HCC as defined by extrahepatic metastasis, lymphadenopathy >2 cm, or gross vascular invasion. Sorafenib therapy was required for inclusion. Survival of patients who received LRTx after progression to advanced stage was compared to those who did not receive LRTx.
Results
Using an intention to treat analysis of 312 eligible patients, a propensity weighted proportional hazards model demonstrated LRTx as a predictor of survival (HR = 0.505, 95% CI: 0.407–0.628; P < 0.001). The greatest benefit was seen in patients with the largest tumor burden (HR = 0.305, 95% CI: 0.236–0.393; P < 0.01). Median survival in the sorafenib arm was 143 days (95% CI: 118–161) vs. 247 days (95% CI: 220–289) in the sorafenib plus LRTx arm (P < 0.001).
Conclusions
These results demonstrate a survival benefit with the addition of LRTx to sorafenib for patients with advanced HCC. These findings should prompt a prospective clinical trial to further assess the role of LRTx in patients with advanced HCC.
Introduction
There are limited therapeutic options for patients with advanced hepatocellular carcinoma (HCC), as defined by locally advanced disease or distant metastases. Despite intense research in the field, the only treatment that has been found to provide a survival benefit for this group of patients is the oral multi-kinase inhibitor sorafenib. In the two randomized controlled trials studying this agent, individuals treated with sorafenib versus placebo experienced a survival benefit of 10.7 versus 7.9 months, and 6.5 versus 4.2 months.1, 2 As a result, sorafenib became the standard of care and is the only treatment recommended by the Barcelona Clinic Liver Cancer (BCLC) classification for patients with advanced HCC.3 According to these widely accepted guidelines, patients with advanced HCC should receive only sorafenib without any additional treatment modalities. Nevertheless, liver-directed locoregional therapies (LRTx) such as embolization, ablation, and radiation therapy are often employed in patients with advanced HCC, although it is unknown whether such treatment improves prognosis over sorafenib alone.4
The benefit of LRTx in treating non-advanced tumors is well established, including level 1 evidence from randomized controlled trials: in candidate patients, the use of liver-directed modalities such as chemoembolization, radioembolization, ablative technologies, and external beam radiation therapy has been shown to significantly extend survival.5, 6, 7, 8, 9, 10, 11, 12 Given the efficacy of LRTx in earlier stage patients, some clinicians continue to use LRTx in advanced patients in order to maintain local control of the primary hepatic tumor. Since the preponderance of the tumor burden in patients with advanced HCC is typically the primary hepatic lesion, it is conceivable that a liver-directed therapy may help prolong survival when combined with systemic treatment with sorafenib.13, 14
Therefore, the primary aim of this study was to assess the effect of locoregional therapies on overall survival in patients with advanced HCC treated with sorafenib.
Methods
This study was a retrospective analysis of patient data at an urban tertiary care center. After obtaining approval from the local Institutional Review Board, a query of the medical records was performed to identify all patients prescribed sorafenib at a single, large, academic, hospital from 2006 to 2014. A manual review of the entire cohort was performed to confirm HCC diagnosis, according to accepted radiologic and/or pathologic criteria.15 Date of diagnosis of advanced stage disease was determined by the presence of extrahepatic metastasis, local lymphadenopathy greater than 2 cm, and/or gross vascular invasion (GVI) on imaging.
Data on clinical variables that were considered most likely to confound treatment decisions were recorded: age (≤55, 56–70, >70 years), gender, number of major comorbidities including hypertension, diabetes, cardiovascular disease, pulmonary disease, or renal insufficiency (0, 1, ≥2), primary liver disease (hepatitis B, hepatitis C, other), liver function (Child-Pugh category), gross vascular invasion (yes, no), the presence of ascites (yes, no), extra-hepatic disease (none, 1 extrahepatic site, ≥2 sites), largest liver tumor (<5 cm, 5–10 cm, ≥10 cm and/or innumerable metastases), alphafetoprotein (< or ≥400), performance status (ECOG score), history of prior liver resection, and history of prior LRTx. Additional data collected included race, income (by quartile using median income corresponding to patient's ZIP code), prior off-label use of sorafenib, treating physician, as well as information when available on degree of tolerance of/compliance with sorafenib – as defined below.
Any duration or dose of prior sorafenib therapy was considered sufficient for study inclusion, and patients were included whether they remained on drug, or if treatment had been discontinued due to tumor progression or intolerable side effects. During data collection it was noted that some patients had received off-label sorafenib therapy for high-risk tumors prior to a radiologic diagnosis of advanced stage disease. To evaluate the contribution of these factors, a binary indicator of whether off-label sorafenib use had occurred was entered as a variable in the analysis.
Next, patients who had undergone LRTx after the date of diagnosis of advanced stage were identified and the cohort was divided into two arms: “sorafenib” versus “sorafenib plus LRTx” on an intent to treat basis as described below. Allowed LRTx types were chemoembolization, radioembolization, ablative therapies, and external beam radiation therapy. All patients were presented at our weekly multidisciplinary tumor board and consensus on treatment was made after taking into account relevant tumor and patient characteristics. In general, patients were selected for ablation if the site of disease was a single intrahepatic metastasis <3 cm in size; chemoembolization was undertaken for multifocal HCC without portal vascular invasion, radioembolization for HCC with portal vascular invasion, and external beam radiation if the disease was not amenable to the other therapies. Patients were excluded from analysis if they were not candidates for LRTx due to Child-Pugh C cirrhosis, or because of adequate control of the primary tumor (e.g. history of resection/transplantation or complete response to prior LRTx).
Patients with a history of LRTx administration prior to their advanced stage were classified into the sorafenib arm if no repeat LRTx was performed after the diagnosis of advanced disease. Efforts were made to avoid potential bias inherent in this classification: firstly, since undergoing LRTx would prompt new imaging which could detect asymptomatic metastases, patients who had LRTx within the 30 days prior to date of advanced diagnosis were classified into included in the LRTx arm; secondly, prior LRTx (preceding 30 days) was included in the set of covariates considered as potential confounders in the later analysis.
The primary objective of the study was to assess the relationship between overall survival from date of diagnosis of advanced stage disease and the use of LRTx in the advanced stage. A secondary objective was the assessment of heterogeneity in this relationship according to liver tumor burden or presence of GVI. This was motivated by the a priori theory that patients with a high liver tumor burden or GVI might benefit most from LRTx. The expression of such heterogeneities was by means of interaction effects in a pre-specified secondary analysis of the interaction of treatment with tumor burden and GVI.
This study utilizes an intention to treat (ITT) principle in an observational study context. This approach, and more broadly that of ‘Designed Observational Studies’, has been advocated for its robustness and conservativeness as an approximation to the results of a randomized trial with an intent to treat analysis.16, 17 Specifically, an ‘as treated’ analysis would suffer from numerous self-selection biases, including that due to death before treatment, as well as ‘immortality time’ biases due to the impossibility of any patient classified as ‘treated’ experiencing mortality before the treatment has occurred. This concern is especially relevant since performance of LRTx can require several weeks of preparatory imaging and insurance clearance so that successful attainment of treatment would itself identify patients with longer survival. Initial treatment intention among the selected cohort was determined by detailed chart review of doctors' notes: if the treatment plan declared intended use of LRTx, such a patient was considered as having ‘intention to treat’. ITT had to be declared within the first 60 days after diagnosis of advanced disease. The impact of any ‘immortal time’ on the primary analysis was assessed in later sensitivity analyses.
The primary analysis of survival used a proportional hazards model for comparison of the ITT groups using propensity score-derived weights to balance measured confounders. Specifically, a first stage propensity score model was used to predict ITT status as a function of the potential confounders above. The model was developed using stepwise selection of predictors in logistic regression with entry criterion P = 0.3 and retention criterion P = 0.2. Inverse predicted probabilities were used to construct the re-weighted sample. Balance between treatment arms after re-weighting was verified by confirming the lack of associations with treatment arm in weighted chi-square tests. Variables with P values >0.1 were assumed to be well-balanced between groups; variables with P values <0.1 were considered to be potentially imbalanced and were included in sensitivity analyses. Secondary analyses of treatment heterogeneity by liver tumor burden and by GVI were performed by adding the corresponding interaction effects into the primary model, since there was a priori suspicion that these two variables might predict benefit to LRTx.
Due to clinical judgment concerning the time period over which a survival advantage might exist, and in order to adhere to the proportional hazards assumption of the statistical models, survival times as analyzed were truncated at 500 days post diagnosis of advanced stage.
Efforts were made to eliminate other potential biases in the primary results. The sensitivity to changes in the propensity score model was assessed by weight trimming. Guarantee time bias was ruled out using the conditional landmark method at cutpoints of 30 (the approximate median time from visit to treatment decision) and 60 days (the maximum allowed time to treatment decision). Specifically, it was verified that there was no significant association of mortality with the ITT group in the period before these cutpoints, and that the primary model retained the direction and significance of the main ITT effect in the following period after these cutpoints.
Other covariates of race, income, previous LRTx, and prior off-label use of sorafenib were each individually added to the primary model to assess changes to the magnitude and significance of the results. Similarly, the effect of treating physician was assessed by adding fixed effects for each of the 8 attending physicians into the model. A final proposed mechanism for confounding – that differing rates of sorafenib compliance or tolerance across the treatment arms could bias the findings – was assessed as follows: compliance status information that could be extracted from documentation was dichotomized into categories of compliance corresponding to use of sorafenib for at least two weeks or less than two weeks; this was deemed the minimum time period over which sorafenib use could affect survival. Among the subset of patients where such a compliance determination was possible, the primary model was re-fit and the effect estimates were compared to those of the primary model.
All analyses were done in SAS version 9.3. Throughout this study significance was decide based on a P-value less than 0.05; P-values less than 0.1 were considered suggestive.
Results
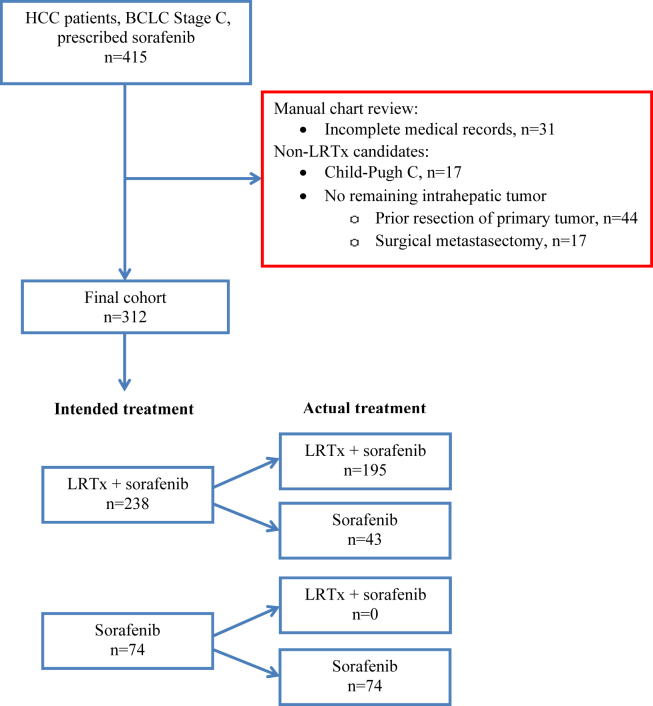
A total of 312 eligible patients were identified with advanced HCC and who met eligibility criteria (Fig. 1). LRTx type delivered was chemoembolization in 143/195 (73.3%), radioembolization in 36/195 (18.5%), external beam radiation in 14/195 (7.2%) and ablation in 1/195 (0.5%) of patients; with more than one type/session used in 105 patients during the study period.
Figure 1.
Criteria for cohort creation
Features of the entire study group were as follows; mean age: 61.6 years; males: 80.4%; hepatitis C: 46.2%; ECOG score 0: 53.5%, score 1: 35.3%, score: 2: 7.7%, score 3: 3.5%; major comorbidities 0: 58.7%, 1: 29.5%, ≥2: 11.9%; Child-Pugh score A: 64.1%, grade B: 35.9%; ascites none: 66.3%, mild/moderate: 29.5%, severe: 4.2%; GVI: 67.3%; extrahepatic metastasis none: 51.2%, 1: 40.7%, ≥2 sites: 8.0%; largest liver tumor <5 cm: 11.9%, 5–10 cm: 27.2%, >10 cm: 60.9%, median AFP 302.1 ng/mL. A history of prior LRTx was noted in 44.2% of patients in the study. Information on compliance with sorafenib therapy was only available on 261 of the patients; out of those, 11.9% received for less than 2 weeks. Off-label treatment with sorafenib prior to radiologic evidence of advanced disease was prescribed in 16% of patients.
Despite propensity score re-weighting, clinical variables were not evenly distributed between the two study arms. Four variables remained unbalanced including number of comorbidities (P = 0.040), gross vascular invasion (P < 0.001), extrahepatic disease (P < 0.001), and AFP level (P < 0.001) (Table 1). This was despite adequate fit of the first stage propensity score model using standard performance metrics (c statistic = 0.84, Hosmer Lemeshow P = 0.75). These factors were included in a sensitivity analysis to determine their impact on all the primary and secondary results.
Table 1.
Patient characteristics by intention to treat: unweighted, and after propensity weighting
| Unweighted |
Weighted |
|||||
|---|---|---|---|---|---|---|
| Sorafenib only, n (%) | Sorafenib + LRTx, n (%) | P-value | Sorafenib only (%) | Sorafenib + LRTx (%) | P-value | |
|
Age ≤55 years 56–70 years ≥70 years |
28 (37.8) 33 (44.6) 13 (17.6) |
62 (26.1) 125 (52.5) 51 (21.4) |
0.147 |
35.1 49.8 15.1 |
27.3 53.9 18.8 |
0.101 |
| Male gender | 56 (75.7) | 195 (81.9) | 0.235 | 82.3 | 83.4 | 0.712 |
|
Major comorbidities 0 1 ≥2 |
45 (60.8) 21 (28.4) 8 (10.8) |
138 (58.0) 71 (29.8) 29 (12.2) |
0.902 |
65.7 26.6 7.7 |
56.1 32.2 11.7 |
0.040 |
|
ECOG status 0 1 2–3 |
25 (33.8) 42 (56.8) 7 (9.5) |
142 (59.7) 92 (38.7) 4 (1.7) |
<0.001 |
58.1 37.9 4.0 |
55.3 42.1 2.6 |
0.414 |
|
Liver disease Hepatitis C Hepatitis B Other |
28 (37.8) 22 (29.7) 24 (34.4) |
116 (48.7) 56 (23.5) 66 (27.7) |
0.052 |
49.3 25.2 25.6 |
45.9 24.6 29.5 |
0.727 |
|
Liver function Child-Pugh A Child-Pugh B |
31 (41.9) 43 (58.1) |
169 (71.0) 69 (29.0) |
<0.001 |
58.0 42.0 |
63.8 36.2 |
0.143 |
|
Ascites None Mild/moderate Severe |
38 (51.4) 28 (37.8) 8 (10.8) |
169 (71.0) 64 (26.9) 5 (2.1) |
<0.001 |
69.2 26.6 4.3 |
67.2 29.4 3.4 |
0.672 |
| GVI | 38 (51.4) | 172 (72.3) | <0.001 | 51.1 | 68.2 | <0.001 |
|
Extrahepatic disease None 1 site ≥2 sites |
26 (35.1) 37 (50.0) 11 (14.9) |
134 (56.3) 90 (37.8) 14 (5.9) |
<0.001 |
38.2 52.0 9.8 |
53.2 40.9 5.9 |
<0.001 |
|
Largest liver tumor <5 cm 5–10 cm >10 cm |
6 (8.1) 23 (31.1) 45 (60.8) |
31 (13.0) 62 (26.1) 145 (60.9) |
0.433 |
15.5 28.4 56.1 |
12.0 27.6 60.4 |
0.386 |
|
Alphafetoprotein <400 ≥400 |
37 (52.1) 34 (47.9) |
124 (52.5) 112 (47.5) |
0.949 |
64.7 35.4 |
51.0 49.0 |
<0.001 |
| Prior LRTx | 39 (52.7) | 99 (41.6) | 0.093 | 50.4 | 44.6 | 0.157 |
| Sorafenib prior to advanced stage | 26 (35.1) | 24 (10.1) | <0.001 | 18.3 | 14.4 | 0.188 |
Multivariate model adjusted for variables with P < 0.1 after weighting.
A univariate analysis by ITT was performed to assess which variables were associated with survival. The predictors with significant effects in a univariate cox proportional hazards models were: LRTx, Child-Pugh score, ascites, extrahepatic disease, liver tumor burden, and ECOG score, all with P < 0.001 (Supplemental Table 1).
A weighted proportional hazards model using intention to treat as the predictor of survival is the primary model of this study (Table 2). The hazard ratio for ITT with LRTx was 0.664 (95% CI: 0.544–0.812; P < 0.001). The results of the weighted ITT model when also adjusted for the imbalanced factors (number of comorbidities, gross vascular invasion, extrahepatic disease, AFP) provided a hazard ratio of 0.505 (0.407–0.628, P < 0.001). An as-treated analysis was also performed similarly by repeating the steps from propensity score model development onward; which again revealed benefit to treatment in a univariate weighted proportional hazards model of 0.556 (95% CI: 0.453–0.681; P < 0.001).
Table 2.
Multivariate model assessing the effect of LRTx in patients with advanced HCC treated with sorafenib
| Hazard ratio (95% CI) | |
|---|---|
| ITT analysis (sorafenib vs. sorafenib + LRT) | 0.664 (0.544–0.812) |
| ITT analysis, corrected for imbalances | 0.505 (0.407–0.628) |
| As treated analysis (sorafenib vs. sorafenib + LRT) | 0.555 (0.453–0.681) |
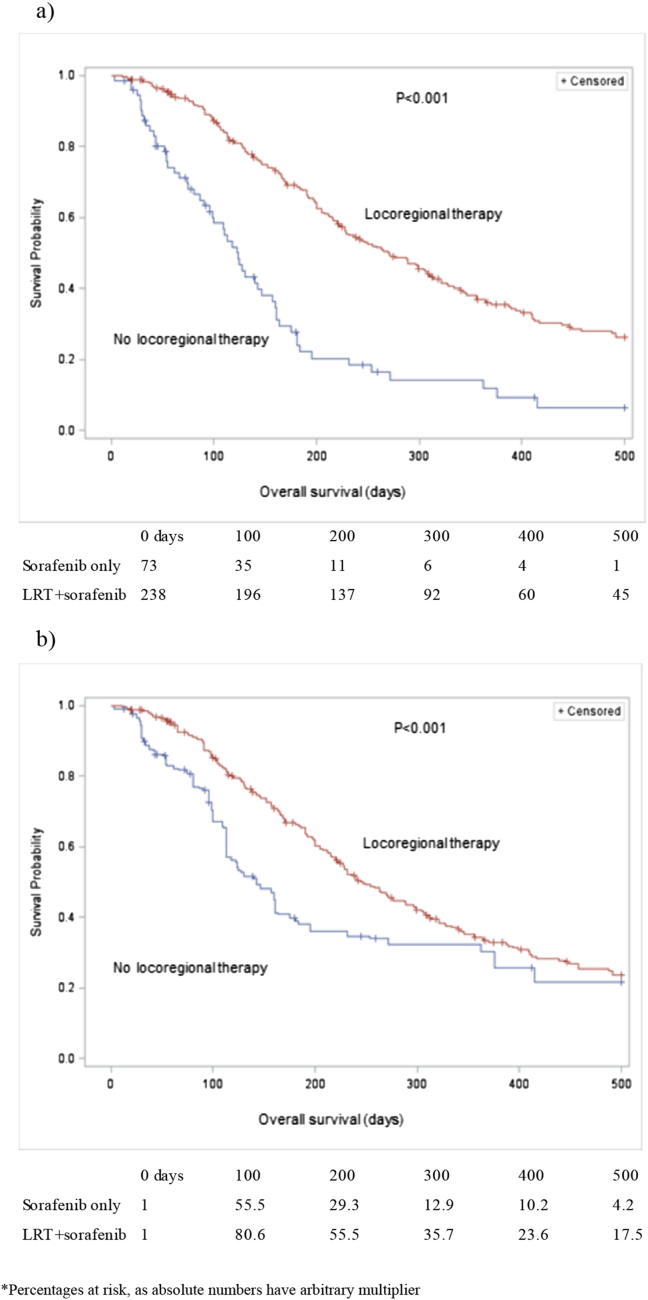
Kaplan Meier curves of survival for both unweighted and weighted ITT arms are shown in Fig. 2a and b, respectively. Median unweighted survival in the sorafenib arm was 123 days (95% CI: 96–146 days) vs. 271 days (95% CI: 228–313 days) in the sorafenib plus intention to treat LRTx arm (P < 0.001) (Fig. 2a). Median weighted survival in the sorafenib arm was 143 days (95% CI: 118–161) vs. 247 days (95% CI: 220–289) in the sorafenib plus intention to treat LRTx arm (P < 0.001) (Fig. 2b).
Figure 2.
a) Unweighted ITT survival curves. b) Weighted ITT survival curves∗
In further sensitivity analyses including individually race, income, previous LRTx, prior off-label use of sorafenib, and treating physician, the results were consistent in direction and significance with those of the primary analysis. Sensitivity analyses for ‘immortality time’ bias as described above also supported the findings across two cutpoints. In the subsample for which information regarding compliance or duration of use of sorafenib was available (261 patients), inclusion of the corresponding covariate in the primary analysis model led to a HR for intended treatment of 0.603 (95% CI: 0.485–0.749; P < 0.001) consistent with the results of the primary analysis (Supplemental Table 2).
The results of two models to assess heterogeneity of effect first by tumor burden and second by GVI status are as follows. In patients whose largest liver tumor was less than 5 cm in size, there was significant survival penalty of LRTx (HR = 3.17, 95% CI: 1.29–7.77; P = 0.012); in those with tumors 5–10 cm in size there was no significant evidence of benefit to LRTx (HR = 0.835, 95% CI: 0.567–1.23; P = 0.36); in patients whose largest liver tumor was greater than 10 cm in size, or in whom there were innumerable tumors, there was a significant benefit to LRTx (HR = 0.305, 95% CI: 0.236–0.393; P < 0.001), test of overall heterogeneity (P < 0.01). It is noted however that after adjustment for all unbalanced covariates the harmful effect of LRT in patients of the lowest tumor burden category lost its significance so that the interpretation of this result may not be clear (HR = 1.96, 95% CI: 0.787–0.492; P > 0.1). With respect to GVI, however, there was no significant evidence of heterogeneity (Test of interaction P > 0.5). In patients without GVI the HR associated with LRTx was 0.630 (95% CI: 0.476–0.833; P = 0.001). In patients with GVI the HR associated with LRTx was 0.660 (95% CI: 0.529–0.824; P < 0.001). These results suggest that the benefit of LRTx was restricted to patients with a large hepatic tumor burden with or without gross vascular invasion.
Discussion
According to current BCLC guidelines, sorafenib is the only recommended treatment in patients with Stage C, or advanced HCC.3 Liver directed LRTx – although validated for earlier stage tumors – is not a recommended therapy in this population. Even after a patient has exhausted sorafenib treatment (either due to intolerable side effects or tumor progression) the use of LRTx is not endorsed by current guidelines.
In the current study, using an intention to treat analysis, a significant survival benefit was seen with the addition of LRTx to prior or current sorafenib use. Patients intended to receive LRTx after the diagnosis with advanced HCC experienced a survival benefit of 3.5 months over patients who were not intended to undergo LRTx. This survival benefit was preserved whether the data were analyzed according to actual or intended treatment, suggesting robustness of the treatment effect.
A secondary analysis of interaction with tumor burden demonstrated heterogeneity in the benefit of LRTx over tumor burden categories, according to which LRTx was estimated to be beneficial among those with the highest tumor burden category, and possibly harmful to those with the lowest tumor burden. Gross vascular invasion did not produce significant evidence of heterogeneity, with both categories experiencing significant benefit. The finding of heterogeneity with respect to tumor burden is biologically plausible since LRTx directly targets the liver, and its beneficial effect would be expected in patients where the preponderance of the tumor burden is within the hepatic parenchyma. “Liver-dominant” disease is the most common type of advanced HCC, suggesting that LRTx can be expected to have a therapeutic effect on most patients with this stage.14 It is difficult to know what ultimately causes death in patients with advanced HCC, and whether it can be attributed to the tumor burden in the liver or metastatic disease. When generalizing to patients with different tumor burdens, prognoses, etc., the drivers of mortality may or may not be the same and there could be different effects to LRTx. Nonetheless, the current results suggest that controlling the primary liver tumor may add a survival benefit to therapy with sorafenib among subgroups within this population.
A recently published study supports the current finding that the use of LRTx may have a therapeutic role in advanced HCC patients. Choi et al. reported that chemoembolization plus sorafenib was superior to sorafenib alone with respect to time to progression in patients with advanced stage HCC, although no improvement in overall survival was detected.18 Other authors have similarly suggested that the BCLC guidelines may be too restrictive in their current form, and that a subset of patients with advanced disease can benefit from more aggressive therapies. For example, in patients with advanced HCC due to portal vein invasion, several publications report on the survival benefit of chemoembolization.19, 20, 21, 22, 23 Similarly in intermediate stage HCC, it has been reported that select patients benefit from surgical resection over the non-curative option of embolization recommended by BCLC.24, 25, 26
This study has some limitations. Sorafenib prescription was required for study inclusion since sorafenib is the standard of care for advanced HCC patients and its use is required for comparison of any other therapeutic option. The majority of patients in this study first received sorafenib after the diagnosis of advanced disease, in accordance with BCLC guidelines. However, also included in this study were patients who received sorafenib off-label prior to progressing to advanced stage. The inclusion of these patients was justified for the following reasons: (i) actual progression of disease precedes the radiologic documentation of advanced stage; (ii) off-label use of sorafenib was presumably elected for patients considered high-risk, many of whom may have had micrometastatic disease at the time; (iii) overly restrictive inclusion criteria would have resulted in a study with limited analytic power; and (iv) this term was analyzed as a separate factor and was confirmed via sensitivity analysis to not alter the primary finding of a LRTx survival benefit.
Another potential criticism of the current findings is that the overall survival in the sorafenib arm was worse than the sorafenib arm of either the SHARP or Asia-Pacific trials.1, 2 However, it is important to note that both of these trials included some patients with earlier stage tumors, and were limited to patients with well-preserved liver function and good performance status. The patients in the current study all had advanced HCC, and additionally had markedly worse prognostic features (e.g. worse liver function and performance status) that limited their overall survival despite treatment with sorafenib. Notably, poor ECOG scores, hepatitis C, BCLC stage C, GVI, higher AFP, and most importantly poor liver function were all more common in the patients of this study than in either the SHARP or Asia Pacific trials (Table 3). This resulted in a shorter survival period that limits direct comparison of this study to prior trials. As a result however, the benefit of LRTx demonstrated in this study may be even greater in some healthier individuals.
Table 3.
Comparison to prior clinical trials of sorafenib, demonstrating the presence of greater poor prognostic features in the current study
| Current study (sorafenib arm) N = 74 | SHARP trial (sorafenib arm) N = 299 | Asia-Pacific trial (sorafenib arm) N = 150 | ||
|---|---|---|---|---|
| Median overall esurvival (ITT, months) | 4.8 | 10.7 | 6.5 | |
| ECOG score | 0 | 33.7% | 54% | 25% |
| 1 | 36.5% | 38% | 69% | |
| 2 | 20.3% | 8% | 5% | |
| 3 | 9.5% | 0% | 0% | |
| Hepatitis C | 37.8% | 29% | 10.7% | |
| BCLC ≥ stage C | 100% | 82% | 95% | |
| Gross vascular invasion | 51.3% | 36% | 36% | |
| Extrahepatic metastases | 64.9% | 53% | 69% | |
| Child Pugh B | 58.1% | 5% | 2.7% | |
| AFP, median (ng/mL) | 332 | 44 | N/A | |
Ultimately, this study is subject to the limitations of any retrospective analysis. Despite rigorous efforts to control for differences between cohorts, it is not possible to account for all the clinical factors (and possible unknown factors) that may affect a clinician's judgment to pursue LRTx in any individual patient. Nevertheless the finding of a significant survival benefit to LRTx is provocative and deserves further investigation. Therefore, these findings should form the basis for a prospective clinical trial to assess the role of LRTx in advanced HCC patients.
In conclusion, the results of the current study suggest that liver-directed locoregional therapies should be considered in addition to the standard of care sorafenib treatment in eligible HCC patients, even in the presence of metastases or gross vascular invasion. This benefit was most notable in patients with “liver-dominant” disease, who represent the largest subgroup of patients with advanced tumor stage. These results require validation and should form the basis for a future prospective controlled trial of the benefit of supplemental LRTx in advanced HCC patients.
Financial support
None.
Conflicts of interest
None declared.
Footnotes
Presented at the International Liver Cancer Association 9th Annual Conference in Paris, France. 4–6 September 2015.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.hpb.2016.02.007.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 2.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 3.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 4.Knox J.J., Cleary S.P., Dawson L.A. Localized and systemic approaches to treating hepatocellular carcinoma. J Clin Oncol. 2015;33:1835–1844. doi: 10.1200/JCO.2014.60.1153. [DOI] [PubMed] [Google Scholar]
- 5.Lo C.M., Ngan H., Tso W.K., Liu C.L., Lam C.M., Poon R.T. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 6.Llovet J.M., Real M.I., Montana X., Planas R., Coll S., Aponte J. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 7.Sangro B., Carpanese L., Cianni R., Golfiere R., Gasparini D., Ezziddin S. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868–878. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 8.Shiina S., Tateishi R., Arano T., Uchino K., Enooku K., Nakagawa H. Radiofrequency ablation for hepatocellular carcinoma; 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shim S.J., Seong J., Han K.H., Chon C.Y., Suh C.O., Lee J.T. Local radiotherapy as a complement to incomplete transarterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005;25:1189–1196. doi: 10.1111/j.1478-3231.2005.01170.x. [DOI] [PubMed] [Google Scholar]
- 10.Cupino A.C., Hair C.D., Angle J.F., Caldwell S.H., Rich T.A., Berg C.L. Does external beam radiation therapy improve survival following transarterial chemoembolization for unresectable hepatocellular carcinoma? Gastrointest Cancer Res. 2012;5:13–17. [PMC free article] [PubMed] [Google Scholar]
- 11.Salem R., Lewandowski R.J., Mulcahy M.F., Riaz A., Ryu R.K., Ibrahim S. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Hilgard P., Hamami M., Fouly A.E., Scherag A., Müller S., Ertle J. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 13.Poon R.T., Fan S.T., Lo C.M., Liu C.L., Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeung Y.P., Lo C.M., Liu C.L., Wong B.C., Fan S.T., Wong J. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100:1995–2004. doi: 10.1111/j.1572-0241.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernán M.A., Alonso A., Logan R., Grodstein F., Michels K.B., Willett W.C. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernán M.A., Hernández-Díaz S., Robins J.M. Randomized trials analyzed as observational studies. Ann Intern Med. 2013;159:560–562. doi: 10.7326/0003-4819-159-8-201310150-00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi G.H., Shim J.H., Kim M.J., Ryu M.H., Ryoo B.Y., Kang Y.K. Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses. Radiology. 2013;269:603–611. doi: 10.1148/radiol.13130150. [DOI] [PubMed] [Google Scholar]
- 19.Chung G.E., Lee J.H., Kim H.Y., Hwang S.Y., Kim J.S., Chung J.W. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627–634. doi: 10.1148/radiol.10101058. [DOI] [PubMed] [Google Scholar]
- 20.Luo J., Guo R.P., Lai E.C., Zhang Y.J., Lau W.Y., Chen M.S. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 21.Lee H.S., Kim J.S., Choi I.J., Chung J.W., Park J.H., Kim C.Y. The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 22.Kim K.M., Kim J.H., Park I.S., Ko G.Y., Yoon H.K., Sung K.B. Reappraisal of repeated transarterial chemoembolization in the treatment of hepatocellular carcinoma with portal vein invasion. J Gastroenterol Hepatol. 2009;24:806–814. doi: 10.1111/j.1440-1746.2008.05728.x. [DOI] [PubMed] [Google Scholar]
- 23.Georgiades C.S., Hong K., D'Angelo M., Geschwind J.F. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16:1653–1659. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 24.Zhong J.H., Xiang B.D., Gong W.F., Ke Y., Mo Q.G., Ma L. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. doi: 10.1371/journal.pone.0068193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin L., Li H., Li A.J., Lau W.Y., Pan Z.Y., Lai E.C. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82–88. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Hsu C.Y., Hsia C.Y., Huang Y.H., Su C.W., Lin H.C., Pai J.T. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol. 2012;19:842–849. doi: 10.1245/s10434-011-2060-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.